Predicting the Potential Suitable Habitat of Solanum rostratum in China Using the Biomod2 Ensemble Modeling Framework
Abstract
1. Introduction
2. Results
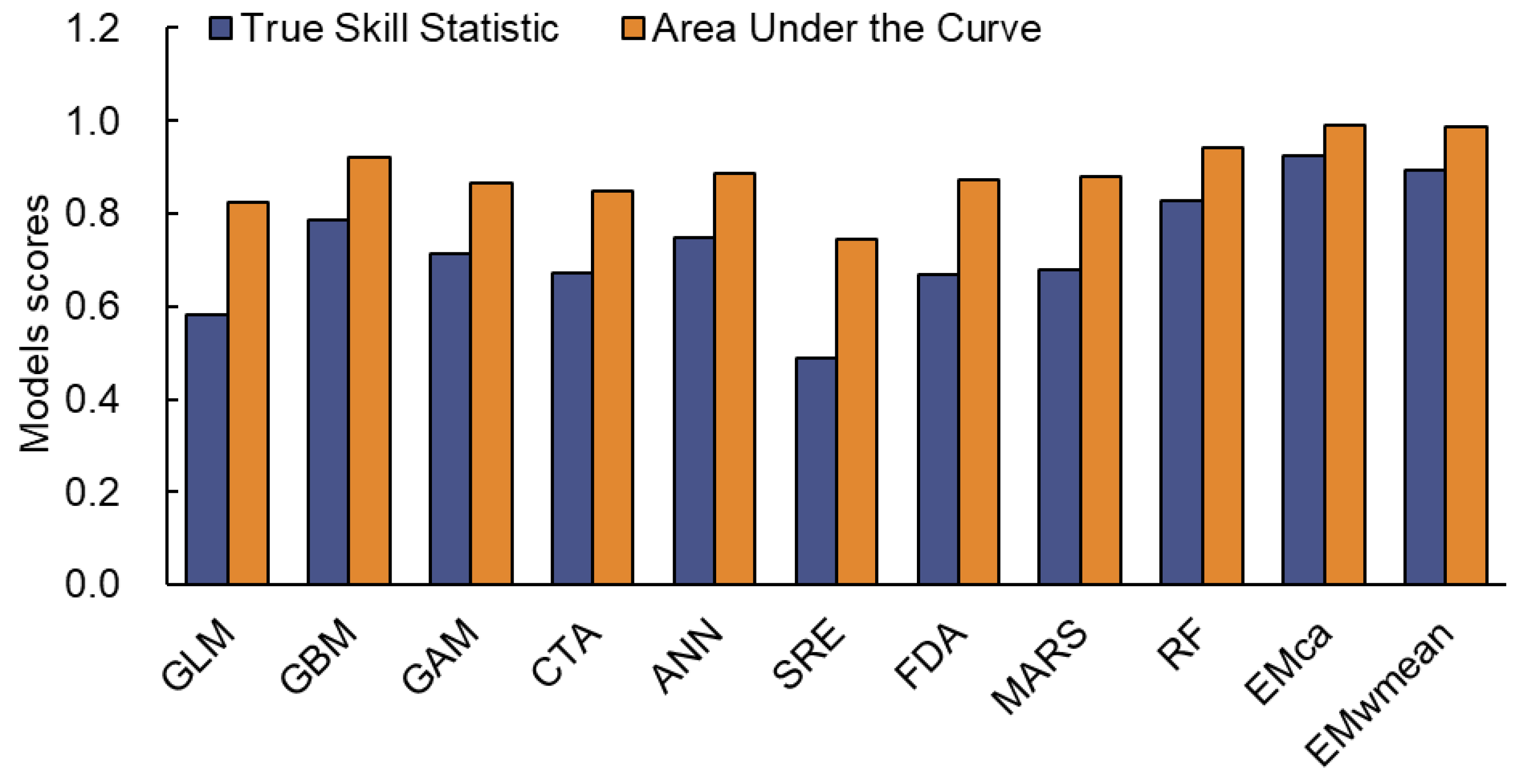
2.1. Comparison of Model Performance
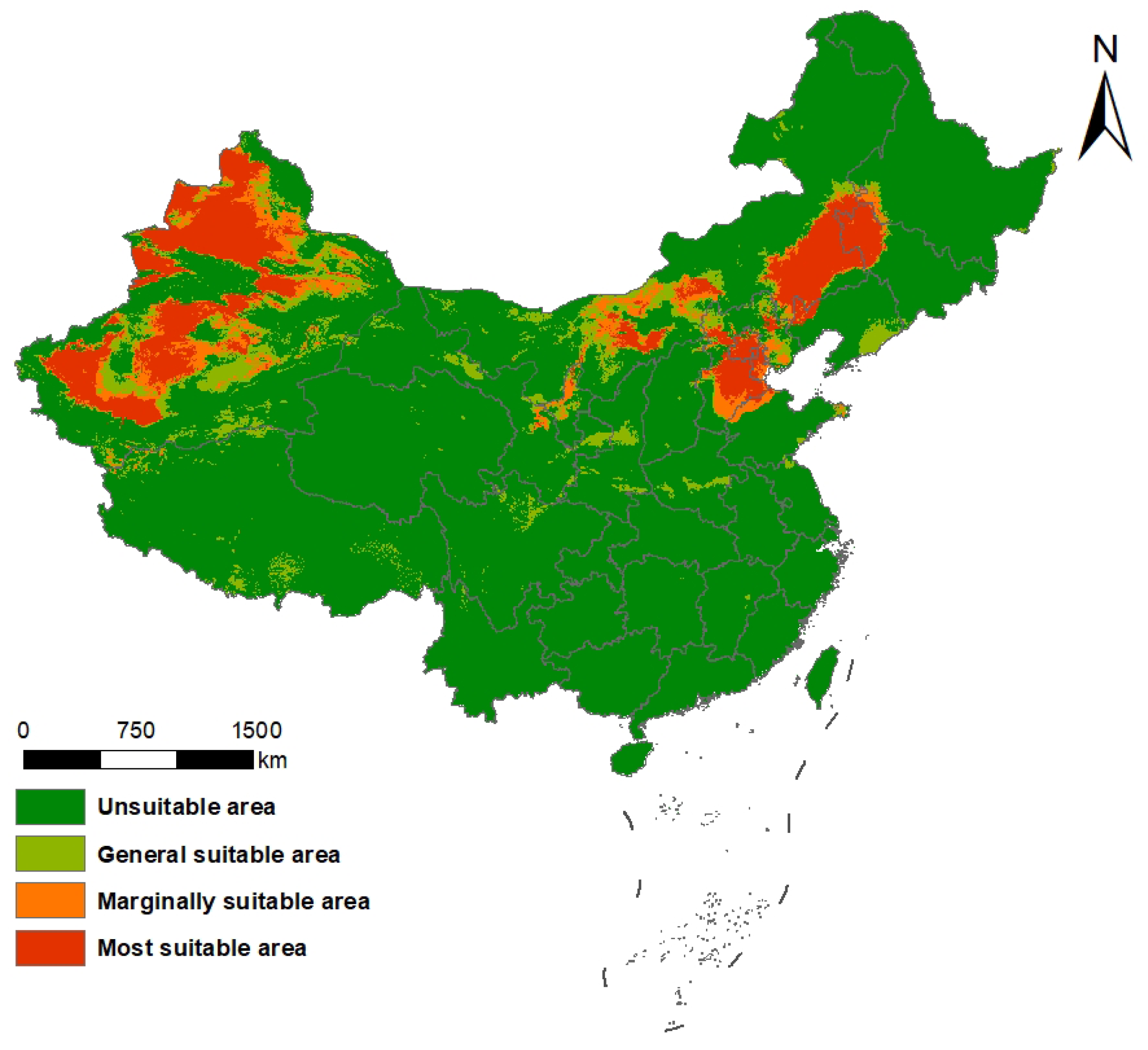
2.2. Current Potential Distribution of S. rostratum in China
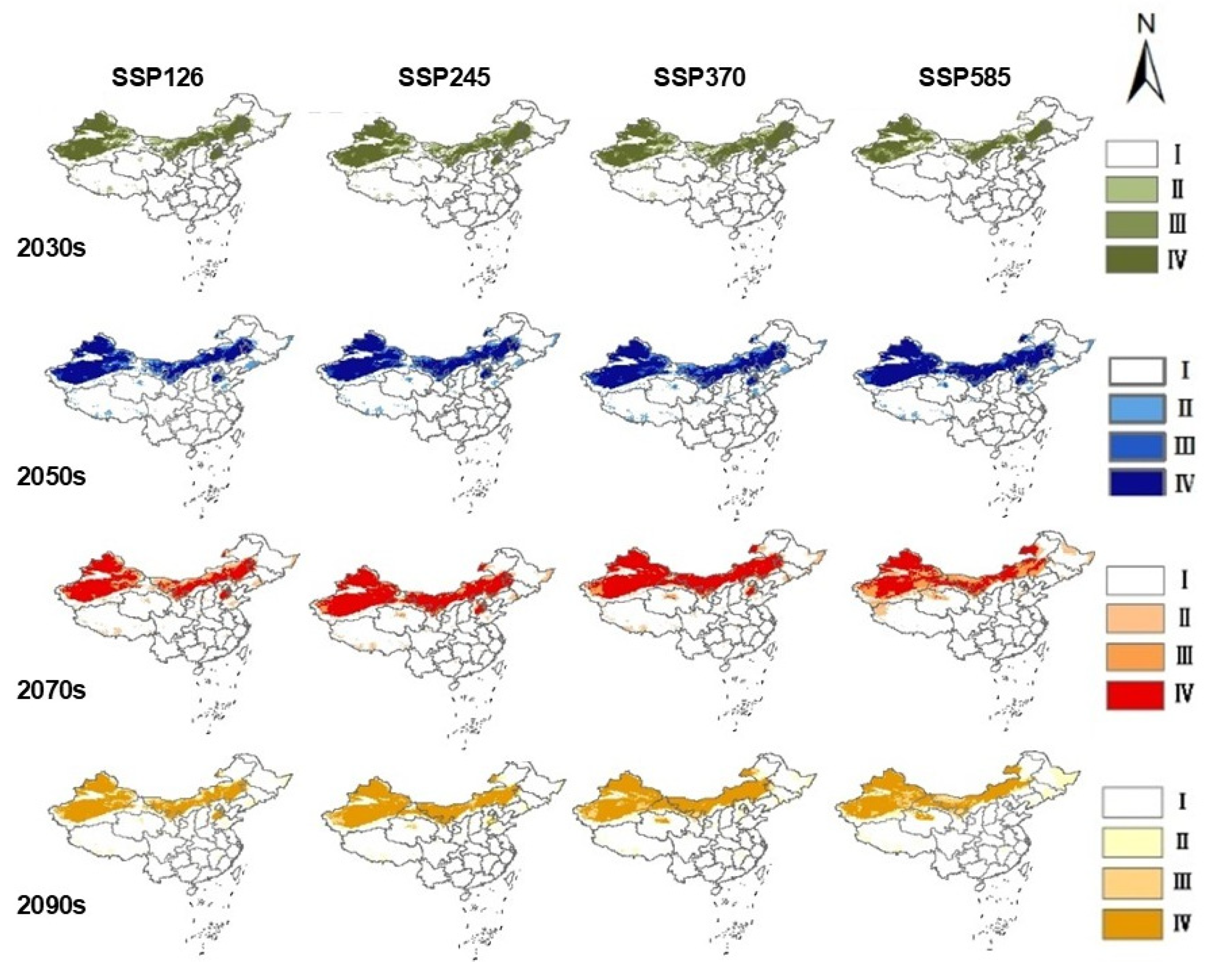
2.3. Projected Future Distribution of S. rostratum Under Climate Change Scenarios
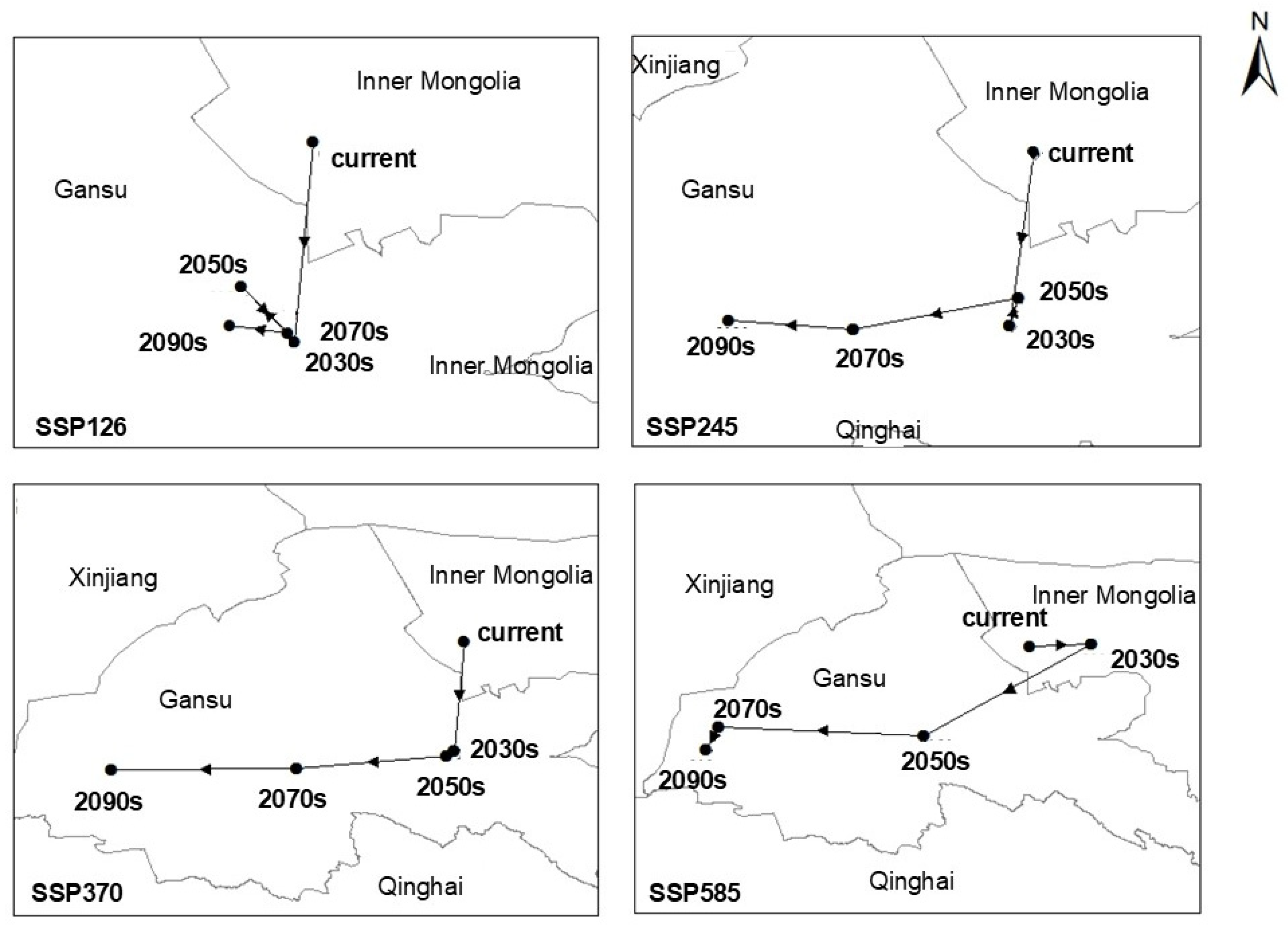
2.4. Habitat Centroid Shift and Spatial Pattern Dynamics
2.5. Habitat Centroid Dynamics Under Future Climate Scenarios
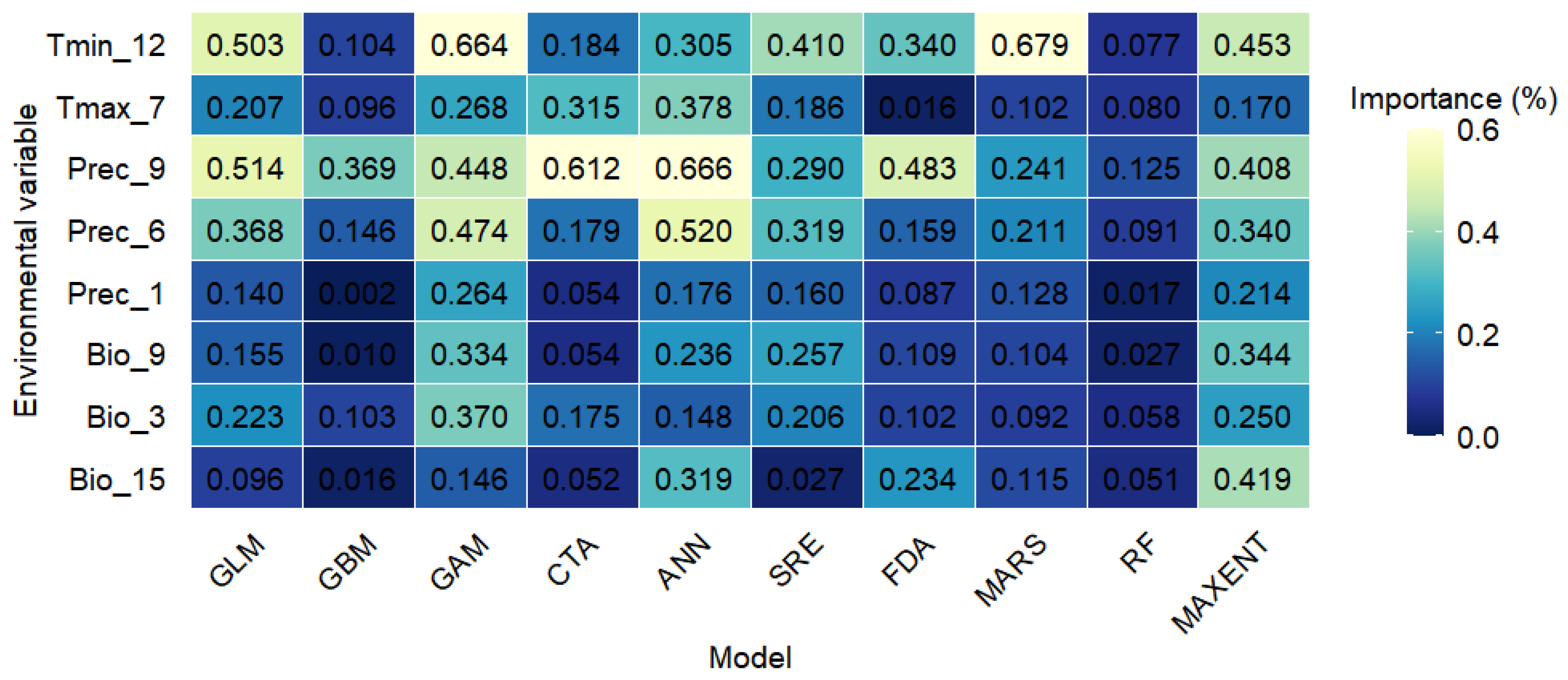
2.6. Key Environmental Predictors of S. rostratum Distribution
3. Discussion
3.1. Dispersal Potential of S. rostratum and Future Changes in Suitable Habitats
3.2. Climatic Drivers of Habitat Suitability
3.3. Model Performance and Comparative Analysis
4. Materials and Methods
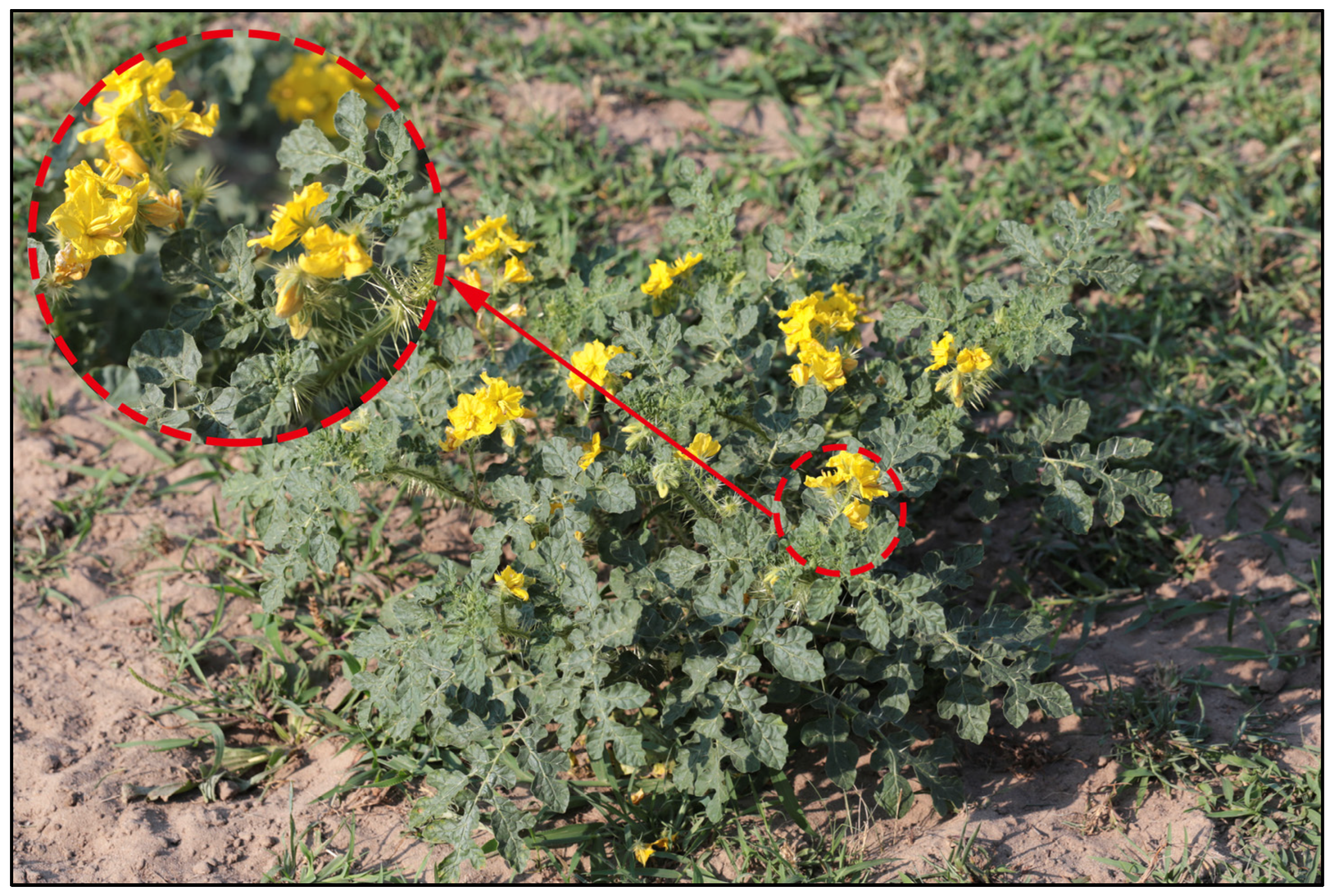
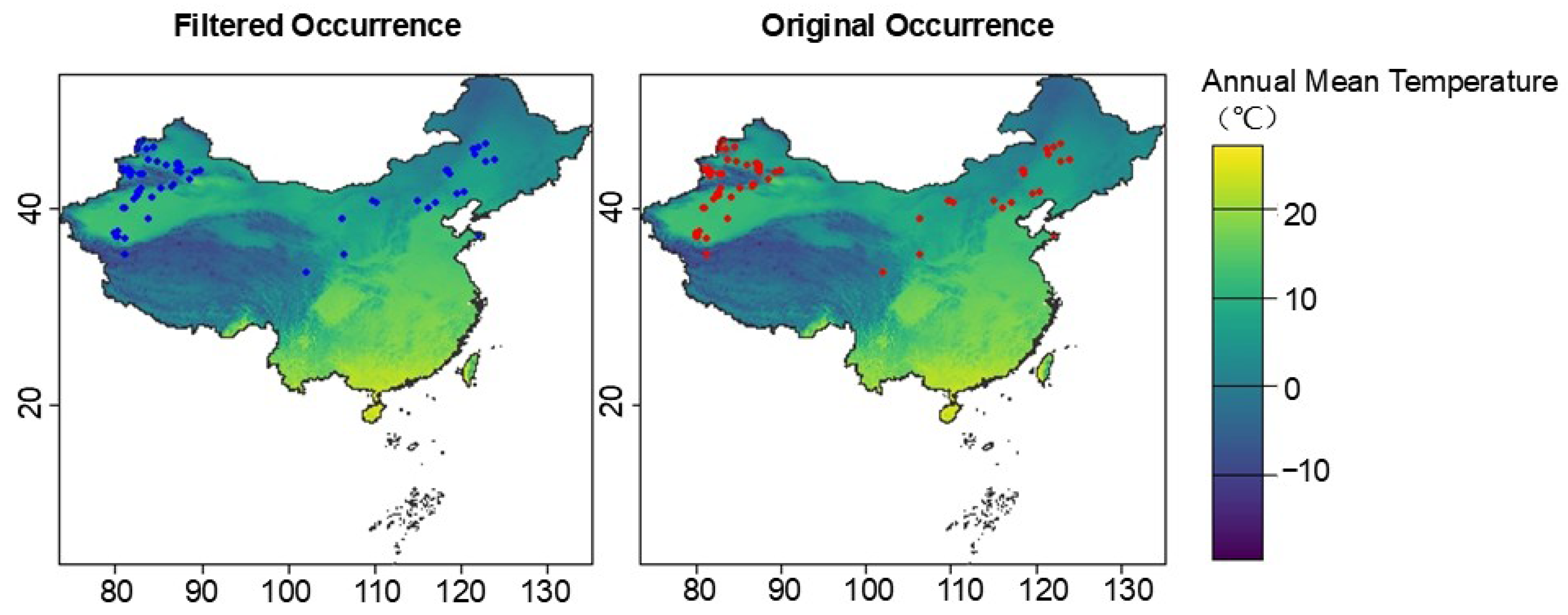
4.1. Occurrence Data Collection
4.2. Environmental Variables Selection
4.3. Species Distribution Modeling
4.4. Data Processing and Visualization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ellstrand, N.C.; Schierenbeck, K.A. Hybridization as a stimulus for the evolution of invasiveness in plants. Euphytica 2006, 148, 35–46. [Google Scholar] [CrossRef]
- Stafford, W.; Birch, C.; Etter, H.; Blanchard, R.; Mudavanhu, S.; Blignaut, J.; Ferreira, L.; Marais, C. The economics of landscape restoration: Benefits of controlling bush encroachment and invasive plant species in South Africa and Namibia. Ecosyst. Serv. 2017, 27, 193–202. [Google Scholar] [CrossRef]
- Dogra, K.S.; Sood, S.K.; Dobhal, P.K.; Sharma, S. Alien plant invasion and their impact on indigenous species diversity at global scale: A review. J. Ecol. Nat. Environ. 2010, 2, 175–186. [Google Scholar]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Xu, H.; Ding, H.; Li, M.; Qiang, S.; Guo, J.; Han, Z.; Huang, Z.; Sun, H.; He, S.; Wu, H.; et al. The distribution and economic losses of alien species invasion to China. Biol. Invasions 2006, 8, 1495–1500. [Google Scholar] [CrossRef]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Segurado, P.; Araujo, M.B. An evaluation of methods for modelling species distributions. J. Biogeogr. 2004, 31, 1555–1568. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Rome, Q.; Villemant, C.; Courchamp, F. Can species distribution models really predict the expansion of invasive species? PLoS ONE 2018, 13, e0193085. [Google Scholar] [CrossRef]
- Radomski, T.; Beamer, D.; Babineau, A.; Wilson, C.; Pechmann, J.; Kozak, K.H. Finding what you don’t know: Testing SDM methods for poorly known species. Divers. Distrib. 2022, 28, 1769–1780. [Google Scholar] [CrossRef]
- El-Barougy, R.F.; Dakhil, M.A.; Halmy, M.W.; Gray, S.M.; Abdelaal, M.; Khedr, A.H.A.; Bersier, L.F. Invasion risk assessment using trait-environment and species distribution modelling techniques in an arid protected area: Towards conservation prioritization. Ecol. Indic. 2021, 129, 107951. [Google Scholar] [CrossRef]
- Pang, S.E.H.; Buitenwerf, R.; Baines, O.; Aćić, S.; Bernhardt-Römermann, M.; Biurrun, I.; Bonari, G.; Bruun, H.H.; Byun, C.; Chacón-Madrigal, E.; et al. Plant Range Disequilibrium in Europe Is Shaped More by Disturbance Than Climate Change. 2025. Available online: https://www.researchsquare.com/article/rs-6681466/v1 (accessed on 15 July 2025).
- Wu, H.; Levinson, D. The ensemble approach to forecasting: A review and synthesis. Transp. Res. Part C-Emer. Technol. 2021, 132, 103357. [Google Scholar] [CrossRef]
- Shao, M.; Ma, Y.; Wang, Y.; Xu, S.; Miao, Q.; Zhai, Q.; Qu, B. A preliminary study on allelopathy and potential allelochemicals of root exudates from Solanum rostratum Dunal. Biotechnol. J. Int. 2022, 26, 31–39. [Google Scholar] [CrossRef]
- Izzo, V.M.; Mercer, N.; Armstrong, J.; Chen, Y.H. Variation in host usage among geographic populations of Leptinotarsa decemlineata, the Colorado potato beetle. J. Pest Sci. 2014, 87, 597–608. [Google Scholar] [CrossRef]
- Barceloux, D.G. Potatoes, tomatoes, and solanine toxicity (Solanum tuberosum L., Solanum lycopersicum L.). Dis.-A-Mon. 2009, 55, 391–402. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, R.; Huang, W.; Liu, W.; Zhan, J.; Wang, R.; Zhao, X.; Feng, Q. Seed Traits and Germination of Invasive Plant Solanum rostratum (Solanaceae) in the Arid Zone of Northern China Indicate Invasion Patterns. Plants 2024, 13, 3287. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, G.; Song, Z.; Zhang, H.; Yan, J.; Zhang, T.; Fu, W. Effects of Solanum rostratum invasion on soil properties in different soil types. Chin. J. Agrometeorol. 2017, 38, 76. [Google Scholar] [CrossRef]
- Zhao, J.; Lou, A. Genetic diversity and population structure of the invasive plant Solanum rostratum in China. Russ. J. Ecol. 2017, 48, 134–142. [Google Scholar] [CrossRef]
- Peerzada, A.M.; Ali, H.H.; Hanif, Z.; Bajwa, A.A.; Kebaso, L.; Frimpong, D.; Iqbal, N.; Namubiru, H.; Hashim, S.; Rasool, G.; et al. Eco-biology, impact, and management of Sorghum halepense (L.) Pers. Biol. Invasions 2023, 25, 955–973. [Google Scholar] [CrossRef]
- Zheng, M.Y.; Song, Y.T.; Li, C.L.; Na, M.H.; Wu, Y.N.; Ma, J.; Yu, Y. Analysis of Potential Geographic Distribution of Solanum rostratum Based on Optimized MaxEnt Model in Agro-pastoral Ecotone of Northern China. Acta Agrestia Sin. 2024, 32, 3905–3914. Available online: https://link.cnki.net/urlid/11.3362.S.20240912.0924.002 (accessed on 15 July 2025).
- Guo, J.; Cao, W.; Zhang, Y.; Gao, Y.; Wang, Y.Y. Prediction of the potential distribution area of Solanum rostratum in northeast China. Pratacultural Sci. 2019, 36, 2476–2484. [Google Scholar] [CrossRef]
- Meng, D.; Dong, J.Y.; Jiang, H.Y.; Zhao, S.G.; Wu, Y.J.; Dang, H.L.; Fang, Y.X. Prediction of the Potential Distribution Area of Solanum rostratum Dunal in Inner Mongolia under Climate Change. Acta Agrestia Sin. 2025, 32, 213–221. Available online: https://link.cnki.net/urlid/11.3362.S.20240903.1812.007 (accessed on 15 July 2025).
- Solís-Montero, L.; Vega-Polanco, M.; Vázquez-Sánchez, M.; Suárez-Mota, M.E. Ecological niche modeling of interactions in a buzz-pollinated invasive weed. Glob. Ecol. Conserv. 2022, 39, e02279. [Google Scholar] [CrossRef]
- Zhao, J.L.; Solis-Montero, L.; Lou, A.R.; Vallejo-Marín, M. Population structure and genetic diversity of native and invasive populations of Solanum rostratum (Solanaceae). PLoS ONE 2013, 8, e79807. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Eminniyaz, A.; Qiu, J.; Tan, D.; Baskin, C.C.; Baskin, J.M.; Nowak, R.S. Dispersal mechanisms of the invasive alien plant species Buffalobur (Solanum rostratum) in cold desert sites of Northwest China. Weed Sci. 2013, 61, 557–563. [Google Scholar] [CrossRef]
- Bowers, K.A.W. The pollination ecology of Solanum rostratum (Solanaceae). Am. J. Bot. 1975, 62, 633–638. [Google Scholar] [CrossRef]
- Huang, T.; Yang, T.; Wang, K.; Huang, W. Assessing the current and future potential distribution of Solanum rostratum Dunal in China using multisource remote sensing data and principal component analysis. Remote Sens. 2024, 16, 271. [Google Scholar] [CrossRef]
- Feller, U.; Vaseva, I.I. Extreme climatic events: Impacts of drought and high temperature on physiological processes in agronomically important plants. Front. Environ. Sci. 2014, 2, 39. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, C.; Li, X.; Cui, H.; Huang, H.; Sui, B.; Meng, Q.; Zhang, H. Factors affecting buffalobur (Solanum rostratum) seed germination and seedling emergence. Weed Sci. 2009, 57, 521–525. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, X.; Huang, W.; Zhan, J.; He, Y. Drought stress influences the growth and physiological characteristics of Solanum rostratum Dunal seedlings from different geographical populations in China. Front. Plant Sci. 2021, 12, 733268. [Google Scholar] [CrossRef] [PubMed]
- Kou, D.; Sun, Y.; Long, L.; Wang, J.; Wu, J.; Long, T.; Li, W. Predicting the suitable habitat of the invasive alien plant Lactuca serriola using Biomod2 model with ArcGIS. Environ. Res. Commun. 2025, 7, 045029. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, J.; Wang, X.; Chen, Y.; Mao, Z.; Lin, F.; Gong, Z.; Wang, X. Habitat suitability evaluation of invasive plant species Datura stramonium in Liaoning Province: Based on Biomod2 combination model. J. Appl. Ecol. 2023, 34, 1272–1280. [Google Scholar] [CrossRef]
- Song, Z.; Tan, D.; Zhou, G. Distribution and community characteristics of the invasive plant Solanum rostratum in Xinjiang. Arid. Zone Res. 2013, 30, 129–134. [Google Scholar] [CrossRef]
- He, J.; Hasbagen; Menggenqiqige; Hu, M. A newly recorded alien invasive plant: Solanum rostratum in Inner Mongolia. Acta Pratacult. J. Inn. Mong. Norm. Univ. (Nat. Sci. Ed.) 2011, 40, 288–290. Available online: https://kns.cnki.net/kcms2/article/abstract?v=5q1osi_AJORLWN4QIC8kRatDJzdMgXczn4mmzOZ00pa8z7xxqFsu-1H8wXFB4EX72n0kYlTBAsk4qUNuCsvRScSfhxEsrWJtZfn3LEp0BcZgqpElVvRjc-KdbQco38ckMDgZGWSrSX-XM15-PKg04NlvQDIdWHPo&uniplatform=NZKPT (accessed on 15 July 2025).
- Jia, X.; Li, B.; Chen, Y. First Record of the Harmful Plant Solanum rostratum in Guyang. Baotou Daily, 2011. Available online: https://kns.cnki.net/kcms2/article/abstract?v=5q1osi_AJORJy-m1nr2cnK8UJBeDSz82g04o3hNUa10Opkd4JCiIVyfh8tOnm4SQ-KzkpkH2pWoyZF5lEix3xsy_rgAUf4tYtV5DJonpgbkmgupKKSi58d_PHOOFIEVHDqBeGIiAPNbblNSBzh9LRHAy_eiCN8rWfW--zfYvvGP-6Aj-lkrkag==&uniplatform=NZKPT (accessed on 15 July 2025).
- Lin, Y.; Tan, D. A potential invasive alien plant: Solanum rostratum. Acta Phytotaxon. Sin. 2007, 5, 675–685. Available online: https://kns.cnki.net/kcms2/article/abstract?v=5q1osi_AJOTHx8BgjhqzPdq56sxz-MaNHRZ17Ql3l8uV2MMwarzSI5qJHwOYGVas2diCKXUgiWUkX1AfIGJZkcbSUcl6E4xZUCMW42F91adiBccmvLOvvaXvKfh3PQMfly2TF5pwXGtHXf6wbM8h2KFK4oh8qX43&uniplatform=NZKPT (accessed on 15 July 2025). [CrossRef]
- Qu, Z. Occurrence and control status of invasive plant Solanum rostratum in Liaoning region. Agric. Sci. Technol. Equip. 2021, 5, 14–15. [Google Scholar] [CrossRef]
- Tian, Z.; Zeng, J.; Wang, Y.; Zhu, Q. Distribution and risk assessment of the invasive plant Solanum rostratum in Ningxia, China. Ningxia J. Agri. Fores. Sci. Tech 2021, 62, 61–64. Available online: https://kns.cnki.net/kcms2/article/abstract?v=5q1osi_AJOTrFB0BeS58JbxmYtucpVmlZibk7FvgmPt00ZCUWwgfaRHbZTsIMqBHUEevvmczH_4PZhPM50jRO24Pq3gXN85xO0eDFTpM_N54b0_PmN4_M0zFL_GlaR3ynTWI7otaeKGcJqD8SZ-Mcu_X2-DBG3PJ3NURDnIyiy0=&uniplatform=NZKPT (accessed on 15 July 2025).
- Akinwande, M.O.; Dikko, H.G.; Samson, A. Variance inflation factor: As a condition for the inclusion of suppressor variable (s) in regression analysis. Open J. Stat. 2015, 5, 754. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Wang, L.; Li, Z.; Shi, Z.; Yang, X.; Yahdjian, L. Plant invasion risk assessment in Argentina’s arid and semi-arid rangelands. J. Environ. Manag. 2025, 377, 124648. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F.; Georges, M.D.; Thuiller, C.W. Package ‘biomod2’. Species Distribution Modeling Within Ensemble Forecasting Framework. 2016. 1600-0587. Available online: https://cran.r-project.org/web/packages/biomod2/index.html (accessed on 15 July 2025).
- Nelder, J.A.; Wedderburn, R.W.M. Generalized linear models. J. R. Stat. Soc. Ser. A Stat. Soc. 1972, 135, 370–384. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. Available online: https://www.jstor.org/stable/2699986 (accessed on 15 July 2025). [CrossRef]
- Hastie, T.J. Generalized Additive Models. In Generalized Additive Models; Taylor & Francis: London, UK, 2017; pp. 249–307. [Google Scholar] [CrossRef]
- Zakeri, I.F.; Adolph, A.L.; Puyau, M.R.; Vohr, F.A.; Butt, N.F. Multivariate adaptive regression splines models for the prediction of energy expenditure in children and adolescents. J. Appl. Physiol. 2010, 108, 128–136. [Google Scholar] [CrossRef]
- Yarnold, P.R.; Soltysik, R.C.; Bennett, C.L. Predicting in-hospital mortality of patients with AIDS-related Pneumocystis carinii pneumonia: An example of hierarchically optimal classification tree analysis. Stat. Med. 1997, 16, 1451–1463. [Google Scholar] [CrossRef]
- Zupan, J. Introduction to artificial neural network (ANN) methods: What they are and how to use them. Acta Chim. Slov. 1994, 41, 327. [Google Scholar]
- Kruithof, N.; Vegter, G. Envelope surfaces. Proc. Twenty-Second. Annu. Symp. Comput. Geom. 2006, 411–420. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Buja, A. Flexible discriminant analysis by optimal scoring. J. Am. Stat. Assoc. 1994, 89, 1255–1270. [Google Scholar] [CrossRef]
- Rigatti, S.J. Random forest. J. Insur. Med. 2017, 47, 31–39. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
| Climate Scenario | Period | Total Suitable Area/km2 | Highly Suitable Area/km2 | Contraction Area/km2 | Expansion Area/km2 | Unchanged Area/km2 | Contraction Rate/% | Expansion Rate/% |
|---|---|---|---|---|---|---|---|---|
| Current | 1,191,586.55 | 600,215.76 | —— | —— | —— | —— | —— | |
| SSP126 | 2030s | 2,353,087.05 | 1,362,399.01 | 16,940 | 360,960 | 491,190 | 4.483 | 129.979 |
| 2050s | 2,419,584.54 | 1,468,682.39 | 77,740 | 774,410 | 143,940 | 9.123 | 16.891 | |
| 2070s | 2,427,033.55 | 1,467,659.71 | 57,860 | 860,490 | 55,400 | 6.3 | 6.033 | |
| 2090s | 2,530,792.36 | 1,636,708.54 | 28,480 | 887,410 | 132,720 | 3.11 | 14.491 | |
| SSP245 | 2030s | 2,357,819.24 | 1,305,520.30 | 34,560 | 343,340 | 474,220 | 9.145 | 125.488 |
| 2050s | 2,544,239.44 | 1,646,379.74 | 41,150 | 776,410 | 250,480 | 5.033 | 30.638 | |
| 2070s | 2,680,518.58 | 1,946,596.01 | 22,520 | 1,004,370 | 212,410 | 2.193 | 20.685 | |
| 2090s | 2,620,111.73 | 1,884,886.90 | 117,840 | 1,098,940 | 80,030 | 9.685 | 6.577 | |
| SSP370 | 2030s | 2,391,533.69 | 1,334,178.87 | 33,910 | 343,990 | 489,980 | 8.973 | 129.659 |
| 2050s | 2,643,836.62 | 1,690,488.06 | 28,470 | 805,500 | 250,350 | 3.414 | 30.019 | |
| 2070s | 2,810,975.55 | 2,053,106.81 | 96,280 | 959,570 | 326,470 | 9.119 | 30.92 | |
| 2090s | 2,939,625.48 | 2,035,003.91 | 133,550 | 1,152,490 | 124,550 | 10.385 | 9.685 | |
| SSP585 | 2030s | 1,933,157.77 | 1,146,210.69 | 47,600 | 330,300 | 385,810 | 12.596 | 102.093 |
| 2050s | 2,680,658.82 | 1,921,309.23 | 26,540 | 689,570 | 51,060 | 3.706 | 71.314 | |
| 2070s | 2,850,610.42 | 1,387,471.26 | 470,890 | 729,370 | 14,570 | 39.232 | 12.153 | |
| 2090s | 2,976,804.38 | 1,822,833.93 | 64,320 | 810,920 | 330,250 | 7.349 | 37.733 | |
| Type | Variable | Description | VIF |
|---|---|---|---|
| Bioclimatic variables | Bio_3 | Isothermality (BIO2/BIO7 × 100) | 2.474489 |
| Bio_9 | Mean temperature of driest quarter | 6.236408 | |
| Bio_15 | Precipitation seasonality (coefficient of variation) | 4.626837 | |
| Precipitation | Prec_1 | Precipitation in January | 3.350388 |
| Prec_6 | Precipitation in June | 5.838437 | |
| Prec_9 | Precipitation in September | 4.605355 | |
| Temperature | Tmax_7 | Maximum temperature in July | 2.787535 |
| Tmin_12 | Minimum temperature in December | 4.164368 |
| Model Name | Model Code | References |
|---|---|---|
| Generalized linear model | GLM | Nelder et al. [46] |
| Gradient boosting machine | GBM | Friedman [47] |
| Generalize additive model | GAM | Hastie [48] |
| Multivariate adaptive regression spline model | MARS | Zakeri et al. [49] |
| Classification tree analysis model | CTA | Yarnold et al. [50] |
| Artificial neural networks model | ANN | Zupan [51] |
| Surface range envelop model | SRE | Kruithof et al. [52] |
| Flexible discriminant analysis model | FDA | Hastie et al. [53] |
| Random forest model | RF | Rigatti [54] |
| Maximum entropy model | MaxEnt | Phillips et al. [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhao, J.; Jiang, L.; Han, X.; Zhu, Y. Predicting the Potential Suitable Habitat of Solanum rostratum in China Using the Biomod2 Ensemble Modeling Framework. Plants 2025, 14, 2779. https://doi.org/10.3390/plants14172779
Wang J, Zhao J, Jiang L, Han X, Zhu Y. Predicting the Potential Suitable Habitat of Solanum rostratum in China Using the Biomod2 Ensemble Modeling Framework. Plants. 2025; 14(17):2779. https://doi.org/10.3390/plants14172779
Chicago/Turabian StyleWang, Jiajie, Jingdong Zhao, Lina Jiang, Xuejiao Han, and Yuanjun Zhu. 2025. "Predicting the Potential Suitable Habitat of Solanum rostratum in China Using the Biomod2 Ensemble Modeling Framework" Plants 14, no. 17: 2779. https://doi.org/10.3390/plants14172779
APA StyleWang, J., Zhao, J., Jiang, L., Han, X., & Zhu, Y. (2025). Predicting the Potential Suitable Habitat of Solanum rostratum in China Using the Biomod2 Ensemble Modeling Framework. Plants, 14(17), 2779. https://doi.org/10.3390/plants14172779






