Abstract
Members of the WRKY transcription factors (TFs) family play crucial roles in biotic and abiotic stress responses in plants, but their roles in response to drought stress in maize (Zea mays L.) have not been fully elucidated. Maize ZmWRKY82, a group IIc WRKY gene, was isolated from maize using reverse transcription polymerase chain reaction (RT-PCR). Using the UniProt online database, we found that ZmWRKY82 encodes a 222-amino protein with conserved WRKYGKK and C-X4-C-X23-H-X1-H motifs. ZmWRKY82 is strongly induced by polyethylene glycol (PEG), abscisic acid (ABA), methyl jasmonate (MeJA), salicylic acid (SA), and ethephon (ETH) treatments. The ZmWRKY82 protein was located in the cell nucleus. ZmWRKY82 had transcriptional activation capability and was able to bind to the W-box element. ZmWRKY82-overexpressing Arabidopsis and maize exhibited stronger drought resilience, which was associated with enhanced antioxidant enzyme activity and altered transcription level of drought-related genes. These findings suggest that ZmWRKY82 plays a central role in conferring drought tolerance in maize and may contribute to crop improvement and sustainable agricultural practices.
1. Introduction
Drought stress has become more frequent due to global warming and altered rainfall patterns, which severely impair reproductive development, vegetative growth, and yield of maize [1,2]. Drought can reduce corn yields by 39.3%, and severe drought can even result in total crop failure [3]. To mitigate its adverse effects, drought resilience in plants is enhanced by modulating physiological, biochemical, and molecular mechanisms. Such mechanisms include stomatal closure, reduced transpiration rate, osmotic adjustment, increased antioxidant enzyme activity, and regulation of drought-responsive gene expression and transcription factor activities [4,5,6,7]. Investigating drought-resistant genes and breeding resilient cultivars are critical for enhancing crop drought tolerance and productivity.
WRKY transcription factors (TFs) are crucial for mediating plant adaptation to environmental stresses through perceiving external signals and regulating the expression of genes encoding mitigating factors [8,9]. Defined by a highly conserved WRKY domain, the WRKY TF family is divided into three subgroups: I, II, and III. Subgroup I possesses dual WRKY domains, whereas subgroups II and III each feature a single WRKY domain [10]. Structurally, WRKY TFs possess dual conserved structural motifs: WRKYGQK/GKK sequence and zinc finger domain (C-X4–5-C-X22–23-H-X1-H or C-X7-C-X23-H-X1-C). The WRKY TF mediates plant stress adaptation and hormonal signaling via cooperating with the W-box sequence of specific target genes [10,11]. For example, Paeonia ostii PoWRKY69 is able to increase plant drought resistance in conjunction with the W-box motif of the PoFBA5 promoter sequence [12]. AtWRKY63/ABO3 regulates drought resilience in Arabidopsis by specifically binding to the W-box cis-regulatory motif of ABF2 [13]. These findings further demonstrate that WRKY TFs play a critical role in plant responses to various stresses by directly modulating the downstream target gene.
WRKY82 is an important WRKY TF that participates in diverse stress responses and in the regulation of plant growth and development. The WRKY82 gene has been identified in multiple plant species, including maize, rice (Oryza sativa), mango (Mangifera indica L.), and Gossypium raimondii [14,15,16,17]. The expression of rice OsWRKY82 is strongly induced by multiple hormones and stresses, including methyl jasmonate (MeJA), ethephon (ETH), wounding, and high-temperature stress [18]. An elevated OsWRKY82 transcription level improves rice resistance to bacterial leaf blight by regulating the transcriptional level of defense marker genes [19]. HbWRKY82-overexpressing Hevea brasiliensis exhibits enhanced drought and salt resilience through coordinately regulating the transcription of stress-responsive genes [20]. These findings suggest that WRKY82 plays a key role in regulating plant growth and development and in responding to adverse environmental stresses. However, the function of WRKY82 in maize drought resilience remains unclear.
Through long-term evolution, plants have established inherent defense system to enhance their adaptability to drought stress [1]. These mechanisms include maintaining reactive oxygen species (ROS) homeostasis, increasing abscisic acid (ABA) levels, increasing calcium ion (Ca2+) concentration and activating relevant transcription factors [21,22,23]. Among these, ROS are key signaling molecules in plant stress responses to external stress [24]. An increasing number of studies suggest that WRKY TFs play a role in regulating ROS homeostasis in plants. For example, overexpression of cotton GhWRKY68 diminishes tobacco’s drought and salt resilience by promoting the ROS accumulation [25]. Cotton GhWRKY17 negatively regulates the drought and salt resilience of cotton by increasing ROS accumulation and diminishing the transcription level of ROS scavenger genes [26]. Sweet potato IbWRKY2-overexpressing Arabidopsis exhibits greater drought and salt resilience, as well as a lower ROS content [27]. Brachypodium distachyon BdWRKY36-overexpressing tobacco exhibits stronger resilience to drought and lower ROS contents [28]. These findings suggest that WRKY TFs can increase plant drought resilience through regulating ROS accumulation.
ABA, a key signaling molecule, plays central roles in mediating plant responses to drought stresses [29]. Many studies show that WRKY TFs mediate plant drought resilience by regulating the ABA signaling pathway [30]. WRKY57-overexpressing Arabidopsis exhibits stronger drought resilience and elevated endogenous ABA level [31]. Loss of function of GhWRKY21 improves cotton drought resilience, mainly by regulating the ABA signaling pathway [32]. Elevated expression of Myrothamnus flabellifolia MfWRKY17 markedly improves Arabidopsis resilience to drought stresses via regulating ABA biosynthesis and the transcriptional level of stress-responsive genes [33]. The GsWRKY20 gene from Glycine soja increases Arabidopsis drought resilience through modulating the closure of stomata and engaging the ABA-dependent signaling pathway [34]. Therefore, WRKY TFs can regulate ABA biosynthesis and signal transduction genes to mediate plant drought resistance.
Although more than 100 unique WRKY TFs have been identified and characterized in maize [35], their drought resilience function has not been fully elucidated. In this study, we identified a WRKY gene that was significantly induced in maize under drought stress based on transcriptome data, designated as ZmWRKY82. To investigate its function in the drought stress response, we analyzed its expression pattern and subcellular localization, along with the physiological indicators and phenotypes of ZmWRKY82-overexpressing plants, after drought stress treatment. This study offers a foundation for advancing analysis of WRKY TFs in maize drought response and is critical for the development of novel drought-tolerant cultivars, ensuring food security and sustainable agriculture.
2. Results
2.1. Cloning and Characterization of ZmWRKY82
We cloned the ZmWRKY82 gene from maize B73 (a maize inbred line). The coding sequence (CDS) of ZmWRKY82 was 666 base pairs long, encoding a protein of 222 amino acids, possessing a predicted molecular mass of 22.96 kDa and a theoretical isoelectric point (pI) of 6.65. Homologous amino acid sequences in Oryza sativa L., Arabidopsis thaliana, and Sorghum bicolor were searched using the phytozome BLAST online tool (https://phytozome-next.jgi.doe.gov/blast-search, accessed on 21 September 2025). Phylogenetic analysis reveals that ZmWRKY82 is closely related to SbWRKY83 (a group IIc WRKY gene) (Figure S1a) [36]. Therefore, ZmWRKY82 is a member of WRKY TF group IIc. Multiple sequence alignment of amino acid homologs demonstrated that ZmWRKY82 contains a (C-X4-C-X23-H-X1-H) zinc finger sequence and a conserved WRKY (WRKYGKK) domain (Figure S1b). These results suggest that ZmWRKY82 is a member of the WRKY TF family and may have the ability to bind the W-box sequence.
2.2. Expression Pattern of ZmWRKY82 in Maize Under Different Stresses
Previous studies have shown that the transcription levels of WRKY TFs are increased by stress treatments with polyethylene glycol (PEG), ABA, MeJA, salicylic acid (SA), and ETH [37,38,39]. To explore the signaling pathways in which ZmWRKY82 may be involved, maize seedlings at the third-leaf stage (V3) were subjected to various hormone and stress treatments, including irrigation with PEG solution or spraying with ABA, MeJA, SA, and ETH solution. The treated seedlings were cultured in an incubator at 28 °C (16 h light/8 h dark cycle). The transcription level of ZmWRKY82 in the V3- stage maize seedling leaves was analyzed with quantitative real-time polymerase chain reaction (qRT-PCR). The transcription level of ZmWRKY82 progressively increased following treatment with 20% PEG6000, reaching its maximum at 72 h (25-fold increase) (Figure 1a). After 200 μM ABA treatment, the transcription level of ZmWRKY82 was notably increased and reached a maximum at 24 h (2.5-fold increase) (Figure 1b). After treatment with 50 μM MeJA, the transcription level of ZmWRKY82 decreased significantly between 6 h and 12 h, followed by a sharp increase, peaking at 24 h (Figure 1c). The transcription level of ZmWRKY82 was significantly upregulated at 6 h after 1 mM SA treatment, reaching a value 9 times higher than that at 0 h (Figure 1d). After 300 mg/L ETH treatment, the transcription level of ZmWRKY82 was down regulated at 6 h and 12 h, followed by an upward trend, and reached its peak at 48 h (Figure 1e). The results show that the ZmWRKY82 gene is induced by PEG, ABA, MeJA, SA, and ETH, with the highest transcription level observed after treatment with 20% PEG6000. Thus, we hypothesize that ZmWRKY82 participates in multiple signaling pathways and contributes to the plant’s response to drought stress.
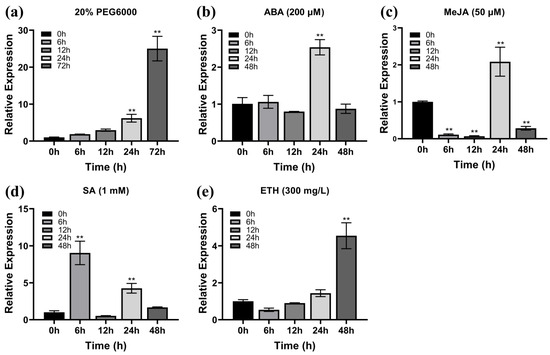
Figure 1.
The relative expression level of ZmWRKY82 under various treatments: (a) 20% PEG6000 treatment, (b) 200 μM ABA treatment, (c) 50 μM MeJA treatment, (d) 1 mM SA treatment and (e) 300 mg/L ETH treatment. ZmTub (GRMZM2G066191) was used as an endogenous reference for data standardization. The relative expression levels were analyzed using the 2−ΔΔCT method. 0 h is set as the control. The significance analysis compared with 0 h was performed using one-way ANOVA (** p < 0.01). Bars indicate standard error of the mean. The experiment was performed using three biological replicates from three independent plants.
2.3. Subcellular Localization of ZmWRKY82
WRKY TF, which acts as a transcriptional activation factor, is commonly localized to the nucleus [40]. Following the method of Zang et al. (2021) [41], we removed the stop codon of ZmWRKY82 CDS and ligated ZmWRKY82 CDS into pCAMBIA1302 to construct the pCAMBIA1302-ZmWRKY82-GFP (35S:: ZmWRKY82-GFP) recombinant vector (Figure 2a). The 35S:: ZmWRKY82-GFP recombinant vector was introduced into tobacco leaves via Agrobacterium-mediated transformation. As shown in Figure 2b, the fluorescence signal of pCAMBIA1302-GFP (35S:: GFP) is detected in both the nucleus and cell membrane. However, 35S:: ZmWRKY82-GFP was detected exclusively in the nucleus, which is consistent with nuclear-localized protein ZmERF061 [41]. This result indicates that ZmWRKY82 may exert transcriptional activation function in the nucleus.
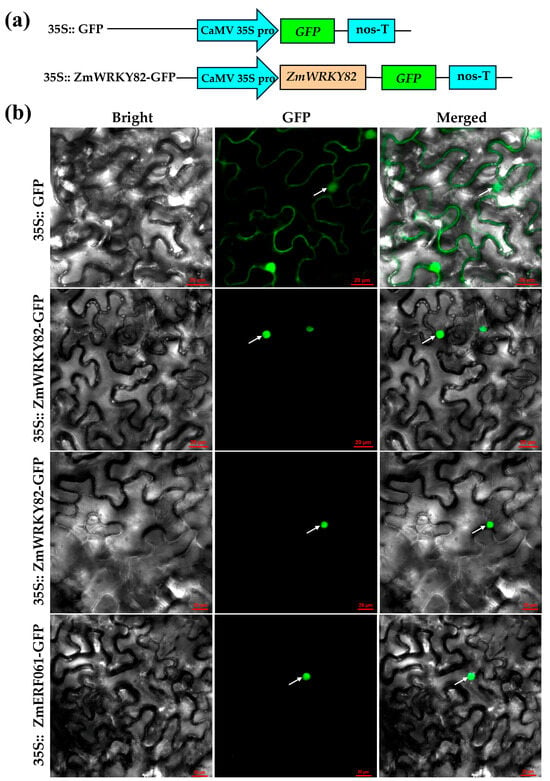
Figure 2.
Subcellular location of ZmWRKY82-GFP in the epidermal cells of Nicotiana benthamiana leaves. (a) Schematic diagram of pCAMBIA1302-GFP (35S:: GFP) and the pCAMBIA1302-ZmWRKY82-GFP (35S:: ZmWRKY82-GFP). (b) ZmWRKY82-GFP, pCAMBIA1302-GFP (35S:: GFP) and ZmERF061-GFP were transiently expressed in the epidermal cells of tobacco leaves, respectively. The white arrow indicated the nucleus of tobacco epidermal cells. Scale bars = 20 μm.
2.4. ZmWRKY82 Has Transcriptional Activation Potential and Specifically Binds to W-Box Elements
To further study the transcriptional activation potential of ZmWRKY82, the recombinant construct of the pGBKT7-ZmWRKY82 was created. pGBKT7-ZmWRKY82 recombinant construct plasmid was transformed into Y2HGold yeast cells. After 3 days (d) of cultivation, all the yeast cells grew well on SD/-Trp medium (synthetic dextrose minimal medium lacking tryptophan) (Figure 3a). In contrast, robust growth and blue coloration on SD/-Trp/-His/-Ade/X-α-Gal medium (synthetic dextrose minimal medium lacking tryptophan, histidine, and adenine, supplemented with α-galactosidase) were exclusively observed in yeast cells carrying pGBKT7-ZmWRKY82 or the pGBKT7-53+pGADT7-T (positive control) plasmids (Figure 3a). These findings suggest that ZmWRKY82 possesses transcriptional activation activity in the yeast expression system.
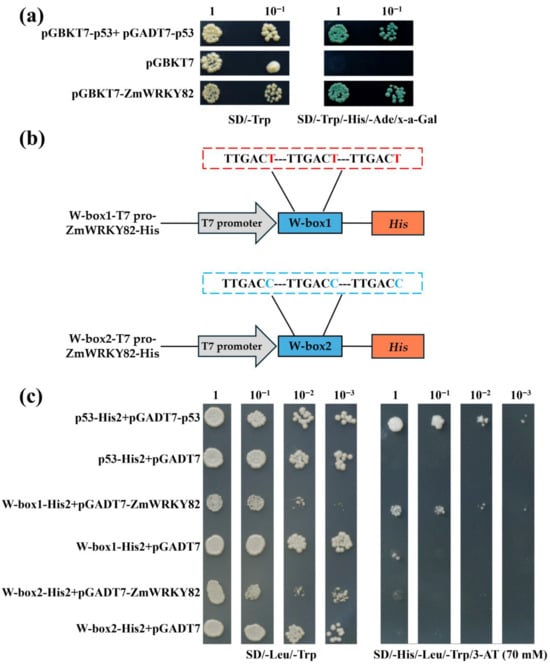
Figure 3.
Transcription activity and DNA-binding activity analysis of ZmWRKY82. (a) Transcriptional activation analysis of ZmWRKY82. (b) Sequence of the triple tandem repeats of the W-box1 and W-box2. Schematic diagram of W-box1 (TTGACT)-T7 pro-ZmWRKY82-His and W-box2 (TTGACC)-T7 pro-ZmWRKY82-His constructs. (c) The DNA-binding activity of ZmWRKY82 was assessed via yeast one-hybrid assay using 3× W-box1 or 3× W-box2 as bait. Positive control p53-His2+pGADT7-p53, negative control p53-His2+pGADT7, W-box1-His2+pGADT7-ZmWRKY82, W-box1-His2+pGADT7, W-box2-His2-+pGADT7-ZmWRKY82, W-box2-His2+pGADT7 were grown on SD/-Leu/-Trp medium or SD/-His/-Leu/-Trp with 3-AT (70 mM) medium.
Subsequently, we created the pGADT7-ZmWRKY82 recombinant construct to verify whether ZmWRKY82 could bind to the W-box sequence. The recombinant reporter plasmid pHis2 was constructed to contain three tandem repeats of the W-box1 (TTGACT) and W-box2 (TTGACC) sequences (Figure 3b). The recombinant reporter plasmids, along with either the pGADT7-ZmWRKY82 or pGADT7, were introduced into the Y187 yeast cells. Following a 3 d incubation, all yeast transformants exhibited normal growth on SD/-Leu/-Trp medium (synthetic dextrose minimal medium lacking leucine and tryptophan) (Figure 3c). Y187 [p53-His2+pGADT7-p53] (positive control), Y187 [W-box1-His2+pGADT7-ZmWRKY82], and Y187 [W-box2-His2+pGADT7-ZmWRKY82] yeast cells were able to grow on SD/-His/-Leu/-Trp/3-AT (70 mM) medium (a synthetic dextrose minimal medium lacking histidine, leucine, and tryptophan, supplemented with 70 mM 3-AT) (Figure 3c). However, the yeast cells of Y187 [p53-His2+pGADT7] (negative control), Y187 [W-box1-His2+pGADT7], and Y187 [W-box2-His2+pGADT7] did not grow or grew poorly on SD/-His/-Leu/-Trp/3-AT (70 mM) medium (Figure 3c). These findings suggest that ZmWRKY82 specifically interacts with the W-box (TTGACT/C) motif.
2.5. Overexpression of ZmWRKY82 Increases Arabidopsis Resilience to Drought Stress
Mannitol-induced osmotic stress is commonly used to assess Arabidopsis thaliana’s drought tolerance [42]. To investigate the drought resilience of ZmWRKY82 in depth, we subjected wild-type (WT) Arabidopsis (Columbia) and T3-generation-overexpressing Arabidopsis strains (OE-1 and OE-2) to mannitol-induced osmotic stress (Figure S2a,b). No notable variation in root length was observed among the WT, OE-1, and OE-2 strains when grown on medium without mannitol for 14 d (Figure 4a,b). The root lengths of the OE-1 and OE-2 strains were notably higher than in the WT plants when grown on medium containing 100 mM or 300 mM mannitol for 14 d (Figure 4a,b). From these results, we hypothesized that ZmWRKY82 enhances the resilience of Arabidopsis to mannitol-induced osmotic stress.
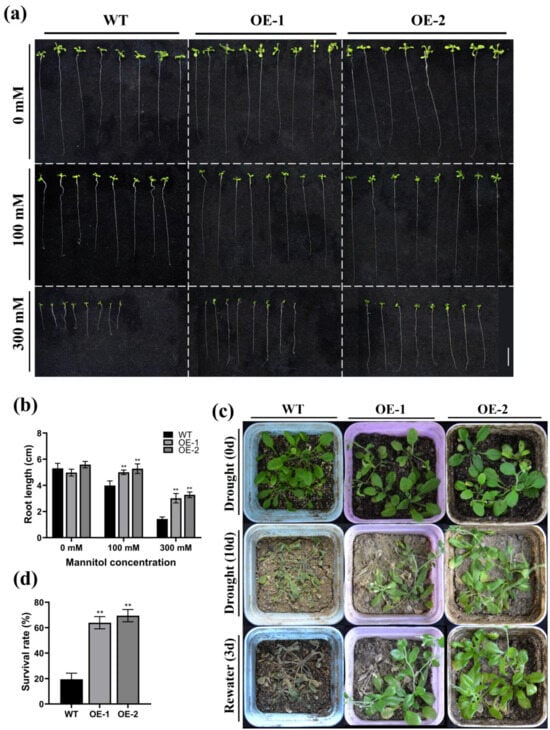
Figure 4.
Overexpression of ZmWRKY82 enhances the drought tolerance of Arabidopsis. (a) Root lengths of ZmWRKY82-overexpressing lines (OE-1 and OE-2) and wild-type (WT) Arabidopsis (Columbia) seedlings were measured on control conditions (1/2 MS medium) and on 1/2 MS medium supplemented with 100 mM or 300 mM mannitol. Scale bars = 1 cm. (b) Root lengths of ZmWRKY82-overexpressing lines and WT were analyzed under 1/2 MS medium, 100 mM or 300 mM mannitol treatment. (c) The 21-day-old plants were subjected to water withholding treatment for 10 d, and then re-watering for 3 d. (d) The survival rate was analyzed after re-watering for 3 d. Each biological replicate in the drought stress experiment comprised 12 plants. The survival rate for each biological replicate was calculated as the mean value of 12 plants. The graphed data were the average (±SD) of three independent biological replicates. The significance analysis compared with WT was performed using one- and two-way ANOVAs (** p < 0.01). The photos show representative plants.
The 21-day-old WT, OE-1, and OE-2 strains were subjected to 10 d of water withholding treatment. After this treatment and 3 d of re-watering, WT plants exhibited more severe wilting compared with the OE-1 and OE-2 plants (Figure 4c). Analysis showed that 63.89% of the OE-1 strain, 69.44% of the OE-2 strain, and 19.44% of the WT strain survived (Figure 4d). The results demonstrate that ZmWRKY82 can increase Arabidopsis drought resilience.
2.6. Overexpression of ZmWRKY82 Increases Arabidopsis Drought Resilience by Affecting Antioxidant Enzyme Activity
We also assessed the relevant physiological parameters in the leaf tissue of the WT, OE-1, and OE-2 Arabidopsis strains at 0 d and after 10 d of water withholding treatment. No notable differences were observed in superoxide dismutase (SOD) and peroxidase (POD) activity, and malondialdehyde (MDA) content among the WT, OE-1, and OE-2 plants before water withholding treatment (Figure 5a–c). After 10 d of this treatment, the OE-1 and OE-2 transgenic strains showed increased SOD and POD activity and reduced MDA level compared to the WT strains (Figure 5a–c). These findings indicate that ZmWRKY82 may increase Arabidopsis drought resilience by increasing SOD and POD activity and decreasing MDA.
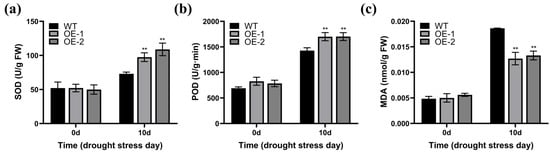
Figure 5.
Determination of physiological indices in the leaf tissue of wild-type (WT) Arabidopsis (Columbia) and ZmWRKY82-overexpressing Arabidopsis at 0 d and after 10 d of water withholding treatment. (a) SOD activity, (b) POD activity and (c) MDA content in ZmWRKY82-overexpressing Arabidopsis and WT plants before and after 10 d of water withholding treatment. Enzyme activities were expressed as enzyme units per gram fresh weight (U/g FW). The experiments were performed with three biological replicates. The data were the average (±SD) of three independent experiments. The significance analysis compared with WT was performed using two-way ANOVA (** p < 0.01).
2.7. Overexpression of ZmWRKY82 Increases Arabidopsis Drought Resilience by Affecting Defense Gene Expression
We further measured the transcription level of the drought-induced genes DREB2A, DREB2B, and RD29A [43,44] in the leaf tissue of the WT and ZmWRKY82-overexpressing Arabidopsis strains at 0 d and after 10 d of water withholding treatment. Prior to water withholding treatment, no notable discrepancies were observed in the transcription levels of DREB2A and DREB2B among the OE-1, OE-2, and WT strains (Figure 6a,b). The transcription level of RD29A was notably up-regulated in the OE-1 and OE-2 strains before treatment (Figure 6c). The transcription level of the drought-induced genes was notably elevated in the OE-1 and OE-2 strains compared to the WT after 10 d of water withholding treatment (Figure 6a–c). These results suggest that ZmWRKY82 may modulate the transcription level of these genes, thereby contributing to the drought stress response in Arabidopsis.
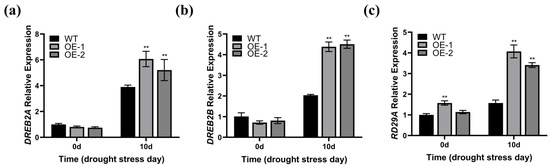
Figure 6.
Determination of transcription level of drought-stress responsive genes in the leaf tissue of wild-type (WT) Arabidopsis (Columbia) and ZmWRKY82-overexpressing Arabidopsis at 0 d and after 10 d of water withholding treatment. Expression analysis of (a) DREB2A (AT5G05410), (b) DREB2B (AT3G11020) and (c) RD29A (AT5G52310) drought-stress responsive genes in ZmWRKY82-overexpressing Arabidopsis and WT lines at 0 d and after 10 d of water withholding treatment. ACTIN2 (At3g18780) was used as an endogenous reference for data standardization. The experiments were performed with three biological replicates. The data were the average (±SD) of three independent experiments. The significance analysis compared with WT was performed using two-way ANOVA (** p < 0.01).
2.8. Overexpression of ZmWRKY82 Enhances Drought Resilience in Maize
To further research the drought resilience performance of ZmWRKY82 in maize, we subjected the maize inbred line B104 and ZmWRKY82-overexpressing maize strains (ZmWRKY82-OE1 and ZmWRKY82-OE2) to drought stress treatment. All maize strains exhibited similar growth before water withholding treatment (Figure 7a). After 7 d and after 14 d of water withholding treatment, WT (B104) exhibited more severe wilting compared with the ZmWRKY82-overexpressing maize strains. After 3 d of re-watering, the ZmWRKY82-overexpressing maize strains exhibited better growth compared with the WT (B104). The survival rates of ZmWRKY82-OE1 and ZmWRKY82-OE2 were 72.22% and 77.78%, respectively, whereas WT (B104) showed a survival rate of only 38.89% (Figure 7b). These findings indicate that ZmWRKY82 enhances drought resilience in maize plants.
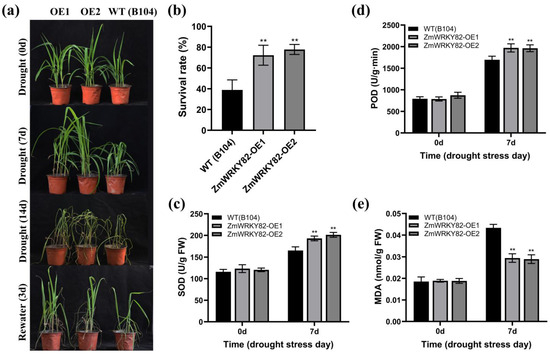
Figure 7.
Overexpression of ZmWRKY82 enhanced the drought tolerance of maize. (a) The phenotype in ZmWRKY82-overexpressing maize lines and WT (B104) lines at 0 d, after 14 d of water withholding treatment, and after 3 d of re-watering. (b) The survival rate was analyzed by re-watering for 3 d. (c) SOD activity, (d) POD activity and (e) MDA content in the leaf tissue of ZmWRKY82-overexpressing maize lines and WT (B104) lines were assessed at 0 d and after 7 d of water withholding treatment. Enzyme activities were expressed as enzyme units per gram fresh weight (U/g FW). The experiments were performed with three biological replicates. The data were the average (±SD) of three independent experiments. The significance analysis compared with was performed using one- and two-way ANOVAs (** p < 0.01). Each biological replicate in the drought stress experiment comprised 12 plants.
We assessed the physiological parameters in the leaf tissue of the WT (B104) and ZmWRKY82-overexpressing maize strains at 0 d and after 7 d of water withholding treatment. No notable discrepancies were observed in SOD and POD activity and MDA content among all maize strains before water withholding treatment (Figure 7c–e). The SOD and POD activity of the ZmWRKY82-OE1 and ZmWRKY82-OE2 strains was notably higher compared to the WT (B104) strains after 7 d of water withholding treatment (Figure 7c,d). The MDA levels of the ZmWRKY82-OE1 and ZmWRKY82-OE2 strains were notably lower than those of the WT (B104) strains after 7 d of treatment (Figure 7e). These findings demonstrate that ZmWRKY82 positively regulates drought resilience in maize by increasing SOD and POD activity and decreasing the MDA level.
2.9. ZmWRKY82 Affects the Transcriptional Level of Many Drought-Related Genes
The transcription level of drought-related genes ZmDREB2A, ZmNCED, ZmERD1, and ZmRD20 [45] was assessed in the leaf tissue of WT (B104) and ZmWRKY82-overexpressing maize strains at 0 d and after 7 d of water withholding treatment. No notable differences were observed in the transcription levels of ZmDREB2A, ZmNCED, and ZmRD20 among the ZmWRKY82-OE1, ZmWRKY82-OE2, and WT (B104) strains before water withholding treatment (Figure 8a,b,d). The transcription level of ZmERD1 was notably higher in ZmWRKY82-overexpressing maize strains compared to WT (B104) at 0 d of water withholding treatment (Figure 8c). The transcription level of ZmDREB2A, ZmNCED, ZmERD1, and ZmRD20 in the ZmWRKY82-overexpressing maize strains is substantially higher than in the WT (B104) strains after 7 d of water withholding treatment (Figure 8a–d). These findings suggest that ZmWRKY82 improves drought resilience in maize via regulating the transcription level of drought response genes.
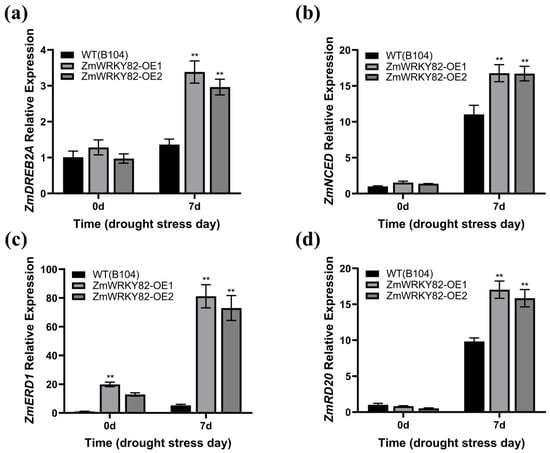
Figure 8.
The relative expression level of the drought-stress-responsive genes were analyzed in the leaf tissue of WT (B104) lines and ZmWRKY82-overexpressing maize lines at 0 d and after 7 d of water withholding treatment, including: (a) ZmDREB2A (GRMZM2G006745). (b) ZmNCED (GRMZM5G838285). (c) ZmERD1 (GRMZM2G172230). (d) ZmRD20 (GRMZM2G342685). ZmTub (GRMZM2G066191) was used as an endogenous reference for data standardization. The experiments were performed with three biological replicates. The data were the average (±SD) of three independent experiments. The significance analysis compared with WT was performed using two-way ANOVA (** p < 0.01).
2.10. Overexpression of ZmWRKY82 Increases Sensitivity to ABA in Maize
To further confirm the response of ZmWRKY82 to ABA treatment, WT (B104) and ZmWRKY82-overexpressing maize seeds were treated with exogenous ABA. Root length was inhibited to varying degrees in all seeds after 4 d of treatment, but the ZmWRKY82-overexpressing maize seeds exhibited notably shorter roots compared to the WT (B104) (Figure 9a,b). These findings indicate that the overexpression of ZmWRKY82 increases maize sensitivity to ABA.
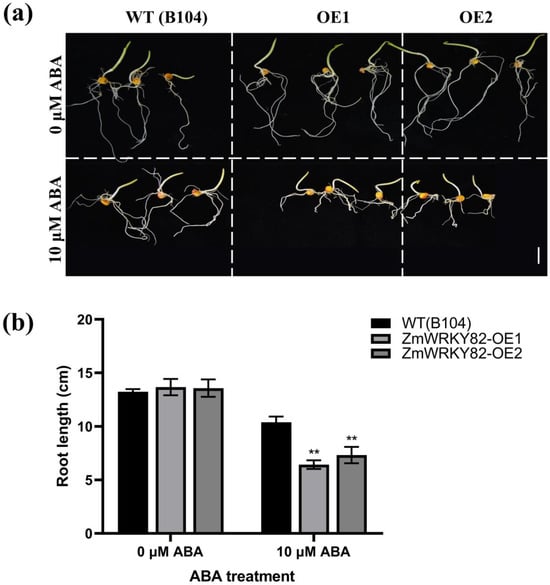
Figure 9.
Root lengths of ZmWRKY82-overexpressing maize lines were significantly longer under 10 μM ABA treatment with WT (B104). (a) Phenotype of root length in ZmWRKY82-overexpressing maize lines (ZmWRKY82-OE1 and ZmWRKY82-OE2) and WT (B104) under 0 μM or 10 μM ABA treatments. Scale bars = 2.5 cm. (b) Root lengths of ZmWRKY82-overexpressing maize lines under 0 μM or 10 μM ABA treatment. The experiments were performed with three biological replicates. The data were the average (±SD) of three independent experiments. The significance analysis compared with WT was performed using two-way ANOVA (** p < 0.01).
3. Discussion
Drought is a notable environmental stressor for plants, and severe drought stress causes irreversible damage to plants [46,47]. The literature suggests that WRKY TFs are crucial in regulating plant responses to drought stress [48], but the function in maize is not yet fully understood. In this study, we found that the TF ZmWRKY82 plays a valuable role in enhancing drought resilience in maize and Arabidopsis.
Much research has shown that WRKY TFs play a key role in mediating plant responses to drought stress [49]. In this study, the transcription level of ZmWRKY82 was significantly increased in response to 20% PEG 6000 treatment (Figure 1a), and overexpression of ZmWRKY82 positively regulated drought resilience of Arabidopsis and maize plants (Figure 4 and Figure 7). These findings demonstrate that ZmWRKY82 increases drought resilience of Arabidopsis and maize. Most WRKY TF members possess transcriptional activation potential and bind to the W-box sequence [49]. In this study, we found that ZmWRKY82 exhibits transcriptional activation activity (Figure 3a) and can bind the W-box (TTGACT/C) sequence (Figure 3c). Therefore, we hypothesize that ZmWRKY82 may enhance plant drought resistance by binding to W-box elements in the promoters of drought-responsive genes and activating their expression.
ROS are important messengers in the plant defense system, and numerous studies have demonstrated that WRKY TFs are closely associated with ROS accumulation in response to drought stress in plants [50]. Overexpression of yellowhorn XsWRKY20 positively regulated drought resilience of tobacco by maintaining ROS homeostasis [51]. ZmWRKY79 improved drought resilience in Arabidopsis via increasing antioxidant enzyme activity, decreasing MDA content and promoting ROS scavenging [42]. In our study, the SOD and POD activity was notably higher in ZmWRKY82-overexpressing Arabidopsis and maize strains than in the WT strains following water withholding treatment (Figure 5a,b and Figure 7c,d). The MDA content was notably lower in ZmWRKY82-overexpressing Arabidopsis and maize strains compared to the WT strains (Figure 5c and Figure 7e). Our results suggest that elevated ZmWRKY82 expression may enhance the ROS scavenging capacity of plants and mitigate drought-induced cellular damage.
The ABA signaling pathway in plants plays a key regulatory role in defense signaling pathways [52]. Previous studies have reported that TaWRKY1 boosts drought resilience in Arabidopsis through participating in the ABA-dependent pathway [53]. Elevated expression of TaWRKY31 positively regulates the drought resilience of wheat by adjusting the transcription level of ABA-responsive genes [54]. Overexpression of OsWRKY45 positively regulates the drought resilience of Arabidopsis via modulating the transcription level of ABA response genes [55]. Our findings suggest that ABA treatment notably up-regulated the transcription level of ZmWRKY82 (Figure 1b), and ZmWRKY82-overexpressing maize plants displayed enhanced sensitivity to exogenous ABA (Figure 9). Our results are consistent with other reports, suggesting that ZmWRKY82 may play a key role in regulating maize drought resilience via the ABA signaling pathway.
4. Materials and Methods
4.1. Plant Material, Growing Conditions, and Treatments
Both the maize seeds (Zea mays inbred lines B73 and B104) and Arabidopsis WT seeds were provided by the Maize Breeding Innovation Team at Jilin Agricultural University. The maize seeds were germinated in an incubator at 28 °C. The maize seedlings were then transplanted into a 1:1 (v/v) mixture of sterile nutrient soil and vermiculite and subsequently cultivated under a 16 h light/8 h dark photoperiod at 28 °C until reaching the V3-stage. Soil moisture was maintained at 80% of field capacity. For PEG treatment, the V3-stage maize plants were placed in containers and irrigated once with 100 mL of a 20% (w/v) PEG6000 solution until the soil was saturated, with water treatment serving as the control. For phytohormone treatments, water treatment (control), 200 μM ABA, 50 μM MeJA, 1 mM SA, and 300 mg/L ETH were sprayed on the leaves of V3-stage maize plants. Treated leaves (similar growth patterns) from three independent plants were collected at 0 h, 6 h, 12 h, 24 h, 48 h, or 72 h and stored at −80 °C.
4.2. RNA Extraction and qRT-PCR Analysis
Total RNA was extracted from maize leaf tissue using the phenol/chloroform-based TRIzol reagent (Tiangen, Beijing, China). Total cDNA was obtained from the RNA using a ReverTra Ace® qPCR RT kit (TOYOBO, Shanghai, China). The maize gene ZmTub (GRMZM2G066191) and the Arabidopsis gene ACTIN2 (At3g18780) were used as internal controls. Three independent biological replicates were conducted for the experiment. Relative transcript abundance was determined using the 2−∆∆CT method [56]. The sequences of all primers are provided in Table S1.
4.3. Subcellular Localization
The ZmWRKY82-GFP recombinant construct was generated by inserting the ZmWRKY82 CDS into the Nco I and Spe I restriction enzyme sites of pCAMBIA1302 using the Seamless Cloning Kit (In-Fusion cloning) (Beyotime, Shanghai, China). The sequences of (ZmWRKY82-1302) primer are provided in Table S1. Agrobacterium competent cells GV3101 (pSoup-p19) (Coolaber, Beijing, China) were transformed with the ZmWRKY82-GFP, pCAMBIA1302, and ZmERF061 plasmids according to the manufacturer’s instructions. Agrobacterium carrying ZmWRKY82-GFP, pCAMBIA1302, or ZmERF061 was separately introduced into 28-day-old tobacco leaves via an Agrobacterium-mediated transformation approach [57]. Inoculated tobacco leaves were incubated in the dark at 22 °C for 24 h. The green fluorescent signal in tobacco leaves was visualized using confocal laser scanning microscopy (CLSM) (ZEISS, Shanghai, China) at 488 nm.
4.4. Transcription Activity Assay of ZmWRKY82
The pGBKT7-ZmWRKY82 recombinant vector was constructed by inserting the full-length ZmWRKY82 CDS into the Nco I and EcoR I restriction sites of pGBKT7 using the In-Fusion cloning method. The sequences of (ZmWRKY82-pGBKT7) primer are provided in Table S1. Three transformation mixtures, including pGBKT7-p53+pGADT7-p53, pGBKT7, and pGBKT7-zmWRKY82, were transformed into the Y2HGold yeast strain (Coolaber, Beijing, China). Yeast transformants were cultured on SD/-Trp medium and SD/-Trp/-His/-Ade medium supplemented with X-α-Gal and incubated at 28 °C for 3 d.
4.5. Yeast One-Hybrid Assay
To generate the pGADT7-ZmWRKY82 recombinant construct, the full-length ZmWRKY82 CDS was inserted into the EcoR I and BamH I restriction sites of pGADT7 vector using the In-Fusion cloning method. The sequences of (ZmWRKY82-pGADT7) primers are provided in Table S1. The sequences of W-box1 (TTG ACT) and W-box2 (TTG ACC) were ligated into pHis2 vectors by repeating the tandem three times. According to the instructions provided with the Y187-pHis2 Yeast One-Hybrid Interaction Verification Kit (Coolaber, Beijing, China), the four plasmid combinations W-box1-His2+pGADT7-ZmWRKY82, W-box1-His2+pGADT7, W-box2-His2+pGADT7-ZmWRKY82, and W-box2-His2+pGADT7 were cotransformed into Y187 competent cells, respectively. The yeast cells were inoculated into SD/-Leu/-Trp yeast medium for 3 d (30 °C). Then, the yeast colonies were subjected to serial dilutions (1:1, 1:10, 1:100, and 1:1000) and inoculated into SD/-Leu/-Trp and SD/-His/-Leu/-Trp/3-AT (70 mM) medium, followed by incubation for 3 d (30 °C).
4.6. Creation of Transgenic Arabidopsis and Maize
pCAMBIA3301-35S-ZmWRKY82 expression construct was generated by inserting the full-length ZmWRKY82 CDS into the Pml I and Nco I restriction sites of pCAMBIA3301 using the In-Fusion cloning method. The sequences of (ZmWRKY82-35S) primers are provided in Table S1. Positive transgenic plants were generated using flower inflorescence immersion method [58].
The full-length CDS of ZmWRKY82 was ligated into the pCAMBIA3301-UBI vector using T4 DNA ligase (TaKaRa, China) to generate the pCAMBIA3301-UBI-ZmWRKY82 expression construct. The pCAMBIA3301-UBI-ZmWRKY82 expression vector was transformed into Agrobacterium tumefaciens strain EHA105 (Coolaber, Beijing, China). The Agrobacterium strain carrying pCAMBIA3301-UBI-ZmWRKY82 was introduced into maize inbred line B104 via the Agrobacterium-mediated transformation method [59]. The positive transgenic strains were confirmed using glufosinate screening, Bar diagnostic strips, and qRT-PCR analysis. The sequences of the (ZmWRKY82-UBI and ZmWRKY82-Q) primers are provided in Table S1.
4.7. Drought and ABA Tolerance Assay
For the mannitol osmotic stress assay [42], ZmWRKY82-overexpressing Arabidopsis and WT Arabidopsis seeds were germinated on 1/2 MS medium supplemented with mannitol (0 mM, 100 mM, and 300 mM) for 14 d (16 h light/8 h dark cycle, 22 °C), and their root length was observed.
For the drought stress assay [60], WT Arabidopsis and T3 generation ZmWRKY82-overexpressing Arabidopsis strains were cultured on 1/2 MS medium for 7 d. Planter boxes were filled to three-quarters of their capacity with a 1:1 (v/v) mixture of sterile nutrient soil and vermiculite. Subsequently, the seedlings were transplanted into planter boxes and grown for 14 d (16 h light/8 h dark cycle, 22 °C). The planter boxes were placed in a propagation tray and watered every 3 d to maintain soil moisture at 80% of field capacity. Subsequently, the plants were subjected to water withholding treatment for 10 d until the soil moisture reached 30% of field capacity. The plants were then subjected to a 3 d re-watering treatment, maintaining soil moisture at 80% of field capacity. The V3-stage of WT (B104) and T2 ZmWRKY82-overexpressing maize strains were placed in propagation trays and watered until the soil moisture reached 80% of field capacity. Subsequently, the maize plants were subjected to 14 d of water withholding treatment until the soil moisture reached 35% of field capacity, and then subjected to 3 d re-watering treatment, maintaining soil moisture at 80% of field capacity. Soil moisture content was measured with a soil moisture meter.
For the ABA stress assay, maize seeds were germinated in an incubator at 28 °C for 2 d, and then treated with 10 μM ABA for 4 d. The root length was photographed using a Nikon D7000 camera (Nikon, Tokyo, Japan). All images were taken with a Nikon D7000 camera.
4.8. Detection of Physiological Indices
Following the method of Wu et al. (2025) [61], the SOD and POD activity and MDA content in the WT Arabidopsis and ZmWRKY82-overexpressing Arabidopsis were evaluated at 0 d and after 10 d of the water withholding treatment. Similarly, the activities of these enzymes were measured at 0 and after 7 d of water withholding in WT (B104) and ZmWRKY82-overexpressing maize lines. Fresh leaf samples (0.1 g) with uniform growth were homogenized in 1 mL extraction buffer to prepare the concentration of extract at 0.1 g/mL. The crude extracts were used to determine SOD and POD activity. Measurement of SOD activity was carried out by using the nitroblue tetrazolium (NBT) method [62]. Measurement of POD activity was carried out by employing the guaiacol assay [63]. Fresh leaf samples (0.1 g) with uniform growth were homogenized in 1 mL of 5% trichloroacetic acid (TCA) to obtain the concentration of extract at 0.1 g/mL for determining the MDA content. The MDA level was measured by reacting with thiobarbituric acid to form a colored complex [64].
4.9. Statistical Analysis
All experimental data were analyzed using one-way or two-way analysis of variance (ANOVA) in GraphPad Prism version 9.0. Three independent biological replicates were conducted for the experiment. A significance level of ** p < 0.01 was considered statistically highly significant.
5. Conclusions
In this study, we found that nuclear-localized ZmWRKY82 has transcriptional activation potential and can specifically bind to the W-box sequence. ZmWRKY82 can increase Arabidopsis and maize drought resilience by participating in the ROS signaling pathway and the ABA signaling pathway. These findings provide new insights into the function of ZmWRKY82 in maize response to drought stress and provide an important theoretical foundation for cultivating drought-resistant varieties.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants14192943/s1, Figure S1: Sequence alignment and phylogenetic analysis of ZmWRKY82.; Figure S2: Detection of T3 generation transgenic Arabidopsis thaliana and T2 generation transgenic maize. Table S1: RT-qPCR Primers used in this study.
Author Contributions
Conceptualization, L.J. and Z.Z.; Methodology, L.J. and Z.Z.; Validation, Z.W., M.L., H.W. and D.L.; Investigation, Z.W., M.L., X.X., H.W., D.L., X.F., D.Y., P.Z. and H.P.; Resources, L.J., Z.Z., W.Y., J.C. and X.R.; Data curation, Z.W., M.L. and X.X.; Writing—original draft preparation, Z.W., M.L., X.X. and H.W.; Writing—review and editing, L.J., Z.Z., Z.W. and M.L.; Visualization, Z.W., M.L., X.X. and H.W.; Supervision, L.J., Z.Z., W.Y., J.C. and X.R.; Project administration, L.J. and Z.Z.; Funding acquisition, L.J., Z.Z., W.Y., J.C. and X.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Jilin Provincial Scientific and Technological Development Program of China (20240303005NC).
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Materials files.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TFs | Transcription factors |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| ABA | Abscisic acid |
| MeJA | Methyl jasmonate |
| SA | Salicylic acid |
| ETH | Ethephon |
| ROS | Reactive oxygen species |
| WT | Wild type |
| h | Hour |
| d | Day |
| V3 | Third-leaf stage |
| CDS | Coding sequence |
| CLSM | Confocal laser scanning microscopy |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| MDA | Malondialdehyde |
| NBT | Nitroblue tetrazolium |
| TCA | Trichloroacetic acid |
References
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Escalona, J.M.; Flexas, J.; Medrano, H. Stomatal and non-stomatal limitations of photosynthesis under water stress in field-grown grapevines. Funct. Plant Biol. 2000, 27, 87. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide Application Improves the Drought Tolerance in Maize Through Modulation of Enzymatic Antioxidants and Leaf Gas Exchange. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Kumar, S.; Sachdeva, S.; Bhat, K.V.; Vats, S. Plant responses to drought stress: Physiological, biochemical and molecular basis. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer: Singapore, 2018; Chapter 14; pp. 1–25. [Google Scholar]
- Haworth, M.; Elliott-Kingston, C.; McElwain, J.C. Co-ordination of physiological and morphological responses of stomata to elevated [CO2] in vascular plants. Oecologia 2013, 171, 71–82. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought stress tolerance in plants: Interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, J.; Li, H.; Wei, F.; Zhang, Y.; Jiang, H.; Peng, X. Identification of the WRKY gene family and characterization of stress-responsive genes in Taraxacum kok-saghyz Rodin. Int. J. Mol. Sci. 2022, 23, 10270. [Google Scholar] [CrossRef]
- Luan, Y.; Chen, Z.; Fang, Z.; Meng, J.; Tao, J.; Zhao, D. PoWRKY69-PoVQ11 module positively regulates drought tolerance by accumulating fructose in Paeonia ostii. Plant J. 2024, 119, 1782–1799. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, Z.; Liu, Y.; Zhang, H.; Zhang, M.; Liu, Q.; Hong, X.; Zhu, J.K.; Gong, Z. ABO3, a WRKY transcription factor, mediates plant responses to abscisic acid and drought tolerance in Arabidopsis. Plant J. 2010, 63, 417–429. [Google Scholar] [CrossRef]
- Hu, W.; Ren, Q.; Chen, Y.; Xu, G.; Qian, Y. Genome-wide identification and analysis of WRKY gene family in maize provide insights into regulatory network in response to abiotic stresses. BMC Plant Biol. 2021, 21, 427. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Liu, Y.; Shen, Q.J. The WRKY gene family in rice (Oryza sativa). J. Integr. Plant Biol. 2007, 49, 827–842. [Google Scholar] [CrossRef]
- Shi, B.; Wu, H.; Zhu, W.; Zheng, B.; Wang, S.; Zhou, K.; Qian, M. Genome-wide identification and expression analysis of WRKY genes during anthocyanin biosynthesis in the mango (Mangifera indica L.). Agriculture 2022, 12, 821. [Google Scholar] [CrossRef]
- Cai, C.; Niu, E.; Du, H.; Zhao, L.; Feng, Y.; Guo, W. Genome-wide analysis of the WRKY transcription factor gene family in Gossypium raimondii and the expression of orthologs in cultivated tetraploid cotton. Crop J. 2014, 2, 87–101. [Google Scholar] [CrossRef]
- Yan, H.E. Isolation and expression patterns of rice WRKY82 transcription factor gene responsive to both biotic and abiotic stresses. Agric. Sci. China 2011, 10, 893–901. [Google Scholar] [CrossRef]
- Yuan, H.; Cheng, M.; Fan, F.; Zheng, X.; Wang, R.; Si, F.; Luo, X.; Li, N.; Li, S. OsGRF6-OsYUCCA1/OsWRKY82 signaling cascade upgrade grain yield and bacterial blight resistance in rice. Adv. Sci. 2024, 11, 2407733. [Google Scholar] [CrossRef]
- Kang, G.; Yan, D.; Chen, X.; Yang, L.; Zeng, R. HbWRKY82, a novel IIc WRKY transcription factor from Hevea brasiliensis associated with abiotic stress tolerance and leaf senescence in Arabidopsis. Physiol. Plant 2021, 171, 151–160. [Google Scholar] [CrossRef]
- Pereira, A. Plant abiotic stress challenges from the changing environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Ma, S.L.; Bai, L.P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Seo, D.H.; Shin, H.; Kim, H.J.; Kim, C.M.; Jang, G. The role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants. Agronomy 2020, 10, 788. [Google Scholar] [CrossRef]
- Garg, N.; Manchanda, G.J.P.B. ROS generation in plants: Boon or bane? Plant Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Jia, H.; Wang, C.; Wang, F.; Liu, S.; Li, G.; Guo, X. GhWRKY68 reduces resistance to salt and drought in transgenic Nicotiana benthamiana. PLoS ONE 2015, 10, e0120646. [Google Scholar] [CrossRef]
- Yan, H.; Jia, H.; Chen, X.; Hao, L.; An, H.; Guo, X. The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production. Plant Cell Physiol. 2014, 55, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhou, Y.; Zhai, H.; He, S.; Zhao, N.; Liu, Q. A novel sweetpotato WRKY transcription factor, IbWRKY2, positively regulates drought and salt tolerance in transgenic Arabidopsis. Biomolecules 2020, 10, 506. [Google Scholar] [CrossRef]
- Sun, J.; Hu, W.; Zhou, R.; Wang, L.; Wang, X.; Wang, Q.; Feng, Z.; Li, Y.; Qiu, D.; He, G.; et al. The Brachypodium distachyon BdWRKY36 gene confers tolerance to drought stress in transgenic tobacco plants. Plant Cell Rep. 2015, 34, 23–35. [Google Scholar] [CrossRef]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The role and regulation of ABI5 (ABA-Insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef]
- Rushton, D.L.; Tripathi, P.; Rabara, R.C.; Lin, J.; Ringler, P.; Boken, A.K.; Langum, T.J.; Smidt, L.; Boomsma, D.D.; Emme, N.J.; et al. WRKY transcription factors: Key components in abscisic acid signalling. Plant Biotechnol. J. 2012, 10, 2–11. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, G.; Yu, D. Activated expression of WRKY57 confers drought tolerance in Arabidopsis. Mol. Plant 2012, 5, 1375–1388. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Yan, Y.; Zhang, S.; Li, H.; Gao, Z.; Wang, C.; Guo, X. GhWRKY21 regulates ABA-mediated drought tolerance by fine-tuning the expression of GhHAB in cotton. Plant Cell Rep. 2021, 40, 2135–2150. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, H.D.; Liu, L.; Jin, S.H.; Zhu, P.L.; Zhang, Y.P.; Jiang, C.Z. Heterologous expression of dehydration-inducible MfWRKY17 of Myrothamnus flabellifolia confers drought and salt tolerance in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 4603. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Bai, X.; Sun, X.; Zhu, D.; Liu, B.; Ji, W.; Cai, H.; Cao, L.; Wu, J.; Hu, M.; et al. Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signalling. J. Exp. Bot. 2013, 64, 2155–2169. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.F.; Chen, J.; Chen, Y.F.; Wu, L.J.; Xie, D.X. Molecular phylogenetic and expression analysis of the complete WRKY transcription factor family in maize. DNA Res. 2012, 19, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Baillo, E.H.; Hanif, M.S.; Guo, Y.; Zhang, Z.; Xu, P.; Algam, S.A. Genome-wide Identification of WRKY transcription factor family members in sorghum (Sorghum bicolor (L.) moench). PLoS ONE 2020, 15, e0236651. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Qayyum, A.; Farooq, S.; Almutairi, S.M.; Rasheed, R.A.; Qadir, M.; Vyhnánek, T.; Sun, Y. Pepper immunity against Ralstonia solanacearum is positively regulated by CaWRKY3 through modulation of different WRKY transcription factors. BMC Plant Biol. 2024, 24, 522. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, S.; Yang, L.; Xiao, R.; Chen, S.; Wang, D.; Wang, S.; Wang, Z. Genome-wide identification of the Hypericum perforatum WRKY gene family implicates HpWRKY85 in drought resistance. Int. J. Mol. Sci. 2022, 24, 352. [Google Scholar] [CrossRef]
- Gu, L.; Li, L.; Wei, H.; Wang, H.; Su, J.; Guo, Y.; Yu, S. Identification of the group IIa WRKY subfamily and the functional analysis of GhWRKY17 in upland cotton (Gossypium hirsutum L.). PLoS ONE 2018, 13, e0191681. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Tan, X.L.; Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. BrWRKY65, a WRKY transcription factor, is involved in regulating three leaf senescence-associated genes in Chinese flowering cabbage. Int. J. Mol. Sci. 2017, 18, 1228. [Google Scholar] [CrossRef]
- Zang, Z.; Wang, Z.; Zhao, F.; Yang, W.; Ci, J.; Ren, X.; Jiang, L.; Yang, W. Maize Ethylene Response Factor ZmERF061 Is Required for Resistance to Exserohilum turcicum. Front. Plant Sci. 2021, 12, 630413. [Google Scholar] [CrossRef]
- Gulzar, F.; Fu, J.; Zhu, C.; Yan, J.; Li, X.; Meraj, T.A.; Shen, Q.; Hassan, B.; Wang, Q. Maize WRKY transcription factor ZmWRKY79 positively regulates drought tolerance through elevating ABA biosynthesis. Int. J. Mol. Sci. 2021, 22, 10080. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef]
- Narusaka, Y.; Nakashima, K.; Shinwari, Z.K.; Sakuma, Y.; Furihata, T.; Abe, H.; Narusaka, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003, 34, 137–148. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Long, Y.; Si, W.; Cheng, B.; Jiang, H. A novel heat shock transcription factor (ZmHsf08) negatively regulates salt and drought stress responses in maize. Int. J. Mol. Sci. 2021, 22, 11922. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. WRKY proteins: Signaling and regulation of expression during abiotic stress responses. Sci. World J. 2015, 2015, 807560. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.H.A.I.; Mittler, R.O.N.; Miller, G.A.D. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Zhao, S.; Yu, X.; Sun, Y.; Li, H.; Ruan, C.; Li, J. Yellowhorn drought-induced transcription factor XsWRKY20 acts as a positive regulator in drought stress through ROS homeostasis and ABA signaling pathway. Plant Physiol. Biochem. 2020, 155, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mao, Y.; Duan, X.; Zhou, H.; Lai, D.; Zhang, Y.; Shen, W. Arabidopsis HY1-modulated stomatal movement: An integrative hub is functionally associated with ABI4 in dehydration-induced ABA responsiveness. Plant Physiol. 2016, 170, 1699–1713. [Google Scholar] [CrossRef]
- Ding, W.; Fang, W.; Shi, S.; Zhao, Y.; Li, X.; Xiao, K. Wheat WRKY type transcription factor gene TaWRKY1 is essential in mediating drought tolerance associated with an ABA-dependent pathway. Plant Mol. Biol. Rep. 2016, 34, 1111–1126. [Google Scholar] [CrossRef]
- Ge, M.; Tang, Y.; Guan, Y.; Lv, M.; Zhou, C.; Ma, H.; Lv, J. TaWRKY31, a novel WRKY transcription factor in wheat, participates in regulation of plant drought stress tolerance. BMC Plant Biol. 2024, 24, 27. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yu, D. Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environ. Exp. Bot. 2009, 65, 35–47. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kokkirala, V.R.; Yonggang, P.; Abbagani, S.; Zhu, Z.; Umate, P. Subcellular localization of proteins of Oryza sativa L. in the model tobacco and tomato plants. Plant Signal. Behav. 2010, 5, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Saito, H.; Ohta, S.; Hiei, Y.; Komari, T.; Kumashiro, T. High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat. Biotechnol. 1996, 14, 745–750. [Google Scholar] [CrossRef]
- Li, D.; Wang, H.; Luo, F.; Li, M.; Wu, Z.; Liu, M.; Wang, Z.; Zang, Z.; Jiang, L. A Maize Calmodulin-like 3 Gene Positively Regulates Drought Tolerance in Maize and Arabidopsis. Int. J. Mol. Sci. 2025, 26, 1329. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, M.; Wang, H.; Li, M.; Liu, X.; Zang, Z.; Jiang, L. ZmCaM2-1, a Calmodulin Gene, Negatively Regulates Drought Tolerance in Transgenic Arabidopsis Through the ABA-Independent Pathway. Int. J. Mol. Sci. 2025, 26, 2156. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Maehly, A.C. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [PubMed]
- Draper, H.H.; Hadley, M. [43] Malondialdehyde determination as index of lipid Peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).