Low-Temperature-Induced Changes in Rice Panicle Architectures and Their Robustness in Extremely Cold-Tolerant Cultivars
Abstract
1. Introduction
2. Results
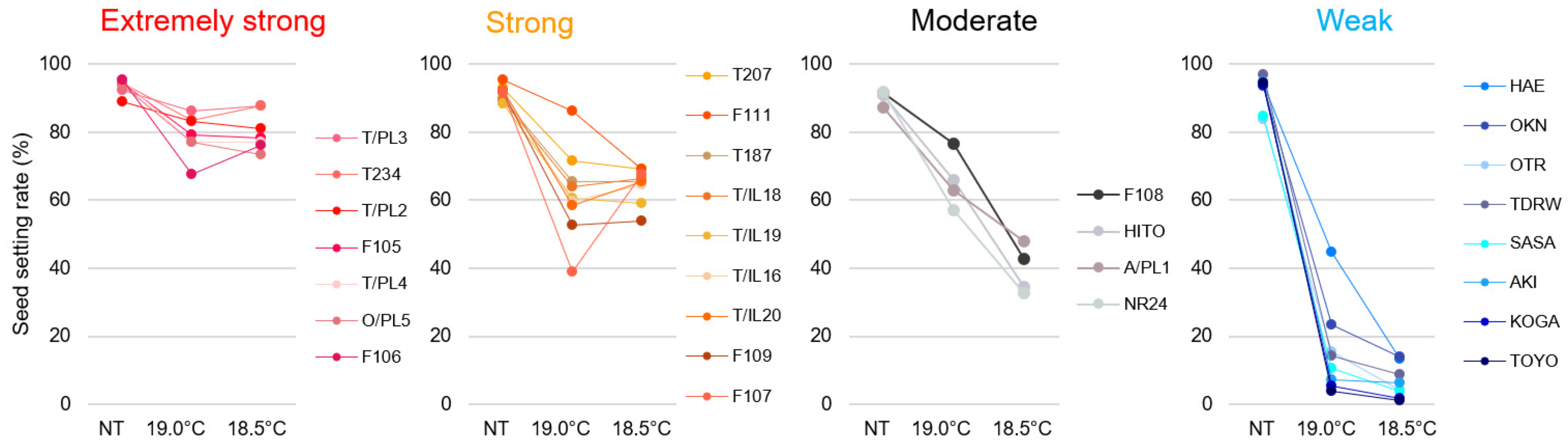
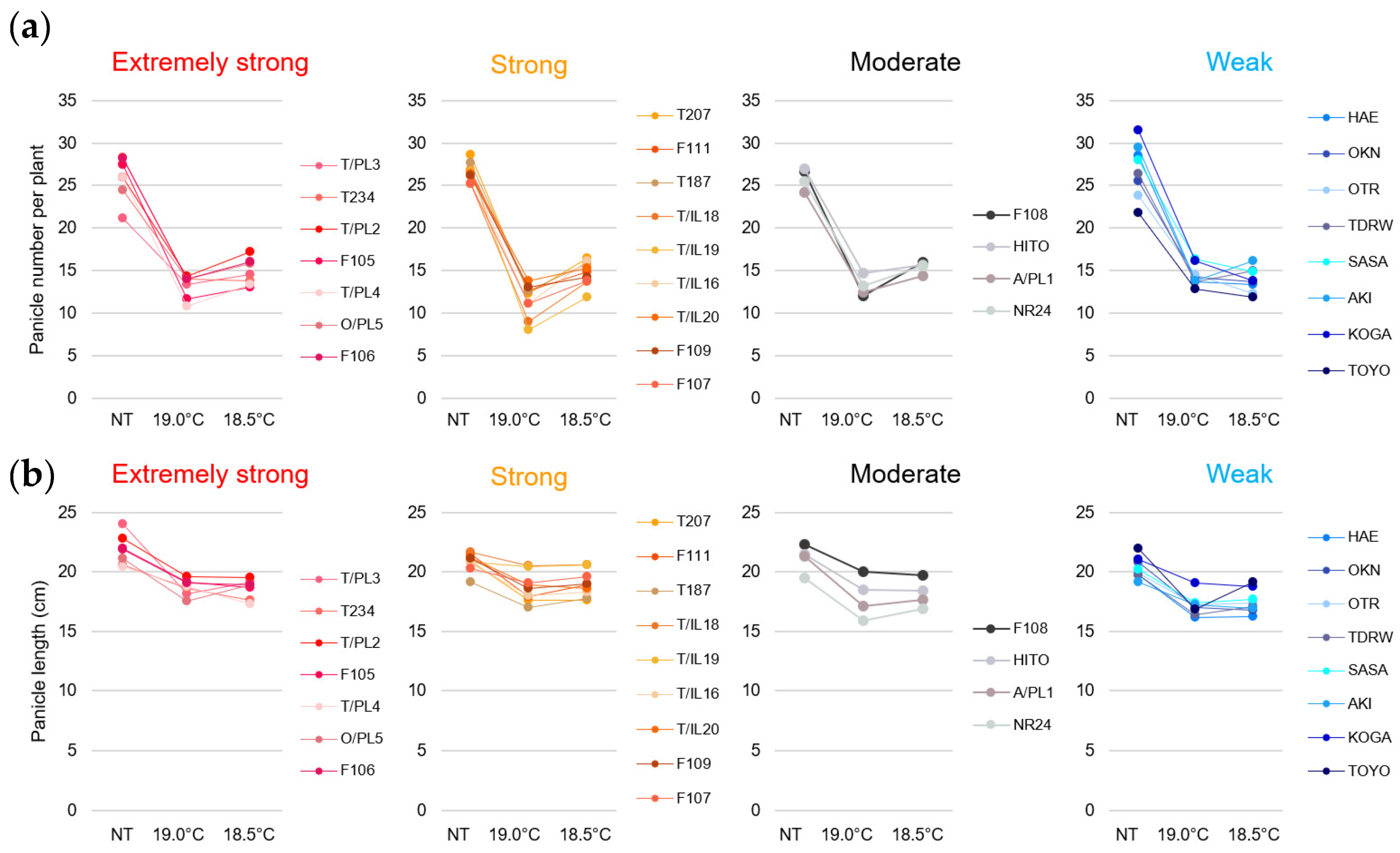
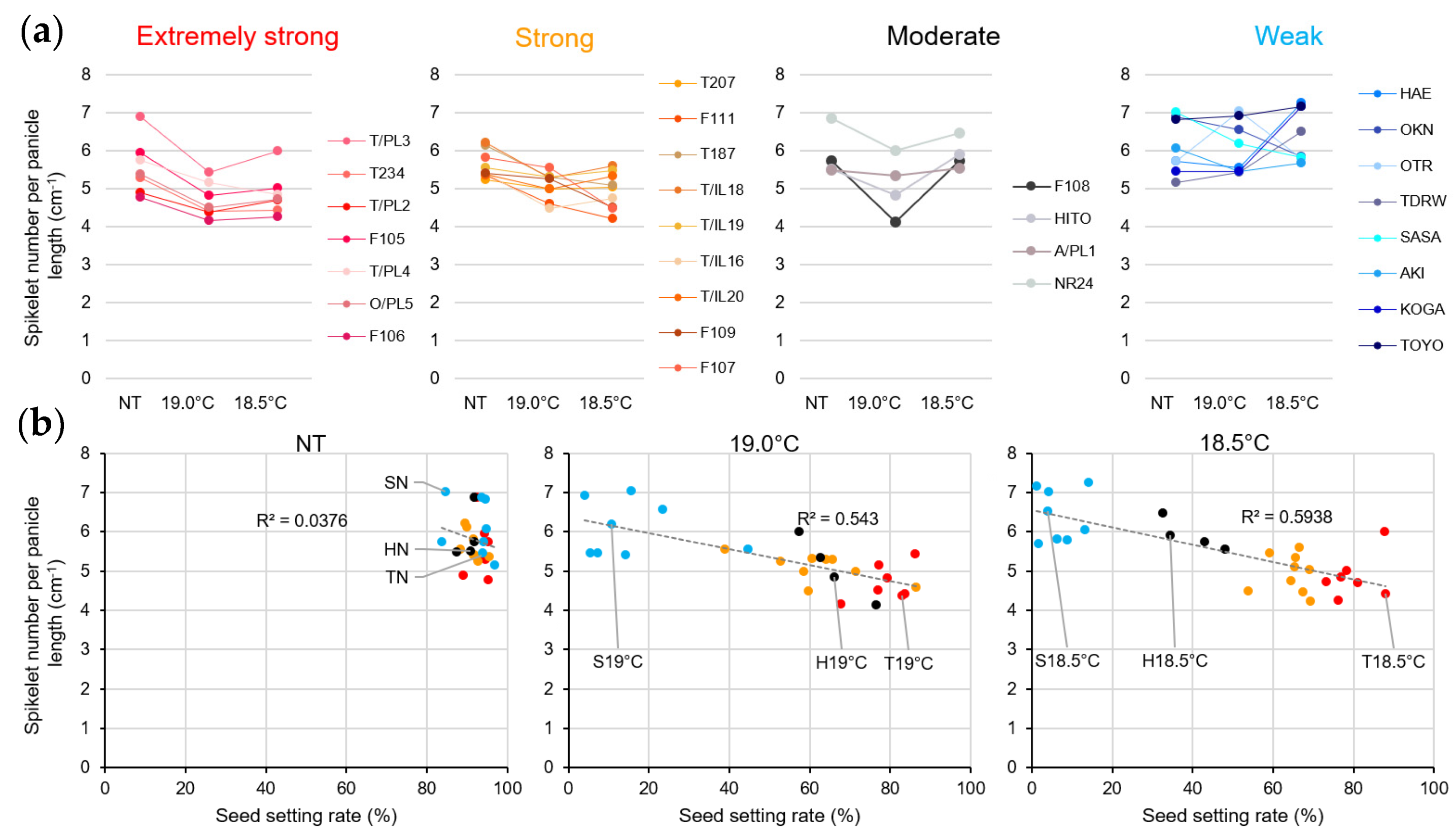
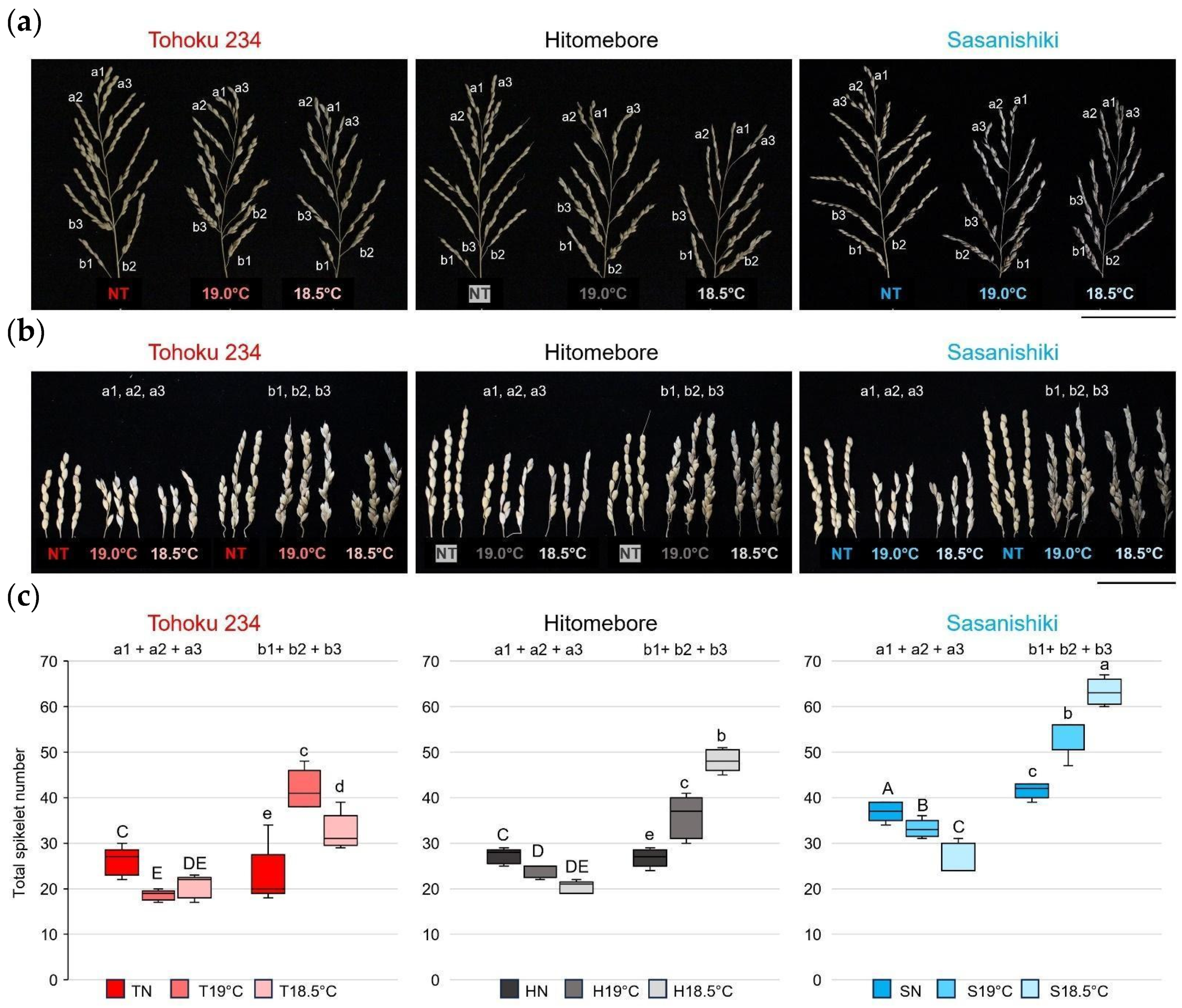
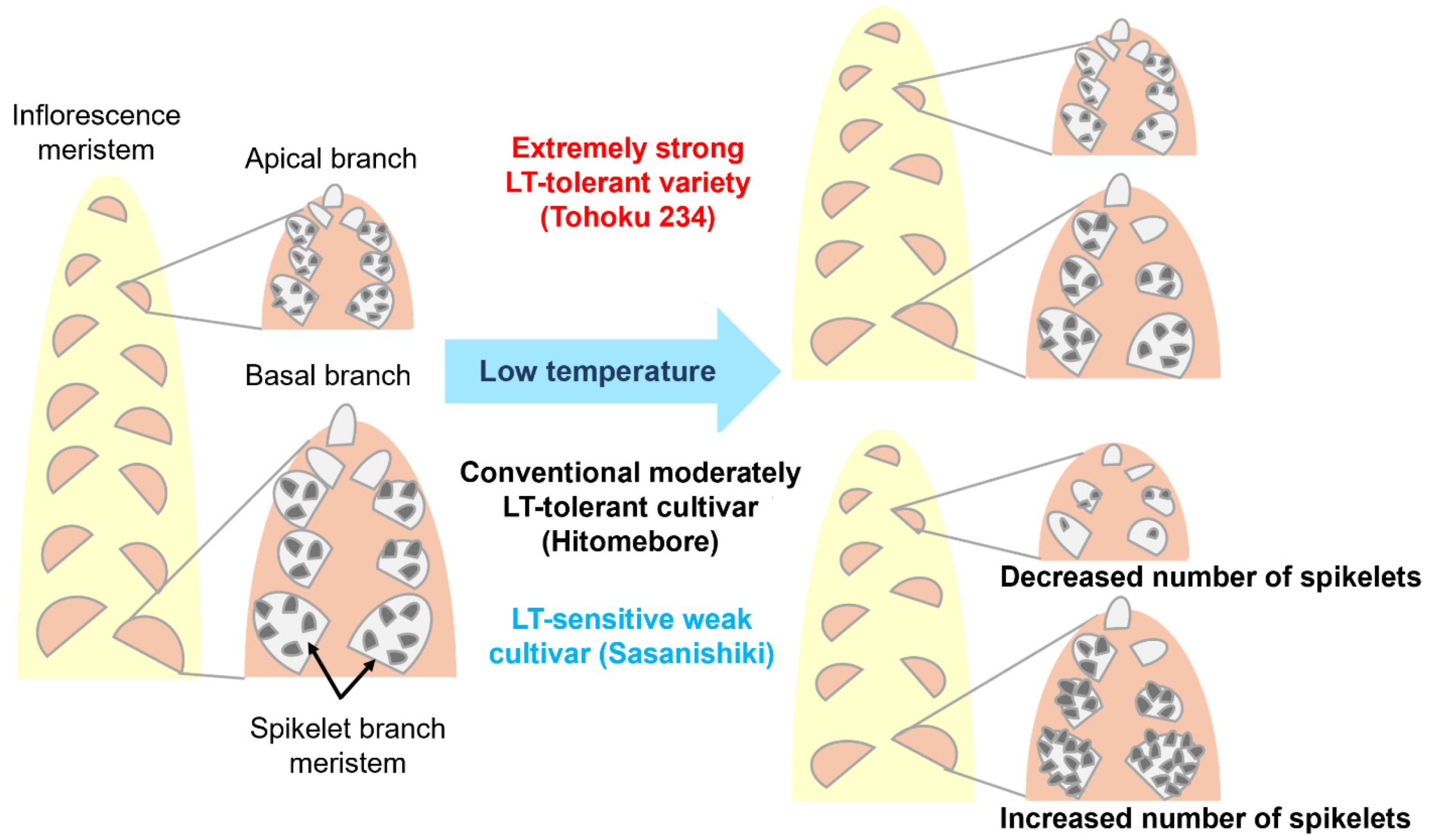
2.1. Panicle Architecture Changes in Response to LT Stress
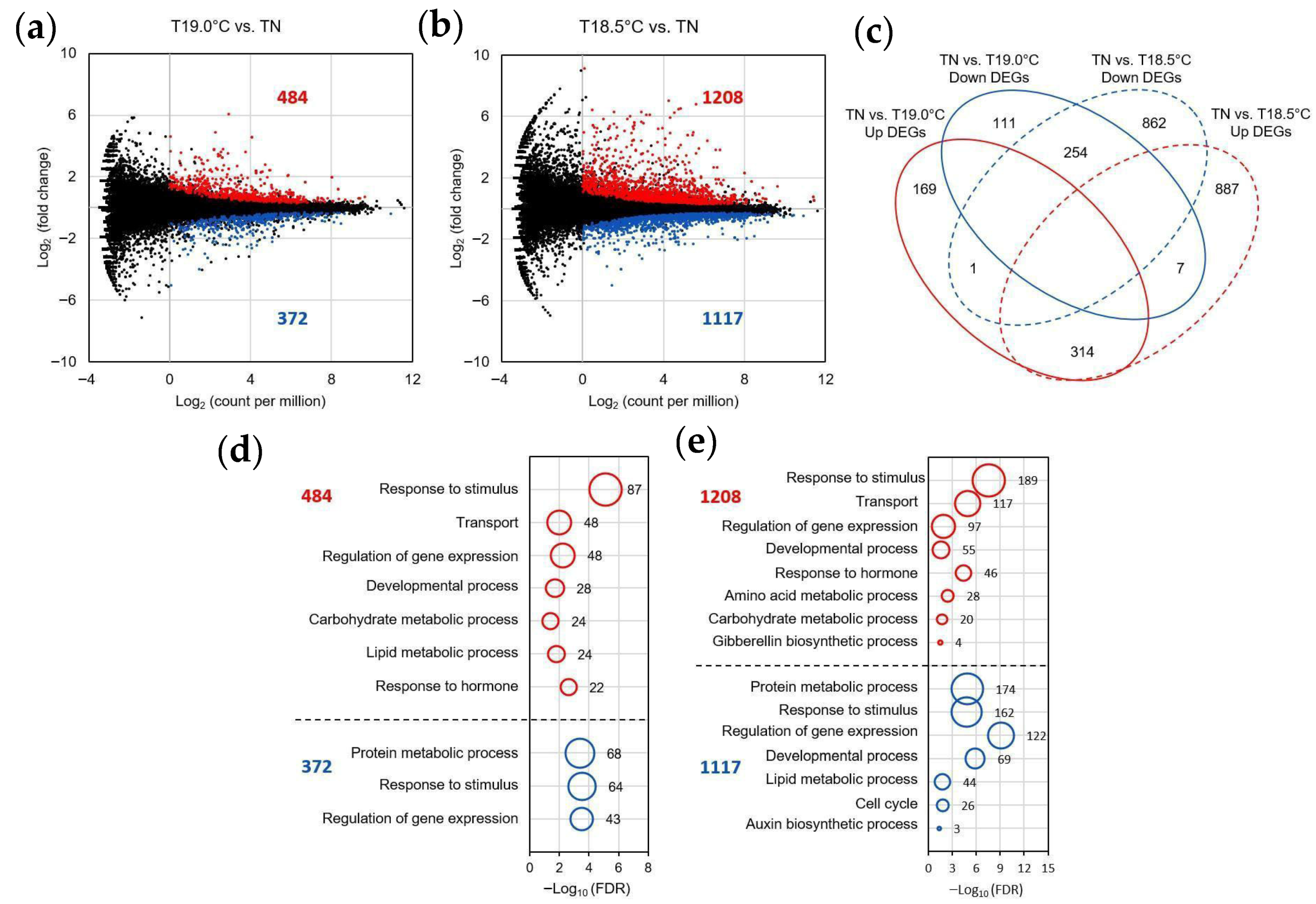
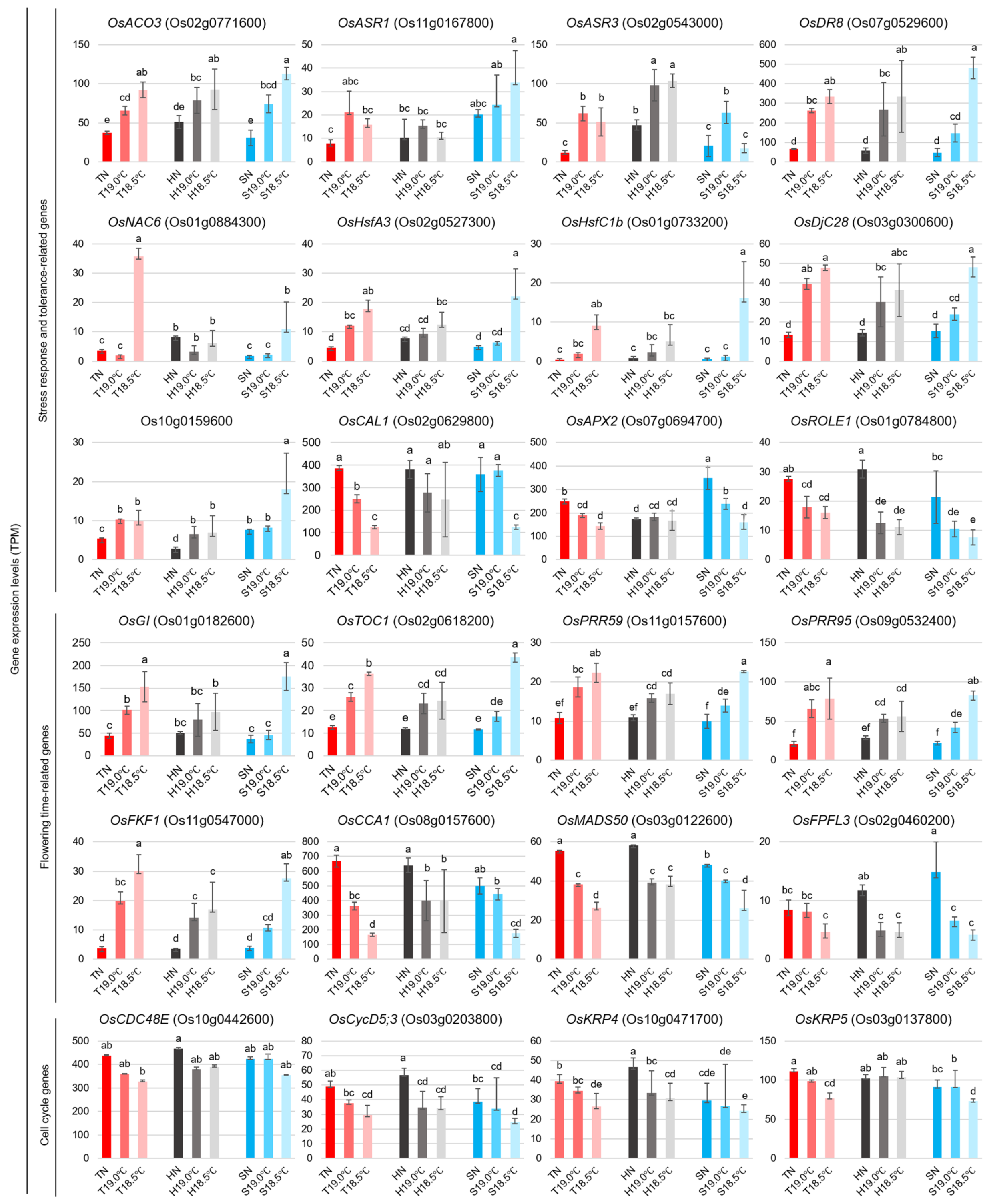
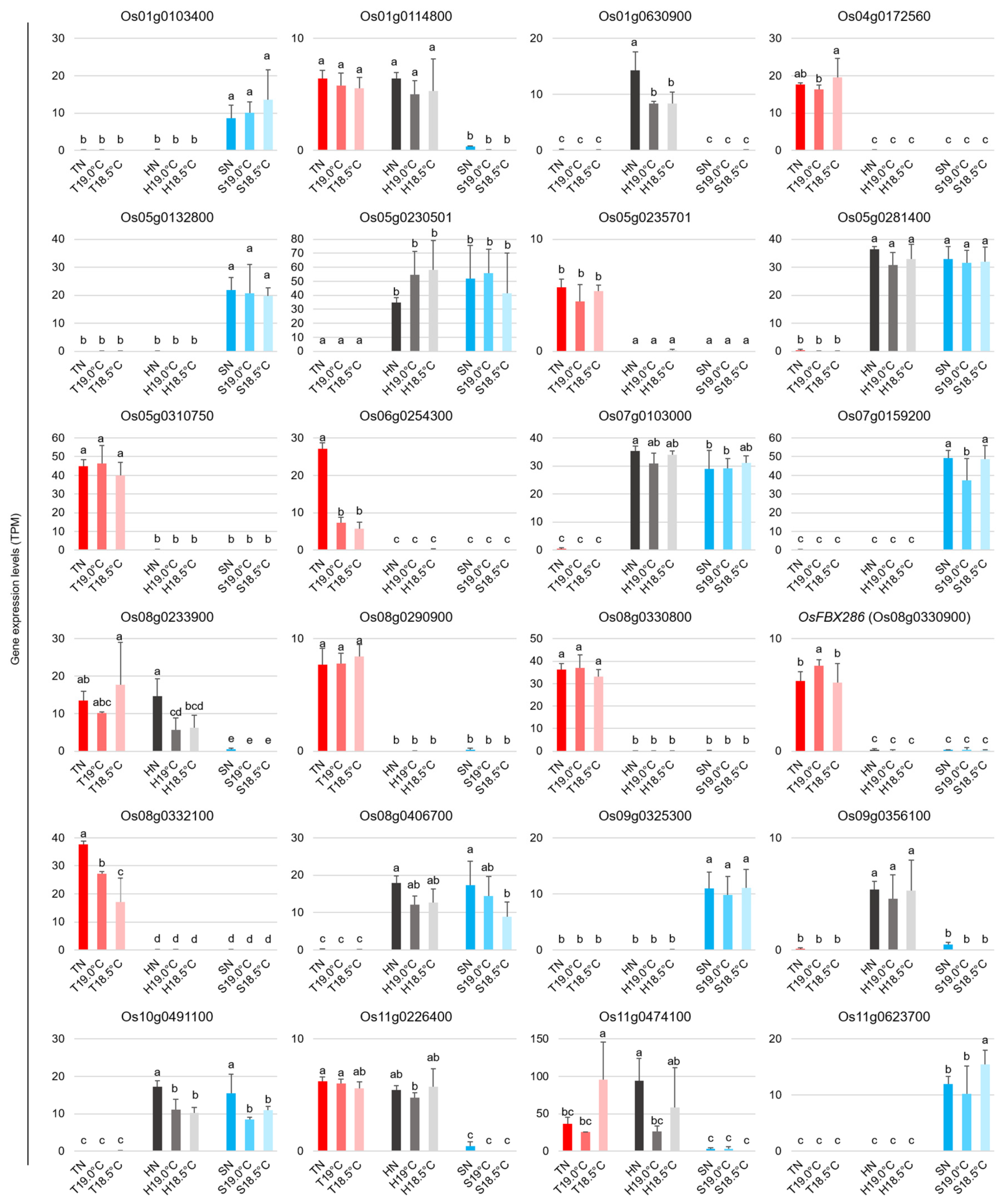
2.2. Transcriptional Changes in Early Panicles in Three Varieties Grown at Different Temperatures
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Expression Analyses in Juvenile Panicles with RNA Sequencing
4.3. Quantitative RT-PCR Analysis
4.4. Statistical Analysis and Data Visualization
4.5. Differentially Expressed Genes (DEGs)
4.6. Functional Categorization of Genes
4.7. Evaluation of Panicle Architecture and Yield-Related Traits
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Chen, Q.; Wang, S.; Hong, Y.; Wang, Z. Rice and cold stress: Methods for its evaluation and summary of cold tolerance-related quantitative trait loci. Rice 2014, 7, 24. [Google Scholar] [CrossRef]
- Hill, C.B.; Li, C. Genetic Improvement of Heat Stress Tolerance in Cereal Crops. Agronomy 2022, 12, 1205. [Google Scholar] [CrossRef]
- Chikoore, H.; Mbokodo, I.L.; Singo, M.V.; Mohomi, T.; Munyai, R.B.; Havenga, H.; Mahlobo, D.D.; Engelbrecht, F.A.; Bopape, M.M.; Ndarana, T. Dynamics of an extreme low temperature event over South Africa amid a warming climate. Weather Clim. Extrem. 2024, 44, 100668. [Google Scholar] [CrossRef]
- Carbon Brief 2021 Mapped: How Climate Change Affects Extreme Weather Around the World. Available online: www.carbonbrief.org/mapped-how-climate-change-affects-extreme-weather-around-the-world (accessed on 28 November 2024).
- Kanda, E.; Kanno, H.; Okubo, S.; Shimada, T.; Yoshida, T.; Kobayashi, T.; Iwasaki, T. Estimation of cool summer damage in the Tohoku region based on the MRI AGCM. J. Agric. Meteorol. 2014, 70, 187–198. [Google Scholar] [CrossRef][Green Version]
- Yan, X.; Guo, Y.; Ma, B.; Zhao, Y.; Guga, S.; Zhang, J.; Liu, X.; Tong, Z.; Zhao, C. Hazard assessment of rice cold damage based on energy balance in paddy field. Agric. For. Meteorol. 2024, 358, 110233. [Google Scholar] [CrossRef]
- Oda, S.; Kaneko, F.; Yano, K.; Fujioka, T.; Masuko, H.; Park, J.; Kikuchi, S.; Hamada, K.; Endo, M.; Nagano, K.; et al. Morphological and gene expression analysis under cool temperature conditions in rice anther development. Genes Genet. Syst. 2010, 85, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Oda, S.; Tsunaga, Y.; Shomura, H.; Kawagishi-Kobayashi, M.; Aya, K.; Saeki, K.; Endo, T.; Nagano, K.; Kojima, M.; et al. Reduction of gibberellin by low temperature disrupts pollen development in rice. Plant Physiol. 2014, 164, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Hayano-Saito, Y.; Kuroki, M.; Sato, Y. Map-based cloning of the rice cold tolerance gene Ctb1. Plant Sci. 2010, 179, 97–102. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Pan, Y.; Li, J.; Zhou, L.; Shi, H.; Zeng, Y.; Guo, H.; Yang, S.; Zheng, W.; et al. Natural variation in CTB4a enhances rice adaptation to cold habitats. Nat. Commun. 2017, 8, 14788. [Google Scholar] [CrossRef]
- Xiao, N.; Gao, Y.; Qian, H.; Gao, Q.; Wu, Y.; Zhang, D.; Zhang, X.; Yu, L.; Li, Y.; Pan, C.; et al. Identification of genes related to cold tolerance and a functional allele that confers cold tolerance. Plant Physiol. 2018, 177, 1108–1123. [Google Scholar] [CrossRef]
- Liu, C.; Schläppi, M.R.; Mao, B.; Wang, W.; Wang, A.; Chu, C. The bZIP73 transcription factor controls rice cold tolerance at the reproductive stage. Plant Biotechnol. J. 2019, 17, 1834–1849. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, R.; Wang, Y.; Zhang, L.; Yao, S. A point mutation in LTT1 enhances cold tolerance at the booting stage in rice. Plant Cell Environ. 2020, 43, 992–1007. [Google Scholar] [CrossRef]
- Li, J.; Zeng, Y.; Pan, Y.; Zhou, L.; Zhang, Z.; Guo, H.; Lou, Q.; Shui, G.; Huang, H.; Tian, H.; et al. Stepwise selection of natural variations at CTB2 and CTB4a improves cold adaptation during domestication of japonica rice. New Phytol. 2021, 231, 1056–1072. [Google Scholar] [CrossRef]
- Tang, J.; Tian, X.; Mei, E.; He, M.; Gao, J.; Yu, J.; Xu, M.; Liu, J.; Song, L.; Li, X.; et al. WRKY53 negatively regulates rice cold tolerance at the booting stage by fine-tuning anther gibberellin levels. Plant Cell 2022, 34, 4495–4515. [Google Scholar] [CrossRef]
- Yang, L.; Lei, L.; Wang, J.; Zheng, H.; Xin, W.; Liu, H.; Zou, D. qCTB7 positively regulates cold tolerance at booting stage in rice. Theor. Appl. Genet. 2023, 136, 135. [Google Scholar] [CrossRef]
- Shimono, H.; Okada, M.; Kanda, E.; Arakawa, I. Low temperature-induced sterility in rice: Evidence for the effects of temperature before panicle initiation. Field Crops Res. 2007, 101, 221–231. [Google Scholar] [CrossRef]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.V.; Prasad, P.V.V.; Siddique, K.H.M. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, C.; Ma, X.; Zhang, Y.; Han, J.; Mei, H.; Yu, S. Comparative analysis of expression profiles of panicle development among tolerant and sensitive rice in response to drought stress. Front. Plant Sci. 2017, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Agata, A.; Ashikari, M.; Sato, Y.; Kitano, H.; Hobo, T. Designing rice panicle architecture via developmental regulatory genes. Breed. Sci. 2023, 73, 86–94. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, Y.; Pan, X.; Cao, R.; Hu, Q.; Fu, R.; Risalat, H.; Shang, B. Effects of increased ozone on rice panicle morphology. iScience 2023, 26, 106471. [Google Scholar] [CrossRef]
- Sasaki, T. A new rice cultivar, Hitomebore and the cultivation technique. Rep. Tohoku Br. Crop Sci. Soc. Japan 1992, 35, 101–104, (Main text is In Japanese). [Google Scholar] [CrossRef]
- Sasaki, T. Identification of rice germplasm for cold tolerance at the booting stage and breeding of Hitomebore, a cold-tolerant rice cultivar having excellent grain and eating quality. Bull. Miyagi Furukawa Agric. Exp. Sta. 2005, 4, 79–128, (In Japanese with English summary). [Google Scholar]
- Sasaki, K.; Nagano, K.; Chiba, B.; Wagatsuma, K. Characteristics of eating quality of principal rice cultivars developed at Miyagi prefectural Furukawa agricultural experiment station. Tohoku J. Crop Sci. 2004, 47, 57–58. [Google Scholar]
- Ishimori, Y.; Endo, T.; Machi, N.; Nakagomi, Y.; Sato, H.; Saeki, K. Development of adaptive rice lines for extreme weather having both cold and high-temperature tolerance. Breed. Res. 2020, 22, 157. [Google Scholar]
- Matsunaga, K. Establishment of an evaluation method for cold tolerance at the booting stage of rice using a deep water irrigation system and development of highly cold-tolerant rice varieties by combining cold tolerance genes. Bull. Miyagi Furukawa Agric. Exp. Sta. 2005, 4, 1–78, (In Japanese with English title). [Google Scholar]
- Xia, J.; Qiu, Y.; Li, W.; Zhang, Y.; Liu, L.; Wang, Y.; Mou, W.; Xue, D. Genome-Wide In Silico Analysis of 1-Aminocyclopropane-1-carboxylate oxidase (ACO) Gene Family in Rice (Oryza sativa L.). Plants 2024, 13, 3490. [Google Scholar] [CrossRef] [PubMed]
- Kurata, N.; Yamazaki, Y. Oryzabase: An integrated biological and genome information database for rice. Plant Physiol. 2006, 140, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Panigrahi, K.C. GIGANTEA—An emerging story. Front. Plant. Sci. 2015, 6, 8. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Jung, K.W.; Yoo, K.S.; Jeung, J.U.; Shin, J.S. A stress-responsive caleosin-like protein, AtCLO4, Acts as a Negative Regulator of ABA Responses in Arabidopsis. Plant Cell Physiol. 2011, 52, 874–884. [Google Scholar] [CrossRef]
- Li, G.; Zhang, H.; Li, J.; Zhang, Z.; Li, Z. Genetic control of panicle architecture in rice. Crop J. 2021, 9, 590–597. [Google Scholar] [CrossRef]
- Liberatore, C.M.; Biancucci, M.; Ezquer, I.; Gregis, V.; Di Marzo, M. Investigating how reproductive traits in rice respond to abiotic stress. J. Exp. Bot. 2025, 76, 2064–2080. [Google Scholar] [CrossRef]
- Panigrahi, R.; Kariali, E.; Panda, B.B.; Lafarge, T.; Mohapatra, P.K. Controlling the trade-off between spikelet number and grain filling: The hierarchy of starch synthesis in spikelets of rice panicle in relation to hormone dynamics. Funct. Plant Biol. 2019, 46, 507–523. [Google Scholar] [CrossRef] [PubMed]
- Yamamori, K.; Ogasawara, K.; Ishiguro, S.; Koide, Y.; Takamure, I.; Fujino, K.; Sato, Y.; Kishima, Y. Revision of the relationship between anther morphology and pollen sterility by cold stress at the booting stage in rice. Ann. Bot. 2021, 128, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.; Han, J.J.; Han, M.J.; An, G. Functional analyses of the flowering time gene OsMADS50, the putative Suppressor of Overexpression of CO 1/Agamous-like 20 (SOC1/AGL20) ortholog in rice. Plant J. 2004, 38, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.H.; Lee, S.; Cho, L.H.; Kim, S.L.; Lee, Y.S.; Choi, S.C.; Jeong, H.J.; Yi, J.; Park, S.J.; Han, C.D.; et al. OsMADS50 and OsMADS56 function antagonistically in regulating long day (LD)-dependent flowering in rice. Plant Cell Environ. 2009, 32, 1412–1427. [Google Scholar] [CrossRef]
- Nakamichi, N. The Transcriptional Network in the Arabidopsis Circadian Clock System. Genes 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Martin, M. NoCutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Joshi, N.A.; Fass, J.N. Sickle: A Windowed Adaptive Trimming Tool for FASTQ Files Using Quality 2011, (Version 1.33) [Software]. Available online: https://github.com/najoshi/sickle (accessed on 4 November 2024).
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 15, 2078–2079. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Wagner, G.P.; Kin, K.; Lynch, V.J. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci. 2012, 131, 281–285. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 1 March 2025).
- Posit Team. RStudio: Integrated Development Environment for R. Boston, MA: 2023.6.1.524. 2025. Available online: https://posit.co/ (accessed on 1 March 2025).
- Microsoft Corporation. Microsoft Excel for Microsoft 365. 2024. Available online: https://www.microsoft.com/de-de/microsoft-365/p/excel/cfq7ttc0pbmf (accessed on 1 March 2025).
- Chen, Y.; Chen, L.; Lun, A.T.L.; Baldoni, P.L.; Symth, G.K. edgeR v4: Powerful differential analysis of sequencing data with expanded functionality and improved support for small counts and larger datasets. Nucleic Acids Res. 2025, 53, 27. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, V.G.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Adler, P.; Vilo, J.; Peterson, H. g: Profiler-interoperable web service for functional enrichment analysis and gene identifier mapping (2023 update). Nucleic Acids Res. 2023, 51, W207–W212. [Google Scholar] [CrossRef]



| Study Area | Variety Name | Sowing Date | Transplanting Date | Heading Date |
|---|---|---|---|---|
| Ambient-condition | Tohoku 234 | 16 April | 22 May | 5 August |
| paddy field (NT) | Hitomebore | 16 April | 22 May | 4 August |
| Sasanishiki | 16 April | 22 May | 5 August | |
| Cold-water | Tohoku 234 | 16 April | 14 May | 15 August |
| paddy field (19.0 °C) | Hitomebore | 16 April | 14 May | 20 August |
| Sasanishiki | 16 April | 14 May | 21 August | |
| Cold-water | Tohoku 234 | 16 April | 14 May | 16 August |
| paddy field (18.5 °C) | Hitomebore | 16 April | 14 May | 23 August |
| Sasanishiki | 16 April | 14 May | 24 August |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisara, M.; Abu, A.A.; Higashitani, A. Low-Temperature-Induced Changes in Rice Panicle Architectures and Their Robustness in Extremely Cold-Tolerant Cultivars. Plants 2025, 14, 2759. https://doi.org/10.3390/plants14172759
Kisara M, Abu AA, Higashitani A. Low-Temperature-Induced Changes in Rice Panicle Architectures and Their Robustness in Extremely Cold-Tolerant Cultivars. Plants. 2025; 14(17):2759. https://doi.org/10.3390/plants14172759
Chicago/Turabian StyleKisara, Masato, Aisha Ahmad Abu, and Atsushi Higashitani. 2025. "Low-Temperature-Induced Changes in Rice Panicle Architectures and Their Robustness in Extremely Cold-Tolerant Cultivars" Plants 14, no. 17: 2759. https://doi.org/10.3390/plants14172759
APA StyleKisara, M., Abu, A. A., & Higashitani, A. (2025). Low-Temperature-Induced Changes in Rice Panicle Architectures and Their Robustness in Extremely Cold-Tolerant Cultivars. Plants, 14(17), 2759. https://doi.org/10.3390/plants14172759







