Colchicine-Induced Polyploidization Influences the Morphological, Physiological, and Biochemical Characteristics of Cyclocarya paliurus
Abstract
1. Introduction
2. Results
2.1. Polyploidy Induction Rate
2.2. Ploidy Determination
2.3. Comparative Analysis of Growth and Morphological Characteristics
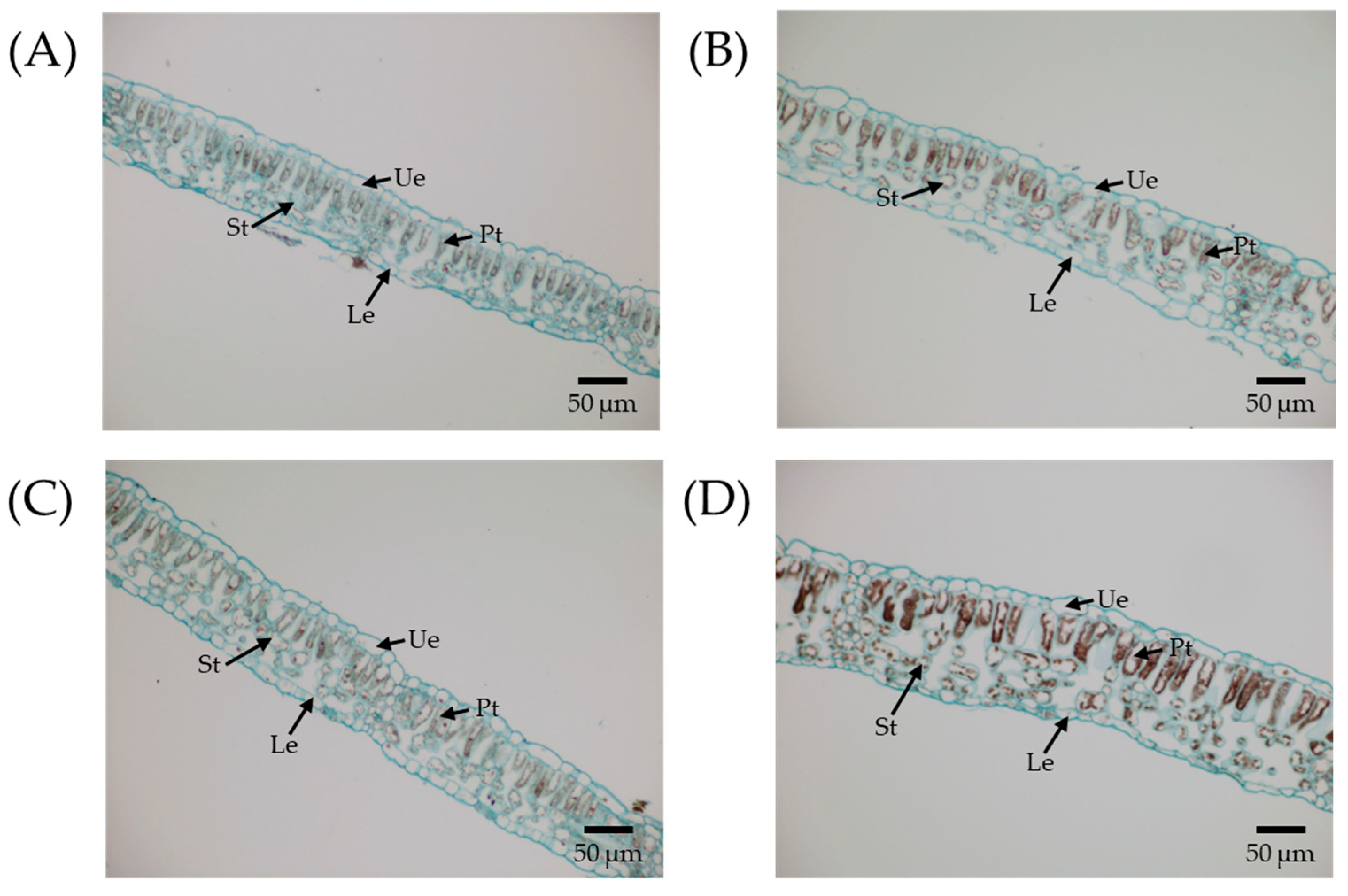
2.4. Analysis of Leaf Anatomical Structure
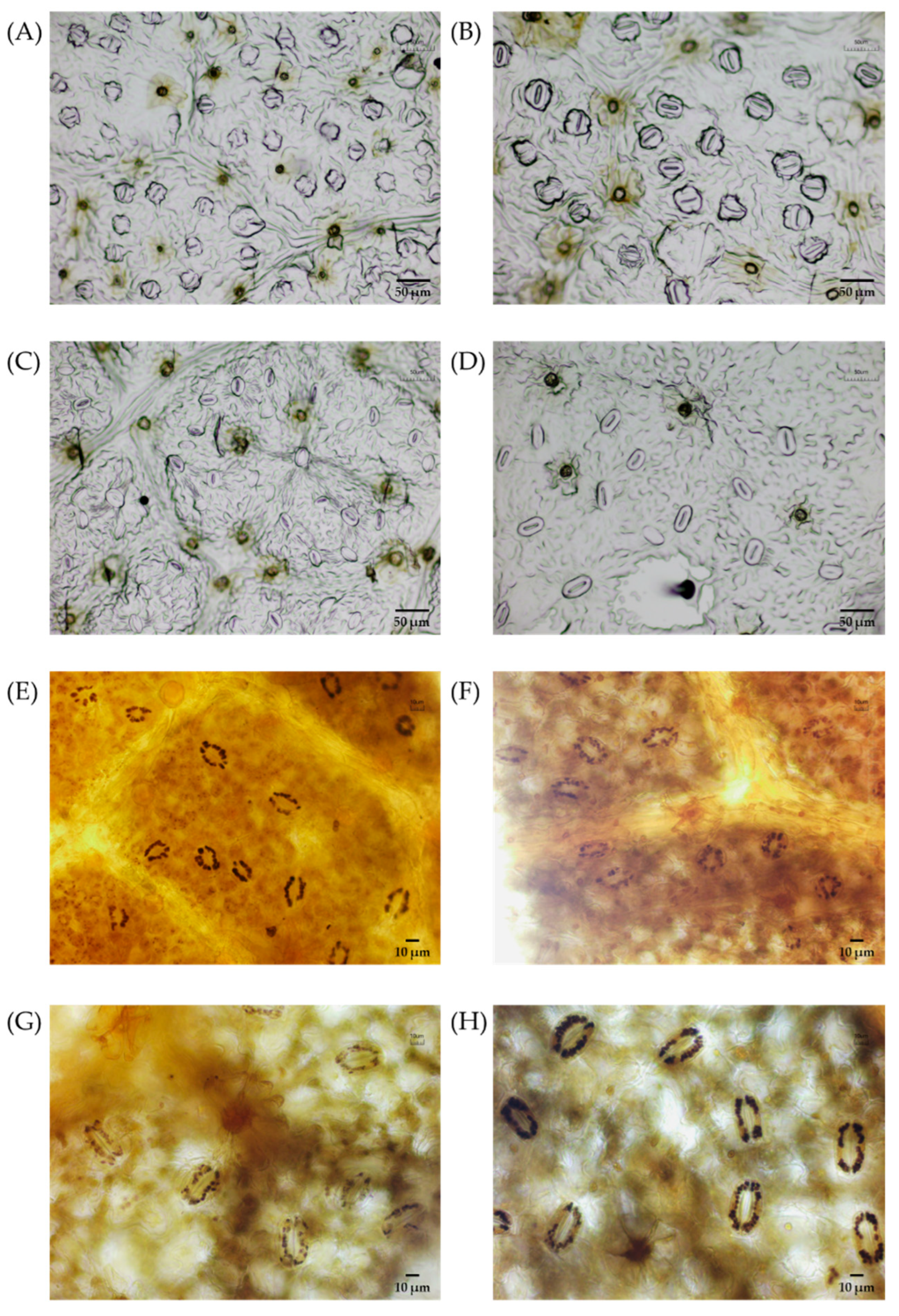
2.5. Analysis of Stomatal Characteristics
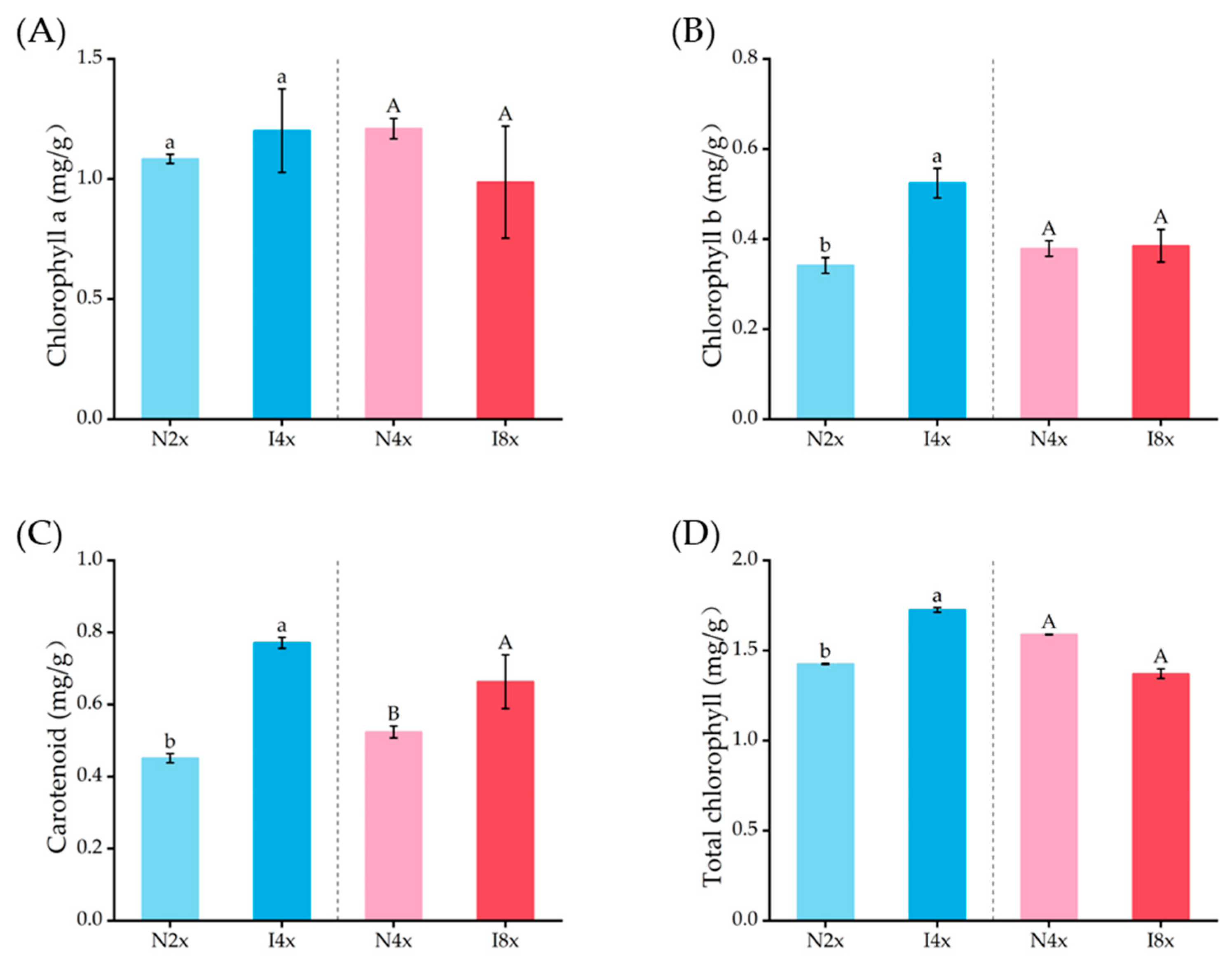
2.6. Analysis of Photosynthetic Pigments in Leaves
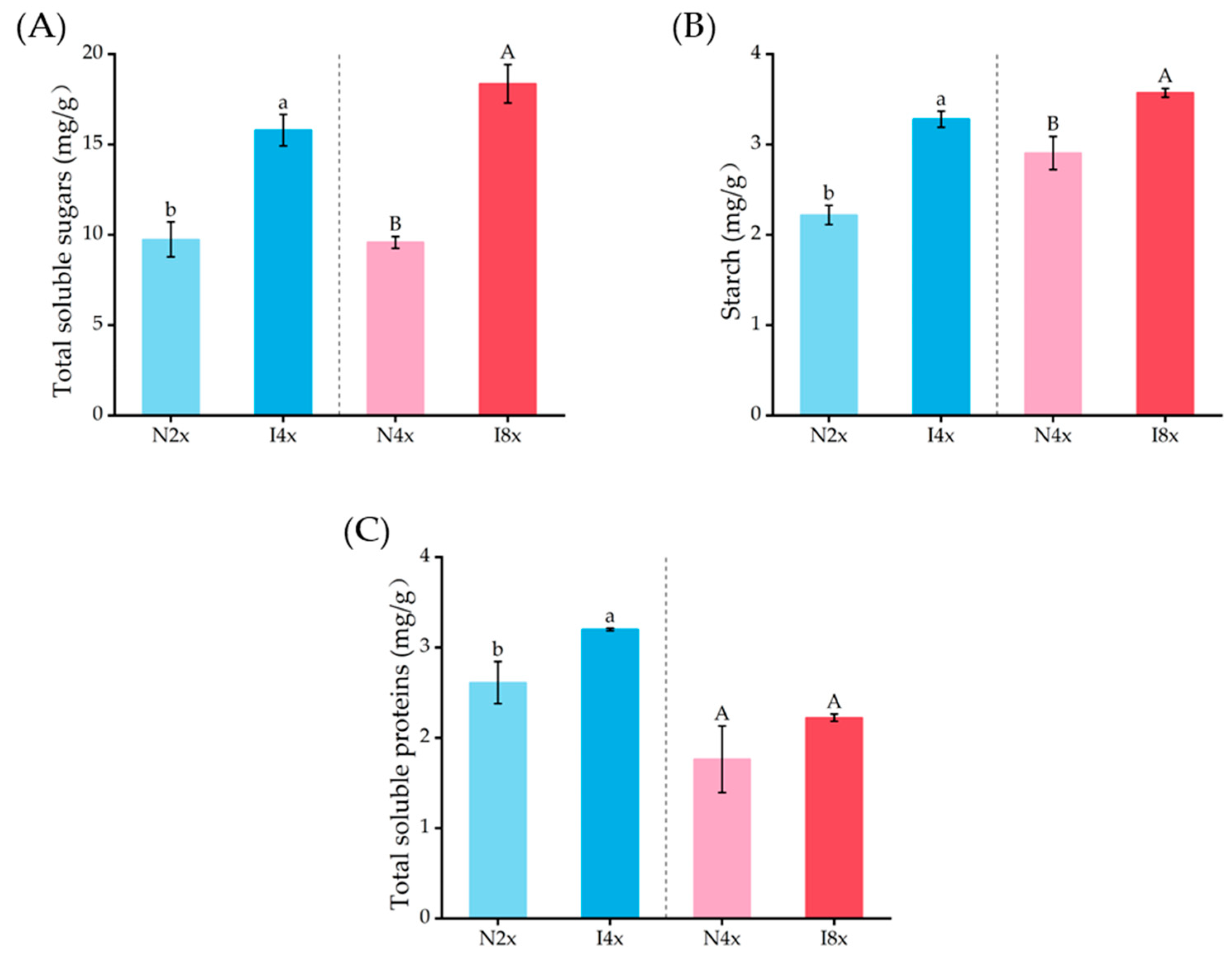
2.7. Analysis of Primary Metabolites in Leaves
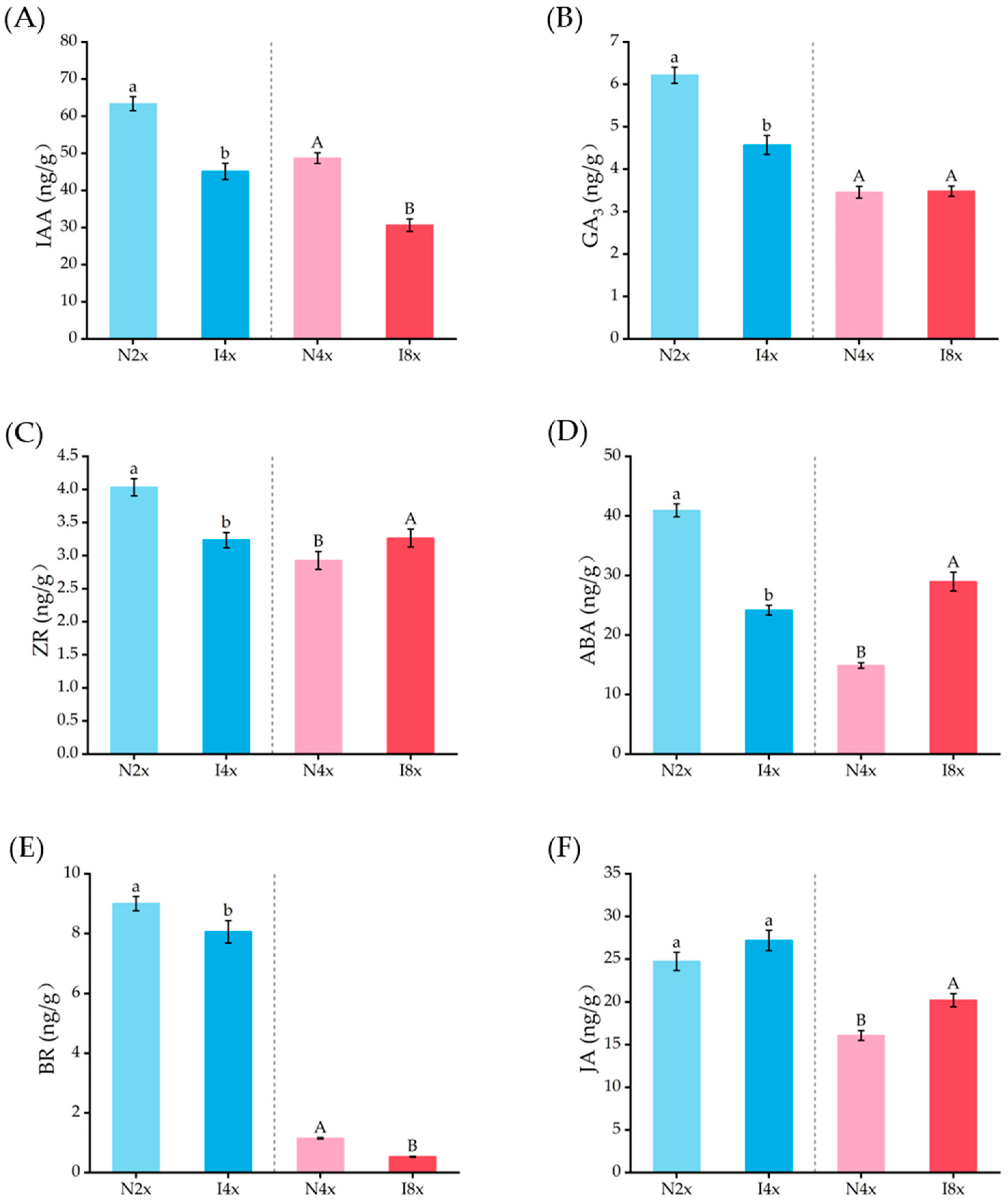
2.8. Analysis of Endogenous Hormones in Leaves
3. Discussion
3.1. Effect of Colchicine on Polyploidy Induction
3.2. Morphological and Anatomical Changes in Polyploidies
3.3. Biochemical Changes in Polyploidies
4. Materials and Methods
4.1. Plant Material
4.2. Colchicine Treatment
4.3. Ploidy Identification
4.3.1. Flow Cytometry (FCM) Analysis
4.3.2. Chromosome Counting
4.4. Growth and Morphological Measurements
4.5. Stomata Observation
- n = number of stomata in the view;
- L = length of view;
- W = width of view.
4.6. Leaf Anatomical Structure Observation
4.7. Physiological and Biochemical Analysis
4.7.1. Chlorophyll Content Measurement
4.7.2. Primary Metabolite Content Measurement
4.7.3. Endogenous Hormone Content Measurement
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| N2x | Natural diploid |
| I4x | Induced tetraploid |
| N4x | Natural tetraploid |
| I8x | Induced octoploid |
| ZR | Zeatin riboside |
| IAA | Indole-3-acetic acid |
| BR | Brassinosteroids |
| GA3 | Gibberellic acid |
| JA | Jasmonic acid |
| ABA | Abscisic acid |
References
- Fang, S.; Wang, J.; Wei, Z.; Zhu, Z. Methods to Break Seed Dormancy in Cyclocarya paliurus (Batal) Iljinskaja. Sci. Hortic. 2006, 110, 305–309. [Google Scholar] [CrossRef]
- Fang, S.; Sun, D.; Shang, X.; Fu, X.; Yang, W. Variation in Radial Growth and Wood Density of Cyclocarya paliurus across Its Natural Distribution. New For. 2019, 51, 453–467. [Google Scholar] [CrossRef]
- Li, C.; Wan, Y.; Shang, X.; Fang, S. Integration of Transcriptomic and Metabolomic Analysis Unveils the Response Mechanism of Sugar Metabolism in Cyclocarya paliurus Seedlings Subjected to PEG-induced Drought Stress. Plant Physiol. Biochem. 2023, 201, 107856. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Yang, W.; Chu, X.; Shang, X.; She, C.; Fu, X. Provenance and Temporal Variations in Selected Flavonoids in Leaves of Cyclocarya paliurus. Food Chem. 2011, 124, 1382–1386. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Chen, Z.; Kowalchuk, G.A.; Fu, X.; Kuramae, E.E. Microbial Inoculants Modulate Growth Traits, Nutrients Acquisition and Bioactive Compounds Accumulation of Cyclocarya paliurus (Batal.) Iljinskaja under degraded field condition. For. Ecol. Manag. 2021, 482, 118897. [Google Scholar] [CrossRef]
- Xie, J.H.; Wang, Z.J.; Shen, M.Y.; Nie, S.P.; Gong, B.; Li, H.S.; Zhao, Q.; Li, W.J.; Xie, M.Y. Sulfated Modification, Characterization and Antioxidant Activities of Polysaccharide from Cyclocarya paliurus. Food Hydrocoll. 2016, 53, 7–15. [Google Scholar] [CrossRef]
- Iannicelli, J.; Guariniello, J.; Tossi, V.E.; Regalado, J.J.; Di Ciaccio, L.; van Baren, C.M.; Pitta Álvarez, S.I.; Escandón, A.S. The “Poly-ploid Effect” in the Breeding of Aromatic and Medicinal Species. Sci. Hortic. 2020, 260, 108854. [Google Scholar] [CrossRef]
- Madani, H.; Escrich, A.; Hosseini, B.; Sanchez-Muñoz, R.; Khojasteh, A.; Palazon, J. Effect of Polyploidy Induction on Natural Metabolite Production in Medicinal Plants. Biomolecules 2021, 11, 8899. [Google Scholar] [CrossRef]
- Fox, D.T.; Soltis, D.E.; Soltis, P.S.; Ashman, T.-L.; Van de Peer, Y. Polyploidy: A Biological Force From Cells to Ecosystems. Trends Cell Biol. 2020, 30, 688–694. [Google Scholar] [CrossRef]
- Corneillie, S.; De Storme, N.; Van Acker, R.; Fangel, J.U.; De Bruyne, M.; De Rycke, R.; Geelen, D.; Willats, W.G.T.; Vanholme, B.; Boerjan, W. Polyploidy Affects Plant Growth and Alters Cell Wall Composition. Plant Physiol. 2019, 179, 74–87. [Google Scholar] [CrossRef]
- Bharati, R.; Severová, L. Artificial Polyploidy as A Tool for Improving Growth and Stress Resilience in Tree Species. Front. For. Glob. Change. 2025, 8, 1569384. [Google Scholar] [CrossRef]
- Mo, L.; Chen, J.-H.; Chen, F.; Xu, Q.-W.; Tong, Z.-K.; Huang, H.-H.; Dong, R.-H.; Lou, X.-Z.; Lin, E.-P. Induction and characterization of polyploids from seeds of Rhododendron fortunei Lindl. J. Integr. Agric. 2020, 19, 2016–2026. [Google Scholar] [CrossRef]
- Abdolinejad, R.; Shekafandeh, A.; Jowkar, A. In vitro Tetraploidy Induction creates Enhancements in Morphological, Physiological and Phytochemical Characteristics in the Fig Tree (Ficus carica L.). Plant Physiol. Biochem. 2021, 166, 191–202. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Yang, W.; Li, X.; Hou, L.; Li, M.; Pang, X.; Li, Y. Hybrid Triploid Induced by Megaspore Chromosome Doubling in Jujube (Ziziphus jujuba Mill.) ‘Maya’ and Its Characteristics. Forests 2021, 12, 112. [Google Scholar] [CrossRef]
- Kara, Z.; Yazar, K. Induction of Polyploidy in Grapevine (Vitis vinifera L.) Seedlings by in Vivo Colchicine Applications. Turk. J. Agric. For. 2022, 46, 152–159. [Google Scholar] [CrossRef]
- Mohammadi, V.; Talebi, S.; Ahmadnasab, M.; Mollahassanzadeh, H. The Effect of Induced Polyploidy on Phytochemistry, Cellular Organelles and the Expression of Genes Involved in Thymol and Carvacrol Biosynthetic Pathway in Thyme (Thymus vulgaris). Front. Plant Sci. 2023, 14, 1228844. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, X.; Kong, B.; Zhou, Q.; Sang, Y.; Zhang, P. In vitro Octaploid Induction of Populus hopeiensis with Colchicine. BMC Plant Biol. 2022, 22, 176. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, L.; Li, J.E.; Lu, M.; An, H. Polyploid Induction and Identification of Rosa roxburghii f. eseiosa. Plants 2023, 12, 2194. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, H.; Wang, Y. Advances in Screening of Horticultural Plant Chemical Mutagenesis and Resistance Mutants. Chin. Agric. Sci. Bull. 2005, 8, 302–305. [Google Scholar]
- Gantait, S.; Mukherjee, E. Induced Autopolyploidy—A Promising Approach for Enhanced Biosynthesis of Plant Secondary Metabolites: An Insight. J. Genet. Eng. Biotechnol. 2021, 19, 4. [Google Scholar] [CrossRef]
- Touchell, D.H.; Palmer, I.E.; Ranney, T.G. In vitro Ploidy Manipulation for Crop Improvement. Front. Plant Sci. 2020, 11, 722. [Google Scholar] [CrossRef]
- Zeng, Q.Q. Induction of Hexaploid and Molecular Mechanism of Its Trait Variation in White Polar. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2020. [Google Scholar] [CrossRef]
- Zhang, R.; Rao, S.; Wang, Y.; Qin, Y.; Qin, K.; Chen, J. Chromosome Doubling Enhances Biomass and Carotenoid Content in Lycium chinense. Plants 2024, 13, 439. [Google Scholar] [CrossRef]
- Bharati, R.; Shmeit, Y.H.; Šedivá, J.H.; Cong, T.T.N.; Kundu, J.K.; Severová, L.; Svoboda, R.; Fernández-Cusimamani, E. Comparative Assessment of Morphological, Cytological, and Photosynthetic Characteristics of the Induced Octoploid and Its Tetraploid Counterpart of Celosia argentea L. BMC Plant Biol. 2024, 24, 1227. [Google Scholar] [CrossRef]
- Van Drunen, W.E.; Husband, B.C. Immediate vs. Evolutionary Consequences of Polyploidy on Clonal Reproduction in An Autopolyploid Plant. Ann. Bot. 2018, 122, 195–205. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, T.; Bashir, M.A.; Baig, M.M.Q.; Quresh, A.A.; Shah, M.K.N.; Hafiz, I.A. Induction and Identification of Colchicine-induced Polyploidy in Gladiolus grandiflorus ‘White Prosperity’. Folia Hortic. 2018, 30, 307–319. [Google Scholar] [CrossRef]
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The Polyploidy and Its Key Role in Plant Breeding. Planta 2015, 243, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Lourkisti, R.; Oustric, J.; Quilichini, Y.; Froelicher, Y.; Herbette, S.; Morillon, R.; Berti, L.; Santini, J. Improved Response of Triploid Citrus Varieties to Water Deficit is Related to Anatomical and Cytological Properties. Plant Physiol. Biochem. 2021, 162, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Di, Z.; Zhang, R.; Mu, Y.; Sun, T.; Tian, Z.; Lu, Y.; Zheng, J. Tetraploid Induction with Leaf Morphology and Sunburn Variation in Sorbus pohuashanensis (Hance) Hedl. Forests 2023, 14, 1589. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Ye, T.; Zhao, J.; Wang, L.; Wei, H.; Liu, P.; Liu, M. Autotetraploidization Alters Morphology, Photosynthesis, Cytological Characteristics and Fruit Quality in Sour Jujube (Ziziphus acidojujuba Cheng et Liu). Plants 2023, 12, 1106. [Google Scholar] [CrossRef]
- Wu, W.; Du, K.; Kang, X.; Wei, H. The Diverse Roles of Cytokinins in Regulating Leaf Development. Hortic. Res. 2021, 8, 118. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of Phytohormones and Their Signalling Pathways in Leaf Development and Stress Responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef]
- Yang, W.X.; Fang, S.Z.; Shang, X.L.; Fu, X.X. A Method to Break the Dormancy of Cyclocarya paliurus. China Patent 2016105155366, 1 March 2019. [Google Scholar]
- Yan, Z.; Bai, L.; Ma, X.; Wei, S.J. Effect of Different Colohicine Treatment on Plants Variation of Siraitia grosvnorii. Seed 2012, 31, 97–98+101. [Google Scholar] [CrossRef]
- Song, Z.Q.; Bian, G.L.; Lin, F.; Hu, F.L.; Shang, X.L. Establishment and Application of A Flow Cytometry Method for Chromosome Ploidy Identification of Cyclocarya paliurus. J. Nanjing For. Uni. Nat. 2024, 48, 61–68. [Google Scholar] [CrossRef]
- Wang, X.K.; Huang, J.L. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2015; pp. 171–182. [Google Scholar]
- Yang, Y.M.; Xu, C.N.; Wang, B.M.; Jia, J.Z. Effects of Plant Growth Regulators on Secondary Wall Thickening of Cotton Fibres. Plant Growth Regul. 2001, 35, 233–237. [Google Scholar] [CrossRef]
- Weiler, E.W.; Jourdan, P.S.; Conrad, W. Levels of Indole-3-acetic Acid in Intact and Decapitated Coleoptiles as Determined by A Specific and Highly Sensitive Solid-phase Enzyme Immunoassay. Planta 1981, 153, 561–571. [Google Scholar] [CrossRef]


| Year | Provenance | Colchicine Concentration (%) | Time of Treatment (d) | Number of Treated Explants | Survival Rate (%) | Polyploid Induction Rate (%) | Mixoploid Induction Rate (%) |
|---|---|---|---|---|---|---|---|
| 2021 | JZS4x | 0.3 | 4 | 60 | 5.00 ± 5.00 b | 1.67 ± 2.87 b | 1.67 ± 2.87 b |
| 0.3 | 8 | 60 | 6.67 ± 2.87 b | 0.00 ± 0.00 b | 5.00 ± 5.00 b | ||
| 0.3 | 12 | 60 | 8.33 ± 2.87 b | 1.67 ± 2.87 b | 5.00 ± 0.00 b | ||
| 0.4 | 4 | 60 | 35.00 ± 13.23 a | 20.00 ± 5.00 a | 10.00 ± 5.00 a | ||
| 0.4 | 7 | 60 | 1.67 ± 2.87 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | ||
| 0.6 | 3 | 60 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | ||
| 0.6 | 6 | 60 | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | ||
| 2022 | WF4x | 0.4 | 4 | 48 | 52.08 ± 7.80 | 39.58 ± 7.80 | 8.33 ± 2.95 |
| WF2x | 0.4 | 4 | 84 | 51.19 ± 4.12 a | 33.33 ± 7.43 a | 5.95 ± 5.46 a | |
| 0.4 | 5 | 84 | 19.05 ± 2.06 b | 14.29 ± 3.57 b | 4.76 ± 2.06 a | ||
| 0.4 | 6 | 84 | 2.38 ± 4.12 c | 0.00 ± 0.00 c | 2.38 ± 4.12 a |
| Characteristics | N2x | I4x | N4x | I8x |
|---|---|---|---|---|
| Seedling height (cm) | 31.57 ± 0.10 a | 25.67 ± 0.06 b | 33.92 ±0.12 A | 31.17 ± 1.81 A |
| Basal diameter (mm) | 6.08 ± 0.04 a | 5.28 ± 0.34 a | 6.31 ±0.04 A | 5.15 ± 0.22 B |
| Compound leaf area (cm2) | 116.73 ± 5.01 a | 95.93 ± 10.73 b | 144.3 ± 2.71 A | 75.43 ± 1.80 B |
| Specific leaf weight (g/m2) | 22.60 ± 0.72 a | 28.83 ± 4.89 a | 25.07 ± 0.83 B | 27.08 ± 0.79 A |
| Number of compound leaves | 9.67 ± 1.53 a | 6.33 ± 0.58 b | 9.33 ± 0.58 A | 6.67 ± 1.15 B |
| Terminal leaflet length (cm) | 7.72 ± 0.25 b | 9.22 ± 0.81 a | 8.41 ± 0.02 B | 10.69 ± 1.30 A |
| Terminal leaflet width (cm) | 2.90 ± 0.20 a | 3.44 ± 0.71 a | 3.59 ± 0.36 B | 4.53 ± 0.37 A |
| Characteristics | N2x | I4x | N4x | I8x |
|---|---|---|---|---|
| Leaflet thickness (μm) | 83.84 ± 3.64 a | 89.02 ± 1.32 a | 92.84 ± 0.66 B | 110.50 ± 1.33 A |
| Upper epidermal thickness (μm) | 11.15 ± 1.70 a | 10.40 ± 0.72 a | 12.12 ± 1.05 A | 11.20 ± 0.83 A |
| Lower epidermal thickness (μm) | 10.07 ± 1.13 a | 11.05 ± 1.73 a | 11.68 ± 0.61 A | 11.12 ± 0.85 A |
| Palisade tissue thickness (μm) | 31.18 ± 2.74 a | 35.48 ± 0.80 a | 36.44 ± 2.45 B | 43.32 ± 1.04 A |
| Spongy tissue thickness (μm) | 31.44 ± 2.25 a | 32.09 ± 1.68 a | 32.60 ± 2.18 B | 44.86 ± 1.11 A |
| Characteristics | N2x | I4x | N4x | I8x |
|---|---|---|---|---|
| Stomatal length (μm) | 25.23 ± 2.40 b | 36.00 ± 1.36 a | 30.68 ± 2.50 B | 35.57 ± 0.80 A |
| Stomatal width (μm) | 17.64 ± 1.87 b | 22.49 ± 2.33 a | 20.42 ± 1.91 B | 24.74 ± 3.36 A |
| Stomatal density (N/mm2) | 156.99 ± 3.75 a | 57.41 ± 1.06 b | 126.93 ± 10.31 A | 50.01 ± 1.35 B |
| Chloroplast number (N/Stoma) | 14.52 ± 0.52 b | 21.42 ± 2.11 a | 23.04 ± 2.06 B | 30.24 ± 0.81 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, G.; Yi, Y.; Song, Z.; Huang, Y.; Mao, Q.; Qin, J.; Shang, X. Colchicine-Induced Polyploidization Influences the Morphological, Physiological, and Biochemical Characteristics of Cyclocarya paliurus. Plants 2025, 14, 2778. https://doi.org/10.3390/plants14172778
Bian G, Yi Y, Song Z, Huang Y, Mao Q, Qin J, Shang X. Colchicine-Induced Polyploidization Influences the Morphological, Physiological, and Biochemical Characteristics of Cyclocarya paliurus. Plants. 2025; 14(17):2778. https://doi.org/10.3390/plants14172778
Chicago/Turabian StyleBian, Guoliang, Yan Yi, Ziqi Song, Yanmeng Huang, Qianxing Mao, Jian Qin, and Xulan Shang. 2025. "Colchicine-Induced Polyploidization Influences the Morphological, Physiological, and Biochemical Characteristics of Cyclocarya paliurus" Plants 14, no. 17: 2778. https://doi.org/10.3390/plants14172778
APA StyleBian, G., Yi, Y., Song, Z., Huang, Y., Mao, Q., Qin, J., & Shang, X. (2025). Colchicine-Induced Polyploidization Influences the Morphological, Physiological, and Biochemical Characteristics of Cyclocarya paliurus. Plants, 14(17), 2778. https://doi.org/10.3390/plants14172778






