The Impact of Storage Conditions on Peanut Seed Quality, Growth, and Yield
Abstract
1. Introduction
2. Results
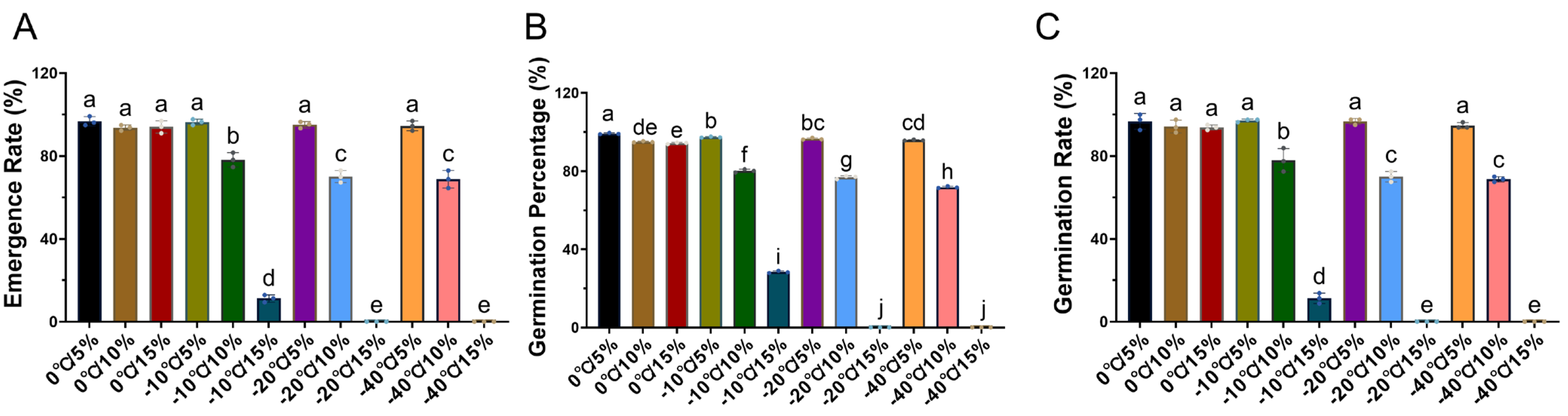
2.1. The Effects of Storage Temperature and Moisture Content on Seed Vitality and Emergence
2.2. Effects of Storage Temperature and Moisture Content on Seed Nutrients
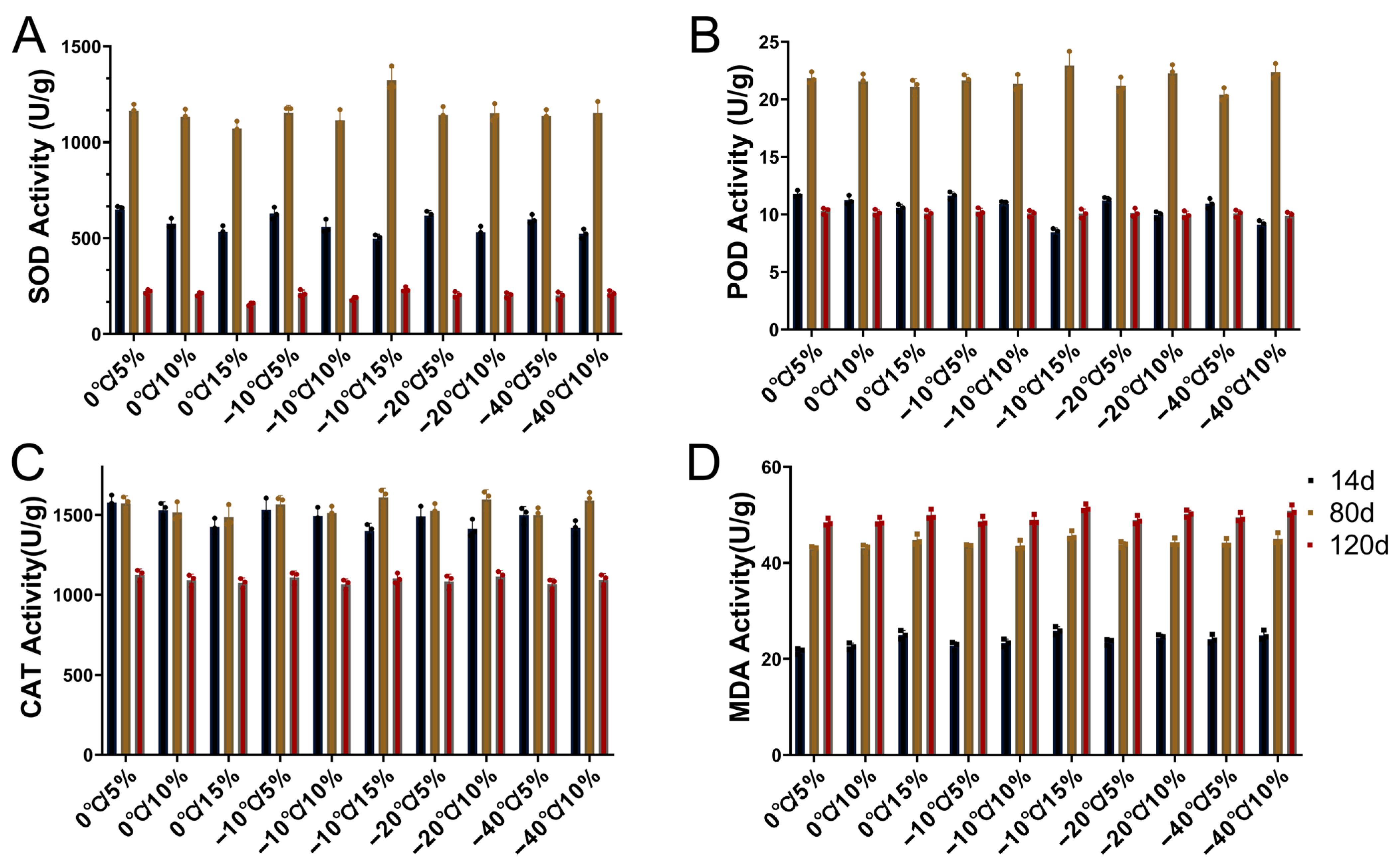
2.3. Effects of Storage Temperature and Moisture Content on Peanut Antioxidant Enzyme Activity
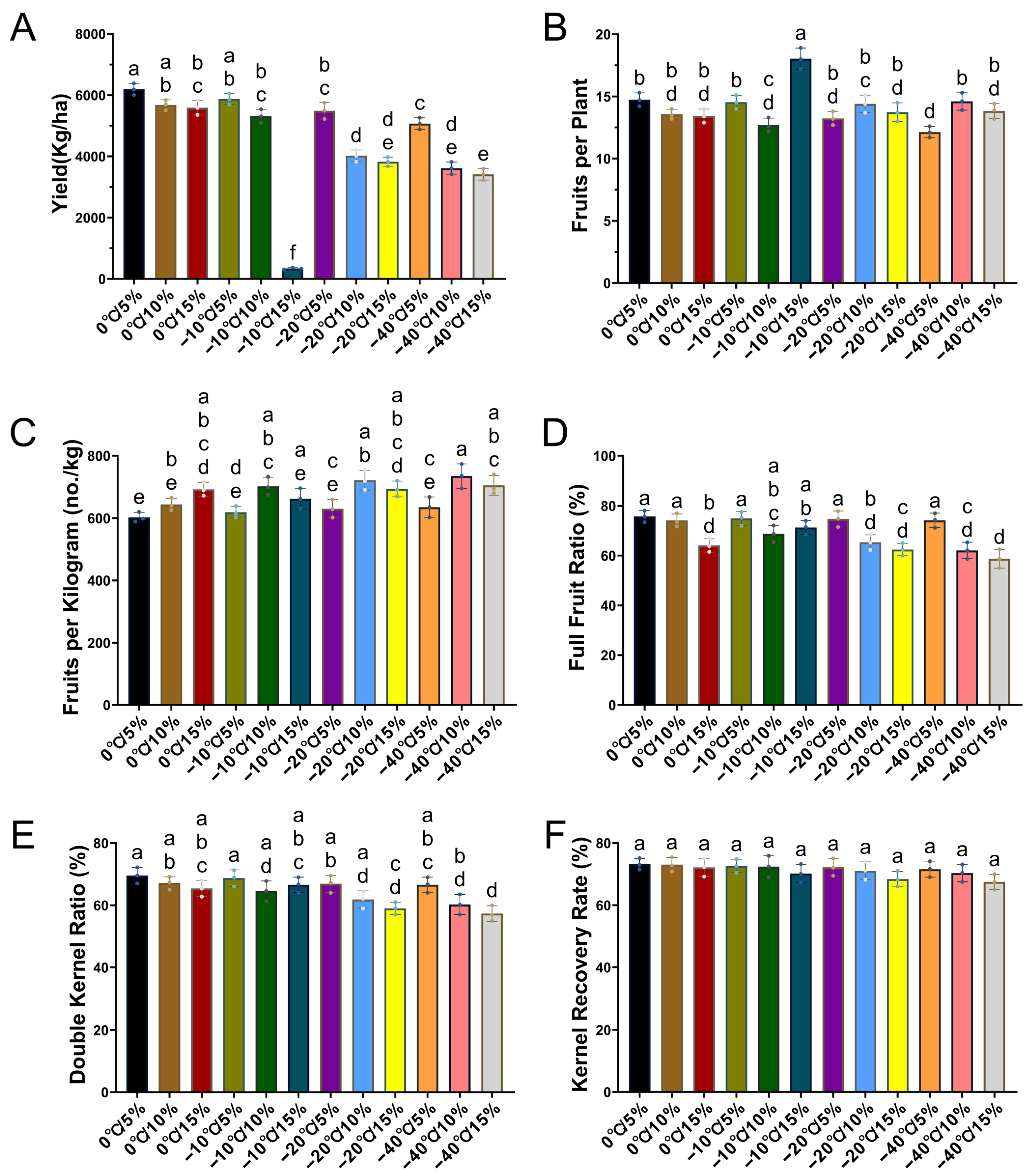
2.4. Positive Effects of Appropriate Storage Temperature and Moisture Content on the Yield of the Next Generation of Plants from Seeds
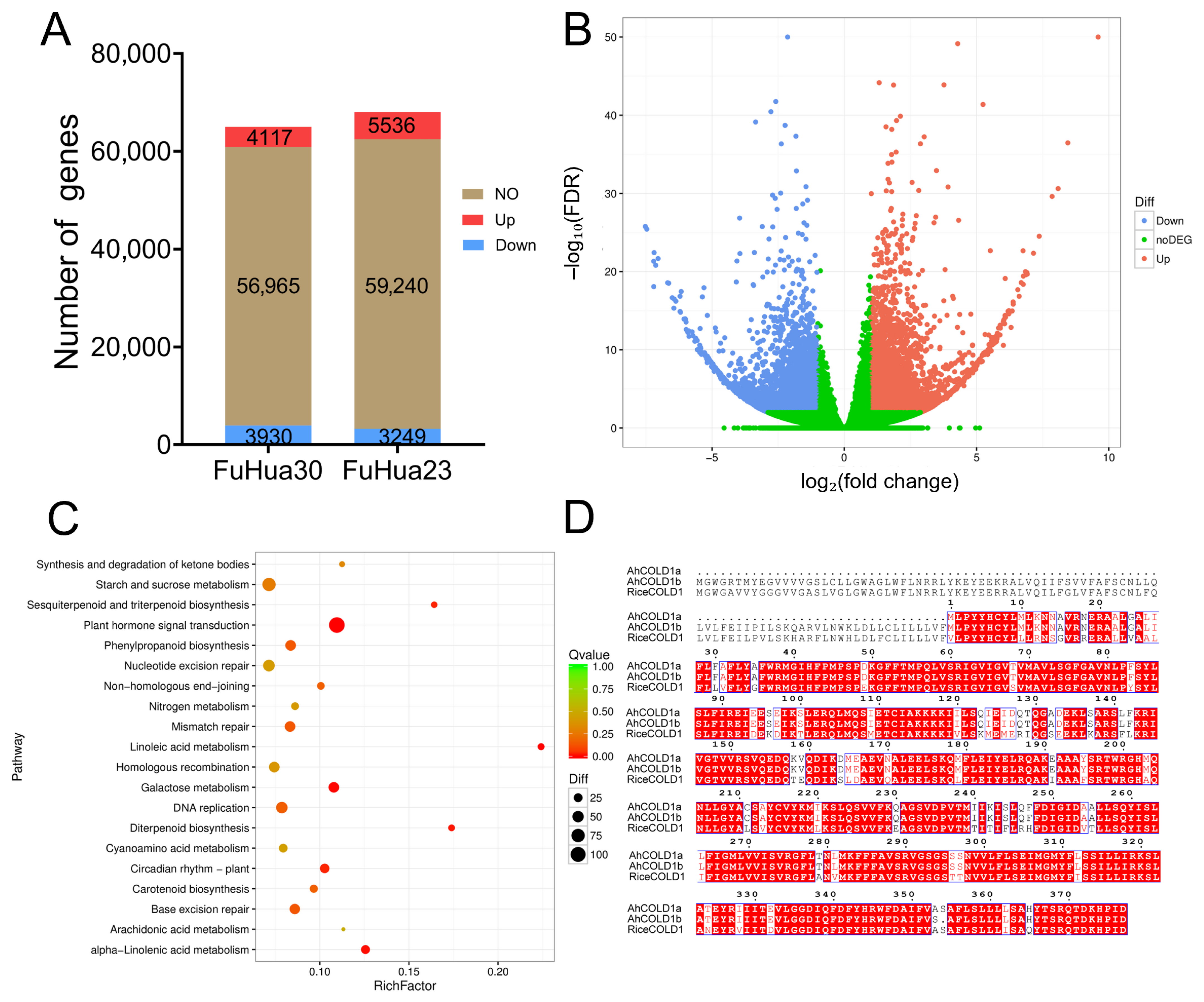
2.5. Response Mechanism of Key Genes in Seeds Based on Transcriptome Analysis of Storage Temperature and Water Content
2.6. Cloning of AhCOLD1a from Peanut and Functional Validation of Its Role in Cold Tolerance Through Overexpression in Arabidopsis thaliana
3. Discussion
3.1. Effects on Seed Vitality
3.2. Effects on Seed Nutrient Composition and Content
3.3. Effects on Antioxidant Enzyme Activity During Peanut Seedling Stage
3.4. Impact on Peanut Yield Composition
3.5. Differential Expression Genes in Response to Cold Stress and the Function of COLD1
3.6. Possible Downstream Regulatory Mechanisms and Application Value of COLD1
4. Materials and Methods
4.1. Plant Materials
4.2. Experimental Sites
4.3. Treatments and Experimental Design
4.4. Indicator Determination and Data Collection
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bertioli, D.J.; Cannon, S.B.; Froenicke, L.; Huang, G.; Farmer, A.D.; Cannon, E.K.; Liu, X.; Gao, D.; Clevenger, J.; Dash, S.; et al. The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat. Genet. 2016, 48, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, Q.; Liu, H.S.; Hong, Y.; Zhang, J. Sequencing of Cultivated Peanut, Arachis hypogaea, Yields Insights into Genome Evolution and Oil Improvement. Mol. Plant 2019, 12, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Chen, J.; Wu, W.; Luo, J.; Long, T.; Wu, Q.; Wang, Q.; Zhen, J.; Zhao, Y.; Wang, Y.; et al. Detection of peanut seed vigor based on hyperspectral imaging and chemometrics. Front. Plant Sci. 2023, 14, 1127108. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Liu, S.; Tan, J.; Huang, Z.; Li, X.; Zhuang, G.; Fang, Z.; Chen, T.; Zhang, L. Combining Hyperspectral Techniques and Genome-Wide Association Studies to Predict Peanut Seed Vigor and Explore Associated Genetic Loci. Int. J. Mol. Sci. 2024, 25, 8414. [Google Scholar] [CrossRef]
- Li, P.; Fan, J.; Song, C.; Dong, X.; Kang, D. Seed Vigour and Morphological and Physiological Characteristics of Epimedium brevicornu Maxim: In Different Stages of Seed Development. Plants 2022, 11, 2399. [Google Scholar] [CrossRef]
- Wang, P.; Li, D.; Wang, L.; Adhikari, B. Effect of High Temperature Intermittent Drying on Rice Seed Viability and Vigor. Int. J. Food Eng. 2017, 13, 20160433. [Google Scholar] [CrossRef]
- Tommasi, F.; Paciolla, C.; Pinto, M.C.D.; Gara, L.D. Effects of storage temperature on viability, germination and antioxidant metabolism in Ginkgo biloba L. seeds. Plant Physiol. Biochem. 2006, 44, 359–368. [Google Scholar] [CrossRef]
- Mbofung, G.C.Y.; Goggi, A.S.; Leandro, L.F.S.; Mullen, R.E. Effects of Storage Temperature and Relative Humidity on Viability and Vigor of Treated Soybean Seeds. Crop Sci. 2013, 53, 1086–1095. [Google Scholar] [CrossRef]
- Devi, P.S.; Botcha, S.; Arundhati, A.; Rao, T.R. Effect of storage temperature and dormancy-breaking treatments on seed germination, moisture content and seed vigor in gum karaya (Sterculia urens Roxb.). For. Sci. Technol. 2012, 8, 11–15. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, Q.; Huang, J.; Wang, Z.; Ren, R. Screening of optimal preservation methods of Elaeagnus mollis seeds and the role of apoptosis-like programmed cell death in seed viability. Plant Cell Tissue Organ. Cult. 2025, 160, 49. [Google Scholar]
- Wei, L.; Zhang, C.; Dong, Q.; Yang, Z.; Yang, X. Effects of temperature and water potential on seed germination of 13 Poa L. species in the Qinghai-Tibetan Plateau. Glob. Ecol. Conserv. 2020, 25, e01442. [Google Scholar] [CrossRef]
- Thirusendura Selvi, D.; Saraswathy, S. Seed viability, seed deterioration and seed quality improvements in stored onion seeds: A review. J. Hortic. Sci. Biotechnol. 2018, 93, 1–7. [Google Scholar] [CrossRef]
- Beardmore, T.; Wang, B.S.P.; Penner, M.; Scheer, G. Effects of seed water content and storage temperature on the germination parameters of white spruce, black spruce and lodgepole pine seed. New For. 2008, 36, 171–185. [Google Scholar] [CrossRef]
- Vertucci, C.W.; Roos, E.E. Theoretical basis of protocols for seed storage. Plant Physiol. 1990, 94, 1019–1023. [Google Scholar] [CrossRef]
- Mohsen, E.; El-Metwally, M.A.; Ibrahim, A.A.; Soliman, M.I. Impact of green antioxidants on decreasing the aflatoxins percentage in peanut oil seed (Arachis hypogaea L.) during storage. Sci. Prog. 2023, 106, 1–18. [Google Scholar] [CrossRef]
- Atungulu, G.G.; Olatunde, G.A. Assessment of new in-bin drying and storage technology for soybean seed. Dry. Technol. 2016, 36, 383–399. [Google Scholar] [CrossRef]
- Kibar, H.; Soydemir, H.E. Storage-induced changes in soybean seeds: Germination, nutritional value, and bioactive compounds. J. Stored Prod. Res. 2025, 111, 102578. [Google Scholar] [CrossRef]
- Buitink, J.; Leprince, O.; Hemminga, M.A.; Hoekstra, F.A. Molecular mobility in the cytoplasm: An approach to describe and predict lifespan of dry germplasm. Proc. Natl. Acad. Sci. USA 2000, 97, 2385–2390. [Google Scholar] [CrossRef]
- Jaganathan, G.K.; Harrison, R.J. Physical dormancy alleviation at room temperature storage is influenced by the initial moisture content of the seeds. Plant Ecol. 2024, 225, 491–497. [Google Scholar] [CrossRef]
- Guillaume, C.; Isabelle, C.; Marc, B.; Thierry, A. Assessing frost damages using dynamic models in walnut trees: Exposure rather than vulnerability controls frost risks. Plant Cell Environ. 2017, 40, 1289–1301. [Google Scholar] [CrossRef]
- Aili, R.; Deng, Y.; Yang, R.; Zhang, X.; Huang, Y.; Li, H.; Jia, S.; Yu, L.; Zhang, T. Molecular Mechanisms of Alfalfa (Medicago sativa L.) in Response to Combined Drought and Cold Stresses. Agronomy 2023, 13, 3002. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, S. COLD1: A cold sensor in rice. Sci. China Life Sci. 2015, 58, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 Confers Chilling Tolerance in Rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Zhang, S.; Zhang, J.; Di, H.; Zhang, L.; Dong, L.; Lu, Q.; Zeng, X.; Liu, X.; et al. The G protein-coupled receptor COLD1 promotes chilling tolerance in maize during germination. Int. J. Biol. Macromol. 2023, 253, 126877. [Google Scholar] [CrossRef]
- Zeng, R.; Shi, Y.; Guo, L.; Fu, D.; Li, M.; Zhang, X.; Li, Z.; Zhuang, J.; Yang, X.; Zuo, J.; et al. A natural variant of COOL1 gene enhances cold tolerance for high-latitude adaptation in maize. Cell 2025, 188, 1315–1329.e13. [Google Scholar] [CrossRef]
- Guo, X.; Chong, K. The teosinte-derived allele COOL1 is a potential target for molecular design of chilling resilience in maize. J. Integr. Plant Biol. 2025, 67, 1205–1207. [Google Scholar] [CrossRef]
- Ducatti, K.R.; Batista, T.B.; Hirai, W.Y.; Luccas, D.A.; Moreno, L.d.A.; Guimarães, C.C.; Bassel, G.W.; da Silva, E.A.A. Transcripts Expressed during Germination Sensu Stricto Are Associated with Vigor in Soybean Seeds. Plants 2022, 11, 1310. [Google Scholar] [CrossRef]
- Maleki Farahani, S.; Rezazadeh, A.; Paravar, A. Influence of seed moisture content and storage period on germination and biochemical indices: Lallemantia iberica and Lallemantia royleana. Sci. Rep. 2025, 15, 4462. [Google Scholar] [CrossRef] [PubMed]
- Tedeschini, E.; Mariani, M.; Frenguelli, G. The effects of cold stress on cypress pollen intine permeability. Aerobiologia 2019, 35, 567–570. [Google Scholar] [CrossRef]
- Kortei, N.K.; Tetteh, R.A.; Wiafe-Kwagyan, M.; Amon, D.N.K.; Odamtten, G.T. Mycobiota profile, phenology, and potential toxicogenic and pathogenic species associated with stored groundnuts (Arachis hypogaea L.) from the Volta Region, Ghana. Food Sci. Nutr. 2022, 10, 888–902. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, S. The relation between vegetable soybean seed vigor and DNA, RNA and protein synthesis. Plant Physiol. 1992, 44, 128–132. (In Chinese) [Google Scholar]
- Wang, L.; Hu, W.; Zahoor, R.; Yang, X.; Wang, Y.; Zhou, Z.; Meng, Y. Cool temperature caused by late planting affects seed vigor via altering kernel biomass and antioxidant metabolism in cotton (Gossypium hirsutum L.). Field Crops Res. 2019, 236, 145–154. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, S.H.; Song, J.H. Evaluation of the inorganic compound leakage and carbohydrates as indicator of physiological potential of Ulmus parvifolia seeds. New For. 2011, 41, 3–11. [Google Scholar] [CrossRef]
- Duan, Z.; Li, Q.; Wang, H.; He, X.; Zhang, M. Genetic regulatory networks of soybean seed size, oil and protein contents. Front. Plant Sci. 2023, 14, 11. [Google Scholar] [CrossRef]
- Qian, X.Z.; Wu, X.M.; Hu, Q.; Tian, Y.L.; Fu, H.H.; Wang, Y. Effect of storage duration on the physiological and chemical characteristics of rapeseeds. J. Oil Crops China 1993, 3, 30–32. [Google Scholar]
- Paravar, A.; Farahani, S.M.; Rezazadeh, A. How storage circumstance alters the quality of seeds of Lallemantia iberica and Lallemantia royleana produced under maternal drought stress. Environ. Exp. Bot. 2024, 217, 12. [Google Scholar] [CrossRef]
- Wang, X.; Yu, C.; Liu, Y.; Yang, L.; Li, Y.; Yao, W.; Cai, Y.; Yan, X.; Li, S.; Cai, Y.; et al. GmFAD3A, A ω-3 Fatty Acid Desaturase Gene, Enhances Cold Tolerance and Seed Germination Rate under Low Temperature in Rice. Int. J. Mol. Sci. 2019, 20, 3796. [Google Scholar] [CrossRef]
- Lv, T.; Li, J.; Zhou, L.; Zhou, T.; Pritchard, H.W.; Ren, C.; Chen, J.; Yan, J.; Pei, J. Aging-Induced Reduction in Safflower Seed Germination via Impaired Energy Metabolism and Genetic Integrity Is Partially Restored by Sucrose and DA-6 Treatment. Plants 2024, 13, 659. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J.; Zhou, L.; Pan, G.; Li, Z.; Zaidi, S.H.R.; Cheng, F. Senescence-specific change in ROS scavenging enzyme activities and regulation of various SOD isozymes to ROS levels in psf mutant rice leaves. Plant Physiol. Biochem. 2016, 109, 248–261. [Google Scholar] [CrossRef]
- Alves, R.D.C.; Oliveira, K.R.; Lucio, J.C.B.; Silva, J.D.S.; Carrega, W.C.; Queiroz, S.F.; Gratao, P.L. Exogenous foliar ascorbic acid applications enhance salt-stress tolerance in peanut plants throughout an increase in the activity of major antioxidant enzymes. S. Afr. J. Bot. 2022, 150, 759–767. [Google Scholar] [CrossRef]
- He, Y.; Ye, Z.; Ying, Q.; Ma, Y.; Zang, Y.; Wang, H.; Yu, o.; Zhu, Z. Glyoxylate cycle and reactive oxygen species metabolism are involved in the improvement of seed vigor in watermelon by exogenous GA(3). Sci. Hortic. 2019, 247, 184–194. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Mu, K.; Cai, W.; Zhao, Y.; Shen, H.; Wang, X.; Ma, H. Phenylalanine Ammonia Lyase GmPAL1.1 Promotes Seed Vigor under High-Temperature and -Humidity Stress and Enhances Seed Germination under Salt and Drought Stress in Transgenic Arabidopsis. Plants 2022, 11, 3239. [Google Scholar] [CrossRef]
- Zhang, J.; Xing, Z.; Gu, F.; Wang, Y.; Wang, T.; Chen, J. Changes in the microflora on the seed surface and seed vigor of maize (Zea mays) under different conditions. PLoS ONE 2024, 19, e0311258. [Google Scholar] [CrossRef]
- Ebone, L.A.; Caverzan, A.; Tagliari, A.; Chiomento, J.L.T.; Silveira, D.C.; Chavarria, G. Soybean Seed Vigor: Uniformity and Growth as Key Factors to Improve Yield. Agronomy 2020, 10, 545. [Google Scholar] [CrossRef]
- Gu, S.; Zhuang, J.; Zhang, Z.; Chen, W.; Xu, H.; Zhao, M.; Ma, D. Multi-omics approach reveals the contribution of OsSEH1 to rice cold tolerance. Front. Plant Sci. 2022, 13, 1110724. [Google Scholar] [CrossRef]
- Hou, Y.; Yan, W.; Deng, R.; Wang, J.; Wang, Y.; Wang, L.; Xing, J.; Jin, P.; Zhao, Y. Multi-omics analysis reveals the role of L-Glutamate in regulating cold tolerance of postharvest prune fruit. Postharvest Biol. Technol. 2025, 223, 113444. [Google Scholar] [CrossRef]
- Malviya, R.; Majumder, S.; Sharma, P.; Gayen, D. Integrated proteomics and metabolomics analysis of chickpea seeds under aging condition. J. Proteom. 2025, 320, 105487. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.M.; Hall, A.; Millar, A.J.; Darrah, C.; Davis, S.J. Protocol: Streamlined sub-protocols for floral-dip transformation and selection of transformants in Arabidopsis thaliana. Plant Methods 2009, 5, 3. [Google Scholar] [CrossRef]
| Treatment | Protein | Fat | Oleic Acid Content | Linoleic Acid Percentage |
|---|---|---|---|---|
| (%) | (%) | (%) | (%) | |
| 0 °C/5% | 25.37 ± 1.26 | 50.13 ± 2.03 | 42.95 ± 0.15 | 39.77 ± 4.06 |
| 0 °C/10% | 25.32 ± 1.07 | 49.92 ± 1.60 | 41.48 ± 1.36 | 39.5 ± 4.13 |
| 0 °C/15% | 25.1 ± 0.69 | 49.85 ± 3.62 | 38.91 ± 0.15 | 38.15 ± 1.28 |
| −10 °C/5% | 25.67 ± 2.01 | 49.91 ± 4.15 | 42.11 ± 0.26 | 39.73 ± 2.34 |
| −10 °C/10% | 25.14 ± 3.04 | 49.88 ± 0.29 | 40.33 ± 1.36 | 39.16 ± 1.29 |
| −10 °C/15% | 25.04 ± 4.26 | 48.82 ± 2.36 | 32.49 ± 2.31 | 35.7 ± 0.38 |
| −20 °C/5% | 24.69 ± 0.98 | 49.99 ± 4.12 | 41.47 ± 2.16 | 39.5 ± 1.66 |
| −20 °C/10% | 25.1 ± 2.34 | 49.93 ± 2.36 | 39.46 ± 2.67 | 39.07 ± 2.34 |
| −20 °C/15% | 24.92 ± 1.26 | 47.8 ± 5.40 | 28.17 ± 5.15 | 33.14 ± 2.06 |
| −40 °C/5% | 25.14 ± 5.10 | 50.64 ± 1.92 | 40.09 ± 2.16 | 38.92 ± 0.34 |
| −40 °C/10% | 25.47 ± 3.08 | 49.73 ± 6.01 | 37.18 ± 3.19 | 38.73 ± 3.01 |
| −40 °C/15% | 24.36 ± 6.05 | 46.52 ± 5.21 | 26.21 ± 4.16 | 32.36 ± 6.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, P.; Sun, H.; Wang, Y.; Han, N.; Ren, L.; Lv, J.; Guo, Q.; He, K.; Wang, H.; Yu, G. The Impact of Storage Conditions on Peanut Seed Quality, Growth, and Yield. Plants 2025, 14, 2944. https://doi.org/10.3390/plants14192944
Shi P, Sun H, Wang Y, Han N, Ren L, Lv J, Guo Q, He K, Wang H, Yu G. The Impact of Storage Conditions on Peanut Seed Quality, Growth, and Yield. Plants. 2025; 14(19):2944. https://doi.org/10.3390/plants14192944
Chicago/Turabian StyleShi, Puxiang, Hongxi Sun, Yibo Wang, Ning Han, Liang Ren, Jinhao Lv, Qing Guo, Kang He, Haixin Wang, and Guoqing Yu. 2025. "The Impact of Storage Conditions on Peanut Seed Quality, Growth, and Yield" Plants 14, no. 19: 2944. https://doi.org/10.3390/plants14192944
APA StyleShi, P., Sun, H., Wang, Y., Han, N., Ren, L., Lv, J., Guo, Q., He, K., Wang, H., & Yu, G. (2025). The Impact of Storage Conditions on Peanut Seed Quality, Growth, and Yield. Plants, 14(19), 2944. https://doi.org/10.3390/plants14192944







