Does the Species Number of Invasive Plants Regulate the Intensity of Interspecific Interactions Among Multiple Plants Under Different Invasion Scenarios?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- (1)
- The uninvaded plots (control; no invasion by any IPS) (n = 92);
- (2)
- The mono-invasion achieved by one IP (n = 827);
- (3)
- The co-invasion achieved by two IPS (n = 280);
- (4)
- The co-invasion achieved by three IPS (n = 71).
2.2. Determination of the Intensity of Interspecific Interactions Among Multiple Plants
2.3. Determination of Plant Taxonomic Diversity, IPS’ Invasion Intensity and the Community Invasibility
2.4. Statistical Analysis
3. Results
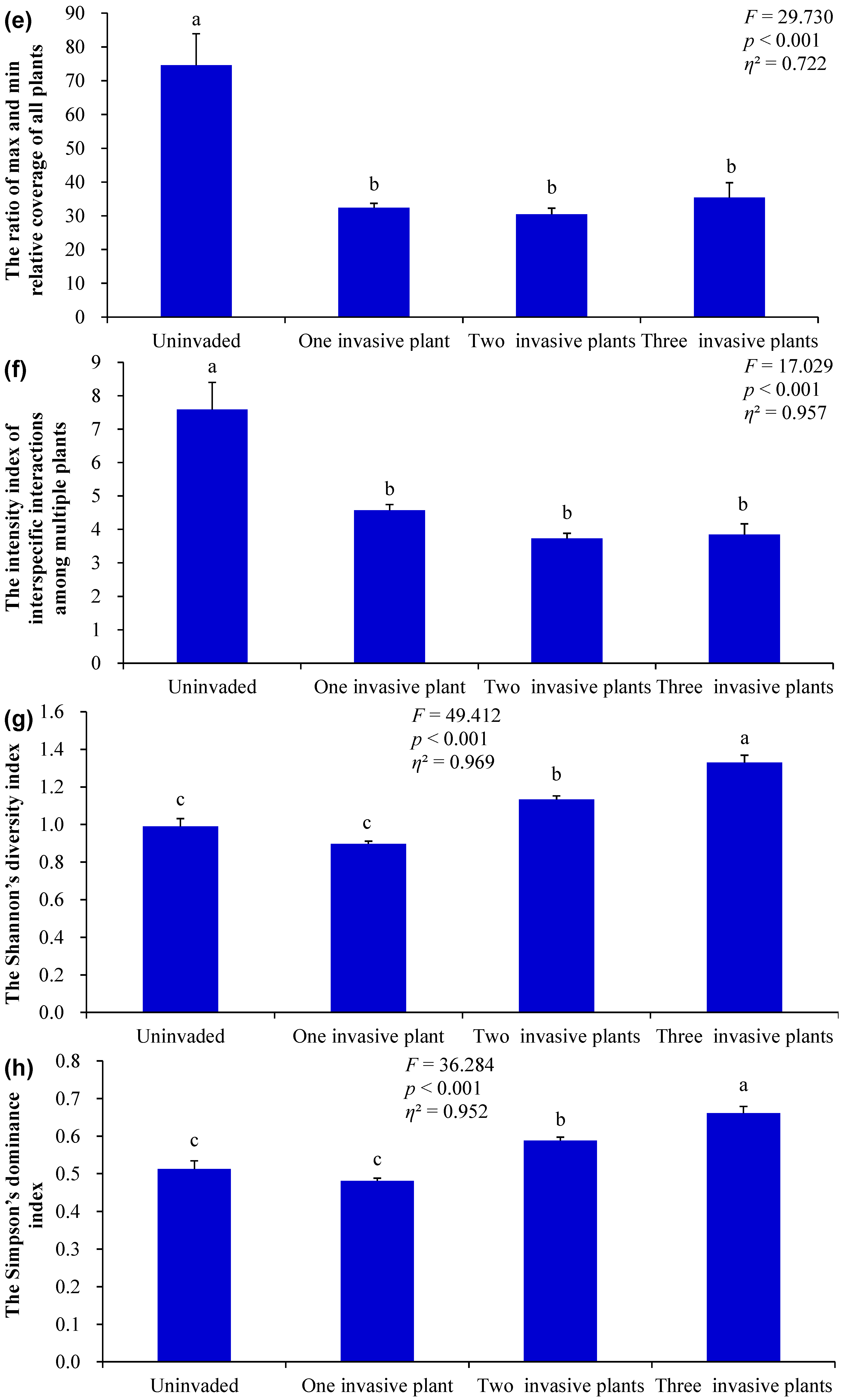
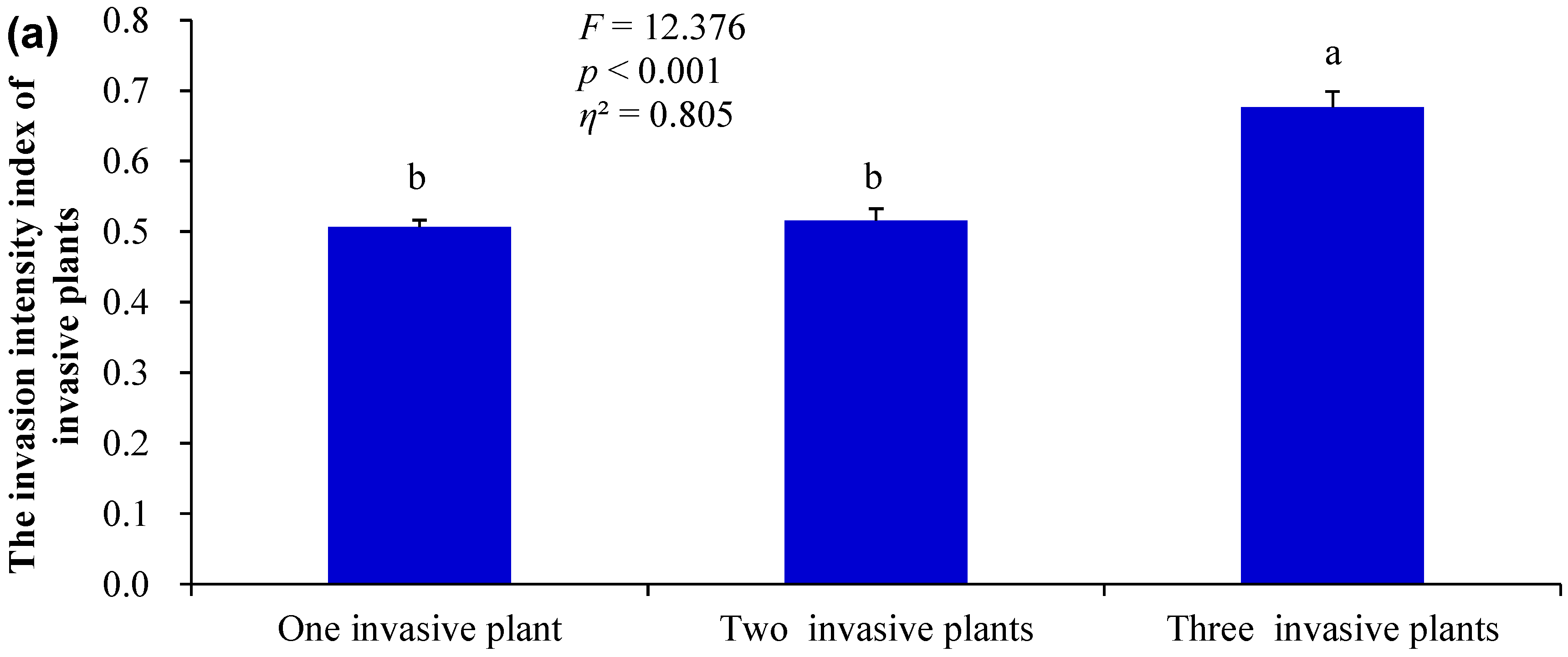
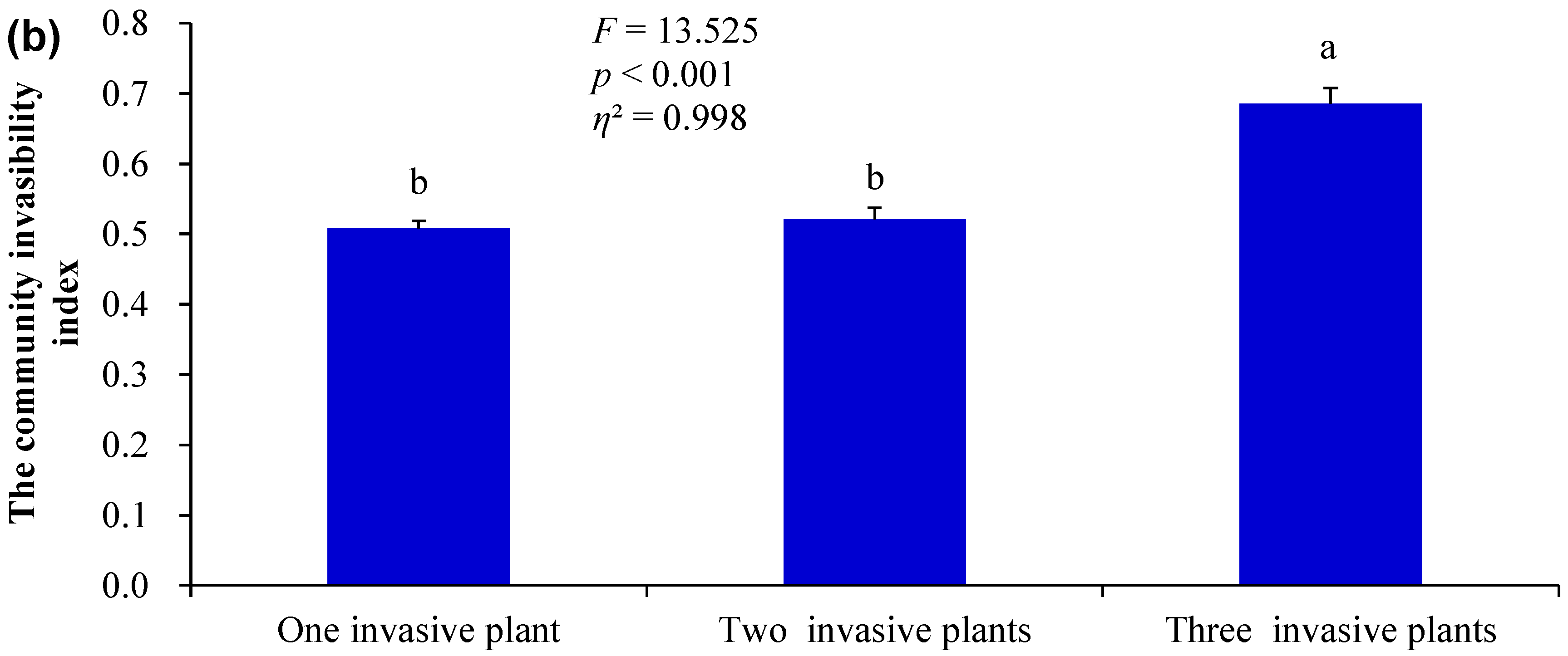
3.1. Differences in the Measured Indices Under Different Invasion Scenarios
3.2. Correlation Between the Measured Indices Under Different Invasion Scenarios
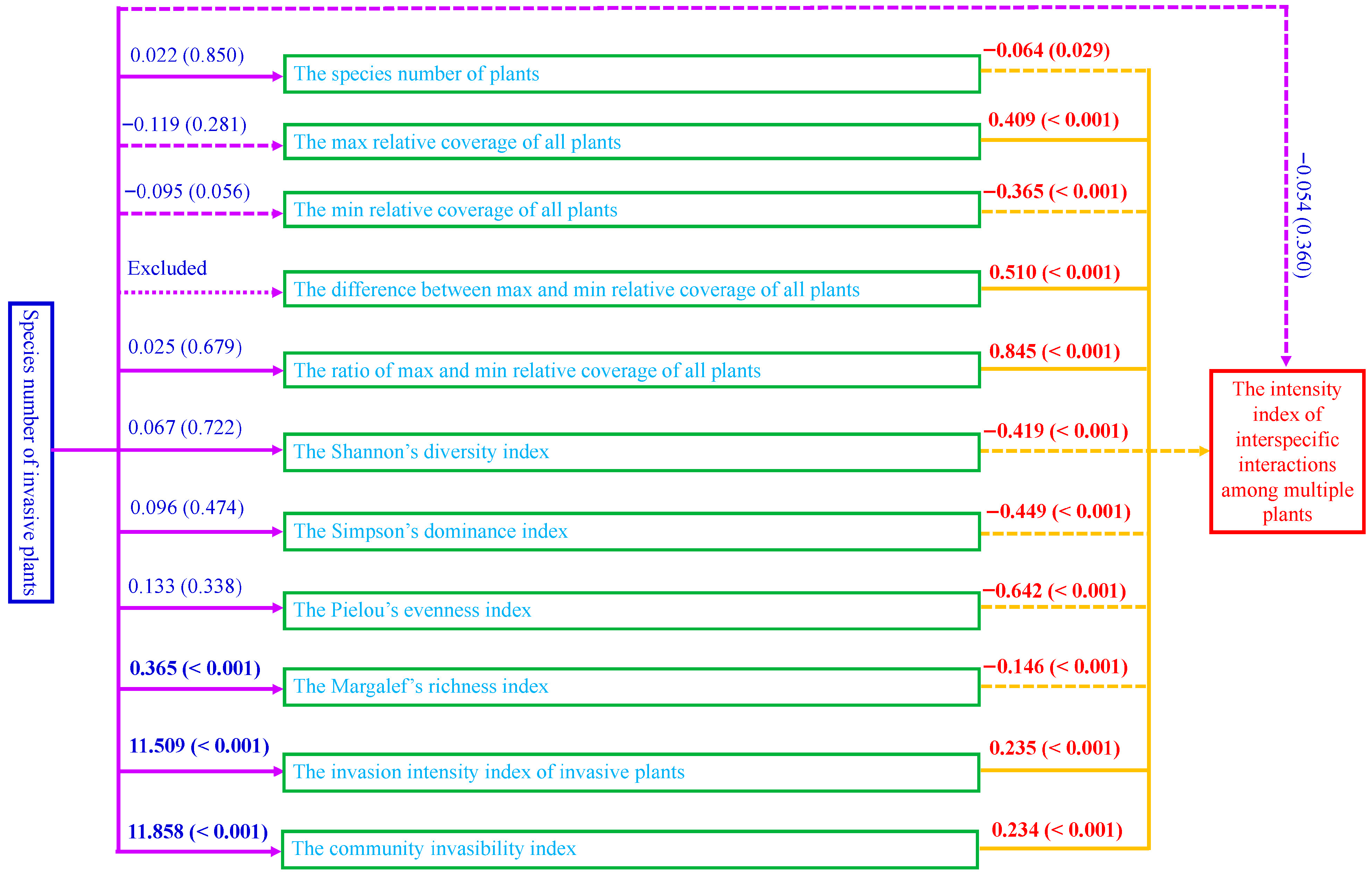
3.3. Influences of Si on the Measured Variances as Well as Influences of the Measured Variances on IIII Under Different Invasion Scenarios
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, R.; Lone, S.A.; Rashid, I.; Khuroo, A.A. A global synthesis of the ecological effects of co-invasions. J. Ecol. 2025, 113, 570–581. [Google Scholar] [CrossRef]
- Du, Y.Z.; Liu, Y.S.; Li, Y.; Li, C.; Wang, C.Y.; Cheng, H.Y.; Du, D.L. The invasive tree Rhus typhina L. decreases plant diversity and richness but increases plant functional diversity in the invaded communities. Biologia 2025, 80, 1647–1658. [Google Scholar] [CrossRef]
- Bhattarai, D.; Lamichhane, S.; Regmi, A.R.; Joshi, K.P.; Pandeya, P.; Dhami, B.; Gautam, A.P.; Adhikari, H. Impact of invasive alien plants on the resident floral diversity in Koshi Tappu Wildlife Reserve, Nepal. Ecol. Evol. 2024, 14, e70316. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.L.; Zhong, S.S.; Yu, Y.L.; Wang, Y.Y.; Cheng, H.Y.; Du, D.L.; Wang, C.Y. Rhus typhina L. triggered greater allelopathic effects than Koelreuteria paniculata Laxm under ammonium fertilization. Sci. Hortic. 2023, 309, 111703. [Google Scholar] [CrossRef]
- Carboni, M.; Livingstone, S.W.; Isaac, M.E.; Cadotte, M.W. Invasion drives plant diversity loss through competition and ecosystem modification. J. Ecol. 2021, 109, 3587–3601. [Google Scholar] [CrossRef]
- Vujanović, D.; Losapio, G.; Milić, S.; Milić, D. The impact of multiple species invasion on soil and plant communities increases with invasive species co-occurrence. Front. Plant Sci. 2022, 13, 875824. [Google Scholar] [CrossRef]
- Rastogi, R.; Qureshi, Q.; Shrivastava, A.; Jhala, Y.V. Multiple invasions exert combined magnified effects on native plants, soil nutrients and alters the plant-herbivore interaction in dry tropical forest. For. Ecol. Manag. 2023, 531, 120781. [Google Scholar] [CrossRef]
- De La Cruz, H.J.; Salgado-Luarte, C.; Stotz, G.C.; Gianoli, E. An exotic plant species indirectly facilitates a secondary exotic plant through increased soil salinity. Biol. Invasions 2023, 25, 2599–2611. [Google Scholar] [CrossRef]
- Green, P.T.; O’Dowd, D.J.; Abbott, K.L.; Jeffery, M.; Retallick, K.; Nally, R.M. Invasional meltdown: Invader-invader mutualism facilitates a secondary invasion. Ecology 2011, 92, 1758–1768. [Google Scholar] [CrossRef]
- Cavieres, L.A. Facilitation and the invasibility of plant communities. J. Ecol. 2021, 109, 2019–2028. [Google Scholar] [CrossRef]
- Guo, X.; Li, M.Y.; Jiang, S.Y.; Yang, L.Y.; Guo, S.X.; Xing, L.J.; Wang, T. Arbuscular mycorrhizal fungi inoculation exerts weak effects on species- and community-level growth traits for invading or native plants under nitrogen deposition. Front. Ecol. Evol. 2023, 11, 1152213. [Google Scholar] [CrossRef]
- Wang, G.N.; Wang, X.F.; Zhang, Y.; Yang, J.; Li, Z.K.; Wu, L.Z.; Wu, J.H.; Wu, N.; Liu, L.X.; Liu, Z.W.; et al. Dynamic characteristics and functional analysis provide new insights into long non-coding RNA responsive to Verticillium dahliae infection in Gossypium hirsutum. BMC Plant Biol. 2021, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.O.; Mohamed, H.Y. Allelopathic interference of the exotic naturalized Paspalum dilatatum Poir. threatens diversity of native plants in urban gardens. Flora 2020, 266, 151593. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949; pp. 1–117. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Margalef, R. Diversidad de Especies en Las Comunidades Naturales; Publicaciones del Instituto de Biologia Aplicada: Barcelona, Spain, 1951; Volume 6, pp. 59–72.
- Wang, C.Y.; Wei, M.; Wang, S.; Wu, B.D.; Cheng, H.Y. Erigeron annuus (L.) Pers. and Solidago canadensis L. antagonistically affect community stability and community invasibility under the co-invasion condition. Sci. Total Environ. 2020, 716, 137128. [Google Scholar] [CrossRef]
- Liu, Y.S.; Du, Y.Z.; Li, C.; Li, Y.; Wang, C.Y.; Du, D.L. Co-invasion of three invasive alien plants increases plant taxonomic diversity and community invasibility. Plant Divers. 2025; in press. [Google Scholar] [CrossRef]
- Trevenen, E.J.; Veneklaas, E.J.; Teste, F.P.; Dobrowolski, M.P.; Mucina, L.; Renton, M. Plant interactions can lead to emergent relationships between plant community diversity, productivity and vulnerability to invasion. Sci. Rep. 2024, 14, 13932. [Google Scholar] [CrossRef]
- Kinlock, N.L.; Munch, S.B. Interaction network structure and spatial patterns influence invasiveness and invasibility in a stochastic model of plant communities. Oikos 2021, 130, 2040–2052. [Google Scholar] [CrossRef]
- Hillebrand, H.; Bennett, D.M.; Cadotte, M.W. Consequences of dominance: A review of evenness effects on local and regional ecosystem processes. Ecology 2008, 89, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, P.N.; Schmitz, M.; Scherber, C.; Roscher, C.; Schumacher, J.; Scherer-Lorenzen, M.; Weisser, W.W.; Schmid, B. Niche preemption increases with species richness in experimental plant communities. J. Ecol. 2007, 95, 65–78. [Google Scholar] [CrossRef]
- Symstad, A.J. A test of the effects of functional group richness and composition on grassland invasibility. Ecology 2000, 81, 99–109. [Google Scholar] [CrossRef]
- Catford, J.A.; Smith, A.L.; Wragg, P.D.; Clark, A.T.; Kosmala, M.; Cavender-Bares, J.; Reich, P.B.; Tilman, D. Traits linked with species invasiveness and community invasibility vary with time, stage and indicator of invasion in a long-term grassland experiment. Ecol. Lett. 2019, 22, 593–604. [Google Scholar] [CrossRef]
- Gherardi, L.A.; Sala, O.E. Enhanced interannual precipitation variability increases plant functional diversity that in turn ameliorates negative impact on productivity. Ecol. Lett. 2015, 18, 1293–1300. [Google Scholar] [CrossRef]
- Byun, C.; de Blois, S.; Brisson, J. Plant functional group identity and diversity determine biotic resistance to invasion by an exotic grass. J. Ecol. 2013, 101, 128–139. [Google Scholar] [CrossRef]
- O’Leary, B.; Burd, M.; Venn, S.E.; Gleadow, R. Integrating the Passenger-Driver hypothesis and plant community functional traits to the restoration of lands degraded by invasive trees. For. Ecol. Manag. 2018, 408, 112–120. [Google Scholar] [CrossRef]
- HilleRisLambers, J.; Yelenik, S.G.; Colman, B.P.; Levine, J.M. California annual grass invaders: The drivers or passengers of change? J. Ecol. 2010, 98, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Ferenc, V.; Merkert, C.; Zilles, F.; Sheppard, C.S. Native and alien species suffer from late arrival, while negative effects of multiple alien species on natives vary. Oecologia 2021, 197, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Lenda, M.; Skórka, P.; Knops, J.; Żmihorski, M.; Gaj, R.; Moroń, D.; Woyciechowski, M.; Tryjanowski, P. Multispecies invasion reduces the negative impact of single alien plant species on native flora. Divers. Distrib. 2019, 25, 951–962. [Google Scholar] [CrossRef]
- Ordonez, A. Functional and phylogenetic similarity of alien plants to co-occurring natives. Ecology 2014, 95, 1191–1202. [Google Scholar] [CrossRef]
- Gianoli, E.; Escobedo, V.M. Phenotypic plasticity may mediate habitat filtering in a forest edge community. Oikos 2021, 130, 1788–1796. [Google Scholar] [CrossRef]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Quiroga, R.E.; Premoli, A.C.; Fernandez, R.J. Niche dynamics in amphitropical desert disjunct plants: Seeking for ecological and species-specific influences. Glob. Ecol. Biogeogr. 2021, 30, 370–383. [Google Scholar] [CrossRef]
- Lambdon, P.W.; Lloret, F.; Hulme, P.E. Do alien plants on Mediterranean islands tend to invade different niches from native species? Biol. Invasions 2008, 10, 703–716. [Google Scholar] [CrossRef]
- Gause, G.F.; Witt, A.A. Behavior of mixed populations and the problem of natural selection. Am. Nat. 1935, 69, 596–609. [Google Scholar] [CrossRef]
- Catford, J.A.; Daehler, C.C.; Murphy, H.T.; Sheppard, A.W.; Hardesty, B.D.; Westcott, D.A.; Rejmánek, M.; Bellingham, P.J.; Pergl, J.; Horvitz, C.C.; et al. The intermediate disturbance hypothesis and plant invasions: Implications for species richness and management. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 231–241. [Google Scholar] [CrossRef]
- Downey, S.S.; Walker, M.; Moschler, J.; Penados, F.; Peterman, W.; Pop, J.; Qin, R.J.; Scaggs, S.A.; Song, S. An intermediate level of disturbance with customary agricultural practices increases species diversity in Maya community forests in Belize. Commun. Earth Environ. 2023, 4, 428. [Google Scholar] [CrossRef]
- Driscoll, D.A. Disturbance maintains native and exotic plant species richness in invaded grassy woodlands. J. Veg. Sci. 2017, 28, 573–584. [Google Scholar] [CrossRef]


| r | S | MaxRCi | MinRCi | Difference Between MaxRCi and MinRCi | Ratio of MaxRCi and MinRCi | IIII | H’ | D | EH | F | III | CII | Si |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | 1 | −0.599 | −0.474 | −0.381 | 0.149 | −0.064 | 0.787 | 0.661 | 0.303 | 0.936 | −0.380 | −0.378 | 0.374 |
| MaxRCi | 1 | −0.022 | 0.932 | 0.367 | 0.409 | −0.928 | −0.967 | −0.864 | −0.617 | 0.481 | 0.478 | −0.276 | |
| MinRCi | 1 | −0.383 | −0.436 | −0.365 | −0.134 | −00.015 | 0.390 | −0.434 | 0.033 | 0.033 | −0.122 | ||
| Difference between MaxRCi and MinRCi | 1 | 0.497 | 0.510 | −0.808 | −0.888 | −0.940 | −0.412 | 0.432 | 0.430 | −0.211 | |||
| Ratio of MaxRCi and MinRCi | 1 | 0.845 | −0.302 | −0.356 | −0.569 | 00.041 | 0.189 | 0.189 | <0.001 | ||||
| IIII | 1 | −0.419 | −0.449 | −0.642 | −0.146 | 0.235 | 0.234 | −0.080 | |||||
| H’ | 1 | 0.955 | 0.794 | 0.789 | −0.493 | −0.490 | 0.335 | ||||||
| D | 1 | 0.865 | 0.677 | −0.505 | −0.502 | 0.292 | |||||||
| EH | 1 | 0.345 | −0.424 | −0.422 | 0.170 | ||||||||
| F | 1 | −0.387 | −0.384 | 0.395 | |||||||||
| III | 1 | 1.000 | 0.110 | ||||||||||
| CII | 1 | 0.118 | |||||||||||
| Si | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Liu, Y.; Geng, X.; Zhang, Y.; Wang, C.; Du, D. Does the Species Number of Invasive Plants Regulate the Intensity of Interspecific Interactions Among Multiple Plants Under Different Invasion Scenarios? Plants 2025, 14, 2767. https://doi.org/10.3390/plants14172767
Du Y, Liu Y, Geng X, Zhang Y, Wang C, Du D. Does the Species Number of Invasive Plants Regulate the Intensity of Interspecific Interactions Among Multiple Plants Under Different Invasion Scenarios? Plants. 2025; 14(17):2767. https://doi.org/10.3390/plants14172767
Chicago/Turabian StyleDu, Yizhuo, Yingsheng Liu, Xiaoxuan Geng, Yulong Zhang, Congyan Wang, and Daolin Du. 2025. "Does the Species Number of Invasive Plants Regulate the Intensity of Interspecific Interactions Among Multiple Plants Under Different Invasion Scenarios?" Plants 14, no. 17: 2767. https://doi.org/10.3390/plants14172767
APA StyleDu, Y., Liu, Y., Geng, X., Zhang, Y., Wang, C., & Du, D. (2025). Does the Species Number of Invasive Plants Regulate the Intensity of Interspecific Interactions Among Multiple Plants Under Different Invasion Scenarios? Plants, 14(17), 2767. https://doi.org/10.3390/plants14172767








