Influence of Nitrogen Fertilization and Cutting Dynamics on the Yield and Nutritional Composition of White Clover (Trifolium repens L.)
Abstract
1. Introduction
2. Results
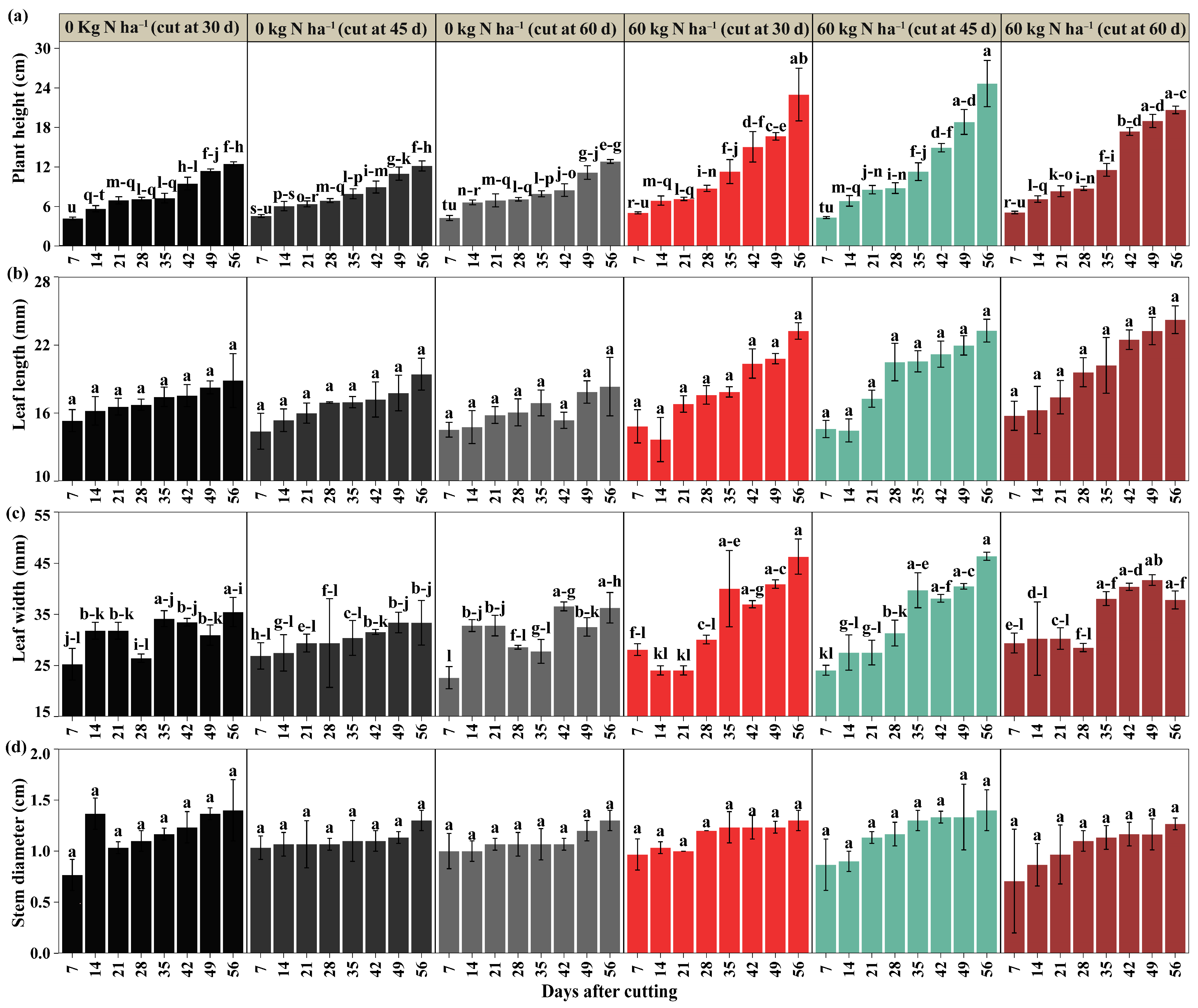
2.1. Morphological Parameters
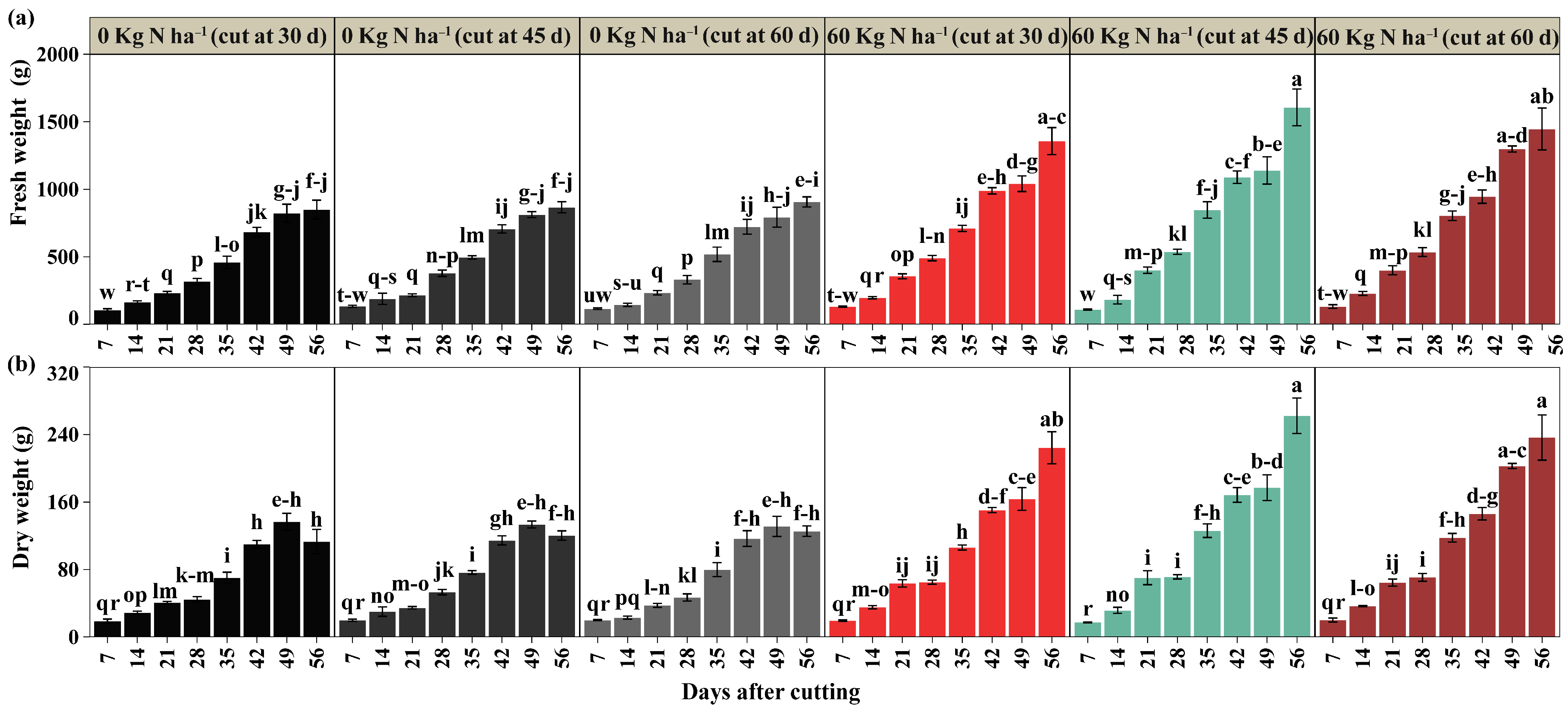
2.2. Performance Parameters
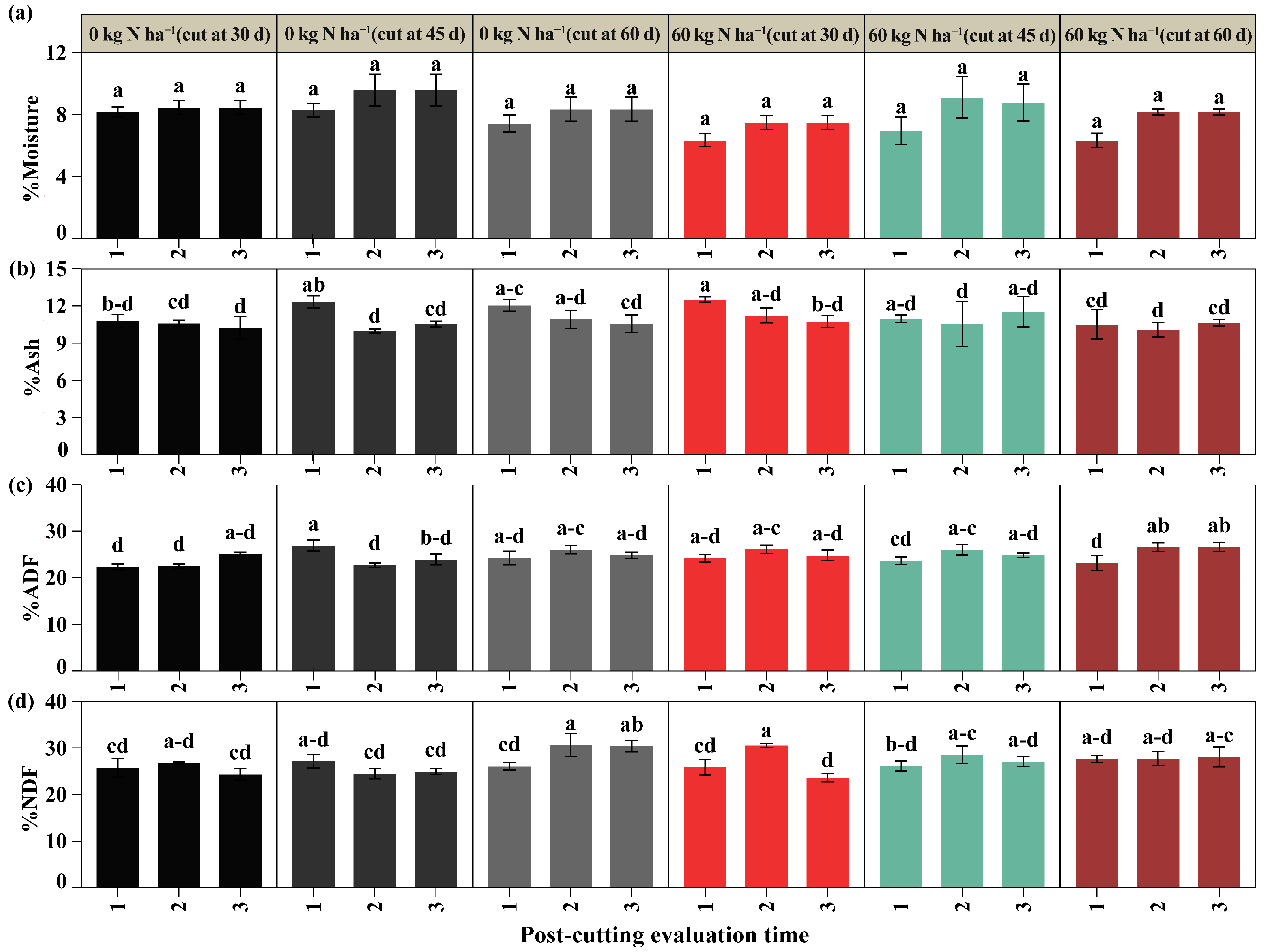
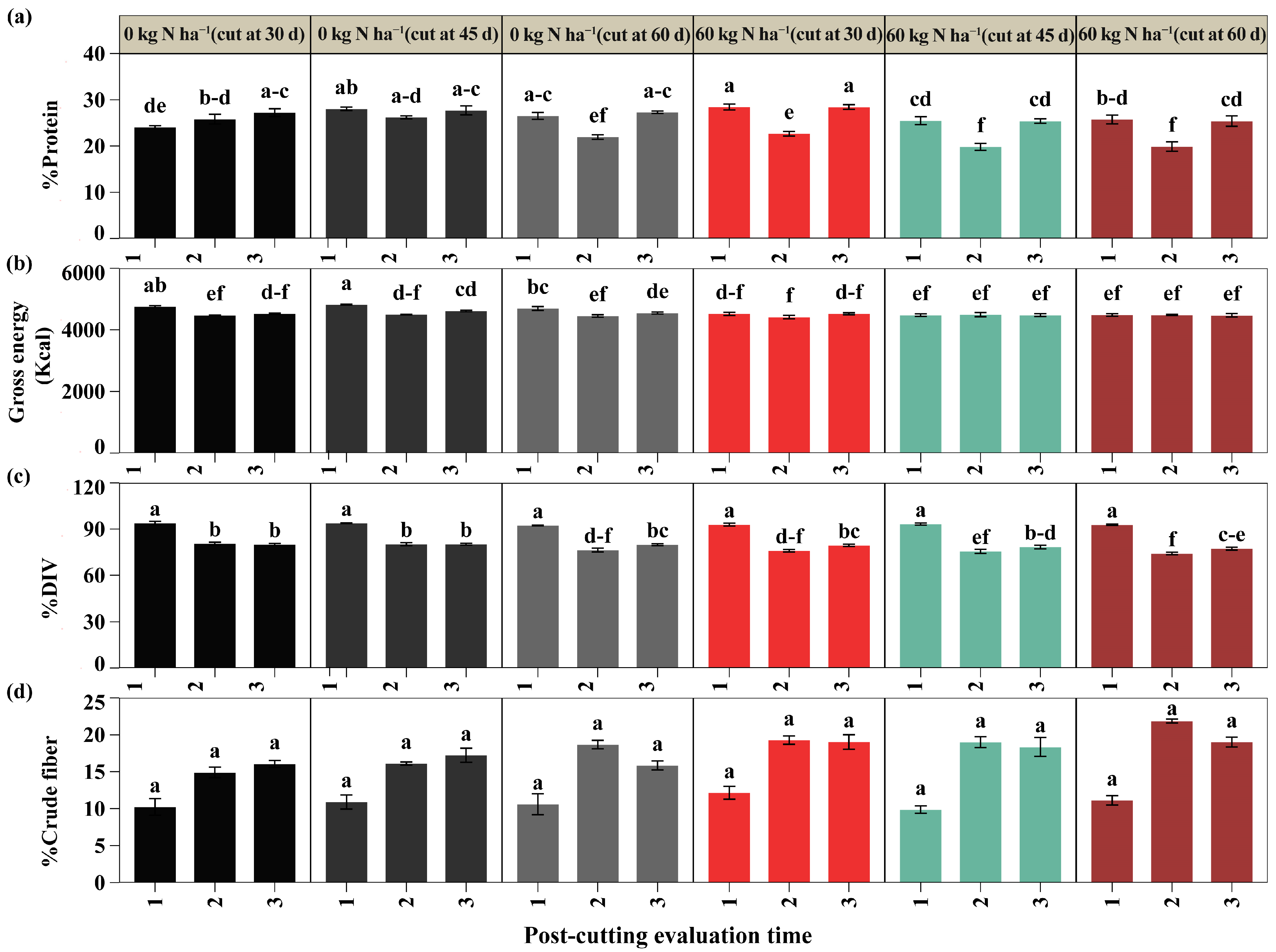
2.3. Bromatological Parameters
3. Discussion
3.1. Morphological Variables
3.2. Fresh and Dry Biomass Yield
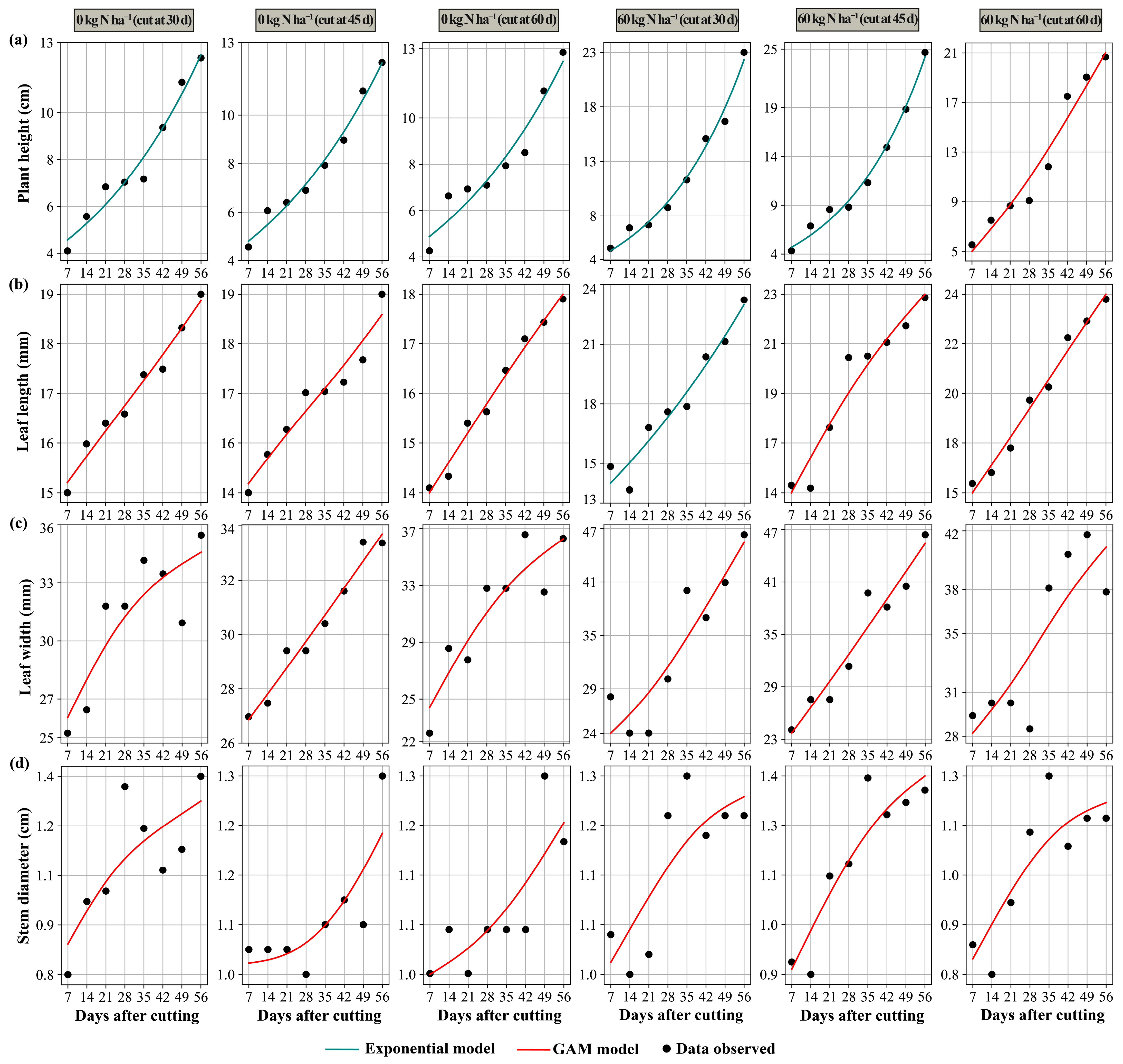
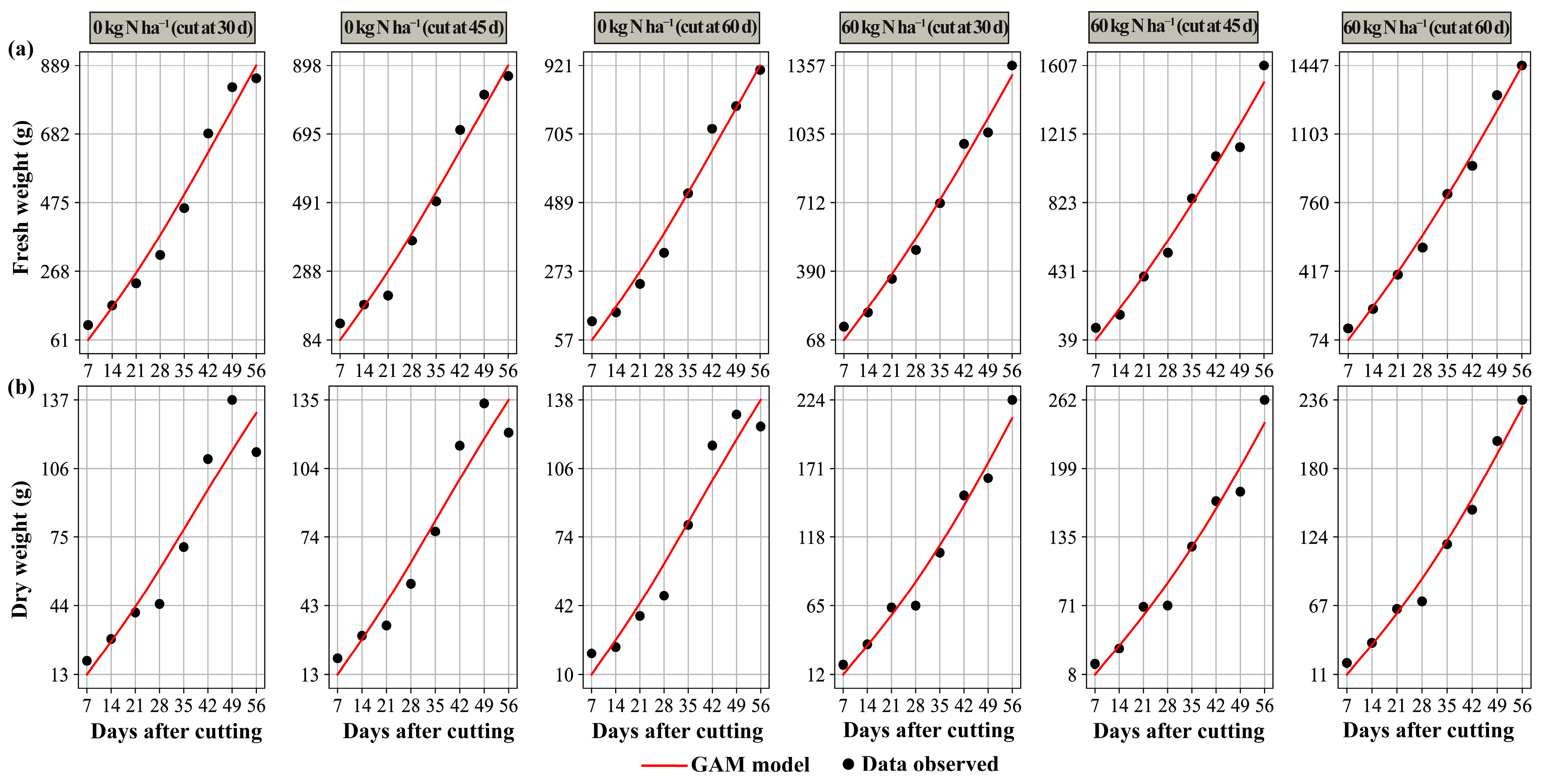
3.3. Prediction Models
3.4. Nutritional Composition
4. Materials and Methods
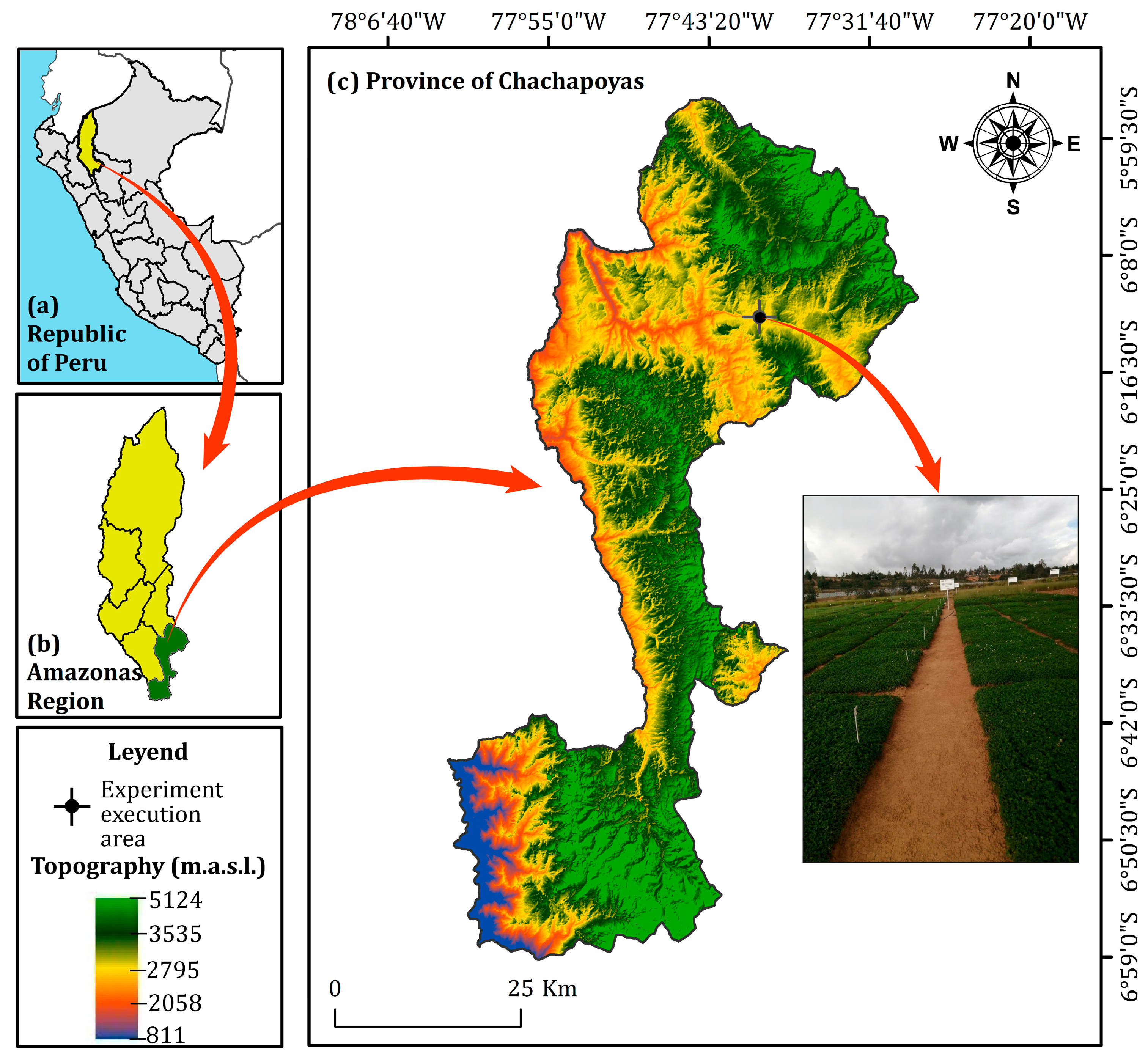
4.1. Research Area
4.2. Experimental Design
4.3. Installation of the Experimental Area
4.4. Evaluation of Indicators
4.4.1. Morphological Parameters
4.4.2. Performance Parameters
4.4.3. Nutritional Composition Parameters
4.5. Predictive Models in Morphology and Yield
4.5.1. Generalized Additive Models
4.5.2. Exponential Model
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Horrocks, R.D.; Valentine, J.F. Cultivar selection. In Harvested Forages, 1st ed.; Academic Press: San Diego, CA, USA, 1999; pp. 125–134. [Google Scholar] [CrossRef]
- Gibson, P.B.; Cope, W.A. White Clover. In Agronomy Monographs; Taylor, N.L., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2015; Volume 25, pp. 471–490. [Google Scholar] [CrossRef]
- Potter, D.A.; Redmond, C.T.; McNamara, T.D.; Munshaw, G.C. Dwarf White Clover Supports Pollinators, Augments Nitrogen in Clover–Turfgrass Lawns, and Suppresses Root-Feeding Grubs in Monoculture but Not in Mixed Swards. Sustainability 2021, 13, 11801. [Google Scholar] [CrossRef]
- Van Eekeren, N.; Van Liere, D.; De Vries, F.; Rutgers, M.; De Goede, R.; Brussaard, L. A mixture of grass and clover combines the positive effects of both plant species on selected soil biota. Appl. Soil Ecol. 2009, 42, 254–263. [Google Scholar] [CrossRef]
- Rutter, S.M. Diet preference for grass and legumes in free-ranging domestic sheep and cattle: Current theory and future application. Appl. Anim. Behav. Sci. 2006, 97, 17–35. [Google Scholar] [CrossRef]
- Sharp, J.M.; Edwards, G.R.; Jeger, M.J. Impact of the spatial scale of grass–legume mixtures on sheep grazing behaviour, preference and intake, and subsequent effects on pasture. Animal 2012, 6, 1848–1856. [Google Scholar] [CrossRef]
- Oliva, M.; Collazos, R.; Vásquez, H.; Rubio, K.; Maicelo, J. Composición florística de especies herbáceas forrajeras en praderas naturales de las principales microcuencas ganaderas de la región Amazonas. Sci. Agropecu. 2019, 10, 109–117. [Google Scholar] [CrossRef]
- Valqui, L.; Saucedo-Uriarte, J.A.; Altamirano-Tantalean, M.A.; Bodadilla, L.G.; Portocarrero Villegas, S.M.; Bardales, W.; Frias, H.; Zagaceta Llanca, L.H.; Valqui-Valqui, L.; Puerta-Chavez, L.J.; et al. Influence of tree species on soil physicochemical composition, macrofauna, and forage production. J. Agric. Food Res. 2025, 23, 102220. [Google Scholar] [CrossRef]
- Egan, M.; Galvin, N.; Hennessy, D. Incorporating white clover (Trifolium repens L.) into perennial ryegrass (Lolium perenne L.) swards receiving varying levels of nitrogen fertilizer: Effects on milk and herbage production. J. Dairy Sci. 2018, 101, 3412–3427. [Google Scholar] [CrossRef]
- Caradus, J.; Woodfield, D.; Stewart, A. Overview and vision for white clover. NZGA Res. Pract. Ser. 1996, 6, 1–6. [Google Scholar] [CrossRef]
- Yang, Z.; Mei, J.; Zheng, W.; Khan, F.S.; Bhuiyan, M.N.; Wang, K.; Rhaman, M.S.; Abe-Kanoh, N.; Ji, W. Identification of Grape NRT Gene Family and Analysis of Its Expression in Leaves Under Nitrogen-Deficiency Stress. Horticulturae 2025, 11, 252. [Google Scholar] [CrossRef]
- Nyfeler, D.; Huguenin-Elie, O.; Frossard, E.; Lüscher, A. Effects of legumes and fertiliser on nitrogen balance and nitrate leaching from intact leys and after tilling for subsequent crop. Agric. Ecosyst. Environ. 2024, 360, 108776. [Google Scholar] [CrossRef]
- Irisarri, P.; Cardozo, G.; Tartaglia, C.; Reyno, R.; Gutiérrez, P.; Lattanzi, F.A.; Rebuffo, M.; Monza, J. Selection of Competitive and Efficient Rhizobia Strains for White Clover. Front. Microbiol. 2019, 10, 768. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Papadopoulos, Y.A.; Rodd, A.V.; Grimmett, M.; Fillmore, S.A.E.; Crouse, M.; Prithiviraj, B. Nitrogen fixation and transfer of red clover genotypes under legume–grass forage based production systems. Nutr. Cycl. Agroecosyst. 2016, 106, 233–247. [Google Scholar] [CrossRef]
- Boller, B.C.; Nösberger, J. Symbiotically fixed nitrogen from field-grown white and red clover mixed with ryegrasses at low levels of 15N-fertilization. Plant Soil 1987, 104, 219–226. [Google Scholar] [CrossRef]
- Williams, W.A.; Graves, W.L.; Cassman, K.G. Nitrogen fixation by irrigated berseem clover versus soil nitrogen supply. J. Agron. Crop Sci. 1990, 164, 202–207. [Google Scholar] [CrossRef]
- Enriquez-Hidalgo, D.; Gilliland, T.J.; Hennessy, D. Herbage and nitrogen yields, fixation and transfer by white clover to companion grasses in grazed swards under different rates of nitrogen fertilization. Grass Forage Sci. 2016, 71, 559–574. [Google Scholar] [CrossRef]
- Kristensen, R.K.; Rasmussen, J.; Eriksen, J. Biological N2-fixation in grass–clover ley in response to N application in cattle slurry vs. mineral fertilizer. Plant Soil 2022, 471, 629–641. [Google Scholar] [CrossRef]
- Kristensen, R.K.; Fontaine, D.; Rasmussen, J.; Eriksen, J. Contrasting effects of slurry and mineral fertilizer on N2-fixation in grass–clover mixtures. Eur. J. Agron. 2022, 133, 126431. [Google Scholar] [CrossRef]
- Oberson, A.; Frossard, E.; Bühlmann, C.; Mayer, J.; Mäder, P.; Lüscher, A. Nitrogen fixation and transfer in grass–clover leys under organic and conventional cropping systems. Plant Soil 2013, 371, 237–255. [Google Scholar] [CrossRef]
- Elgersma, A.; Schlepers, H.; Nassiri, M. Interactions between perennial ryegrass (Lolium perenne L.) and white clover (Trifolium repens L.) under contrasting nitrogen availability: Productivity, seasonal patterns of species composition, N2 fixation, N transfer and N recovery. Plant Soil 2000, 221, 281–299. [Google Scholar] [CrossRef]
- Thers, H.; Jensen, J.L.; Rasmussen, J.; Eriksen, J. Grass–clover response to cattle slurry N-rates: Yield, clover proportion, protein concentration and estimated N2-fixation. Field Crops Res. 2022, 287, 108675. [Google Scholar] [CrossRef]
- Tucak, M.; Čupić, T.; Horvat, D.; Ravlić, M.; Krizmanić, G.; Maćešić, D.; Žnidaršič, T.; Meglič, V. Changes in agronomic and forage nutritive values of red clover in response to different development stage. Rom. Agric. Res. 2023, 40, 215–224. [Google Scholar] [CrossRef]
- De Santis, G.; Iannucci, A.; Dantone, D.; Chiaravalle, E. Changes during growth in the nutritive value of components of berseem clover (Trifolium alexandrinum L.) under different cutting treatments in a Mediterranean region. Grass Forage Sci. 2004, 59, 378–388. [Google Scholar] [CrossRef]
- Djordjevic, S.; Mandic, V.; Djordjevic, N. Effects of cutting stage and bacterial inoculant on quality of the red clover silage. Biotechnol. Anim. Husb. 2021, 37, 65–73. [Google Scholar] [CrossRef]
- Vallejos-Cacho, R.; Vallejos-Fernández, L.A.; Alvarez-García, W.Y.; Tapia-Acosta, E.A.; Saldanha-Odriozola, S.; Quilcate-Pairazaman, C.E. Sustainability of Lolium multiflorum L. ‘Cajamarquino Ecotype’, associated with Trifolium repens L., at three cutting frequencies in the northern highlands of Peru. Sustainability 2024, 16, 6927. [Google Scholar] [CrossRef]
- Salcedo-Mayta, S.; Canihua-Rojas, J.; Samaniego-Vivanco, T.; Cruz-Luis, J.; Pérez-Porras, W.; Cosme De La Cruz, R.C. Cover crops associated with quinoa (Chenopodium quinoa Willd) in the Peruvian Altiplano: Erosion reduction, improved soil health and agricultural yield. Sci. Agropecu. 2022, 13, 265–274. [Google Scholar] [CrossRef]
- Bucksch, A.; Atta-Boateng, A.; Azihou, A.F.; Battogtokh, D.; Baumgartner, A.; Binder, B.M.; Braybrook, S.A.; Chang, C.; Coneva, V.; DeWitt, T.J.; et al. Morphological Plant Modeling: Unleashing Geometric and Topological Potential within the Plant Sciences. Front. Plant Sci. 2017, 8, 900. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ivey, C.E.; Blanchard, C.L.; Do, K.; Lee, S.-M.; Russell, A.G. Emissions and meteorological impacts on PM2.5 species concentrations in Southern California using generalized additive modeling. Sci. Total Environ. 2023, 891, 164464. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Tang, J.; Li, T.; Zhang, A.; Mao, L. Evaluating the relative importance of predictors in Generalized Additive Models using the gam.hp R package. Plant Divers. 2024, 46, 542–546. [Google Scholar] [CrossRef]
- Sun, J.-M.; Lu, L.; Liu, K.-K.; Yang, J.; Wu, H.-X.; Liu, Q.-Y. Forecast of severe fever with thrombocytopenia syndrome incidence with meteorological factors. Sci. Total Environ. 2018, 626, 1188–1192. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Y.; Hu, Z.; Wu, Y.; Sun, W.; Li, Y.; Song, Y. Modelling maize silk extension using segmented exponential and linear functions. Eur. J. Agron. 2024, 159, 127269. [Google Scholar] [CrossRef]
- Nesan, D.; Chan, D.J.C. Exponential decay: An approach to model nutrient uptake rates of macrophytes. Int. J. Phytoremediat. 2021, 23, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, M.; Capasso, V.; Montagna, M.; Venturino, E. A mathematical model for Xylella fastidiosa epidemics in the Mediterranean regions. Promoting good agronomic practices for their effective control. Ecol. Model. 2020, 432, 109204. [Google Scholar] [CrossRef]
- Dumont, Y. Maths for Plants and Plants for Maths. Mathematics applied to Agronomy and Crop Protection. In Proceedings of the BIOMATH-S Conference, Prétoria, South Africa, 12–17 July 2015; Volume 1. Available online: https://biomath.math.bas.bg/biomath/index.php/conference/article/view/545 (accessed on 5 April 2025).
- Fonseca López, D.; Bohórquez Masmela, I.A.; Rodríguez Molano, C.E.; Vivas-Quila, N.J. Efecto del periodo de recuperación en la producción y calidad nutricional de algunas especies forrajeras. Biotecnol. Sect. Agropecu. Agroind. 2020, 18, 135–144. [Google Scholar] [CrossRef]
- Simon, J.C.; Jacquet, A.; Decau, M.L.; Goulas, E.; Le Dily, F. Influence of cutting frequency on the morphology and the C and N reserve status of two cultivars of white clover (Trifolium repens L.). Eur. J. Agron. 2004, 20, 341–350. [Google Scholar] [CrossRef]
- Herbert, D.B.; Ekschmitt, K.; Wissemann, V.; Becker, A. Cutting reduces variation in biomass production of forage crops and allows low-performers to catch up: A case study of Trifolium pratense L. (red clover). Plant Biol. 2018, 20, 465–473. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, K.; Guo, J.; Zang, J.; Liu, S. Elevated nitrogen supply enhances the recovery capability of alfalfa following rewatering by regulating carbon allocation. Environ. Exp. Bot. 2025, 231, 106095. [Google Scholar] [CrossRef]
- Kim, S.; Albrecht, K.; Sheaffer, C.; Lee, D.; Subramanian, S.; Owens, V. Biomass production of prairie cordgrass (Spartina pectinata Link.) using urea and kura clover (Trifolium ambiguum Bieb.) as a source of nitrogen. BioEnergy Res. 2020, 13, 1095–1107. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, Y. Contribution of mycorrhizal symbiosis and root strategy to red clover aboveground biomass under nitrogen addition and phosphorus distribution. Mycorrhiza 2024, 34, 489–502. [Google Scholar] [CrossRef]
- Reis, V.M.; Alves, B.J.R.; Hartmann, A.; James, E.K.; Zilli, J.E. Beneficial microorganisms in agriculture: The future of plant growth-promoting rhizobacteria. Plant Soil 2020, 451, 1–3. [Google Scholar] [CrossRef]
- Wen, A.; Havens, K.L.; Bloch, S.E.; Shah, N.; Higgins, D.A.; Davis-Richardson, A.G.; Sharon, J.; Rezaei, F.; Mohiti-Asli, M.; Johnson, A.; et al. Enabling Biological Nitrogen Fixation for Cereal Crops in Fertilized Fields. ACS Synth. Biol. 2021, 10, 3264–3277. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current Progress in Nitrogen Fixing Plants and Microbiome Research. Plants 2020, 9, 97. [Google Scholar] [CrossRef]
- Lindström, K.; Mousavi, S.A. Effectiveness of Nitrogen Fixation in Rhizobia. Microb. Biotechnol. 2020, 13, 1314–1335. [Google Scholar] [CrossRef]
- Tang, J.; Li, W.; Wei, T.; Huang, R.; Zeng, Z. Patterns and Mechanisms of Legume Responses to Nitrogen Enrichment: A Global Meta-Analysis. Plants 2024, 13, 3244. [Google Scholar] [CrossRef]
- Gómez-Rubio, V. Generalized Additive Models: An Introduction with R. Stat. Softw. 2018, 86, 1–5. [Google Scholar] [CrossRef]
- Reyes-Santías, F.; Cordova-Arevalo, O.; Rivo-Lopez, E. Using Flexible Regression Models for Calculating Hospital’s Production Functions. BMC Health Serv. Res. 2020, 20, 1110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Mao, B.; Guo, S. Mathematical Modeling of Tumor Growth in Preclinical Mouse Models with Applications in Biomarker Discovery and Drug Mechanism Studies. Cancer Res. Commun. 2024, 4, 2267–2281. [Google Scholar] [CrossRef]
- Zhou, H.; Mao, B.; Guo, S. Abstract 2743: Tumor Growth Modeling and Its Applications in Preclinical Pharmacology Studies to Improve Translatability of Animal Models. Cancer Res. 2022, 82, 2743. [Google Scholar] [CrossRef]
- Cooney, P.; White, A. Extending Beyond Bagust and Beale: Fully Parametric Piecewise Exponential Models for Extrapolation of Survival Outcomes in Health Technology Assessment. Value Health 2023, 26, 1510–1517. [Google Scholar] [CrossRef]
- Shi, H.; Xiao, Z. Exploring Topographic Effects on Surface Parameters over Rugged Terrains at Various Spatial Scales. IEEE Trans. Geosci. Remote Sens. 2022, 60, 1–16. [Google Scholar] [CrossRef]
- Uuemaa, E.; Ahi, S.; Montibeller, B.; Muru, M.; Kmoch, A. Vertical Accuracy of Freely Available Global Digital Elevation Models (ASTER, AW3D30, MERIT, TanDEM-X, SRTM, and NASADEM). Remote Sens. 2020, 12, 3482. [Google Scholar] [CrossRef]
- Ebinne, E.; Apeh, O.; Moka, E.; Abah, E. Comparative Analysis of Freely Available Digital Elevation Models for Applications in Multi-Criteria Environmental Modeling over Data Limited Regions. Remote Sens. Appl. Soc. Environ. 2022, 27, 100795. [Google Scholar] [CrossRef]
- Sun, X.; Luo, N.; Longhurst, B.; Luo, J. Fertiliser Nitrogen and Factors Affecting Pasture Responses. Open Agric. J. 2008, 2, 35–42. [Google Scholar] [CrossRef]
- Jacobs, J.L.; McKenzie, F.R.; Ryan, M.J.; Kearney, G. Effect of Rate and Time of Nitrogen Application from Autumn to Midwinter on Perennial Ryegrass—White Clover Dairy Pastures in Western Victoria. 1. Growth and Composition. Aust. J. Agric. Res. 1999, 50, 1059. [Google Scholar] [CrossRef]
- Karbivska, U.M. Feed Quality of Feed Grassland Agrophytocenoses Depending on Their Species Composition and Fertilization in Precarpathian Conditions. Feeds Feed Prod. 2019, 88, 91–98. [Google Scholar] [CrossRef]
- Ajayi, F.T. Intake and Digestibility of N’Dama Cattle Fed Concentrate Diets Containing Varying Levels of Corncob. Niger. J. Anim. Prod. 2024, 51, 563–565. [Google Scholar] [CrossRef]
- Schaepe, K. Untersuchungen an Hunden zur Rohnährstoffverdaulichkeit Sowie Kot- und Harnzusammensetzung bei Variation der Rohaschegehalte im Futter durch Unterschiedlich Knochenreiche Schlachtnebenprodukte. Available online: https://elib.tiho-hannover.de/receive/etd_mods_00001012 (accessed on 14 August 2025).
- Valqui, L.; Lopez, E.L.; Lopez, C.A.; Valqui-Valqui, L.; Bobadilla, L.G.; Vigo, C.N.; Vásquez, H.V. Influence of the arboreal component in the productive and nutritional parameters of Brachiaria mutica grass in northeastern Peru. Environ. Sci. Proc. 2022, 22, 69. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, W.; Ali, S.; Chang, S.; Jia, Q.; Hou, F. Legume/Maize Intercropping and N Application for Improved Yield, Quality, Water and N Utilization for Forage Production. Agronomy 2022, 12, 1777. [Google Scholar] [CrossRef]
- Schnellmann, L.P.; Verdoljak, J.J.O.; Bernadis, A.; Martínez-Gonzalez, J.C.; Castillo-Rodríguez, S.P.; Limas-Martínez, A.G. Frecuencia y Altura de Corte sobre la Calidad del Megathyrsus maximus (cv. Gatton panic). Cienc. Tecnol. Agropecu. 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Geren, H.; Kavut, Y.; Unlu, H. Effect of Different Cutting Intervals on the Forage Yield and Some Silage Quality Characteristics of Giant King Grass (Pennisetum hybridum) under Mediterranean Climatic Conditions. Turk. J. Field Crops 2020, 25, 1–8. [Google Scholar] [CrossRef]
- Nguyen, H.T.D.; Schonewille, J.T.; Pellikaan, W.F.; Nguyen, T.X.; Hendriks, W.H. In Vitro Gas Production of Common Southeast Asian Grasses in Response to Variable Regrowth Periods in Vietnam. Fermentation 2024, 10, 280. [Google Scholar] [CrossRef]
- Mir, N.H.; Ahmad, S.; Bhat, S.S. Orchard Grass (Dactylis glomerata L.) Yield and Nutritive Characteristics in Response to Different Cutting Regimes in a Temperate Region. Range Manag. Agrofor. 2024, 45, 128–133. [Google Scholar] [CrossRef]
- Schulze, H.; Van Leeuwen, P.; Verstegen, M.W.A.; Huisman, J.; Souffrant, W.B.; Ahrens, F. Effect of Level of Dietary Neutral Detergent Fiber on Ileal Apparent Digestibility and Ileal Nitrogen Losses in Pigs. J. Anim. Sci. 1994, 72, 2362–2368. [Google Scholar] [CrossRef]
- Fruet, A.P.B.; Stefanello, F.S.; Trombetta, F.; De Souza, A.N.M.; Rosado Júnior, A.G.; Tonetto, C.J.; Flores, J.L.C.; Scheibler, R.B.; Bianchi, R.M.; Pacheco, P.S.; et al. Growth performance and carcass traits of steers finished on three different systems including legume–grass pasture and grain diets. Animal 2019, 13, 1552–1562. [Google Scholar] [CrossRef]
- Morales, A.G.; Cockrum, R.R.; Teixeira, I.A.M.A.; Ferreira, G.; Hanigan, M.D. Graduate Student Literature Review: System, plant, and animal factors controlling dietary pasture inclusion and their impact on ration formulation for dairy cows. J. Dairy Sci. 2023, 106, 9151–9165. [Google Scholar] [CrossRef]
- Luthfi, N.; Restitrisnani, V.; Umar, M. The Optimation of Crude Fiber Content of Diet for Fattening Madura Beef Cattle to Achieve Good A:P Ratio and Low Methane Production. IOP Conf. Ser. Earth Environ. Sci. 2018, 119, 012056. [Google Scholar] [CrossRef]
- MacGregor, C.A.; Owen, F.G.; McGill, L.D. Effect of Increasing Ration Fiber with Soybean Mill Run on Digestibility and Lactation Performance. J. Dairy Sci. 1976, 59, 682–689. [Google Scholar] [CrossRef]
- Batista, H.A.M.; Autrey, K.M.; Von Tiesenhausen, I.M.E.V. Comparative in vitro digestibility of forages by buffalo, zebu, and Holstein cattle. J. Dairy Sci. 1982, 65, 746–748. [Google Scholar] [CrossRef]
- Šidlauskaitė, G.; Kadžiulienė, Ž. The effect of inorganic nitrogen fertilizers on the quality of forage composed of various species of legumes in the northern part of a temperate climate zone. Plants 2023, 12, 3676. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, X. Responses of nitrogen metabolism pathways to low-phosphorus stress: Decrease in nitrogen accumulation and alterations in protein metabolism in soybeans. Agronomy 2025, 15, 836. [Google Scholar] [CrossRef]
- Kashyap, S.; Varkey, A.; Shivakumar, N.; Devi, S.; Reddy, R.B.H.; Thomas, T.; Preston, T.; Sreeman, S.; Kurpad, A.V. True ileal digestibility of legumes determined by dual-isotope tracer method in Indian adults. Am. J. Clin. Nutr. 2019, 110, 873–882. [Google Scholar] [CrossRef]
- Mecha, E.; Alves, M.L.; Bento da Silva, A.; Pereira, A.B.; Rubiales, D.; Vaz Patto, M.C.; Bronze, M.R. High Inter- and Intra- Diversity of Amino Acid Content and Protein Digestibility Disclosed in Five Cool Season Legume Species with a Growing Market Demand. Foods 2023, 12, 1383. [Google Scholar] [CrossRef]
- Gallo, A.; Moschini, M.; Cerioli, C.; Masoero, F. Use of principal component analysis to classify forages and predict their calculated energy content. Animal 2013, 7, 930–939. [Google Scholar] [CrossRef]
- Blaser, R.E. Symposium on forage utilization: Effects of fertility levels and stage of maturity on forage nutritive value. J. Anim. Sci. 1964, 23, 246–253. [Google Scholar] [CrossRef]
- Long, R.J.; Apori, S.O.; Castro, F.B.; Ørskov, E.R. Feed value of native forages of the Tibetan Plateau of China. Anim. Feed Sci. Technol. 1999, 80, 101–113. [Google Scholar] [CrossRef]
- Vásquez, H.V.; Valqui, L.; Bobadilla, L.G.; Meseth, E.; Trigoso, M.J.; Zagaceta, L.H.; Valqui-Valqui, L.; Saravia-Navarro, D.; Barboza, E.; Maicelo, J.L. Agronomic and nutritional evaluation of INIA 910–Kumymarca ryegrass (Lolium multiflorum Lam.): An alternative for sustainable forage production in the Department of Amazonas (NW Peru). Agronomy 2025, 15, 100. [Google Scholar] [CrossRef]
- Caballero, R.; López Goicoechea, E. Efecto de la Fertilización Nitrogenada Sobre los Rendimientos, Composición y Valor Nutritivo del Ray-Grass Italiano (Lolium multiflorum, variedad Westerwoldicum). Available online: https://polired.upm.es/index.php/pastos/article/download/696/689/2332 (accessed on 10 July 2025).
- Villegas, D.; Valbuena Torres, N.; Milla Pino, M.E.; Terán, Y.; Pérez Pérez, Y.; Villegas Rivas, S.; Ruiz Camacho, W.; Paredes Guerrero, Á. Comparación de modelos para estimar el área foliar en pasto Caymán (Brachiaria híbrida). Rev. Investig. Agroproducción Sustentable 2019, 3, 71. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. (Eds.) Official Methods of Analysis of AOAC INTERNATIONAL, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005; Available online: https://www.researchgate.net/publication/292783651_AOAC_2005 (accessed on 16 June 2025).
- ANKOM Technology. ANKOM A200 Fiber Analyzer. Available online: https://www.ankom.com/?srsltid=AfmBOoqOv_HvQ_qn7yYG-l3FSCd5dekfQtCPBwOkQGdwkR5LxzZZGyJK (accessed on 16 June 2025).
- Mejía, F.; Yoplac, I.; Bernal, W.; Castro, W. Evaluación de modelos de predicción de composición química y energía bruta de kikuyo (Pennisetum clandestinum) usando espectroscopía en infrarrojo cercano (NIRS). Rev. Investig. Vet. Perú 2019, 30, 1068–1076. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchetti, J.M. Mathematical modelling of the drying kinetics of Hass avocado seeds. Ind. Crops Prod. 2016, 91, 76–87. [Google Scholar] [CrossRef]
- Taylor, J. Introduction to Error Analysis: The Study of Uncertainties in Physical Measurements, 2nd ed.; University Science Books: Sausalito, CA, USA, 1997; Available online: https://ui.adsabs.harvard.edu/abs/1997ieas.book.....T (accessed on 8 June 2025).
- Vaidya, H.N.; Breininger, R.D.; Madrid, M.; Lazarus, S.; Kachouie, N.N. Generalized Additive Models for Predicting Sea Level Rise in Coastal Florida. Geosciences 2023, 13, 310. [Google Scholar] [CrossRef]
- Banks, D.L.; Fienberg, S.E. Statistics, Multivariate. In Encyclopedia of Physical Science and Technology; Elsevier: Amsterdam, The Netherlands, 2003; pp. 851–889. [Google Scholar] [CrossRef]
- Posada, S.; Rosero Noruega, R. Comparison of mathematical models: An application for evaluation of animal food. Rev. Colomb. Cienc. Pecu. 2007, 20, 163–171. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-06902007000200006 (accessed on 13 June 2025).
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org (accessed on 13 June 2025).
- de Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 13 February 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 13 February 2025).
- Servén, D.; Brummitt, C. pyGAM: Generalized Additive Models in Python; Zenodo: Geneva, Switzerland, 2018. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.R.; Millman, K.J.; van der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Treatments | GAM | Exponential Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose (kg N ha−1) | Cutting Time (Days) | MBE | RMSE | R2 | EF | MBE | RMSE | R2 | EF | |
| Plant height (cm) | 0 | 30 | 0 | 0.5266 | 0.9799 | 0.9601 | 0.0001 | 0.5021 | 0.9817 | 0.9638 |
| 45 | 0 | 0.3616 | 0.9887 | 0.9775 | 0.0034 | 0.3129 | 0.9916 | 0.9832 | ||
| 60 | 0 | 0.6547 | 0.9660 | 0.9330 | 0.0077 | 0.6395 | 0.9676 | 0.9361 | ||
| 60 | 30 | 0 | 1.0346 | 0.9836 | 0.9671 | 0.0305 | 0.6890 | 0.9928 | 0.9854 | |
| 45 | 0 | 1.1101 | 0.9848 | 0.9695 | 0.0337 | 0.6546 | 0.9947 | 0.9894 | ||
| 60 | 0 | 1.1307 | 0.9792 | 0.9588 | −0.0361 | 1.1953 | 0.9768 | 0.9539 | ||
| Leaf length (mm) | 0 | 30 | 0 | 0.1630 | 0.9883 | 0.9767 | 0 | 0.1680 | 0.9876 | 0.9753 |
| 45 | 0 | 0.3542 | 0.9691 | 0.9392 | 0 | 0.3723 | 0.9658 | 0.9328 | ||
| 60 | 0 | 0.1568 | 0.9929 | 0.9859 | −0.0003 | 0.1890 | 0.9897 | 0.9795 | ||
| 60 | 30 | 0 | 0.7126 | 0.9718 | 0.9443 | 0.0014 | 0.6987 | 0.9729 | 0.9465 | |
| 45 | 0 | 0.8049 | 0.9667 | 0.9344 | −0.0073 | 1.0589 | 0.9417 | 0.8865 | ||
| 60 | 0 | 0.3906 | 0.9916 | 0.9833 | −0.0019 | 0.4424 | 0.9893 | 0.9786 | ||
| Leaf width (mm) | 0 | 30 | 0 | 1.6090 | 0.8802 | 0.7730 | −0.004 | 1.9983 | 0.8063 | 0.6499 |
| 45 | 0 | 0.4107 | 0.9836 | 0.9675 | −0.0001 | 0.4138 | 0.9833 | 0.9669 | ||
| 60 | 0 | 1.7426 | 0.9183 | 0.8426 | −0.0092 | 2.1970 | 0.8661 | 0.7499 | ||
| 60 | 30 | 0 | 3.0548 | 0.9213 | 0.8486 | 0.0111 | 3.1219 | 0.9176 | 0.8419 | |
| 45 | 0 | 1.8365 | 0.9684 | 0.9378 | −0.0076 | 1.8817 | 0.9668 | 0.9347 | ||
| 60 | 0 | 2.6924 | 0.8502 | 0.7226 | −0.0029 | 2.8373 | 0.8318 | 0.6919 | ||
| Stem diameter (cm) | 0 | 30 | 0 | 0.1142 | 0.8088 | 0.6522 | −0.0004 | 0.1313 | 0.7353 | 0.5404 |
| 45 | 0 | 0.0416 | 0.8489 | 0.7131 | 0 | 0.0525 | 0.7369 | 0.5429 | ||
| 60 | 0 | 0.0556 | 0.8172 | 0.6673 | 0 | 0.0590 | 0.7904 | 0.6248 | ||
| 60 | 30 | 0 | 0.0693 | 0.8176 | 0.6673 | −0.0001 | 0.0779 | 0.7617 | 0.5801 | |
| 45 | 0 | 0.0718 | 0.9292 | 0.8627 | −0.0006 | 0.0947 | 0.8726 | 0.7609 | ||
| 60 | 0 | 0.0780 | 0.8619 | 0.7408 | −0.0003 | 0.0960 | 0.7798 | 0.6078 | ||
| Parameters | Treatments | GAM | Exponential Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose (kg N ha−1) | Cutting Time (Days) | MBE | RMSE | R2 | EF | MBE | RMSE | R2 | EF | |
| fresh weight (g m−2) | 0 | 30 | 0 | 46.4716 | 0.986 | 0.9721 | −6.9601 | 68.0347 | 0.971 | 0.9403 |
| 45 | 0 | 43.2283 | 0.9874 | 0.9749 | −6.1257 | 65.604 | 0.9719 | 0.9423 | ||
| 60 | 0 | 41.9226 | 0.9894 | 0.9790 | −7.4969 | 68.1581 | 0.9732 | 0.9444 | ||
| 60 | 30 | 0 | 50.7474 | 0.9924 | 0.9848 | −9.5011 | 80.3657 | 0.9819 | 0.9620 | |
| 45 | 0 | 71.5865 | 0.9892 | 0.9785 | −12.1286 | 102.1064 | 0.9791 | 0.9563 | ||
| 60 | 0 | 46.1199 | 0.9948 | 0.9897 | −10.6269 | 82.6629 | 0.9843 | 0.9668 | ||
| dry weight (g m−2) | 0 | 30 | 0 | 13.1246 | 0.9483 | 0.8992 | −1.0854 | 16.1611 | 0.9223 | 0.8472 |
| 45 | 0 | 10.9155 | 0.9657 | 0.9325 | −1.1370 | 14.7676 | 0.9381 | 0.8764 | ||
| 60 | 0 | 10.6767 | 0.9698 | 0.9405 | −1.2808 | 14.8332 | 0.9429 | 0.8852 | ||
| 60 | 30 | 0 | 10.3831 | 0.9877 | 0.9755 | −1.0195 | 10.6328 | 0.9875 | 0.9743 | |
| 45 | 0 | 14.4083 | 0.9829 | 0.9660 | −1.3948 | 14.9280 | 0.9822 | 0.9636 | ||
| 60 | 0 | 9.2666 | 0.9920 | 0.9840 | −1.3514 | 10.7129 | 0.9899 | 0.9786 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vásquez, H.V.; Valqui, L.; Valqui-Valqui, L.; Bobadilla, L.G.; Reyna, M.; Maravi, C.; Pajares, N.; Altamirano-Tantalean, M.A. Influence of Nitrogen Fertilization and Cutting Dynamics on the Yield and Nutritional Composition of White Clover (Trifolium repens L.). Plants 2025, 14, 2765. https://doi.org/10.3390/plants14172765
Vásquez HV, Valqui L, Valqui-Valqui L, Bobadilla LG, Reyna M, Maravi C, Pajares N, Altamirano-Tantalean MA. Influence of Nitrogen Fertilization and Cutting Dynamics on the Yield and Nutritional Composition of White Clover (Trifolium repens L.). Plants. 2025; 14(17):2765. https://doi.org/10.3390/plants14172765
Chicago/Turabian StyleVásquez, Héctor V., Leandro Valqui, Lamberto Valqui-Valqui, Leidy G. Bobadilla, Manuel Reyna, Cesar Maravi, Nelson Pajares, and Miguel A. Altamirano-Tantalean. 2025. "Influence of Nitrogen Fertilization and Cutting Dynamics on the Yield and Nutritional Composition of White Clover (Trifolium repens L.)" Plants 14, no. 17: 2765. https://doi.org/10.3390/plants14172765
APA StyleVásquez, H. V., Valqui, L., Valqui-Valqui, L., Bobadilla, L. G., Reyna, M., Maravi, C., Pajares, N., & Altamirano-Tantalean, M. A. (2025). Influence of Nitrogen Fertilization and Cutting Dynamics on the Yield and Nutritional Composition of White Clover (Trifolium repens L.). Plants, 14(17), 2765. https://doi.org/10.3390/plants14172765






