Integrated Transcriptome and Metabolome Analysis of Mature Stage Sand Pear Fruit Response to High-Temperature Stress
Abstract
1. Introduction
2. Results
2.1. The Effect of High-Temperature Treatment on the Temperature, Firmness, and Intrinsic Phenotype of Pear Fruits
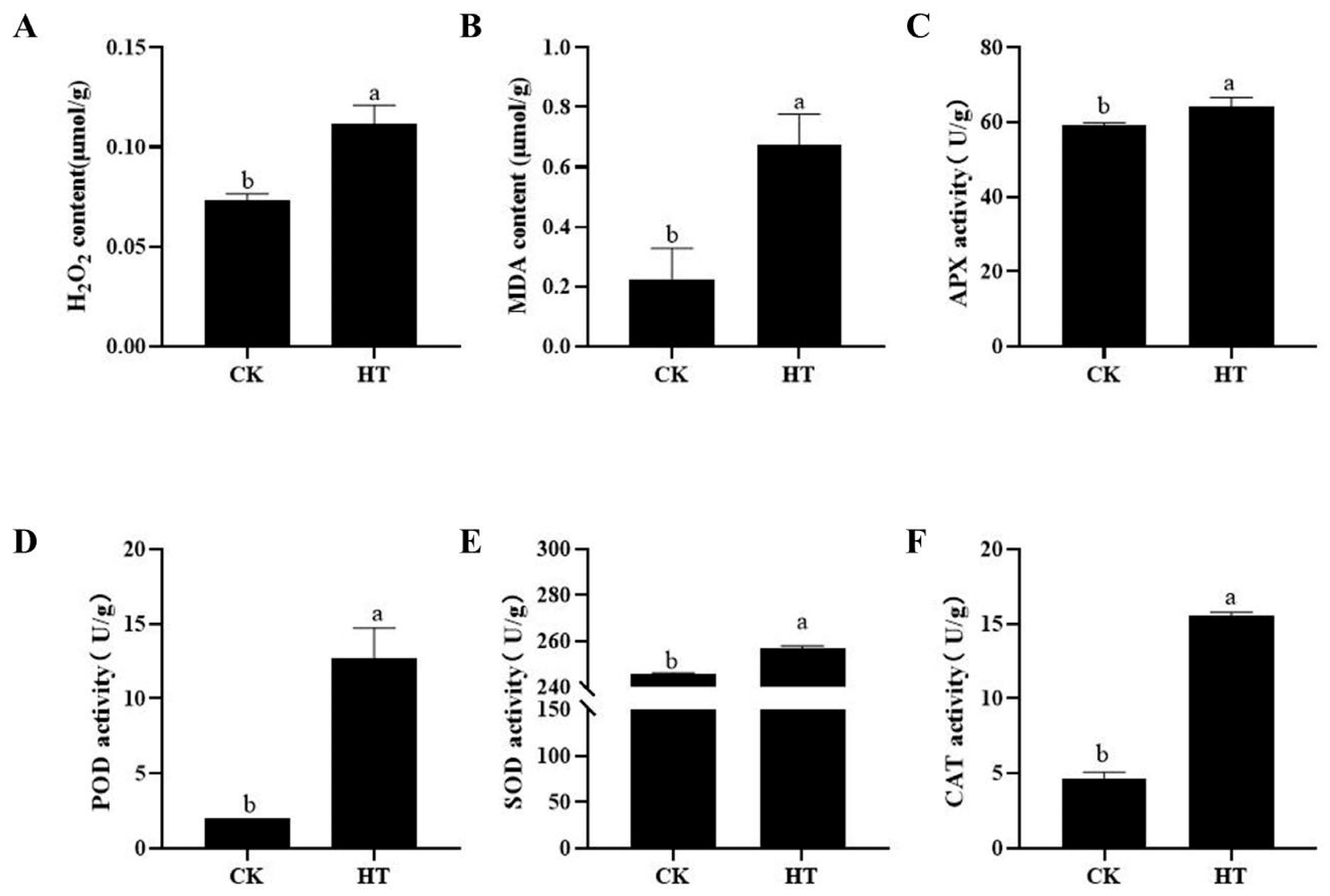
2.2. The Effect of High Temperature on the MDA, H2O2, and Activity of Antioxidant Enzymes in Fruits
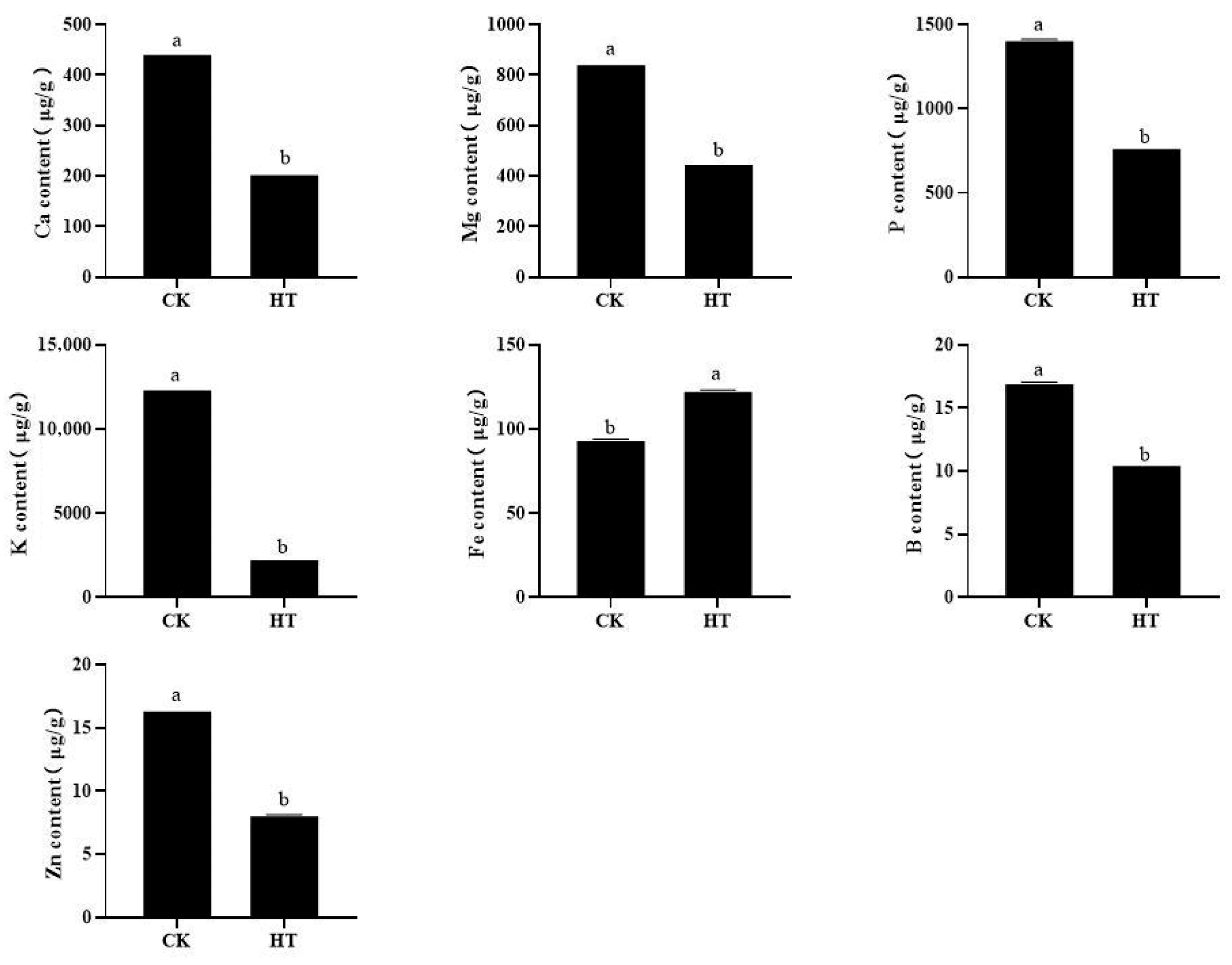
2.3. The Effect of High Temperature on the Content of Mineral Elements in Fruits
2.4. Analysis of Differential Metabolites in Fruits Under High-Temperature Stress
2.5. Changes in Main Sugar and Acid Components in Fruits Under High-Temperature Stress
2.6. Changes in Bioactive Substances in Fruits Under High-Temperature Stress
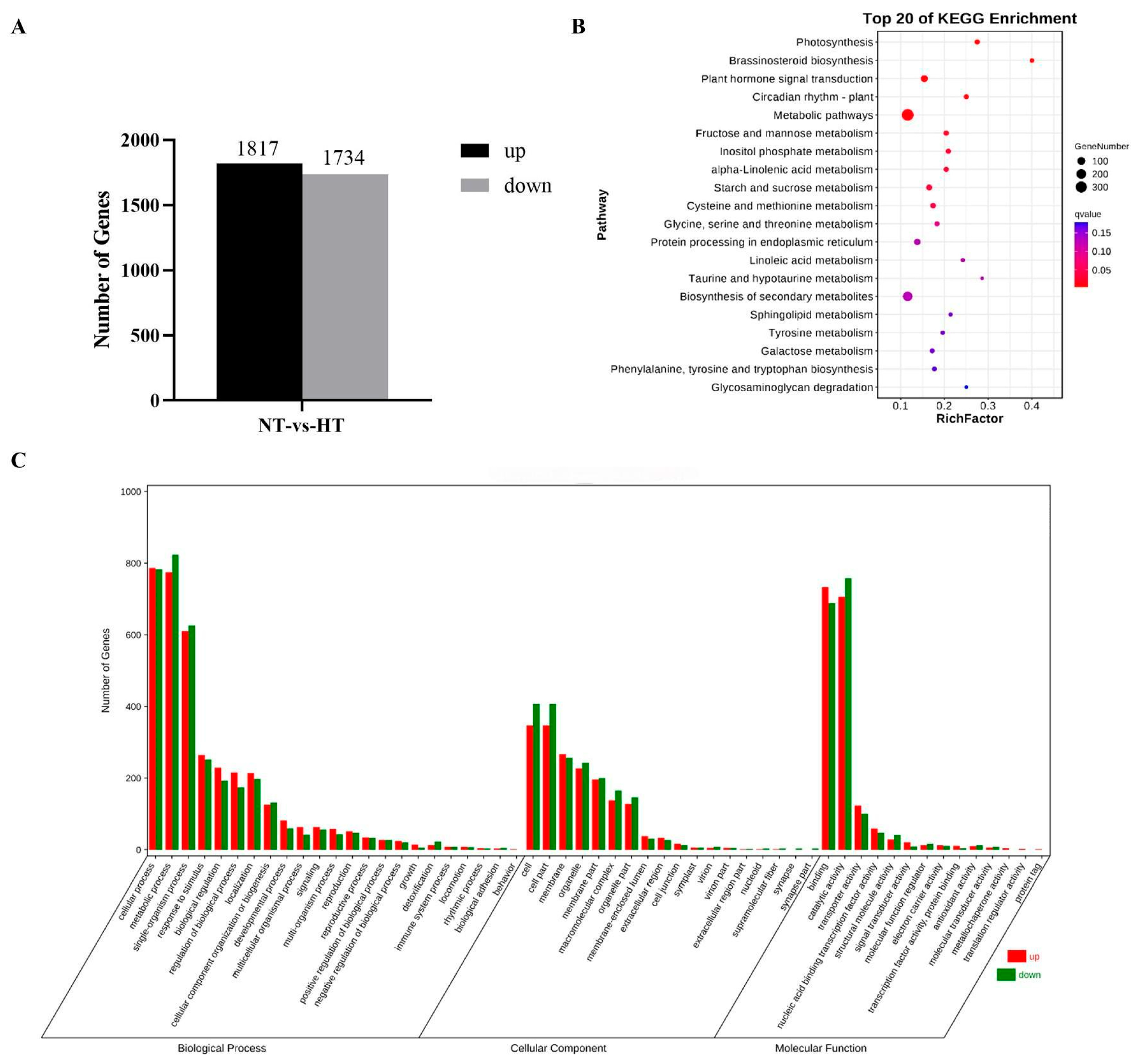
2.7. RNA Sequencing and Analysis of Differentially Expressed Genes
2.8. Transcriptome Changes in Antioxidant Enzyme Genes in High-Temperature-Treated Fruits
2.9. Transcriptome Changes in Sugar Metabolism-Related Genes in High-Temperature-Treated Fruits
2.10. Differential Genes Related to Ethylene and Abscisic Acid Synthesis
2.11. Exploration of Differentially Expressed Heat Shock Proteins and Heat Shock Transcription Factors
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatment
4.2. Physiological Assay
4.3. RNA Extraction and RNA Sequencing
4.4. Statistical Analysis
4.5. LC-MS/MS Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Wu, Y.Q.; Wu, W.L.; Lyu, L.F.; Li, W.L. A review of changes at the phenotypic, physiological, biochemical, and molecular levels of plants due to high temperatures. Planta 2024, 259, 57. [Google Scholar] [CrossRef]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Li, J.; Zhang, R.; Lin, Y.X.; Xiong, A.S.; Tan, G.F.; Luo, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; et al. Combined analysis of the metabolome and transcriptome to explore heat stress responses and adaptation mechanisms in celery (Apium graveolens L.). Int. J. Mol. Sci. 2022, 23, 3367. [Google Scholar] [CrossRef] [PubMed]
- Janni, M.; Gullì, M.; Maestri, E.; Marmiroli, M.; Valliyodan, B.; Nguyen, H.T.; Marmiroli, N. Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J. Exp. Bot. 2020, 71, 3780–3802. [Google Scholar] [CrossRef]
- Kopecká, R.; Kameniarová, M.; Cerny, M.; Brzobohaty, B.; Novák, J. Abiotic stress in crop production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef]
- Haider, S.; Iqbal, J.; Naseer, S.; Yaseen, T.; Shaukat, M.; Bibi, H.; Ahmad, Y.; Daud, H.; Abbasi, N.L.; Mahmood, T. Molecular mechanisms of plant tolerance to heat stress: Current landscape and future perspectives. Plant Cell Rep. 2021, 40, 2247–2271. [Google Scholar] [CrossRef]
- Racsko, J.; Schrader, L.E. Sunburn of apple fruit: Historical background, recent advances and future perspectives. Crit. Rev. Plant Sci. 2012, 31, 455–504. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Li, Z.G.; Tchuenbou-Magaia, F.; Liu, Y.D. Investigation on the environmental causes of tomato fruit cracking and its propagation prediction in greenhouse. J. Texture Stud. 2024, 55, e12845. [Google Scholar] [CrossRef] [PubMed]
- Do, V.; Lee, Y.; Park, J.; Win, N.M.; Kwon, S.I.; Yang, S.; Kim, S. Heat stress and water irrigation management effects on the fruit color and quality of ‘Hongro’ apples. Agriculture 2024, 14, 761. [Google Scholar] [CrossRef]
- Hayama, H.; Iwatani, A.; Nishimoto, T.; Oya, Y.; Nakamura, Y. Watercore disorder in Japanese pear ‘Niitaka’ is increased by high fruit temperatures during fruit maturation. Sci. Hortic. 2014, 175, 27–32. [Google Scholar] [CrossRef]
- Kim, S.K.; Choi, D.G.; Choi, Y.M. Relationship between the temperature characteristics and the occurrence of watercore at various altitudes in ‘Hongro’ and ‘Fuji’ apples. Hortic. Sci. Technol. 2023, 41, 595–604. [Google Scholar] [CrossRef]
- Privitera, G.F.; Treccarichi, S.; Nicotra, R.; Branca, F.; Pulvirenti, A.; Piero, A.R.L.; Sicilia, A. Comparative transcriptome analysis of B. oleracea L. var. italica and B. macrocarpa Guss. genotypes under drought stress: De novo vs reference genome assembly. Plant Stress 2024, 14, 100657. [Google Scholar] [CrossRef]
- Xu, H.; Chen, L.Z.; Song, B.; Fan, X.X.; Yuan, X.H.; Chen, J.F. De novo transcriptome sequencing of pakchoi (Brassica rapa L. chinensis) reveals the key genes related to the response of heat stress. Acta Physiol. Plant. 2016, 38, 252. [Google Scholar] [CrossRef]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef]
- Hassan, M.R.; Arakawa, O.; Nissato, K.; Ito, D. Changes in the harvesting window and quality of apple fruit cultivated under long-term high temperature and CO2. Sci. Hortic. 2024, 338, 113611. [Google Scholar] [CrossRef]
- Sun, M.M.; Khan, A.; Wang, J.H.; Ding, L.C.; Yang, X.H.; Xiong, J.; Sun, Z.Y.; Feng, N.J.; Zheng, D.F. Exogenous hemin increases the yield, phenolic compound content, and antioxidant enzyme activity of dragon fruit during the high-temperature period. Agronomy 2024, 14, 1850. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Yang, Z.Q.; Wei, T.T.; Zhao, H.L. Response of tomato sugar and acid metabolism and fruit quality under different high temperature and relative humidity vonditions. Phyton-Int. J. Exp. Bot. 2022, 91, 2033–2054. [Google Scholar] [CrossRef]
- Liu, D.F.; Ni, J.B.; Wu, R.Y.; Teng, Y.W. High temperature alters sorbitol metabolism in Pyrus pyrifolia leaves and fruit flesh during late stages of fruit enlargement. J. Am. Soc. Hortic. Sci. 2013, 138, 443–451. [Google Scholar] [CrossRef]
- Liu, M.C.; Pirrello, J.; Chervin, C.; Roustan, J.P.; Bouzayen, M. Ethylene control of fruit fipening: Revisiting the complex network of transcriptional regulation. Plant Physiol. 2015, 169, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.C.; Chen, J.Y.; Yu, W.M.; Kuang, J.F.; Chen, W.X.; Li, X.P.; Lu, W.J. Expression of genes associated with ethylene-signalling pathway in harvested banana fruit in response to temperature and 1-MCP treatment. J. Sci. Food Agric. 2011, 91, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, R.; Ali, A.; Gururani, M. Abscisic acid and ethylene coordinating fruit ripening under abiotic stress. Plant Sci. 2024, 349, 112243. [Google Scholar] [CrossRef]
- Tan, Y.; Wen, B.B.; Xu, L.; Zong, X.J.; Sun, Y.G.; Wei, G.Q.; Wei, H.R. High temperature inhibited the accumulation of anthocyanin by promoting ABA catabolism in sweet cherry fruits. Front. Plant Sci. 2023, 14, 1079292. [Google Scholar] [CrossRef] [PubMed]
- Pedreschi, R.; Franck, C.; Lammertyn, J.; Erban, A.; Kopka, J.; Hertog, M.; Verlinden, B.; Nicolai, B. Metabolic profiling of ‘Conference’ pears under low oxygen stress. Postharvest Biol. Technol. 2009, 51, 123–130. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, Y.M.; Li, Y.; Zhao, J.L.; Cheng, Y.D.; Wang, Y.X.; Guan, J.F. Changes of bioactive components and antioxidant capacity of pear ferment in simulated gastrointestinal digestion in vitro. Foods 2023, 12, 1211. [Google Scholar] [CrossRef]
- Guo, Z.M.; Wang, M.M.; Agyekum, A.A.; Wu, J.Z.; Chen, Q.S.; Zuo, M.; El-Seedi, H.R.; Tao, F.; Shi, J.; Ouyang, Q.; et al. Quantitative detection of apple watercore and soluble solids content by near infrared transmittance spectroscopy. J. Food Eng. 2020, 279, 109955. [Google Scholar] [CrossRef]
- Anil Kumar, S.; Kaniganti, S.; Hima Kumari, P.; Sudhakar Reddy, P.; Suravajhala, P.; Suprasanna, P.; Kishor, P.B.K. Functional and biotechnological cues of potassium homeostasis for stress tolerance and plant development. Biotechnol. Genet. 2024, 40, 3527–3570. [Google Scholar] [CrossRef]
- Ullah, I.; Toor, M.D.; Yerlikaya, B.A.; Mohamed, H.I.; Yerlikaya, S.; Basit, A.; Rehman, A.U. High-temperature stress in strawberry: Understanding physiological, biochemical and molecular responses. Planta 2024, 260, 118. [Google Scholar] [CrossRef]
- Pimenta, T.M.; Souza, G.A.; BritoF, A.L.; Teixeira, L.S.; Arruda, R.S.; Henschel, J.M.; Zsögön, A.; Ribeiro, D.M. The impact of elevated CO2 concentration on fruit size, quality, and mineral nutrient composition in tomato varies with temperature regimen during growing season. Plant Growth Regul. 2023, 100, 519–530. [Google Scholar] [CrossRef]
- Itai, A. Watercore in Fruits. In Abiotic Stress Biology in Horticultural Plants; Kanayama, Y., Kochetov, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 10, pp. 127–145. [Google Scholar]
- Gao, Z.; Jayanty, S.; Beaudry, R.; Loescher, W. Sorbitol transporter expression in apple sink tissues: Implications for fruit sugar accumulation and watercore development. J. Am. Soc. Hortic. Sci. 2005, 130, 261–268. [Google Scholar] [CrossRef]
- Yu, C.Y.; Cheng, H.Y.; Cheng, R.; Qi, K.J.; Gu, C.; Zhang, S.L. Expression analysis of sorbitol transporters in pear tissues reveals that PbSOT6/20 is associated with sorbitol accumulation in pear fruits. Sci. Hortic. 2019, 243, 595–601. [Google Scholar] [CrossRef]
- Fan, R.C.; Peng, C.C.; Xu, Y.H.; Wang, X.F.; Li, Y.; Shang, Y.; Du, S.Y.; Zhao, R.; Zhang, X.Y.; Zhang, L.Y.; et al. Apple Sucrose transporter SUT1 and sorbitol transporter SOT6 interact with cytochrome b5 to regulate their affinity for substrate sugars. Plant Physiol. 2009, 150, 1880–1901. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, Y.; Liu, D.H.; Liu, J.; Zhang, J.; Wei, J.; Wang, C.L. A sorbitol transporter gene plays specific role in the occurrence of watercore by modulating the level of intercellular sorbitol in pear. Plant Sci. 2022, 317, 111179. [Google Scholar] [CrossRef]
- Cukrov, D. Progress toward understanding the molecular basis of fruit response to hypoxia. Plants 2018, 7, 78. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, D.H.; Chen, T.; Zhang, J.; Wang, C.L. Watercore pear fruit respiration changed and accumulated γ-aminobutyric acid (GABA) in response to inner hypoxia stress. Genes 2022, 13, 977. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Euring, D.J.; Cha, J.Y.; Lin, Z.; Lu, M.Z.; Huang, L.J.; Kim, W.Y. Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Ohama, N.; Kusakabe, K.; Mizoi, J.; Zhao, H.M.; Kidokoro, S.; Koizumi, S.; Takahashi, F.; Ishida, T.; Yanagisawa, S.; Shinozaki, K.; et al. The transcriptional cascade in the heat stress response of arabidopsis is strictly regulated at the level of transcription factor expression. Plant Cell 2016, 28, 181–201. [Google Scholar] [CrossRef]
- Cao, J.K.; Jiang, W.B.; Zhao, Y.M. Experiment Guidance of Postharvest Physiology and Biochemistry of Fruits and Vegetables; China Light Industry Press: Beijing, China, 2013. [Google Scholar]
- Liu, C.; Li, H.L.; Ren, A.H.; Chen, G.Y.; Ye, W.J.; Wu, Y.X.; Ma, P.; Yu, W.Q.; He, T.M. A comparison of the mineral element content of 70 different varieties of pear fruit (Pyrus ussuriensis) in China. Peer J. 2023, 11, e15328. [Google Scholar] [CrossRef]


| Sugars and Organic Acid Metabolites | Metabolite | Log2FC | VIP |
|---|---|---|---|
| Sugars | Fructose | 0.24 | 1.83 |
| Sucrose | 3.35 | 33.36 | |
| Sorbitol | 0.32 | 32.71 | |
| Organic Acids | Malic acid | −0.62 | 19.88 |
| Shikimic acid | −0.47 | 2.81 | |
| Succinic acid | 1.67 | 8.04 | |
| Quinic acid | −0.09 | 7.83 | |
| Fumaric acid | −0.64 | 4.90 |
| Metabolite | Log2FC | VIP |
|---|---|---|
| Pipecolic acid | −1.11 | 3.69 |
| γ-aminobutyric acid | −0.46 | 7.16 |
| Methionine | −0.72 | 4.04 |
| Alanine | −1.11 | 2.99 |
| Asparagine | −3.28 | 14.22 |
| Aspartic acid | −1.85 | 8.97 |
| Serine | −0.44 | 1.19 |
| Glutamic acid | −0.33 | 1.34 |
| Isoleucine | 0.311 | 2.023 |
| Quercetin | −0.83 | 1.66 |
| Chlorogenate | −1.10 | 1.06 |
| Gallic acid | −9.03 | 6.86 |
| Function | ID | Log2FC |
|---|---|---|
| Peroxidase, POD | LOC103966973 | −2.58 |
| LOC103934252 | −2.34 | |
| LOC103964015 | 1.04 | |
| LOC103938713 | 3.24 | |
| LOC103958862 | 4.91 | |
| Superoxide dismutase, SOD | LOC103947719 | −2.28 |
| LOC103956225 | 1.12 | |
| Ascorbate peroxidase, APX | LOC103934327 | 1.58 |
| LOC103948906 | 1.83 | |
| Glutathione S-transferase, GST | LOC103930887 | 1.48 |
| LOC103958810 | 8.74 |
| Function | ID | Log2FC |
|---|---|---|
| Sucrose-phosphate synthase, SPS | LOC103952486 | 2.08 |
| Sucrose synthase, SS | LOC103935319 | 1.27 |
| LOC103959924 | −1.73 | |
| LOC103939687 | −3.60 | |
| Neutral invertase, NI | LOC103952542 | −1.45 |
| LOC103954772 | −1.06 | |
| LOC103928220 | 1.38 | |
| Sorbitol dehydrogenase, SDH | LOC103933317 | −1.05 |
| LOC103933318 | −3.64 | |
| LOC103933319 | −2.05 | |
| LOC103960508 | −2.31 | |
| LOC103960512 | −2.25 | |
| LOC103960507 | −2.05 | |
| LOC103960513 | −1.69 | |
| LOC103933316 | −3.14 | |
| LOC103933312 | −2.58 | |
| LOC103933314 | −3.70 | |
| Sorbitol transporter, SOT | LOC103929477 | −3.41 |
| LOC103964608 | −1.23 | |
| LOC103934620 | −1.38 | |
| LOC103936910 | −1.49 |
| Function | ID | Log2FC |
|---|---|---|
| 1-Aminocyclopropane-1-carboxylate Oxidase, ACO | LOC103946002 | 2.42 |
| LOC103958907 | 3.64 | |
| LOC103939367 | 2.96 | |
| LOC103954990 | −1.62 | |
| LOC103943975 | 14.23 | |
| LOC103967481 | 1.20 | |
| LOC103958927 | 2.13 | |
| LOC103958906 | 2.71 | |
| LOC103943060 | 1.23 | |
| Zeaxanthin Epoxidase, ZEP | LOC103937745 | −1.50 |
| 9-cis-Epoxycarotenoid Dioxygenase, NCED | LOC103945979 | −2.07 |
| Abscisic Aldehyde Oxidase, AAO | LOC103959275 | 2.40 |
| LOC103952077 | −4.43 |
| Function | ID | Log2FC |
|---|---|---|
| Heat shock protein, HSPs | LOC103927502 | 2.50 |
| LOC103927503 | 2.81 | |
| LOC103927504 | 3.71 | |
| LOC103936175 | 1.78 | |
| LOC103936185 | 3.11 | |
| LOC103936191 | 2.94 | |
| LOC103936226 | 4.62 | |
| LOC103942700 | 1.70 | |
| LOC103945002 | 1.26 | |
| LOC103945384 | 2.51 | |
| LOC103948411 | 2.46 | |
| LOC103952522 | 3.24 | |
| LOC103955091 | 1.51 | |
| LOC103956001 | 4.71 | |
| LOC103956628 | 1.86 | |
| LOC103958215 | 2.11 | |
| LOC103963260 | 2.11 | |
| LOC103964327 | 5.40 | |
| LOC103966141 | 5.32 | |
| LOC103936199 | 3.66 | |
| LOC103940225 | 3.04 | |
| LOC103946415 | 5.03 | |
| LOC103955267 | 2.89 | |
| LOC103942709 | 2.67 | |
| LOC103942692 | 1.31 | |
| LOC103945383 | 3.70 | |
| LOC103927498 | 5.73 | |
| LOC103953301 | 9.52 | |
| LOC103960954 | 3.45 | |
| LOC103949874 | 11.39 | |
| LOC103960307 | 11.04 | |
| LOC103961953 | 3.50 | |
| LOC103966169 | 5.84 | |
| LOC103959824 | 3.00 | |
| Heat shock transcription factors, HSFs | LOC103944300 | 4.22 |
| LOC103952243 | 2.67 | |
| LOC103955174 | 2.49 | |
| LOC103944219 | 2.24 | |
| LOC103960090 | 1.67 | |
| LOC103936188 | −1.12 | |
| LOC103945943 | −1.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-X.; Cai, J.-B.; Liu, X. Integrated Transcriptome and Metabolome Analysis of Mature Stage Sand Pear Fruit Response to High-Temperature Stress. Plants 2025, 14, 2776. https://doi.org/10.3390/plants14172776
Li Y-X, Cai J-B, Liu X. Integrated Transcriptome and Metabolome Analysis of Mature Stage Sand Pear Fruit Response to High-Temperature Stress. Plants. 2025; 14(17):2776. https://doi.org/10.3390/plants14172776
Chicago/Turabian StyleLi, Yu-Xuan, Jia-Bei Cai, and Xiao Liu. 2025. "Integrated Transcriptome and Metabolome Analysis of Mature Stage Sand Pear Fruit Response to High-Temperature Stress" Plants 14, no. 17: 2776. https://doi.org/10.3390/plants14172776
APA StyleLi, Y.-X., Cai, J.-B., & Liu, X. (2025). Integrated Transcriptome and Metabolome Analysis of Mature Stage Sand Pear Fruit Response to High-Temperature Stress. Plants, 14(17), 2776. https://doi.org/10.3390/plants14172776





