Profiling Environmental Variations in Condensed Tannins and Other Metabolites of Birdsfoot Trefoil (Lotus corniculatus L.) Genotypes
Abstract
1. Introduction
2. Results and Discussion
2.1. Geographical Growth Location Influence on Condensed Tannin Levels in BFT Genotypes
2.2. Identification of BFT Metabolites Using Untargeted Metabolomic Analysis
2.3. LC-MS Characterization of CT Compositions in BFT Genotypes
2.4. Influence of Geographical Location, Seasonal Factor, and Genotype on BFT Metabolomes
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Sample Collection
3.3. Condensed Tannin Analysis
3.4. LC-MS Metabolome Profiling
3.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| AAFC | Agriculture and Agri-Food Canada |
| AGC | Automatic gain control |
| ANOVA | Analysis of variance |
| ANR | Anthocyanidin reductase |
| BFT | Birdsfoot trefoil |
| CTs | Condensed tannins |
| DAM | Differentially accumulated metabolite |
| DDA | Data-dependent acquisition |
| HESI | Heated electrospray ionization |
| LAR | Leucoanthocyanidin reductase |
| LC-MS | Liquid chromatography–mass spectrometry |
| LSD | Least significant difference |
| MW | Molecular weight |
| PCs | Procyanidins |
| PC1 | Principal component 1 |
| PC2 | Principal component 2 |
| PCA | Principal component analysis |
| PDs | Prodelphinidins |
References
- Ball, D.M.; Collins, M.; Lacefield, G.; Martin, N.; Mertens, D.; Olson, K.; Putnam, D.; Undersander, D.; Wolf, M. Understanding Forage Quality. Am. Farm Bur. Fed. Publ. 2001, 1, 1–15. [Google Scholar]
- Khatiwada, B.; Acharya, S.N.; Larney, F.J.; Lupwayi, N.Z.; Smith, E.G.; Islam, M.A.; Thomas, J.E. Benefits of Mixed Grass–Legume Pastures and Pasture Rejuvenation Using Bloat-Free Legumes in Western Canada: A Review. Can. J. Plant Sci. 2020, 100, 463–476. [Google Scholar] [CrossRef]
- Hamacher, M.; Malisch, C.S.; Reinsch, T.; Taube, F.; Loges, R. Evaluation of Yield Formation and Nutritive Value of Forage Legumes and Herbs with Potential for Diverse Grasslands Due to Their Concentration in Plant Specialized Metabolites. Eur. J. Agron. 2021, 128, 126307. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-Legume Symbiosis and Nitrogen Fixation under Severe Conditions and in an Arid Climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef]
- Wang, Y.; Majak, W.; McAllister, T.A. Frothy Bloat in Ruminants: Cause, Occurrence, and Mitigation Strategies. Anim. Feed Sci. Technol. 2012, 172, 103–114. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wang, Z.; Xue, B.; Peng, Q.; Hu, R.; Yan, T. Recent Advances in Research in the Rumen Bloat of Ruminant Animals Fed High-Concentrate Diets. Front. Vet. Sci. 2023, 10, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Caro, D.; Davis, S.J.; Bastianoni, S.; Caldeira, K. Global and Regional Trends in Greenhouse Gas Emissions from Livestock. Clim. Change 2014, 126, 203–216. [Google Scholar] [CrossRef]
- Grossi, G.; Goglio, P.; Vitali, A.; Williams, A.G. Livestock and Climate Change: Impact of Livestock on Climate and Mitigation Strategies. Anim. Front. 2019, 9, 69–76. [Google Scholar] [CrossRef]
- McMahon, L.R.; McAllister, T.A.; Berg, B.P.; Majak, W.; Acharya, S.N.; Popp, J.D.; Coulman, B.E.; Wang, Y.; Cheng, K.-J. A Review of the Effects of Forage Condensed Tannins on Ruminal Fermentation and Bloat in Grazing Cattle. Can. J. Plant Sci. 2000, 80, 469–485. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.-P.; et al. Benefits of Condensed Tannins in Forage Legumes Fed to Ruminants: Importance of Structure, Concentration, and Diet Composition. Crop Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef]
- Barry, T.N.; Manley, T.R. Interrelationships between the Concentrations of Total Condensed Tannin, Free Condensed Tannin and Lignin in Lotus Sp. and Their Possible Consequences in Ruminant Nutrition. J. Sci. Food Agric. 1986, 37, 248–254. [Google Scholar] [CrossRef]
- Roberts, C.A.; Beuselinck, P.R.; Ellersieck, M.R.; Davis, D.K.; McGraw, R.L. Quantification of Tannis in Birdsfoot Trefoil Germplasm. Crop Sci. 1993, 33, 675–679. [Google Scholar] [CrossRef]
- Marshall, A.; Bryant, D.; Latypova, G.; Hauck, B.; Olyott, P.; Morris, P.; Robbins, M. A High-Throughput Method for the Quantification of Proanthocyanidins in Forage Crops and Its Application in Assessing Variation in Condensed Tannin Content in Breeding Programmes for Lotus corniculatus and Lotus uliginosus. J. Agric. Food Chem. 2008, 56, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, M.J.; McGraw, R.L. Lotus Adaptation, Use, and Management. In CSSA Special Publications; Beuselinck, P.R., Ed.; Crop Science Society of America and American Society of Agronomy: Madison, WI, USA, 1999; pp. 97–119. ISBN 978-0-89118-607-6. [Google Scholar]
- Sleugh, B.; Moore, K.J.; George, J.R.; Brummer, E.C. Binary Legume–Grass Mixtures Improve Forage Yield, Quality, and Seasonal Distribution. Agron. J. 2000, 92, 24–29. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Ulyatt, M.J.; John, A.; Fisher, M.T. The Effect of Condensed Tannins on the Site of Digestion of Amino Acids and Other Nutrients in Sheep Fed on Lotus corniculatus L. Br. J. Nutr. 1987, 57, 115–126. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Douglas, G.B.; Niezen, J.H.; Mcnabb, W.C.; Foote, A.G. Forages with Condensed Tannins—Their Management and Nutritive Value for Ruminants. Proc. N. Z. Grassl. Assoc. 1998, 60, 89–98. [Google Scholar] [CrossRef]
- Wang, Y.; Douglas, G.B.; Waghorn, G.C.; Barry, T.N.; Foote, A.G. Effect of Condensed Tannins in Lotus corniculatus upon Lactation Performance in Ewes. J. Agric. Sci. 1996, 126, 353–362. [Google Scholar] [CrossRef]
- Hymes-Fecht, U.C.; Broderick, G.A.; Muck, R.E.; Grabber, J.H. Replacing Alfalfa or Red Clover Silage with Birdsfoot Trefoil Silage in Total Mixed Rations Increases Production of Lactating Dairy Cows. J. Dairy Sci. 2013, 96, 460–469. [Google Scholar] [CrossRef]
- Barry, T.N.; McNabb, W.C. The Implications of Condensed Tannins on the Nutritive Value of Temperate Forages Fed to Ruminants. Br. J. Nutr. 1999, 81, 263–272. [Google Scholar] [CrossRef]
- Majak, W.; Hall, J.W.; McCaughey, W.P. Pasture Management Strategies for Reducing the Risk of Legume Bloat in Cattle. J. Anim. Sci. 1995, 73, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Marley, C.L.; Cook, R.; Keatinge, R.; Barrett, J.; Lampkin, N.H. The Effect of Birdsfoot Trefoil (Lotus corniculatus) and Chicory (Cichorium intybus) on Parasite Intensities and Performance of Lambs Naturally Infected with Helminth Parasites. Vet. Parasitol. 2003, 112, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Mata-Padrino, D.J.; Belesky, D.P.; Crawford, C.D.; Walsh, B.; MacAdam, J.W.; Bowdridge, S.A. Effects of Grazing Birdsfoot Trefoil–Enriched Pasture on Managing Haemonchus contortus Infection in Suffolk Crossbred Lambs. J. Anim. Sci. 2019, 97, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Heckendorn, F.; Häring, D.A.; Maurer, V.; Senn, M.; Hertzberg, H. Individual Administration of Three Tanniferous Forage Plants to Lambs Artificially Infected with Haemonchus contortus and Cooperia curticei. Vet. Parasitol. 2007, 146, 123–134. [Google Scholar] [CrossRef]
- He, F.; Pan, Q.-H.; Shi, Y.; Duan, C.-Q. Biosynthesis and Genetic Regulation of Proanthocyanidins in Plants. Molecules 2008, 13, 2674–2703. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef]
- Rue, E.A.; Rush, M.D.; Van Breemen, R.B. Procyanidins: A Comprehensive Review Encompassing Structure Elucidation via Mass Spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological Function of Plant Tannin and Its Application in Animal Health. Front. Vet. Sci. 2022, 8, 803657. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Jones, W.T.; Shelton, I.D.; Mcnabb, W.C. Condensed Tannins and the Nutritive Value of Herbage. Proc. N. Z. Grassl. Assoc. 1990, 51, 171–176. [Google Scholar] [CrossRef]
- Jones, W.T.; Mangan, J.L. Complexes of the Condensed Tannins of Sainfoin (Onobrychis viciifolia Scop.) with Fraction 1 Leaf Protein and with Submaxillary Mucoprotein, and Their Reversal by Polyethylene Glycol and pH. J. Sci. Food Agric. 1977, 28, 126–136. [Google Scholar] [CrossRef]
- Min, B.R.; Pinchak, W.E.; Anderson, R.C.; Fulford, J.D.; Puchala, R. Effects of Condensed Tannins Supplementation Level on Weight Gain and in Vitro and in Vivo Bloat Precursors in Steers Grazing Winter Wheat. J. Anim. Sci. 2006, 84, 2546–2554. [Google Scholar] [CrossRef]
- Costa, E.D.; Ribiero, C.V.; Silva, T.M.; Ribeiro, R.D.; Vieira, J.F.; Lima, A.D.; Barbosa, A.M.; da Silva Júnior, J.M.; Bezerra, L.R.; Oliveira, R.L. Intake, Nutrient Digestibility, Nitrogen Balance, Serum Metabolites and Growth Performance of Lambs Supplemented with Acacia mearnsii Condensed Tannin Extract. Anim. Feed Sci. Technol. 2021, 272, 114744. [Google Scholar] [CrossRef]
- Besharati, M.; Maggiolino, A.; Palangi, V.; Kaya, A.; Jabbar, M.; Eseceli, H.; De Palo, P.; Lorenzo, J.M. Tannin in Ruminant Nutrition: Review. Molecules 2022, 27, 8273. [Google Scholar] [CrossRef]
- Cardoso-Gutierrez, E.; Aranda-Aguirre, E.; Robles-Jimenez, L.E.; Castelán-Ortega, O.A.; Chay-Canul, A.J.; Foggi, G.; Angeles-Hernandez, J.C.; Vargas-Bello-Pérez, E.; González-Ronquillo, M. Effect of Tannins from Tropical Plants on Methane Production from Ruminants: A Systematic Review. Vet. Anim. Sci. 2021, 14, 100214. [Google Scholar] [CrossRef]
- Barry, T.N.; Duncan, S.J. The Role of Condensed Tannins in the Nutritional Value of Lotus pedunculatus for Sheep: 1. Voluntary Intake. Br. J. Nutr. 1984, 51, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Hatew, B.; Stringano, E.; Mueller-Harvey, I.; Hendriks, W.H.; Carbonero, C.H.; Smith, L.M.J.; Pellikaan, W.F. Impact of Variation in Structure of Condensed Tannins from Sainfoin (Onobrychis viciifolia) on in Vitro Ruminal Methane Production and Fermentation Characteristics. J. Anim. Physiol. Anim. Nutr. 2016, 100, 348–360. [Google Scholar] [CrossRef]
- Gebrehiwot, L.; Beuselinck, P.R.; Roberts, C.A. Seasonal Variations in Condensed Tannin Concentration of Three Lotus Species. Agron. J. 2002, 94, 1059–1065. [Google Scholar] [CrossRef]
- Tibe, O.; Meagher, L.P.; Fraser, K.; Harding, D.R.K. Condensed Tannins and Flavonoids from the Forage Legume Sulla (Hedysarum coronarium). J. Agric. Food Chem. 2011, 59, 9402–9409. [Google Scholar] [CrossRef] [PubMed]
- Malisch, C.S.; Lüscher, A.; Baert, N.; Engström, M.T.; Studer, B.; Fryganas, C.; Suter, D.; Mueller-Harvey, I.; Salminen, J.-P. Large Variability of Proanthocyanidin Content and Composition in Sainfoin (Onobrychis viciifolia). J. Agric. Food Chem. 2015, 63, 10234–10242. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Salminen, J.-P.; Taube, F.; Malisch, C.S. Large Inter- and Intraspecies Variability of Polyphenols and Proanthocyanidins in Eight Temperate Forage Species Indicates Potential for Their Exploitation as Nutraceuticals. J. Agric. Food Chem. 2021, 69, 12445–12455. [Google Scholar] [CrossRef]
- Morris, P.; Carter, E.B.; Hauck, B.; Lanot, A.; Theodorou, M.K.; Allison, G. Responses of Lotus corniculatus to Environmental Change 3: The Sensitivity of Phenolic Accumulation to Growth Temperature and Light Intensity and Effects on Tissue Digestibility. Planta 2021, 253, 35. [Google Scholar] [CrossRef]
- Morris, P.; Carter, E.B.; Hauck, B.; Hughes, J.-W.; Allison, G.; Theodorou, M.K. Responses of Lotus corniculatus to Environmental Change 4: Root Carbohydrate Levels at Defoliation and Regrowth Climatic Conditions Are Major Drivers of Phenolic Content and Forage Quality. Planta 2021, 253, 38. [Google Scholar] [CrossRef]
- Trush, K.; Gavurová, M.; Monje-Rueda, M.D.; Kolarčik, V.; Bačovčinová, M.; Betti, M.; Paľove-Balang, P. Low Nitrogen Status Affects Isoflavonoid Production and Flavonol Decoration in Lotus corniculatus. Plant Stress 2024, 11, 100336. [Google Scholar] [CrossRef]
- Barry, T.N.; Forss, D.A. The Condensed Tannin Content of Vegetative Lotus pedunculatus, Its Regulation by Fertiliser Application, and Effect upon Protein Solubility. J. Sci. Food Agric. 1983, 34, 1047–1056. [Google Scholar] [CrossRef]
- Anuraga, M.; Duarsa, P.; Hill, M.; Lovett, J. Soil Moisture and Temperature Affect Condensed Tannin Concentrations and Growth in Lotus corniculatus and Lotus pedunculatus. Aust. J. Agric. Res. 1993, 44, 1667. [Google Scholar] [CrossRef]
- Grabber, J.H.; Riday, H.; Cassida, K.A.; Griggs, T.C.; Min, D.H.; MacAdam, J.W. Yield, Morphological Characteristics, and Chemical Composition of European- and Mediterranean-derived Birdsfoot Trefoil Cultivars Grown in the Colder Continental United States. Crop Sci. 2014, 54, 1893–1901. [Google Scholar] [CrossRef]
- Sivakumaran, S.; Rumball, W.; Lane, G.A.; Fraser, K.; Foo, L.Y.; Yu, M.; Meagher, L.P. Variation of Proanthocyanidins in Lotus Species. J. Chem. Ecol. 2006, 32, 1797–1816. [Google Scholar] [CrossRef]
- Gruber, M.; Skadhauge, B.; Yu, M.; Muir, A.; Richards, K. Variation in Morphology, Plant Habit, Proanthocyanidins, and Flavonoids within a Lottus Germplasm Collection. Can. J. Plant Sci. 2008, 88, 121–132. [Google Scholar] [CrossRef]
- Berard, N.C.; Wang, Y.; Wittenberg, K.M.; Krause, D.O.; Coulman, B.E.; McAllister, T.A.; Ominski, K.H. Condensed Tannin Concentrations Found in Vegetative and Mature Forage Legumes Grown in Western Canada. Can. J. Plant Sci. 2011, 91, 669–675. [Google Scholar] [CrossRef]
- Azuhnwi, B.N.; Boller, B.; Dohme-Meier, F.; Hess, H.D.; Kreuzer, M.; Stringano, E.; Mueller-Harvey, I. Exploring Variation in Proanthocyanidin Composition and Content of Sainfoin (Onobrychis viciifolia). J. Sci. Food Agric. 2013, 93, 2102–2109. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, E.J.; Goplen, B.P.; Howarth, R.E. Inheritance of Tannins in Birdsfoot Trefoil 1. Crop Sci. 1984, 24, 921–923. [Google Scholar] [CrossRef]
- Miller, P.R.; Ehlke, N.J. Inheritance of Condensed Tannins in Birdsfoot Trefoil. Can. J. Plant Sci. 1997, 77, 587–593. [Google Scholar] [CrossRef]
- Papadopoulos, Y.A.; Kelman, W.M. Traditional Breeding of Lotus Species. In CSSA Special Publications; Beuselinck, P.R., Ed.; Crop Science Society of America and American Society of Agronomy: Madison, WI, USA, 1999; pp. 187–198. ISBN 978-0-89118-607-6. [Google Scholar]
- Escaray, F.J.; Passeri, V.; Babuin, F.M.; Marco, F.; Carrasco, P.; Damiani, F.; Pieckenstain, F.L.; Paolocci, F.; Ruiz, O.A. Lotus tenuis x L. corniculatus Interspecific Hybridization as a Means to Breed Bloat-Safe Pastures and Gain Insight into the Genetic Control of Proanthocyanidin Biosynthesis in Legumes. BMC Plant Biol. 2014, 14, 40. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the Conundrum of Tannins in Animal Nutrition and Health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Terrill, T.H.; Rowan, A.M.; Douglas, G.B.; Barry, T.N. Determination of Extractable and Bound Condensed Tannin Concentrations in Forage Plants, Protein Concentrate Meals and Cereal Grains. J. Sci. Food Agric. 1992, 58, 321–329. [Google Scholar] [CrossRef]
- Reynaud, J.; Lussignol, M. The Flavonoids of Lotus corniculatus. Lotus Newsl. 2005, 35, 75–82. [Google Scholar]
- Li, X.; Yang, Y.; Chen, L.; Zhang, Y.; Chen, Y. Compounds from Lotus corniculatus. Chem. Nat. Compd. 2019, 55, 719–721. [Google Scholar] [CrossRef]
- Kaducová, M.; Eliašová, A.; Trush, K.; Bačovčinová, M.; Sklenková, K.; Pal’ove-Balang, P. Accumulation of Isoflavonoids in Lotus corniculatus after UV-B Irradiation. Theor. Exp. Plant Physiol. 2022, 34, 53–62. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental Factors Regulate Plant Secondary Metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Foo, L.Y.; Newman, R.; Waghorn, G.; McNabb, W.C.; Ulyatt, M.J. Proanthocyanidins from Lotus corniculatus. Phytochemistry 1996, 41, 617–624. [Google Scholar] [CrossRef]
- Jun, J.H.; Xiao, X.; Rao, X.; Dixon, R.A. Proanthocyanidin Subunit Composition Determined by Functionally Diverged Dioxygenases. Nat. Plants 2018, 4, 1034–1043. [Google Scholar] [CrossRef]
- Carter, E.B.; Theodorou, M.K.; Morris, P. Responses of Lotus corniculatus to Environmental Change 2: Effect of Elevated CO2, Temperature and Drought on Tissue Digestion in Relation to Condensed Tannin and Carbohydrate Accumulation. J. Sci. Food Agric. 1999, 79, 1431–1440. [Google Scholar] [CrossRef]
- Popović, B.M.; Štajner, D.; Ždero-Pavlović, R.; Tumbas-Šaponjac, V.; Čanadanović-Brunet, J.; Orlović, S. Water Stress Induces Changes in Polyphenol Profile and Antioxidant Capacity in Poplar Plants (Populus Spp.). Plant Physiol. Biochem. 2016, 105, 242–250. [Google Scholar] [CrossRef]
- Lee, J.-E.; Lee, B.-J.; Chung, J.-O.; Hwang, J.-A.; Lee, S.-J.; Lee, C.-H.; Hong, Y.-S. Geographical and Climatic Dependencies of Green Tea (Camellia sinensis) Metabolites: A 1H NMR-Based Metabolomics Study. J. Agric. Food Chem. 2010, 58, 10582–10589. [Google Scholar] [CrossRef] [PubMed]
- Grabber, J.H.; Zeller, W.E. Direct versus Sequential Analysis of Procyanidin- and Prodelphinidin-Based Condensed Tannins by the HCl–Butanol–Acetone–Iron Assay. J. Agric. Food Chem. 2020, 68, 2906–2916. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. Tannins and Lignins. In Herbivores: Their Interactions with Secondary Plant Metabolites; Elsevier: Amsterdam, The Netherlands, 1991; Volume 1, pp. 355–388. ISBN 978-0-12-597183-6. [Google Scholar]
- Yu, X.; Xiao, J.; Chen, S.; Yu, Y.; Ma, J.; Lin, Y.; Li, R.; Lin, J.; Fu, Z.; Zhou, Q.; et al. Metabolite Signatures of Diverse Camellia sinensis Tea Populations. Nat. Commun. 2020, 11, 5586. [Google Scholar] [CrossRef]
- Kelman, M.; Renaud, J.; McCarron, P.; Hoogstra, S.; Chow, W.; Wang, J.; Varga, E.; Patriarca, A.; Vaya, A.; Visintin, L.; et al. International Interlaboratory Study to Normalize Liquid Chromatography-Based Mycotoxin Retention Times through Implementation of a Retention Index System. J. Chromatogr. A 2025, 1745, 465732. [Google Scholar] [CrossRef]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: Open Source Software for Rapid Proteomics Tools Development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Tautenhahn, R.; Böttcher, C.; Neumann, S. Highly Sensitive Feature Detection for High Resolution LC/MS. BMC Bioinform. 2008, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Nyamundanda, G.; Brennan, L.; Gormley, I.C. Probabilistic Principal Component Analysis for Metabolomic Data. BMC Bioinform. 2010, 11, 571. [Google Scholar] [CrossRef] [PubMed]
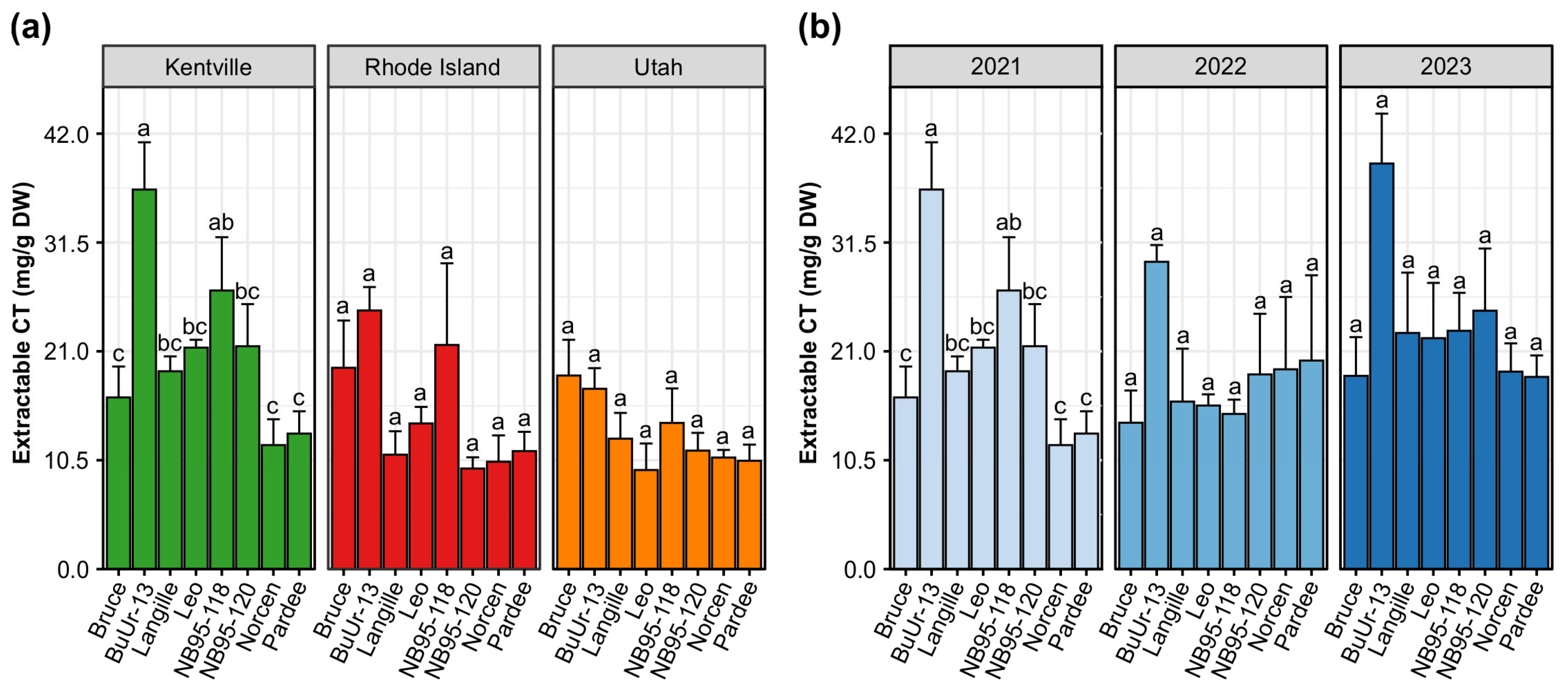

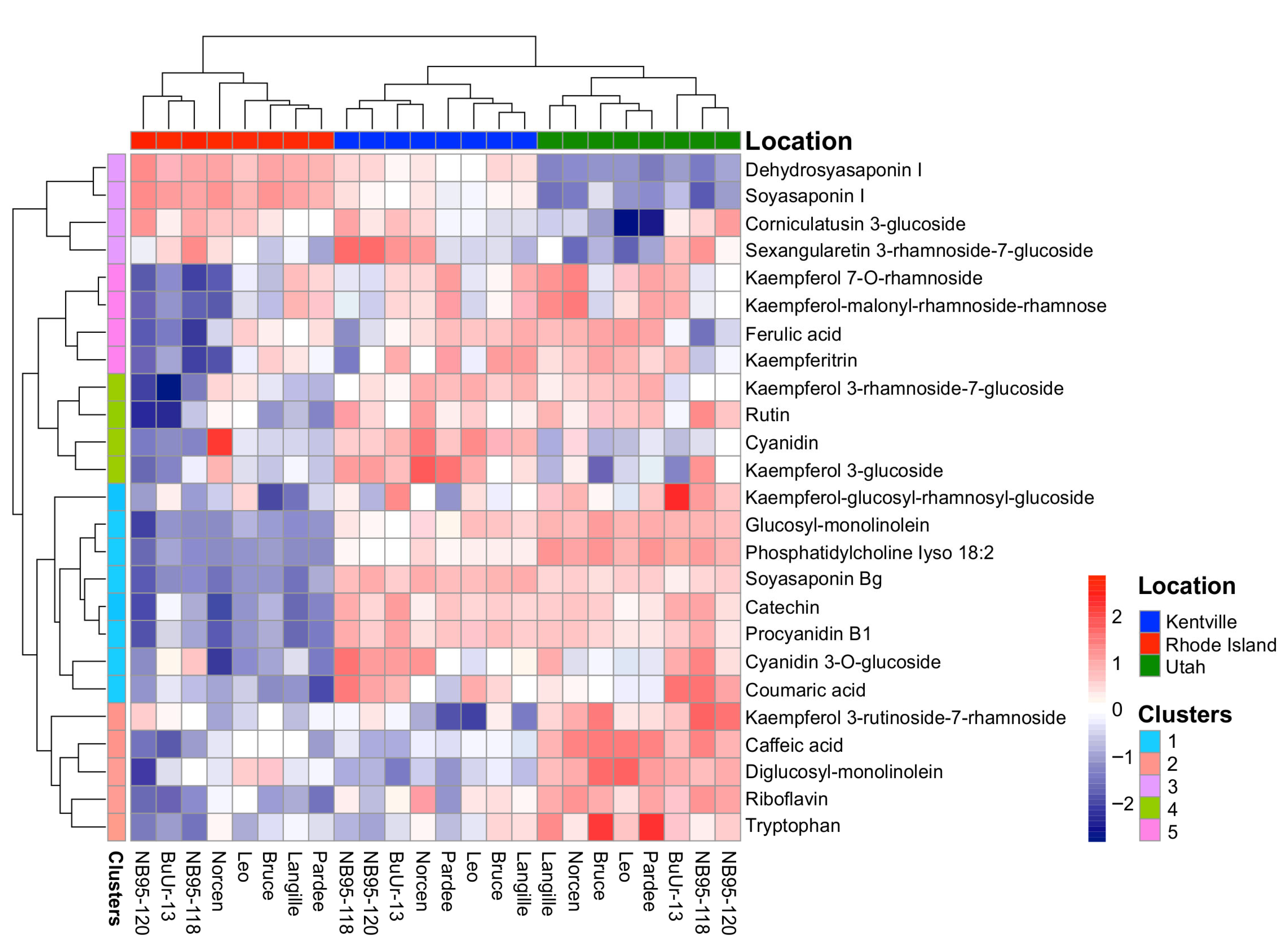

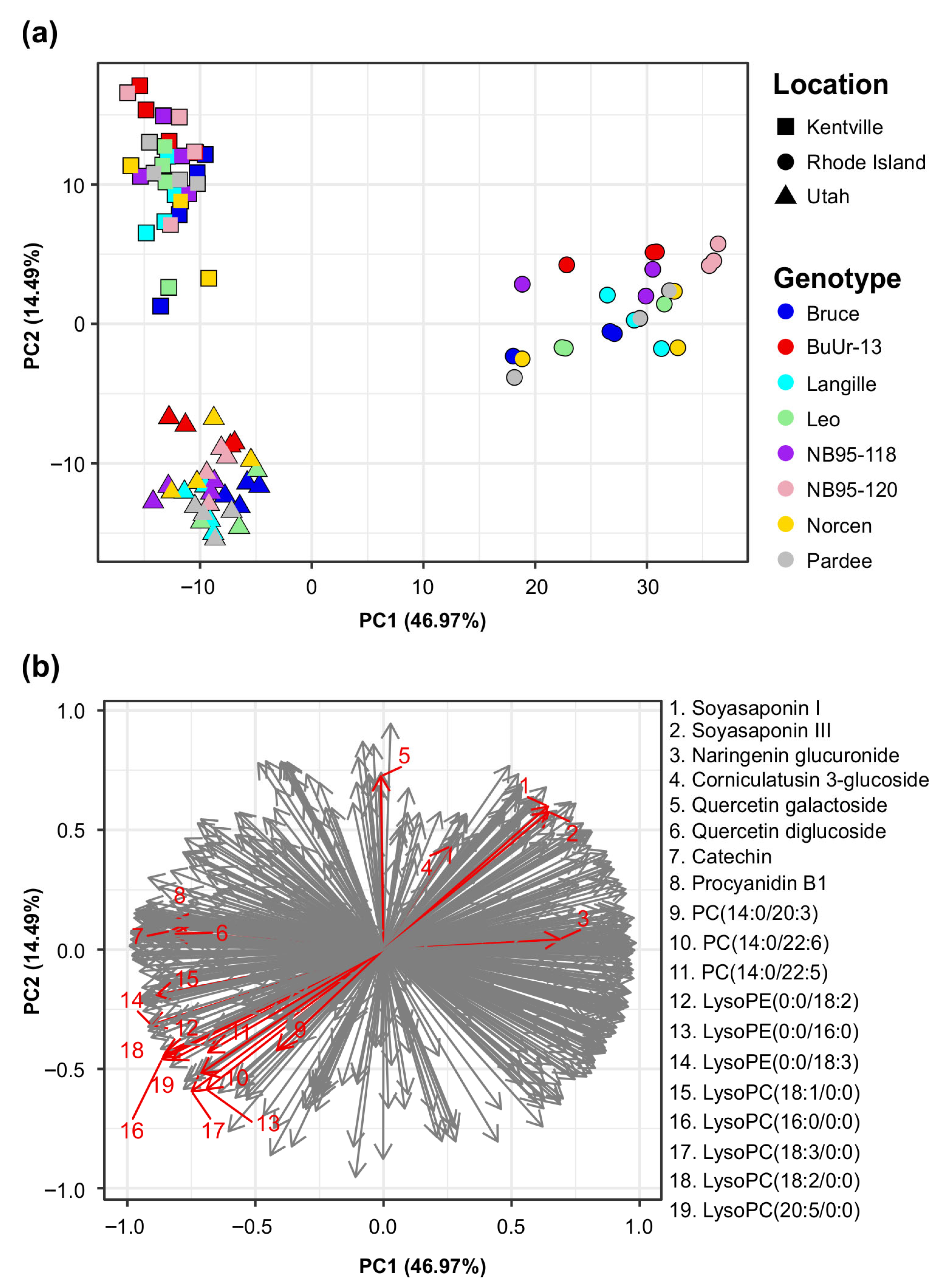

| Source of Variation | df | F Statistic | p Value |
|---|---|---|---|
| Location by genotype in 2021 | |||
| Location | 2 | 14.018 | 1.01 × 10−5 *** |
| Genotype | 7 | 7.035 | 4.01 × 10−6 *** |
| Location × genotype | 14 | 1.580 | 0.112 |
| Residuals | 60 | ||
| Year by genotype in Kentville | |||
| Year | 2 | 3.007 | 0.00569 |
| Genotype | 7 | 5.315 | 9.14 × 10−54 *** |
| Year × genotype | 14 | 0.524 | 0.9096 |
| Residuals | 60 |
| # | Compound Name | Formula | NAPS RI | Ion Type | m/z | Mass Error (ppm) | rt (min) | Confidence |
|---|---|---|---|---|---|---|---|---|
| 1 | Tryptophan | C11H12N2O2 | 493 | [M+H]+ | 205.0972 | 0.22 | 2.32 | 1 |
| 2 | Rutin | C27H30O16 | 528 | [M+H]+ | 611.1606 | −0.13 | 2.52 | 2 |
| 3 | Kaempferol 3-rhamnoside-7-glucoside | C27H30O15 | 548 | [M+H]+ | 595.1657 | −0.06 | 2.63 | 2 |
| 4 | Kaempferitrin | C27H30O14 | 584 | [M+H]+ | 579.1709 | 0.01 | 2.83 | 1 |
| 5 | Coumaric acid | C9H8O3 | 612 | [M+H]+ | 165.0547 | 0.09 | 2.99 | 2 |
| 6 | Kaempferol 7-O-rhamnoside | C21H20O10 | 642 | [M+H]+ | 433.1128 | −0.31 | 3.16 | 2 |
| 7 | Soyasaponin I | C48H78O18 | 1003 | [M+H]+ | 943.5254 | −0.74 | 5.77 | 2 |
| 8 | Soyasaponin βg | C54H84O21 | 1087 | [M+H]+ | 1069.5570 | −0.73 | 6.08 | 2 |
| 9 | Glucosyl-monolinolein | C27H46O9 | 1358 | [M+Na]+ | 537.3040 | 1.03 | 6.97 | 2 |
| 10 | Diglucosyl-monolinolein | C33H56O14 | 1185 | [M+Na]+ | 699.3565 | 0.32 | 6.47 | 2 |
| 11 | Caffeic acid | C9H8O4 | 1022 | [M+H]+ | 181.0494 | −0.86 | 5.84 | 2 |
| 12 | Ferulic acid | C10H10O4 | 660 | [M+H]+ | 195.0651 | −0.49 | 3.26 | 2 |
| 13 | Cyanidin | C15H11O6 | 625 | [M+] | 287.0548 | −0.61 | 3.06 | 2 |
| 14 | Catechin | C15H14O6 | 518 | [M+H]+ | 291.0863 | −0.05 | 2.46 | 1 |
| 15 | Riboflavin | C17H20N4O6 | 526 | [M+H]+ | 377.1455 | −0.06 | 2.51 | 2 |
| 16 | Kaempferol 3-glucoside | C21H20O11 | 641 | [M+H]+ | 449.1076 | −0.6 | 3.15 | 2 |
| 17 | Cyanidin 3-O-glucoside | C21H21O11 | 555 | [M+] | 449.1079 | 0.16 | 2.67 | 2 |
| 18 | Corniculatusin 3-glucoside | C22H22O13 | 589 | [M+H]+ | 495.1133 | −0.07 | 2.86 | 2 |
| 19 | Phosphatidylcholine lyso 18:2 | C26H50NO7P | 1310 | [M+H]+ | 520.3402 | 0.74 | 6.82 | 2 |
| 20 | Procyanidin B1 | C30H26O12 | 495 | [M+H]+ | 579.1501 | 0.67 | 2.33 | 1 |
| 21 | Sexangularetin 3-rhamnoside-7-glucoside | C28H32O16 | 555 | [M+H]+ | 625.1764 | 0.13 | 2.67 | 2 |
| 22 | Kaempferol-malonyl-rhamnose-rhamnose | C30H32O17 | 644 | [M+H]+ | 665.1707 | −0.76 | 3.17 | 2 |
| 23 | Kaempferol 3-rutinoside-7-rhamnoside | C33H40O19 | 511 | [M+H]+ | 741.2235 | −0.2 | 2.42 | 2 |
| 24 | PC(C16:0/C18:3) | C42H78NO8P | 1611 | [M+] | 756.5537 | −0.17 | 7.74 | 2 |
| 25 | Kaempferol-glucosyl-rhamnosyl-glucoside | C33H40O20 | 504 | [M+H]+ | 757.2178 | −0.98 | 2.38 | 2 |
| # | Compound Name | Formula | NAPS RI | Ion Type | m/z | Mass Error (ppm) | rt (min) | Confidence |
|---|---|---|---|---|---|---|---|---|
| 1 | Catechin | C15H14O6 | 518 | [M+H]+ | 291.0863 | −0.05 | 2.46 | 1 |
| 2 | Epicatechin | C15H14O6 | 541 | [M+H]+ | 291.08664 | 1.12 | 2.59 | 1 |
| 3 | Gallocatechin | C15H14O7 | 476 | [M+H]+ | 307.08139 | 0.52 | 2.22 | 1 |
| 4 | Epigallocatechin | C15H14O7 | 500 | [M+H]+ | 307.08137 | 0.45 | 2.35 | 1 |
| 5 | Procyanidin B1 | C30H26O12 | 495 | [M+H]+ | 579.15009 | 0.67 | 2.33 | 1 |
| 6 | Procyanidin B4 | C30H26O12 | 521 | [M+H]+ | 579.1499 | 0.34 | 2.48 | 1 |
| 7 | Procyanidin trimer-1 | C45H38O18 | 512 | [M+H]+ | 867.21338 | 0.33 | 2.43 | 2 |
| 8 | Procyanidin trimer-2 | C45H38O18 | 541 | [M+H]+ | 867.2135 | 0.47 | 2.59 | 2 |
| 9 | Procyanidin trimer-3 | C45H38O18 | 463 | [M+H]+ | 867.2136 | 0.59 | 2.15 | 3 |
| 10 | Prodelphinidin trimer | C45H38O20 | 482 | [M+H]+ | 899.2028 | −0.11 | 2.26 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakariyahu, S.K.; McDowell, T.; Renaud, J.B.; Papadopoulos, Y.; Glover, K.; Brown, R.N.; Peel, M.D.; Riday, H.; Kohalmi, S.E.; Hannoufa, A. Profiling Environmental Variations in Condensed Tannins and Other Metabolites of Birdsfoot Trefoil (Lotus corniculatus L.) Genotypes. Plants 2025, 14, 2766. https://doi.org/10.3390/plants14172766
Sakariyahu SK, McDowell T, Renaud JB, Papadopoulos Y, Glover K, Brown RN, Peel MD, Riday H, Kohalmi SE, Hannoufa A. Profiling Environmental Variations in Condensed Tannins and Other Metabolites of Birdsfoot Trefoil (Lotus corniculatus L.) Genotypes. Plants. 2025; 14(17):2766. https://doi.org/10.3390/plants14172766
Chicago/Turabian StyleSakariyahu, Solihu Kayode, Tim McDowell, Justin B. Renaud, Yousef Papadopoulos, Kathleen Glover, Rebecca Nelson Brown, Michael D. Peel, Heathcliffe Riday, Susanne E. Kohalmi, and Abdelali Hannoufa. 2025. "Profiling Environmental Variations in Condensed Tannins and Other Metabolites of Birdsfoot Trefoil (Lotus corniculatus L.) Genotypes" Plants 14, no. 17: 2766. https://doi.org/10.3390/plants14172766
APA StyleSakariyahu, S. K., McDowell, T., Renaud, J. B., Papadopoulos, Y., Glover, K., Brown, R. N., Peel, M. D., Riday, H., Kohalmi, S. E., & Hannoufa, A. (2025). Profiling Environmental Variations in Condensed Tannins and Other Metabolites of Birdsfoot Trefoil (Lotus corniculatus L.) Genotypes. Plants, 14(17), 2766. https://doi.org/10.3390/plants14172766







