In Vitro Techniques for Seed Potato (Solanum tuberosum L.) Tuber Production: A Systematic Review
Abstract
1. Introduction
2. Literature Review
3. In Vitro Potato Seed Tuber Cultivation Techniques
3.1. Meristem Culture
3.2. In Vitro Micropropagation
3.3. Microtubers Culture
3.4. Propagation in Bioreactors
3.5. Synthetic Seed Production
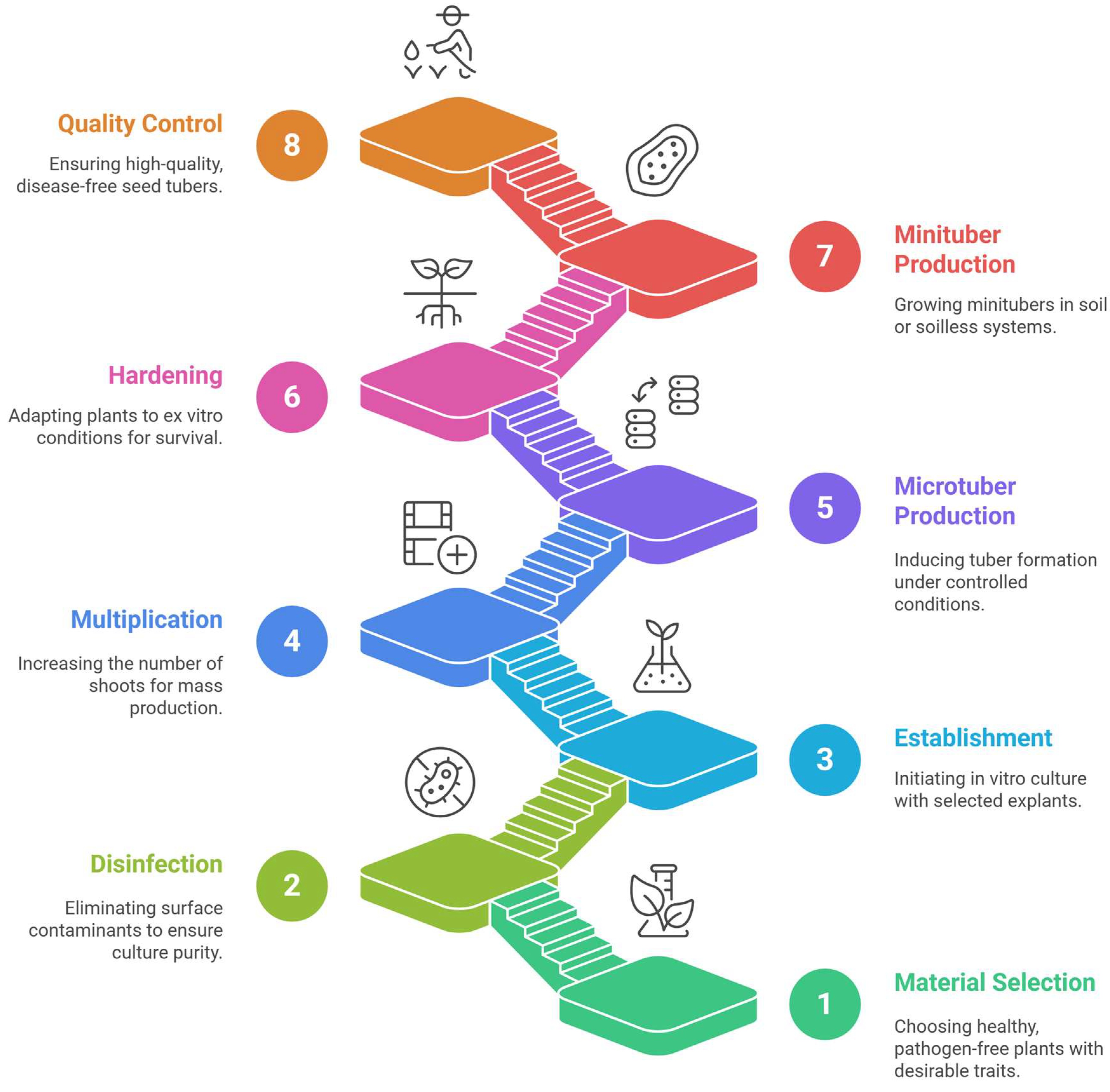
4. Key Procedure for the In Vitro Cultivation of Seed Potato Tubers
4.1. Selection and Preparation of Initial Planting Material
4.2. Establishment and Multiplication (Micropropagation)
4.2.1. Composition of the Culture Medium and Plant Growth Regulators (PGRs)
- Auxins, such as 1-naphthaleneacetic acid (NAA), indole-3-acetic acid (IAA), and indole-3-butyric acid (IBA), are incorporated for the purpose of optimizing various developmental parameters, exerting a significant influence on shoot length, the number of nodes, and the number of leaves [39,64]. IBA is used in certain systems for the formation of nodes and the induction of root primordia, which favors rooting of explants [37,42].
- Cytokinins, such as 6-benzylaminopurine (BAP) and kinetin, have been shown to induce essential processes for cell development, such as cell division, growth, and differentiation, contributing significantly to tissue regeneration. BAP is essential for the establishment of tuber shoot culture and shoot proliferation [45], while kinetin is incorporated in media designed for meristems or in temporary immersion systems, where it optimizes explant response [35]. In the field of molecular and cell biology, zeatin emerges as a cytokinin of significant relevance in the context of embryogenesis. Its research has been characterized by its remarkable impact on somatic embryo formation, highlighting its importance in embryonic developmental processes [43].
- Gibberellins, in particular, gibberellic acid (GA3), play a crucial role in the in vitro culture process, particularly in the stem elongation phase [52]. Their combination with auxins, such as naphthaleneacetic acid (NAA), is decisive in stimulating explant growth and development [23]. A 20:1 ratio (0.2 mg/L GA3 and 0.01 mg/L NAA) has been shown to induce longer stems with more nodes [52]. In botany, gibberellic acid (GA3) is used to induce the breakdown of dormancy in potato (Solanum tuberosum L.) seeds [61]. Furthermore, in media designed for the proliferation of root primordia or meristems, GA3 has been shown to favor their regeneration and development [35].
- Other combinations: The interaction between auxins and gibberellic acid (GA3) is crucial to achieve efficient regeneration and adequate axillary bud growth in binodal explants. This combination optimizes the formation and elongation of vegetative structures. Regarding the differentiation of aerial parts from shoot apices, the use of a medium presenting a higher concentration of cytokinins than auxins is recommended, resulting in increased cell proliferation and shoot development in vitro culture [39].
4.2.2. Other Additives
4.2.3. Environmental Conditions
4.3. Induction and Production of Microtubers In Vitro
4.3.1. Culture Medium
4.3.2. Plant Growth Regulators (PGRs)
4.3.3. Lighting Conditions
4.3.4. Type of Explant
4.3.5. Culture Systems
4.3.6. Yield
4.4. Hardening and Acclimatization
4.5. Ex Vitro Seed Tuber Production (Minitubers)
- Soil or substrates: Seedlings can be transplanted directly into soil or different types of substrates [71]. It has been documented that the use of mineral substrates, such as coarse sand, optimizes the acclimatization process and increases the survival rate in the field [26]. Soil-less substrate configurations, growing beds, or containers are also implemented [1].
- Soilless systems: Technologies such as hydroponics, semi-hydroponics, and aeroponics have been used to optimize growth [1]. Aeroponics is considered a modern and efficient technique for minituber production, providing a soilless environment where roots and subway stems develop in a dark chamber. In this system, water and nutrients are supplied by a sprayed solution, which facilitates the formation of minitubers in subway stolons [11].
4.6. Quality Control and Sanitation
5. Economic Impact and Sustainability
6. Practical Applications and Future Research
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 2,4-D | 2,4-Dichlorophenoxyacetic acid |
| AgNO3 | Silver thiosulfate |
| BA | Benzyladenine |
| BAP | 6-Benzylaminopurine |
| TIBs | Temporary immersion bioreactor systems |
| CaCl2 | Calcium chloride |
| EC | Electrical conductivity |
| G0 | Minitubers obtained from acclimatized in vitro plants |
| GA3 | Gibberellic acid |
| HPI-T | Metal halide tubular lamp |
| IAA | Indole-3-acetic acid |
| IBA | Indole-3-butyric acid |
| LED | Light emitting diode |
| IoT | Internet of Things |
| MS | Murashige and Skoog |
| NAA | Naphthaleneacetic acid |
| PGRs | Plant growth regulators |
| PVY | Potato virus Y |
| SETIS™ | A type of temporary immersion bioreactor |
| SON-T | High pressure sodium tubular lamp |
| TDZ | Thidiazuron |
| TM | Thiophanate methyl |
| TPS | Botanical seeds |
References
- Boubaker, H.; Saadaoui, W.; Dasgan, H.Y.; Tarchoun, N.; Gruda, N.S. Enhancing Seed Potato Production from In Vitro Plantlets and Microtubers through Biofertilizer Application: Investigating Effects on Plant Growth, Tuber Yield, Size, and Quality. Agronomy 2023, 13, 2541. [Google Scholar] [CrossRef]
- Ahlawat, Y.; Yadav, K.; Samani, M.; Chaudhary, D. Harnessing In-Vitro Propagation for the Sustainable Conservation of Medicinal Plants: Challenges and Prospects. In Medicinal and Aromatic Plants; Spring: Berlin/Heidelberg, Germany, 2024; pp. 27–37. [Google Scholar]
- Song, Y.; Spooren, J.; Jongekrijg, C.D.; Manders, E.J.H.H.; de Jonge, R.; Pieterse, C.M.J.; Bakker, P.A.H.M.; Berendsen, R.L. Seed tuber imprinting shapes the next-generation potato microbiome. Environ. Microbiome 2024, 19, 12. [Google Scholar] [CrossRef]
- Hasnain, A.; Naqvi, S.A.H.; Ayesha, S.I.; Khalid, F.; Ellahi, M.; Iqbal, S.; Hassan, M.Z.; Abbas, A.; Adamski, R.; Markowska, D.; et al. Plants in vitro propagation with its applications in food, pharmaceuticals and cosmetic industries; current scenario and future approaches. Front. Plant Sci. 2022, 13, 1009395. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Wang, M.-R.; Wang, Q.-C. In Vitro Regeneration, Micropropagation and Germplasm Conservation of Horticultural Plants. Horticulturae 2024, 10, 45. [Google Scholar] [CrossRef]
- Kulus, D.; Tymoszuk, A. Advancements in In Vitro Technology: A Comprehensive Exploration of Micropropagated Plants. Horticulturae 2024, 10, 88. [Google Scholar] [CrossRef]
- Del Mar, M.; Curtin, S.J.; Gutiérrez, J.J. Potato improvement through genetic engineering. GM Crops Food 2022, 12, 479. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, B.V.; Zebrin, S.N.; Blinkov, E.G.; Gracheva, I.A. Potato seed quality control system development in Russia. Res. Crops 2021, 22, 91–95. [Google Scholar]
- Awati, R.; Bhattacharya, A.; Char, B. Rapid multiplication technique for production of high-quality seed potato (Solanum tuberosum L.) tubers. J. Appl. Biol. Biotechnol. 2019, 7, 1–5. [Google Scholar] [CrossRef]
- Gutarra, D.M.; Chagua, C.P.C. Characteristics and yield of basic seeds of native potato (Solanum goniocalyx) obtained in temporary immersion bioreactor under greenhouse conditions. Rev. Faculty. Agron. lniv. Zulia 2021, 38, 322–341. [Google Scholar]
- Broćić, Z.; Momčilović, I.; Poštić, D.; Oljača, J.; Veljković, B. Production of High-Quality Seed Potato by Aeroponics. In The Potato Crop: Management, Production, and Food Security; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; pp. 25–59. [Google Scholar]
- Veli-Matti, R.; Terttu, K.K.; Elina, V.; Maria, P.A. Bioreactor technologies for mass propagation of potato: Future prospects. In Bulbous Plants: Biotechnology; CRC Press: Boca Raton, FL, USA, 2016; pp. 37–49. [Google Scholar]
- Devaux, A.; Kromann, P.; Ortiz, O. Potatoes for Sustainable Global Food Security. Potato Res. 2014, 57, 185–199. [Google Scholar] [CrossRef]
- Tokbergenova, Z.A.; Babayev, S.A.; Togayeva, D.U.; Kudusbekova, D.Z.; Zagurskii, A.V. Efficiency of microtubers application in the production of original potato seeds. Online J. Biol. Sci. 2017, 17, 316–322. [Google Scholar] [CrossRef]
- Belguendouz, A.; Kaide Harche, M.; Benmahioul, B. Evaluation of different culture media and activated charcoal supply on yield and quality of potato microtubers grown in vitro. J. Plant. Nutr. 2021, 44, 2123–2137. [Google Scholar] [CrossRef]
- Pervaiz, A.; Sajid, Z.A.; Yousaf, S.; Aftab, F. Microtuberization Potential of Jasmonic Acid, Kinetin and Putrescine in Potato (Solanum tuberosum L.). Am. J. Potato Res. 2023, 100, 184–191. [Google Scholar] [CrossRef]
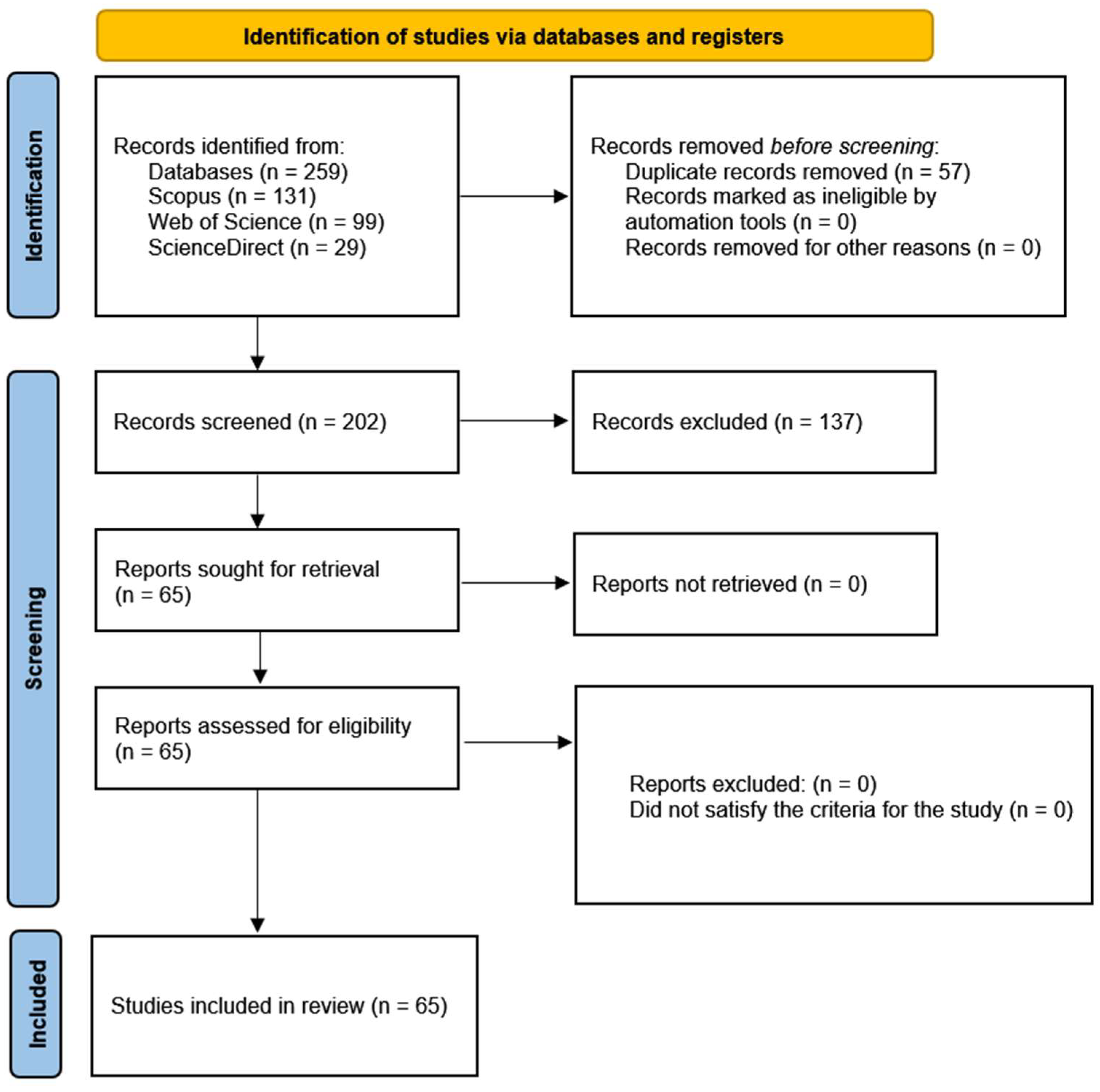
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Morel, G.; Martin, C. Guérison de dahlias atteints d’une maladie á virus. Comptes Rendus Acad. Sci. 1952, 235, 1324–1325. [Google Scholar]
- Macdonald, D.M. Heat treatment and meristem culture as a means of freeing potato varieties from viruses X and S. Potato Res. 1973, 16, 263–269. [Google Scholar] [CrossRef]
- Silva Agurto, C.; Leiva Mora, M.; Sanchez Ortiz, N.; Del Castillo Bastidas, D. Influence of lighting conditions on the in vitro bud set of Solanum tuberosum L. var. Cecilia. Bionatura 2023, 8, 1–9. Available online: http://revistabionatura.com/2023.08.03.9.html (accessed on 26 April 2025).
- Lugovtsova, S.Y.; Stupko, V.Y.; Neshumaeva, N.A.; Zobova, N.V. Potato phytopathogene bank and in vitro seed farming: Illumination, medium. IOP Conf. Ser. Earth Environ. Sci. 2021, 677, 022094. [Google Scholar] [CrossRef]
- Ariste, A.; Ojeda Zacarías, M.D.C.; Lozoya Saldaña, H.; Olivares Sáenz, E.; García Zambrano, E.A.; Ibarra López, A.; Cham, A.K. Optimized in vitro micropropagation and microtuber production in potato (Solanum tuberosum L.) through apical buds using hormone regulation and tissue culture techniques. J. Exp. Biol. Agric. Sci. 2025, 13, 86–96. [Google Scholar] [CrossRef]
- Murashige, T. Plant Propagation Through Tissue Cultures. Annu. Rev. Plant Biol. 1974, 25, 135–166. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- de Morais, T.P.; Asmar, S.A.; de Jesus SILVA, H.F.; Luz, J.M.Q.; de Melo, B. Application of tissue culture techniques in Potato [Aplicação da cultura de tecidos vegetais em Batata]. Biosci. J. 2018, 34, 952–969. [Google Scholar] [CrossRef]
- Hussey, G.; Stacey, N.J. In Vitro Propagation of Potato (Solanum tuberosum L.). Ann. Bot. 1981, 48, 787–796. [Google Scholar] [CrossRef]
- Akita, M.; Takayama, S. Mass Propagation of Potato Tubers Using Jar Fermentor Techniqes. Acta Hortic. 1988, 230, 55–62. [Google Scholar] [CrossRef]
- Sausen, D.; Carvalho, I.R.; Schorr, M.R.W.; da Silva Tavares, M.; Schwalbert, R.; Marques, A.C.R.; Tarouco, C.P.; Mambrin, R.B.; Lautenchleger, F.; Nicoloso, F.T. Potato plants micropropagated and grown from mini tubers: Nutritional efficiency to phosphorus. Aust. J. Crop Sci. 2022, 16, 436–440. [Google Scholar] [CrossRef]
- Sidibe, A.; Dembele, K.; Toure, M.; Diarra, M.M.; Ag Sid Ahmed, I.A.A.; Traore, F. Possible alternative for national supply of seed potatoes (Solanum tuberosum L.) from in vitro culture at the Agro-physio-genetic and Plants Biotechnology laboratory of IPR / IFRA of Katibougou, Mali. Int. J. Biol. Chem. Sci. 2020, 14, 3117–3128. [Google Scholar] [CrossRef]
- Wang, P.-J.; Hu, C.-Y. In vitro mass tuberization and virus-free seed-potato production in Taiwan. Am. Potato J. 1982, 59, 33–37. [Google Scholar] [CrossRef]
- Naik, P.S.; Buckseth, T. Recent advances in virus elimination and tissue culture for quality potato seed production. In Biotechnologies of Crop Improvement; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 131–158. [Google Scholar]
- Li, R.; Long, J.; Yan, Y.; Luo, J.; Xu, Z.; Liu, X. Addition of White Light to Monochromatic Red and Blue Lights Alters the Formation, Growth, and Dormancy of In Vitro-grown Solanum tuberosum L. Microtubers. HortScience 2020, 55, 71–77. [Google Scholar] [CrossRef]
- Teisson, C.; Alvard, D. In vitro production of potato microtubers in liquid medium using temporary immersion. Potato Res. 1999, 42, 499–504. [Google Scholar] [CrossRef]
- Daurov, D.; Daurova, A.; Sapakhova, Z.; Kanat, R.; Akhmetzhanova, D.; Abilda, Z.; Toishimanov, M.; Raissova, N.; Otynshiyev, M.; Zhambakin, K.; et al. The Impact of the Growth Regulators and Cultivation Conditions of Temporary Immersion Systems (TISs) on the Morphological Characteristics of Potato Explants and Microtubers. Agronomy 2024, 14, 1782. [Google Scholar] [CrossRef]
- Andriani, S.; Siregar, L.A.M.; Safni, I. Microtubers production by using Temporary Immersion System (TIS) bioreactor to potato varieties. IOP Conf. Ser. Earth Environ. Sci. 2021, 886, 012005. [Google Scholar] [CrossRef]
- Tang, L.; Syed, A.-u.-A.; Otho, A.R.; Junejo, A.R.; Tunio, M.H.; Hao, L.; Asghar Ali, M.N.H.; Brohi, S.A.; Otho, S.A.; Channa, J.A. Intelligent Rapid Asexual Propagation Technology—A Novel Aeroponics Propagation Approach. Agronomy 2024, 14, 2289. [Google Scholar] [CrossRef]
- Neysi, B.; Pour Mohammadi, P.; Salehi Salmi, M.R. Cell Suspension Culture, Somatic Embryo Genetic and Synthetic Seed Production in Different Cultivars of Potato. J. Crop Breed. 2022, 14, 42–48. [Google Scholar] [CrossRef]
- Kumlay, A.M. Combination of the Auxins, NAA.; IBA, and IAA with GA3 Improves the Commercial Seed-Tuber Production of Potato (Solanum tuberosum L.) under In Vitro Conditions. Biomed Res. Int. 2014, 2014, 439259. [Google Scholar] [CrossRef]
- Kenar, S.; Baysal Furtana, G.; Şebnem Ellialtioğlu, Ş.; Tipirdamaz, R. Patateste (Solanum tuberosum L.) in vitro Mikrotuberizasyon Üzerine Fotoperiyot, Jasmonik Asit ve Aktif Kömürün Etkileri. Yüzüncü Yıl Üniversitesi Tarım Bilimleri Dergisi 2017, 27, 318–325. [Google Scholar] [CrossRef][Green Version]
- Adly, W.M.R.M.; Niedbała, G.; EL-Denary, M.E.; Mohamed, M.A.; Piekutowska, M.; Wojciechowski, T.; Abd El-Salam, E.-S.T.; Fouad, A.S. Somaclonal Variation for Genetic Improvement of Starch Accumulation in Potato (Solanum tuberosum) Tubers. Plants 2023, 12, 232. [Google Scholar] [CrossRef]
- Kalsoom, T.; Ahmed, T.; Khan, M.A.; Hasanuzzaman, M.; Ahmed, M.; Werbrouck, S.P.O. Synthetic Seed Production of Potato under Different Fungicide Levels and Storage Intervals. Phyton-Int. J. Exp. Bot. 2023, 92, 2429–2450. [Google Scholar] [CrossRef]
- Molla, M.M.H.; Akhter, F.; Naznin, S.; Islam, S.; Salam, M.A.; Hossain, M.Z. Regeneration efficiency of five high yielding potato varieties of Bangladesh. Bangladesh J. Bot. 2022, 51, 637–645. [Google Scholar] [CrossRef]
- Harun, M.; Islam, S.M.S.; Miah, M.A.B.; Subramaniam, S. In vitro screening of calli and evaluation their physiological states for the enhancement of regeneration efficiency in various potato (Solanum tuberosum L.) genotypes. Biocatal. Agric. Biotechnol. 2020, 28, 101715. [Google Scholar] [CrossRef]
- Kujeke, G.T.; Chitendera, T.C.; Masekesa, R.T.; Mazarura, U.; Ngadze, E.; Rugare, J.T.; Matikiti, A. Micropropagation of Livingstone Potato (Plectranthus esculentus N.E.Br). Adv. Agric. 2020, 2020, 8364153. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Petropoulos, S.A. Tissue Culture of Potato for Seed Production. In The Potato Crop: Management, Production, and Food Security; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; pp. 61–89. [Google Scholar]
- Betancur, N.A.B.; Giraldo, D.T.; de Jesús Montoya Pérez, N.; González, J.M.G. Production of potato (Solanum tuberosum L.) elite seed of the Diacol Capiro variety in an aeroponic system. Acta Agronómica 2023, 72, 252–257. [Google Scholar]
- Bychkova, O.V.; Brovko, E.S.; Mironenko, O.N.; Khlebova, L.P.; Nebylitsa, A.V. Improvement of approaches to elimination of viruses in potatoes using in vitro culture. Sib. J. Life Sci. Agric. 2023, 15, 74–91. [Google Scholar] [CrossRef]
- Iveta, M.; Maia, K.; Ekaterine, B.; Tamar, S. Formation in vitro potato collection and regeneration under modified conditions. Res. J. Biotechnol. 2020, 15, 98–103. [Google Scholar]
- Hajare, S.T.; Chauhan, N.M.; Kassa, G. Effect of Growth Regulators on in Vitro Micropropagation of Potato (Solanum tuberosum L.) Gudiene and Belete Varieties from Ethiopia. Sci. World J. 2021, 2021, 5928769. [Google Scholar] [CrossRef]
- Parveen, F.; Khatun, M.; Islam, A. Micropropagation of environmental stress tolerant local potato (Solanum tuberosum L.) varieties of Bangladesh. Plant Tissue Cult. Biotechnol. 2014, 24, 101–109. [Google Scholar] [CrossRef]
- Stupko, V.Y.; Lugovtsova, S.Y. The use of phytohormones to increase the efficiency of potato propagation in nodal cuts culture. BIO Web Conf. 2023, 66, 01006. [Google Scholar] [CrossRef]
- Abeuova, L.S.; Kali, B.R.; Rakhimzhanova, A.O.; Bekkuzhina, S.S.; Manabayeva, S.A. High frequency direct shoot regeneration from kazakh commercial potato cultivars. PeerJ 2020, 8, e9447. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Subrahmanyeswari, T.; Mallick, J.; Dey, S.; Bhattacharyya, S.; Gantait, S. A mono-phasic protocol for micropropagation of potato cv. Cooch Behar local, its acclimatization, on-field evaluation, and fidelity analysis. 3 Biotech 2025, 15, 50. [Google Scholar] [CrossRef]
- Mohapatra, P.P.; Batra, V.K.; Kajla, S.; Poonia, A.K. In vitro multiplication and microtuberization of Solanum tuberosum using different growth regulators. Vegetos 2018, 31, 114–122. [Google Scholar] [CrossRef]
- Kim, I.; Barsukova, E.; Fisenko, P.; Chekushkina, T.; Chibizova, A.; Volkov, D.; Klykov, A. Applying methods of replication and recovery of potato microplants (Solanum tuberosum L.) in seed production. E3S Web Conf. 2020, 203, 02003. [Google Scholar] [CrossRef]
- Kane, M. Micropropagation of Potato by Node Culture and Microtuber Production. In Plant Tissue Culture, Development, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2016; pp. 207–212. [Google Scholar]
- Tuba Surmer, B.; Metin Kumlay, A.; Kaya, C. Determination of Salinity Tolerance of Some Potato (Solanum tuberosum L.) Varieties under in vitro Conditions. KSÜ Tarim Doga Dergisi 2023, 26, 107–117. [Google Scholar]
- Lekamge, D.; Sasahara, T.; Yamamoto, S.I.; Hatamoto, M.; Yamaguchi, T.; Maki, S. Effect of enhanced CaCl2, MgSO4, and KH2PO4 on improved in vitro growth of potato. Plant Biotechnol. 2021, 38, 401–408. [Google Scholar] [CrossRef]
- Song, H.; Jin, R.; Quan, X.; Zhang, J.; Qi, W.; Cui, X. Shoot Production of Solanum tuberosum L. ‘Yansi’ Using a Simple Continuous Immersion Airlift Bioreactor Culture System. Potato Res. 2023, 66, 1203–1213. [Google Scholar]
- Mora, V.; Ramasamy, M.; Damaj, M.B.; Irigoyen, S.; Ancona, V.; Avila, C.A.; Vales, M.I.; Ibanez, F.; Mandadi, K.K. Identification and Characterization of Potato Zebra Chip Resistance Among Wild Solanum Species. Front. Microbiol. 2022, 13, 857493. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, K.; Tanabe, K.; Onishi, N. Production of potato (Solanum tuberosum, L.) microtubers using plastic culture bags. Plant Biotechnol. 2020, 37, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Husen, S.; Purnomo, A.E.; Wedyan, M.A.; Susilowati, E.; Nurfitriani, R. Optimization of Potato Cuttings of Granola Kembang Cultivars with the Application of Auxin and Paclobutrazol for Tuber Production. BIO Web Conf. 2024, 104, 00045. [Google Scholar] [CrossRef]
- Sevostyanova, E.P.; Akimova, S.V.; Sevostyanov, M.A.; Nasakina, E.O.; Korshunov, A.V.; Glinushkin, A.P. Obtaining micro cuttings of potatoes by clonal micropropagation. IOP Conf. Ser. Earth Environ. Sci. 2021, 663, 012061. [Google Scholar] [CrossRef]
- Patel, P.; Raval, D.; Joshi, C.; Joshi, M.; Patel, A.; Patel, F. Enhanced clonal propagation for elite potato varieties: Implications for growth performance and plant transformation studies. S. Afr. J. Bot. 2025, 184, 517–532. [Google Scholar] [CrossRef]
- Salem, J.; Hassanein, A.M. In vitro propagation, microtuberization, and molecular characterization of three potato cultivars. Biol. Plant. 2017, 61, 427–437. [Google Scholar] [CrossRef]
- Aslam, A.; Iqbal, J. Combined effect of cytokinin and sucrose on in vitro tuberization parameters of two cultivars i.e., diamant and red norland of potato (Solanum tuberosum). Pak. J. Bot. 2010, 42, 1093–1102. [Google Scholar]
- Chiru, N.; Nistor, A.; Karacsonyi, D.; Chiru, R.N. Researches regarding production of in vitro microtubers >10 mm on potato. Ann. Rom. Soc. Cell Biol. 2010, 15, 73–78. [Google Scholar]
- Szarvas, P.; Dobránszki, J. Aeration and Chemical Additives Prevent Hyperhydration and Allow the Production of High-Quality In Vitro Potato Plantlets. Agronomy 2025, 15, 1470. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhang, K.; Gong, X.C.; Wang, H.Y.; Gao, Y.H.; Wang, X.Q.; Zeng, Z.H.; Hu, Y.G. Effects of different LEDs light spectrum on the growth, leaf anatomy, and chloroplast ultrastructure of potato plantlets in vitro and minituber production after transplanting in the greenhouse. J. Integr. Agric. 2020, 19, 108–119. [Google Scholar] [CrossRef]
- Lommen, W.J.M. Effects of Age of In Vitro-Derived Potato Plantlets on Early Above- and Below-Ground Development After Planting in Different Cultivars. Potato Res. 2024, 67, 93–115. [Google Scholar] [CrossRef]
- Aghajanyan, A.; Mikaelyan, A.; Martirosyan, H.; Melyan, G. The role of plant-derived melanin in enhancing in vitro growth and nutrient accumulation in potato varieties. Bioact. Compd. Health Dis. 2024, 7, 511–524. [Google Scholar] [CrossRef]
- Batta, S.; Thakur, A.K.; Singh, R.; Singh, S.; Thakur, P.; Gupta, R. In vitro induction and plant regeneration in potato (Solanum tuberosum L.) cv. Kufri Sangam. Vegetos 2025, 1–8. [Google Scholar] [CrossRef]
- Zapata-Arias, F.J.; Akter, S.; Asadul Haque, S.M.; Akther, S.; Khatun, F.; Mazid, M.A.; Hossain, M. The Significance of Non-controlled Natural Light, Temperature and Humidity in the Commercial Micro-propagation of Solanum tuberosum L. Cultivar Diamant. Plant Tissue Cult. Biotechnol. 2014, 24, 131–139. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Tunio, M.H.; Ahmad, F.; Solangi, K.A. Overview of the aeroponic agriculture—An emerging technology for global food security. Int. J. Agric. Biol. Eng. 2020, 13, 1–10. [Google Scholar] [CrossRef]
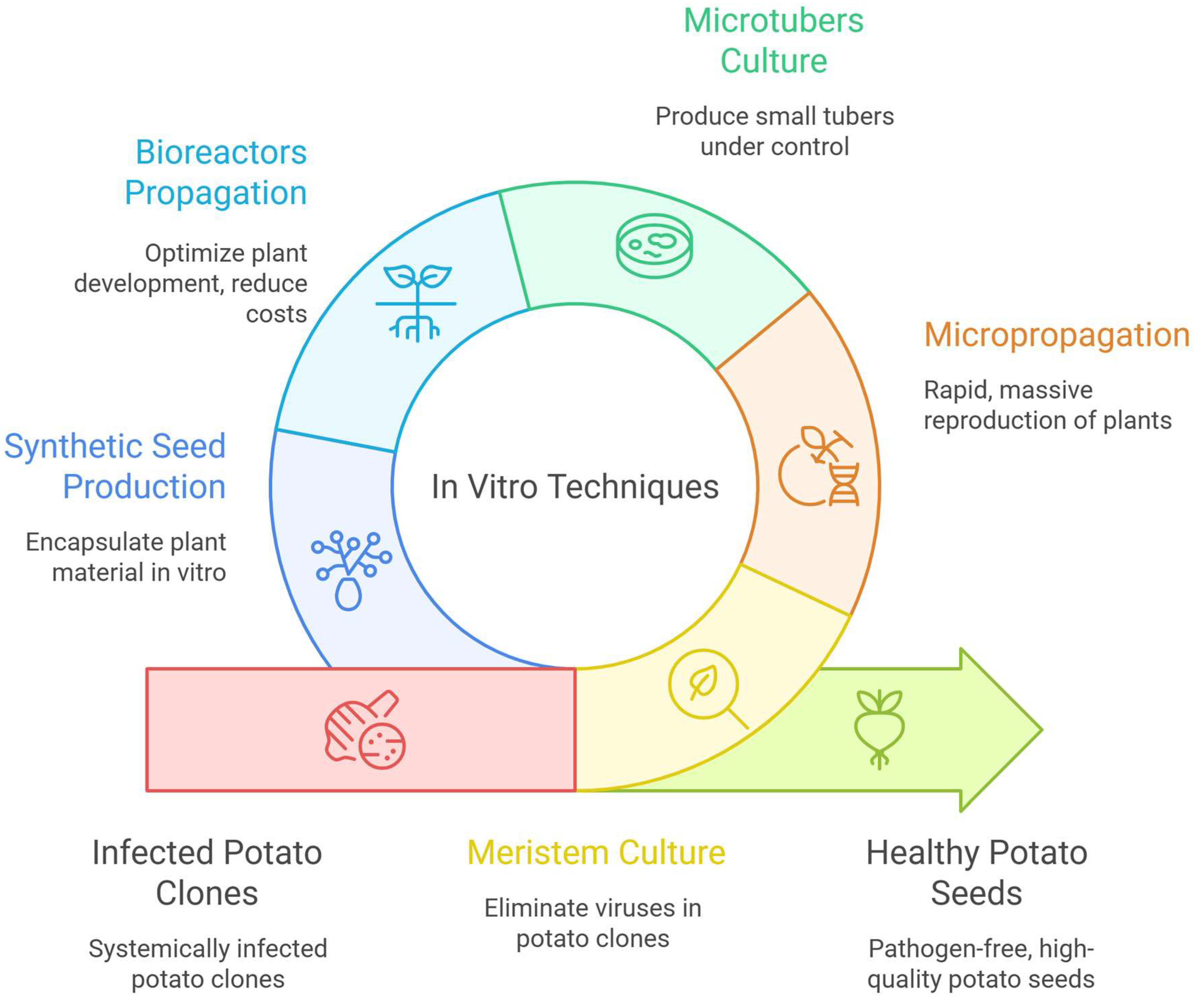
| Technique | |
|---|---|
| Meristem culture; References [21,22,26,35,39,40] | Description This technique involves isolating 0.1–0.3 mm meristematic apices with leaf primordia and cultivating them under sterile conditions using nutrient media such as MS. Its main goal is to regenerate virus-free plants while maintaining the genetic uniformity of the original clone. |
| Primary use Virus elimination of infected potato clones. Production of healthy and pathogen-free seed. | |
| Source material Apical meristems, apices, or buds are isolated from tuber shoots or from plants grown under controlled conditions. Single-node explants can also be used. | |
| Conditions/Key Means In vitro culture is performed in MS medium with salts, vitamins, and sucrose (20–30 g/L, up to 80 g/L for tuberization), solidified with agar or Gelrite. Growth regulators, such as kinetin, GA3, IAA, BAP, IBA, jasmonic acid or daminozide, and, optionally, ribavirin, as an antiviral, are added. The pH is adjusted between 5.7 and 6.0, with temperatures of 21–26 °C. Light is essential, applying a 16/8 h photoperiod (light/dark) or short day (8/16 h) with fluorescent illumination. | |
| Advantages First biotechnological approach to eliminate viruses. Obtaining healthy clones. Provides initial material of high health. | |
| Disadvantages A laborious technique with low initial yield. It requires high specialization and strict aseptic conditions. | |
| In vitro micropropagation; References [6,21,26,29,41] | Description A rapid multiplication method that uses plant tissue fragments (such as nodal segments or shoots) cultivated in artificial media under sterile conditions. It enables the large-scale production of healthy and uniform plants, essential for certified seed production. |
| Primary use Mass production of plants allows for obtaining healthy and uniform planting material, essential for certified seed. It also facilitates the production of microtubers, improving efficiency and eliminating pathogens. | |
| Source material The source materials include nodal segments, cauline apices, apical buds, tuber shoots and discs, callus, leaves, stems, and axillary buds. Botanical seeds (TPS) and virus-free tubers can also be used. The seedlings obtained are used for multiplication by nodal cuttings. | |
| Conditions/Key Means The artificial culture medium, commonly MS or 1/2 MS, is supplemented with sucrose, gelling agents (agar or Gelrite), and growth regulators, such as BAP, kinetin, GA3, NAA, IAA, and IBA, as well as supplements, such as myo-inositol, vitamins, activated charcoal, and fungicides. Strict asepsis is maintained, with the pH adjusted between 5.7 and 5.8, a controlled temperature (20–27 °C), and day/night cycles. Lighting is provided by fluorescent, LED, or SON-T/HPI-T lamps, with specific intensities and a usual photoperiod of 16 h of light. Ventilation and containers vary according to the type of culture. | |
| Advantages Alternative for mass production of plants. Rapid multiplication of healthy clones favors the efficient multiplication of microtubers, optimizing costs and improving germplasm conservation. | |
| Disadvantages Risk of somaclonal variability. Requires specialized infrastructure and rigorous control of environmental conditions. | |
| Microtubers culture; References [10,11,21,29,33,34] | Description A process that induces the formation of small potato tubers under in vitro sterile conditions, simulating field-like environments. It involves shoot proliferation followed by tuberization and is useful for basic seed production and germplasm conservation. |
| Primary use Basic and certified seed production. Facilitates germplasm conservation, genetic, and molecular research. It serves as a basis for the production of minitubers in aeroponic systems. It also has the potential to generate artificial seeds from somatic embryos. | |
| Source material Tuber shoots, nodal sections of etiolated shoots, meristematic apices, and leaf explants can be used. | |
| Conditions/Key Means It uses nutrient media such as Murashige and Skoog (MS), with sucrose up to 9% for tuberization. Gelling agents, such as agar and plant growth regulators (PGRs), such as BAP, auxins (NAA, IAA, IBA), gibberellins (GA3), kinetin, Zeatin, 2,4-D, and TDZ, are used, adjusting their proportions according to the objective. Liquid media are preferred for large-scale production, although solid media are also used. Factors such as light (photoperiod of 16 h light or continuous darkness for tuberization), temperature (18 °C for tuberization, 25 °C for initial culture, 21 °C in controlled room, 20 °C for pre-browning), and humidity influence development. Efficiency is improved with temporary immersion bioreactor systems (TIBs) and aerated bags. Nitrogen, potassium phosphate, and activated carbon adjustments optimize tuberization. | |
| Advantages Alternative for the mass production of plants. Rapid multiplication of healthy clones favors the efficient multiplication of microtubers, optimizing costs and improving germplasm conservation. | |
| Disadvantages It requires precise adjustments to tuberization conditions. Production can be limited by environmental and physiological factors. | |
| Propagation in bioreactors (temporary immersion systems—TIBs); References [5,42,43,44] | Description An automated system that uses temporary immersion bioreactor systems to cultivate plant material in liquid media, enhancing gas exchange and culture efficiency. It is designed for the mass production of microtubers and cuttings for basic seed generation. |
| Primary use Micropropagation and mass production of plants and, specifically, of potato microtubers and cuttings. They are an alternative to produce basic seed (basic category or G0). | |
| Source material It is initiated from propagated material, such as single-node explants, shoots, microtubers, or cuttings. | |
| Conditions/Key Means Temporary immersion bioreactor systems (TIBs), such as SETIS™, optimize in vitro culture by periodically immersing plant material in a liquid medium, which improves gas exchange. Their effectiveness depends on factors such as the frequency and duration of immersion cycles (every 3 h for 2 min), the composition of the medium (modified MS with 9% sucrose, low nitrogen concentration, and growth regulators, such as kinetin, GA, and IBA), and the environmental conditions, which include specific temperatures (22 °C for growth and 18 °C for microtuberization) and appropriate photoperiods (16 h of light for growth and total darkness for microtuberization). | |
| Advantages They offer high yield efficiency, favoring the formation of more tubers than solid media, with greater size and weight. In addition, tubers derived from microtubers in TIBs stand out for their superior performance in the production of basic seed, compared to those obtained from cuttings. | |
| Disadvantages High equipment costs. Requires specialized technical management and constant monitoring. | |
| Synthetic seed production; References [26,42,43,45] | Description A technique that encapsulates potato somatic embryos in calcium alginate matrices, derived from cell cultures. It facilitates the regeneration of complete plants and allows for efficient handling and transport of pathogen-free artificial seeds. |
| Primary use Production of artificial seed potato from somatic embryos. | |
| Source material Somatic embryos, which can be obtained from explants such as leaves or cell cultures in suspension. Uninodal cuttings (3–4 mm) with root primordia. | |
| Conditions/Key Means Callus induction is performed from leaves grown on MS medium with 10 mg/L 2,4-D and TDZ, promoting high callus formation. Somatic embryogenesis is carried out on solid medium or in suspension, using combinations of regulators such as zeatin and GA3 (0.1 mg/L each) or BAP and GA3 (2.5 and 5 mg/L, respectively) to enhance development. Embryos obtained in suspension are encapsulated in 3.5% calcium or sodium alginate, with charcoal and fungicide, and supplemented with 1.1% CaCl2 in medium with artificial endosperm (1/2 MS). | |
| Advantages Mass production of somatic embryos allows for the generation of artificial seeds, which can regenerate plants and provide virus-free material. In addition, synthetic seeds are easy to handle and transport. | |
| Disadvantages Risk of somaclonal variation. Low rate of embryo conversion into viable plants. Technique still under development for commercial use. | |
| Type of Explant | Characteristics | Main Use | References |
|---|---|---|---|
| Apical meristems |
|
| [21,48,49] |
| Activated axillary buds |
|
| [21,50] |
| Nodal segments (with inhibited axillary buds) |
|
| [27,51,52,53] |
| Tuber sprouts |
|
| [10,54,55] |
| Type of Explant | Characteristics | References |
|---|---|---|
| Apical meristems | MS + 30 g/L sucrose (3% MS) | [35,49,65] |
| Axillary buds | MS + 1 mg/L BAP + 0.5 mg/L GA3 | [66] |
| Nodal segments | MS + 5 mg/L BAP + 80 g/L sucrose (8% MS) + 0.75 mg/L GA3 | [23,67] |
| Stem segments | MS + 1 mg/L BAP + 0.5 mg/L NAA | [68] |
| Tuber sprouts | MS + 2 mg/L BAP + 1 mg/L NAA + 60 g/L sucrose (6% MS) | [55,66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jácome Sarchi, G.A.; Coronel Montesdeoca, N.T.; Hernández, F.; Martínez, R.T.S. In Vitro Techniques for Seed Potato (Solanum tuberosum L.) Tuber Production: A Systematic Review. Plants 2025, 14, 2777. https://doi.org/10.3390/plants14172777
Jácome Sarchi GA, Coronel Montesdeoca NT, Hernández F, Martínez RTS. In Vitro Techniques for Seed Potato (Solanum tuberosum L.) Tuber Production: A Systematic Review. Plants. 2025; 14(17):2777. https://doi.org/10.3390/plants14172777
Chicago/Turabian StyleJácome Sarchi, Guillermo Alexander, Nataly Tatiana Coronel Montesdeoca, Francisca Hernández, and Rafael Todos Santos Martínez. 2025. "In Vitro Techniques for Seed Potato (Solanum tuberosum L.) Tuber Production: A Systematic Review" Plants 14, no. 17: 2777. https://doi.org/10.3390/plants14172777
APA StyleJácome Sarchi, G. A., Coronel Montesdeoca, N. T., Hernández, F., & Martínez, R. T. S. (2025). In Vitro Techniques for Seed Potato (Solanum tuberosum L.) Tuber Production: A Systematic Review. Plants, 14(17), 2777. https://doi.org/10.3390/plants14172777








