Dynamic Field Assessment of Canopy Development and Periderm Maturation in Potato (Solanum tuberosum L.)
Abstract
1. Introduction
2. Results
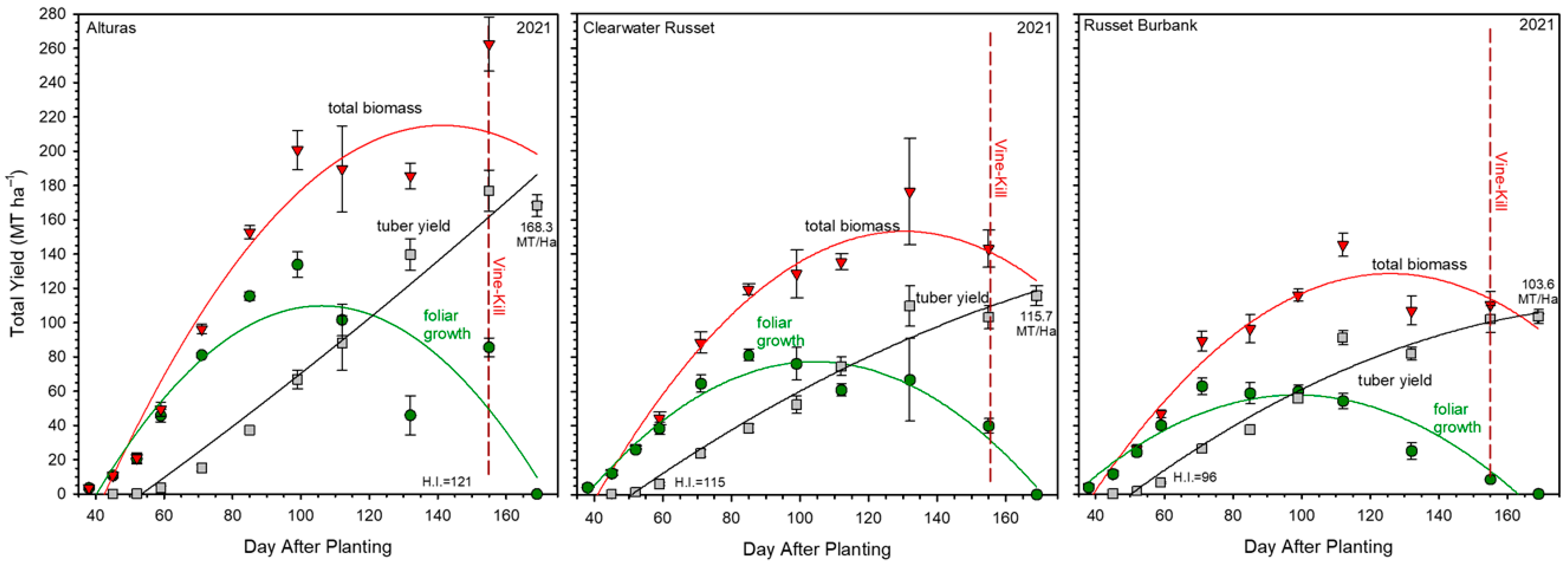
2.1. Foliar Development
2.2. Tuber Development
2.3. Total Biomass
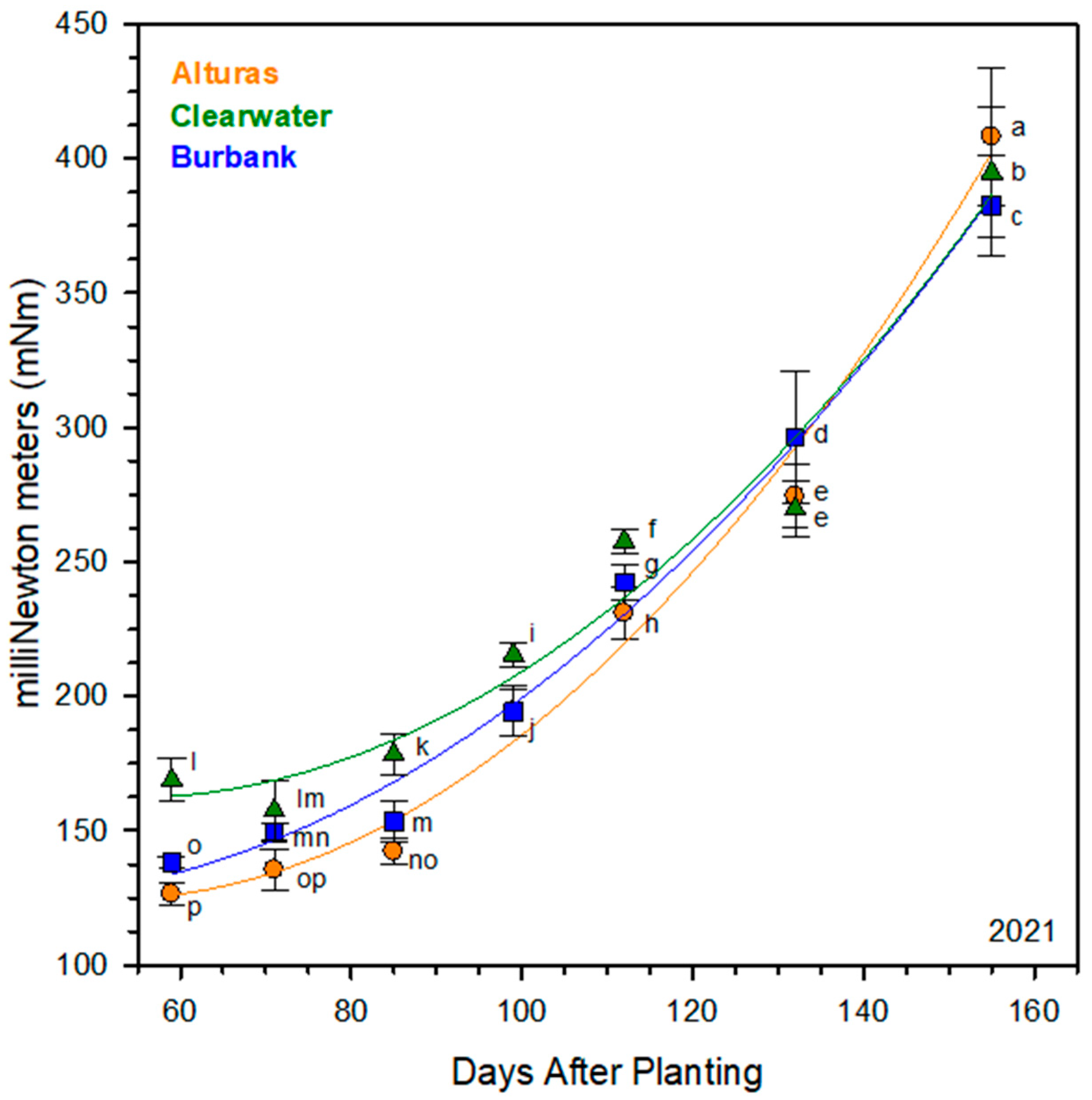
2.4. Shear Resistance
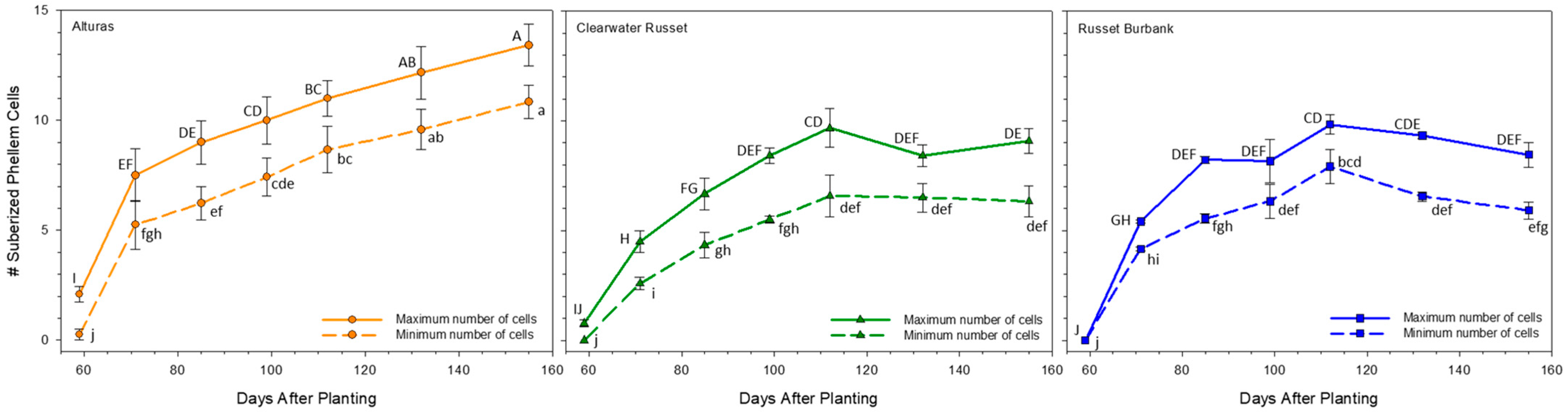
2.5. Phellem Cell Count
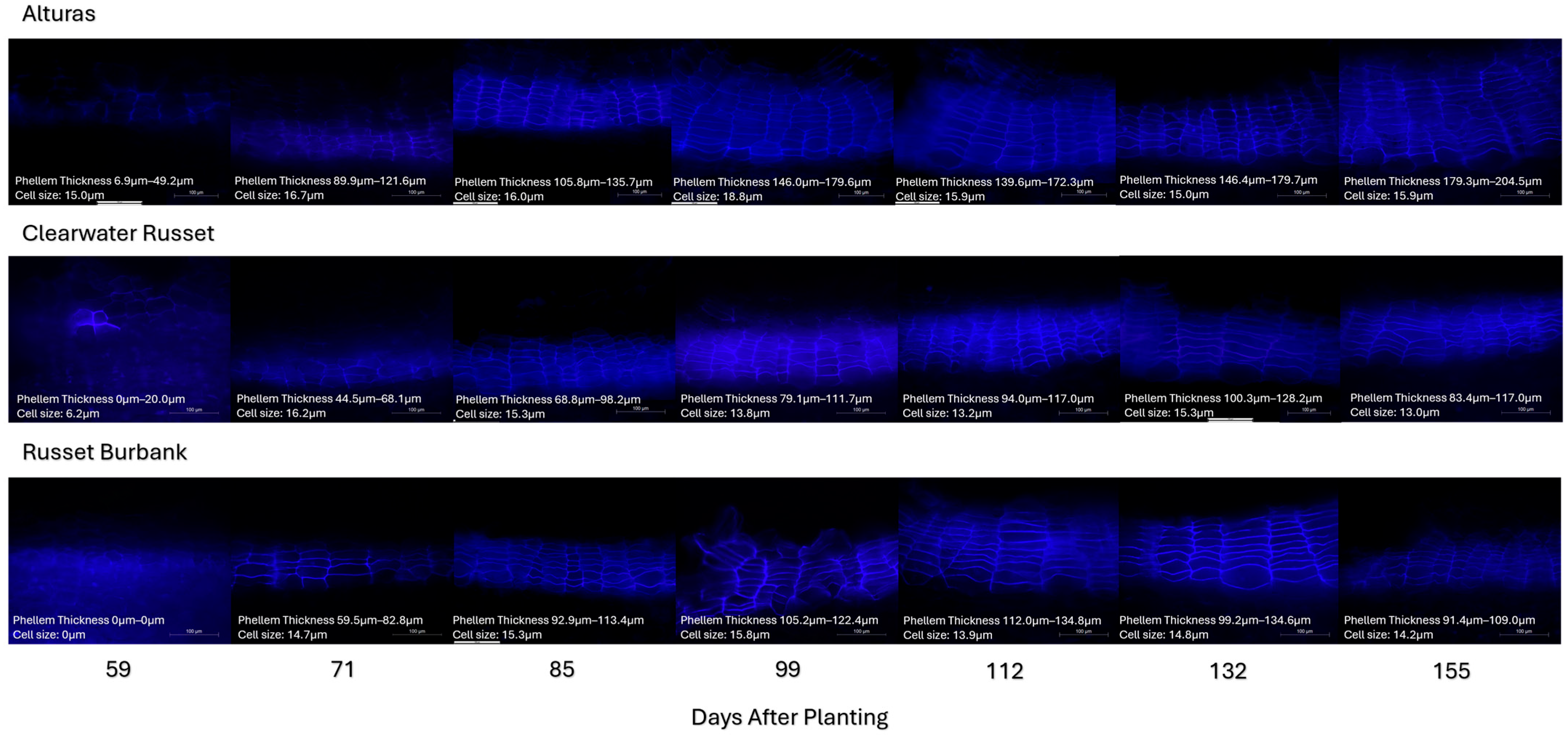
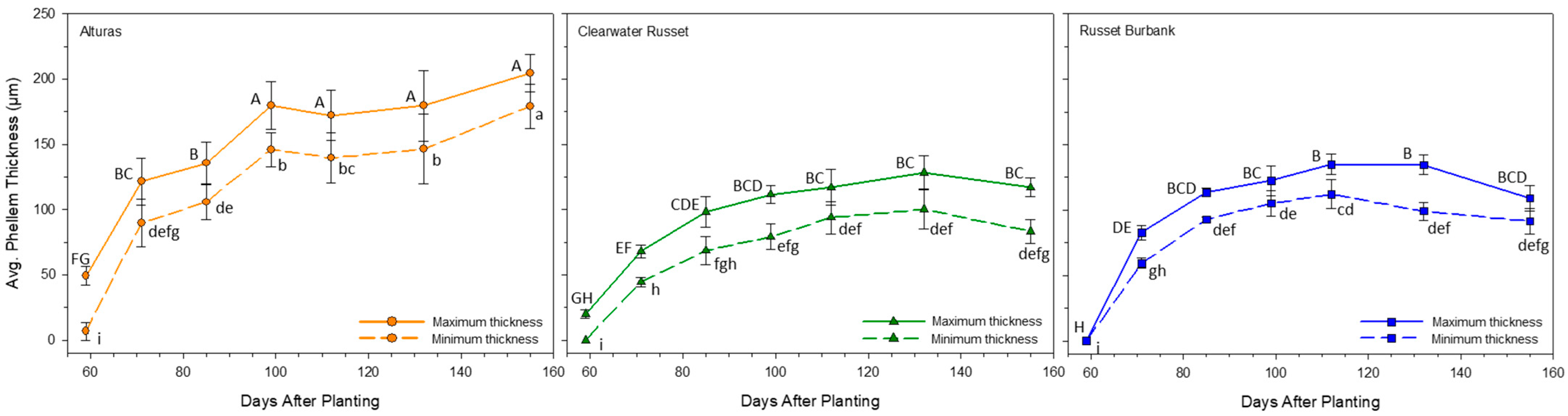
2.6. Phellem Thickness
3. Discussion
3.1. Canopy and Tuber Development in Relation to Biomass Partitioning
3.2. Periderm Development and Shear Strength
3.3. Timing of Skin Set and Cultivar-Specific Implications
4. Materials and Methods
4.1. Plant Material and Field Conditions
4.2. Phenotyping and Sampling
4.2.1. Yield and Tuber Size
4.2.2. Periderm Shear Strength
4.2.3. Histological Sampling
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAP | Days After Planting |
| HI | Harvest Index |
| UV | Ultra-violet |
| SPP | Polyphenolic Domain |
| SPA | Polyaliphatic Domain |
| USA | United States of America |
| WA | Washington State |
| WSU | Washington State University |
| USDA | United State Department of Agriculture |
| RH | Relative Humidity |
| in.oz. | Inch Ounces |
| in.lbs. | Inch Pounds |
| N | Newtons |
| mNm | Millinewton meter |
| FAA | Formaldehyde; Alcohol; Acetic Acid Fixative |
| CV | Cultivar |
| MT | Metric Ton |
| Ha | Hectare |
| cm | Centimeter |
| µm | Micrometer |
References
- Zhang, H.; Xu, F.; Wu, Y.; Hu, H.; Dai, X.F. Progress of potato staple food research and industry development in China. J. Integr. Agric. 2017, 16, 2924–2932. [Google Scholar] [CrossRef]
- Devaux, A.; Goffart, J.-P.; Kromann, P.; Andrade-Piedra, P.; Polar, V.; Hareau, G. The Potato of the Future: Opportunities and Challenges in Sustainable Agri-food Systems. Potato Res. 2021, 64, 681–720. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Colwell, F.; Souter, J.; Bryan, G.J.; Compton, L.J.; Boonham, N.; Prashar, A. Development and Validation of Methodology for Estimating Potato Canopy Structure for Field Crop Phenotyping and Improved Breeding. Front. Plant Sci. 2021, 12, 612843. [Google Scholar] [CrossRef]
- Firman, D.M.; Allen, E. Relationship between light interception, ground cover and leaf area index in potatoes. J. Agric. Sci. 1989, 113, 355–359. [Google Scholar] [CrossRef]
- Dahal, K.; Li, X.; Vanlerberghe, G.C. The enhancement of photosynthetic performance, water use efficiency and tuber yield of potatoes under elevated CO2 is cultivar dependent. Front. Plant Sci. 2019, 10, 1287825. [Google Scholar] [CrossRef]
- Li, S.; Kupriyanovich, Y.; Wagg, C.; Zheng, F.; Hann, S. Water deficit duration affects potato plant growth, yield and tuber quality. Agriculture 2023, 13, 2007. [Google Scholar] [CrossRef]
- Baritelle, A.L.; Hyde, G.M. Specific gravity and cultivar effects on potato tuber impact sensitivity. Postharvest Bio. Technol. 2003, 29, 279–286. [Google Scholar] [CrossRef]
- Kumar, P.; Ginzberg, I. Potato Periderm Development and Tuber Skin Quality. Plants 2022, 11, 2099. [Google Scholar] [CrossRef] [PubMed]
- Lulai, E.C.; Sabba, R.P.; Nolte, P.; Gudmestad, N.C.; Secor, G.A. “Periderm Disorder Syndrome”: A New Name for the Syndrome Formerly Referred to as Pink Eye. Am. J. Potato Res. 2018, 95, 435–440. [Google Scholar] [CrossRef]
- Artschwager, E.F. Anatomy of the potato plant, with special reference to the ontogeny of the vascular system. J. Agric. Res. 1918, 14, 221–264. [Google Scholar]
- Peterson, R.I.; Barker, W.G. Early tuber development from explanted stolon nodes of Solanum tuberosum var. Kennebec [Potato]. Bot. Gaz. 1979, 140, 398–406. [Google Scholar] [CrossRef]
- Neubauer, J.D.; Lulai, E.C.; Thompson, A.L.; Suttle, J.C.; Bolton, M.D.; Campbell, L.G. Molecular and cytological aspects of native periderm maturation in potato tubers. J. Plant Physiol. 2013, 170, 413–423. [Google Scholar] [CrossRef]
- Bernards, M.A. Demystifying suberin. Can. J. Bot. 2002, 80, 227–240. [Google Scholar] [CrossRef]
- Sabba, R.P.; Lulai, E.C. Histological Analysis of the Maturation of Native and Wound Periderm in Potato (Solanum tuberosum L.) Tuber. Ann. Bot. 2002, 90, 1–10. [Google Scholar] [CrossRef]
- Lendzian, K.J. Survival strategies of plants during secondary growth: Barrier properties of phellems and lenticels towards water, oxygen, and carbon dioxide. J. Exp. Bot. 2006, 57, 2535–2546. [Google Scholar] [CrossRef]
- Lulai, E.C.; Freeman, T.P. The Importance of Phellogen Cells and their Structural Characteristics in Susceptibility and Resistance to Excoriation in Immature and Mature Potato Tuber (Solanum tuberosum L.) Periderm. Ann. Bot. 2001, 88, 555–561. [Google Scholar] [CrossRef]
- Schreiber, L.; Franke, R.; Hartmann, K. Effects of NO3 deficiency and NaCl stress on suberin deposition in rhizo-and hypodermal (RHCW) and endodermal cell walls (ECW) of castor bean (Ricinus communis L.) roots. Plant Soil 2005, 269, 333–339. [Google Scholar] [CrossRef]
- Schreiber, L.; Franke, R.; Hartmann, K. Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpiration. Plant Soil 2005, 220, 520–530. [Google Scholar] [CrossRef]
- Lulai, E.C.; Orr, P.H. Determining the feasibility of measuring genotypic differences in skin-Set. Am. J. Potato Res. 1993, 70, 599–610. [Google Scholar] [CrossRef]
- Bowen, S.A.; Muir, A.Y.; Dewar, C.T. Investigations into skin strength in potatoes: Factors affecting skin adhesion strength. Potato Res. 1996, 39, 313–321. [Google Scholar] [CrossRef]
- Boydston, R.A.; Navarre, D.A.; Collins, H.P.; Chaves-Cordoba, B. The Effect of Nitrogen Rate on Vine Kill, Tuber Skinning Injury, Tuber Yield and Size Distribution, and Tuber Nutrients and Phytonutrients in Two Potato Cultivars Grown for Early Potato Production. Am. J. Potato Res. 2017, 94, 425–436. [Google Scholar] [CrossRef]
- Makani, M.N.; Sargent, S.A.; Zotarelli, L.; Huber, D.J. Harvest Interval Has Greater Effect on Periderm Maturity and Storage Quality of Early-maturing, Tablestock Potato than Nitrogen Rate. Hortic. Sci. 2017, 52, 1390–1395. [Google Scholar] [CrossRef]
- Pavek, J.J.; Corsini, D.L. Alturas: A high-yielding, processing potato cultivar with long tubers, high specific gravity, and resistant to late-blight. Am. J. Potato Res. 2021, 78, 37–45. [Google Scholar] [CrossRef]
- Novy, R.G.; Whitworth, J.L.; Stark, J.C.; Love, S.L.; Corsini, D.L.; Pavek, J.J.; Vales, M.I.; James, S.R.; Hane, D.C.; Shock, C.C.; et al. Clearwater russet: A dual-purpose potato cultivar with cold sweetening resistance, high protein content, and low incidence of external defects and sugar ends. Am. J. Potato Res. 2010, 87, 458–471. [Google Scholar] [CrossRef]
- Stark, J.C.; Love, S.L.; Knowles, N.R. Tuber quality. In Potato Production Systems; Stark, J., Thornton, M., Nolte, P., Eds.; Springer: Cham, Switzerland, 2020; pp. 479–497. [Google Scholar] [CrossRef]
- Tekalign, T.; Hammes, P.S. Growth and productivity of potato as influenced by cultivar and reproductive growth. II. Growth analysis, tuber yield and quality. Sci. Hortic. 2005, 105, 29–44. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with drought: Stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, T.F.; Pavek, M.J.; Knowles, N.R.; Holden, Z. Reduced Late-Season Irrigation Improves Potato Quality, Often at the Expense of Yield and Economic Return. Am. J. Potato Res. 2024, 101, 202–225. [Google Scholar] [CrossRef]
- Amjadi, H.; Heidari, G.; Babaei, S.; Sharifi, Z. Evaluation of yield, yield components and some quality traits of tuber of potato (Solanum tuberosum L.) under different weed and nutritional management practices. Front. Plant Sci. 2025, 15, 1495541. [Google Scholar] [CrossRef] [PubMed]
- Vulavala, V.K.R.; Fogelman, E.; Faigenboim, A.; Shoseyov, O.; Ginzberg, I. The transcriptome of potato tuber phellogen reveals cellular functions of cork cambium and genes involved in periderm formation and maturation. Sci. Rep. 2019, 9, 10216. [Google Scholar] [CrossRef]
- Vulavala, V.K.R.; Fogelman, E.; Rozental, L.; Faigenboim, A.; Tanami, Z.; Shoseyov, O.; Ginzberg, I. Identification of genes related to skin development in potato. Plant Mol. Biol. 2017, 94, 481–494. [Google Scholar] [CrossRef]
- Sabba, R.P.; Bussan, A.J. Comparison of Skin-set and Periderm Maturation in ‘Red Norland’ Potatoes Grown in Two Soil Types in Wisconsin. Am. J. Potato Res. 2012, 89, 508–511. [Google Scholar] [CrossRef]
- Krupek, F.S.; Dittmar, P.J.; Sargent, S.A.; Zotarelli, L.; Rowland, D. Impact of Early Potato Desiccation Method on Crop Growth, Skinning Injury, and Storage Quality Maintenance. Am. J. Potato Res. 2021, 98, 218–231. [Google Scholar] [CrossRef]
- Lulai, E.C. The Roles of Phellem (Skin) Tensile-related Fractures and Phellogen Shear-related Fractures in Susceptibility to Tuber-skinning Injury and Skin-set Development. Am. J. Potato Res. 2002, 79, 241–248. [Google Scholar] [CrossRef]
- Lulai, E.C.; Corsini, D.L. Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound-healing. Physiol. Mol. Plant Pathol. 1998, 53, 209–222. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, G.; Murphy, A.; De Koeyer, D.; Tai, H.; Bizimungu, B.; Si, H.; Li, X.Q. Differences between the Bud End and Stem End of Potatoes in Dry Matter Content, Starch Granule Size, and Carbohydrate Metabolic Gene Expression at the Growing and Sprouting Stages. J. Agric. Food Chem. 2016, 64, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Lenfesty, C.M. Soil survey: Adams County; Conservation Service: Washington, DC, USA, 1967.
- Bohl, W.H.; Johnson, S.B. Commercial Potato Production in North America; The Potato Association of America Handbook, Eds.; Potato Association of America: Livonia, MI, USA, 2010; Available online: https://vric.ucdavis.edu/pdf/POTATOES/Commercial%20Potato%20Production%20in%20North%20America%202010.pdf (accessed on 3 September 2024).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
| Observation | Cultivar | * bx2 | * ax | * c | Peak DAP | Peak Value a | Growth Rate b | Decay Rate b | R-Sq. |
|---|---|---|---|---|---|---|---|---|---|
| Foliar Weight | Alturas | −0.025 | 5.36 | −175.03 | 106 | 109.87 | 1.04 | −1.23 | 0.78 |
| Foliar Weight | Clearwater Russet | −0.0174 | 3.64 | −112.31 | 104 | 77.00 | 0.74 | −0.79 | 0.94 |
| Foliar Weight | Russet Burbank | −0.0138 | 2.72 | −76.22 | 99 | 57.74 | 0.58 | −0.89 | 0.87 |
| Total Biomass | Alturas | −0.022 | 6.23 | −225.05 | 140 | 215.06 | 1.54 | - | 0.92 |
| Total Biomass | Clearwater Russet | −0.0191 | 4.99 | −171.97 | 130 | 152.82 | 1.18 | −0.39 | 0.96 |
| Total Biomass | Russet Burbank | −0.0171 | 4.31 | −142.93 | 126 | 128.13 | 1.02 | −0.61 | 0.94 |
| Tuber Weight | Alturas | 0.0015 | 1.28 | −72.75 | 169 | 186.4 | 1.00 | - | 0.97 |
| Tuber Weight | Clearwater Russet | −0.0032 | 1.71 | −78.85 | 169 | 118.54 | 0.68 | - | 0.97 |
| Tuber Weight | Russet Burbank | −0.0049 | 1.96 | −86.25 | 169 | 116.51 | 0.61 | - | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buckley, C.L.; Gonzalez-Tapia, F.; Navarre, D.A.; Blauer, J.M. Dynamic Field Assessment of Canopy Development and Periderm Maturation in Potato (Solanum tuberosum L.). Plants 2025, 14, 2780. https://doi.org/10.3390/plants14172780
Buckley CL, Gonzalez-Tapia F, Navarre DA, Blauer JM. Dynamic Field Assessment of Canopy Development and Periderm Maturation in Potato (Solanum tuberosum L.). Plants. 2025; 14(17):2780. https://doi.org/10.3390/plants14172780
Chicago/Turabian StyleBuckley, Connor L., Fransico Gonzalez-Tapia, Duroy A. Navarre, and Jacob M. Blauer. 2025. "Dynamic Field Assessment of Canopy Development and Periderm Maturation in Potato (Solanum tuberosum L.)" Plants 14, no. 17: 2780. https://doi.org/10.3390/plants14172780
APA StyleBuckley, C. L., Gonzalez-Tapia, F., Navarre, D. A., & Blauer, J. M. (2025). Dynamic Field Assessment of Canopy Development and Periderm Maturation in Potato (Solanum tuberosum L.). Plants, 14(17), 2780. https://doi.org/10.3390/plants14172780






