Effects of Foliar Organic Selenium Application During the Main Season on Ratoon Rice Yield, Grain Quality, and Selenium Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Materials
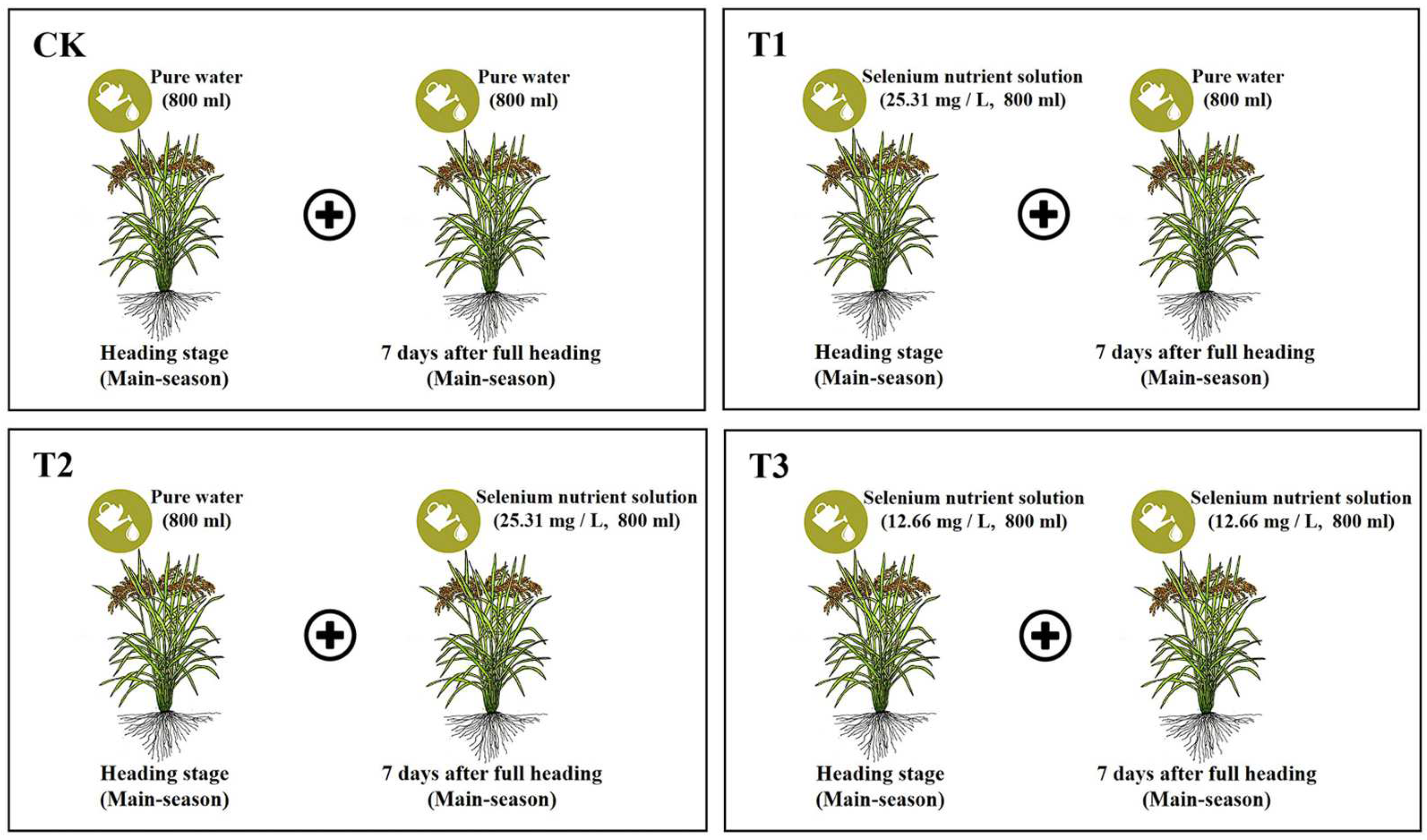
2.2. Experimental Treatments
2.3. Determination Indexes and Methods
2.3.1. Agronomic Character
2.3.2. Grain Quality
Processing Quality
Appearance Quality
Eating Quality and Nutritional Quality
2.3.3. Tetravalent Se (Se IV), Hexavalent Se (Se VI), and Total Se Content (TS)
2.3.4. Selenomethionine Content (SM)
2.4. Statistical Analysis
3. Results
3.1. Yield and Yield Components
3.2. Processing Quality and Appearance Quality
3.3. Nutritional Quality and Eating Quality
3.4. Se Content in Grains
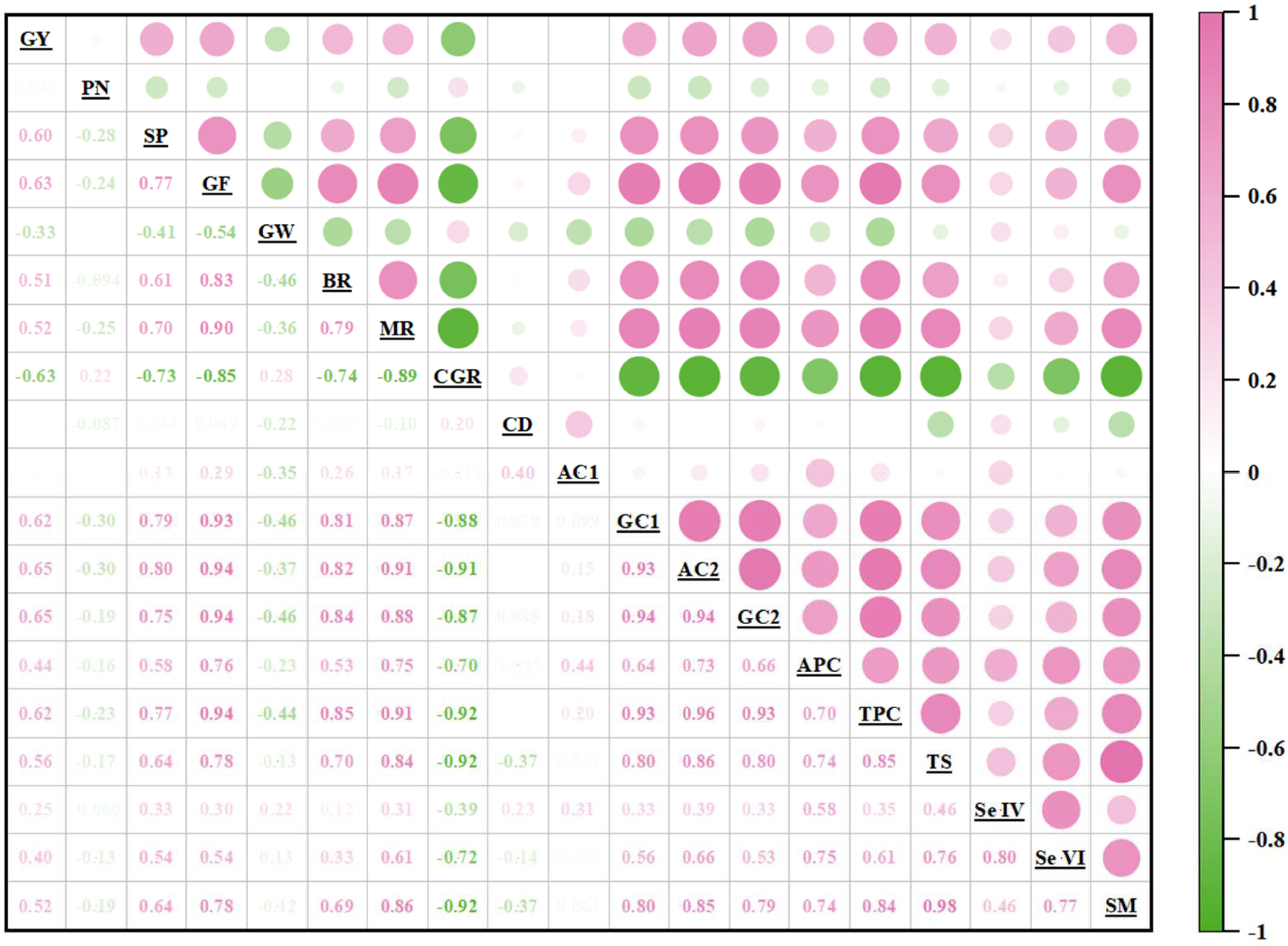
3.5. Correlation Analysis
4. Discussion
4.1. Accumulation and Form of Se
4.2. Effects of Se Application on Yield and Yield Components
4.3. Effects of Se Application on Grain Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Maurya, M.K.; Sarma, A.; Tamuly, G.; Shukla, V.K.; Behera, P.; Bora, S.S.; Chakravarthi, B. Rice ratooning: A pioneering strategy for enhancing rice productivity and embracing climate change adaptation and mitigation. Int. J. Plant Soil Sci. 2023, 35, 1047–1059. [Google Scholar] [CrossRef]
- Wang, W.; He, A.; Jiang, G.; Sun, H.; Jiang, M.; Man, J.; Ling, X.; Cui, K.; Huang, J.; Peng, S. Ratoon rice technology: A green and resource-efficient way for rice production. Adv. Agron. 2020, 159, 135–167. [Google Scholar]
- Yamano, T.; Arouna, A.; Labarta, R.A.; Huelgas, Z.M.; Mohanty, S. Adoption and impacts of international rice research technologies. Glob. Food Secur. 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Peng, S.; Tang, Q.; Zou, Y. Current status and challenges of rice production in China. Plant Prod. Sci. 2009, 12, 3–8. [Google Scholar] [CrossRef]
- Ray, D.; Foley, J. Increasing global crop harvest frequency: Recent trends and future directions. Environ. Res. Lett. 2013, 8, 4041. [Google Scholar] [CrossRef]
- Lal, D.B.; Gautam, P.; Nayak, A.K.; Raja, R.; Panda, B.; Tripathi, R.; Shahid, M.; Chatterjee, D.; Bhattacharyya, P.; Bihari, P.; et al. Agronomic manipulation in main season and ratoon rice influences growth, productivity, and regeneration ability in tropical lowlands. Field Crops Res. 2023, 294, 108872. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Anticarcinogenic effects of selenium. Cell Mol. Life Sci. CMLS 2000, 57, 1864–1873. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food sources of selenium and its relationship with chronic diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, Y.; Dun, X.; Wang, X.; Wang, H. Research progress of selenium-enriched foods. Nutrients 2023, 15, 4189. [Google Scholar] [CrossRef]
- Song, T.; Su, X.; He, J.; Liang, Y.; Zhou, T.; Liu, C. Selenium (Se) uptake and dynamic changes of Se content in soil–plant systems. Environ. Sci. Pollut. Res. 2018, 25, 34343–34350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-H.; Shi, W.-M.; Wang, X.-C. Difference in Selenium Accumulation in Shoots of Two Rice Cultivars1 1Project supported by the National Natural Science Foundation of China (No. 40371063) and the National Key Basic Research Support Foundation of China (No. G1999011808). Pedosphere 2006, 16, 646–653. [Google Scholar] [CrossRef]
- Deng, X.; Liu, K.; Li, M.; Zhang, W.; Zhao, X.; Zhao, Z.; Liu, X. Difference of selenium uptake and distribution in the plant and selenium form in the grains of rice with foliar spray of selenite or selenate at different stages. Field Crops Res. 2017, 211, 165–171. [Google Scholar] [CrossRef]
- Shen, J.; Jiang, C.; Yan, Y.; Zu, C. Selenium distribution and translocation in rice (Oryza sativa L.) under different naturally seleniferous soils. Sustainability 2019, 11, 520. [Google Scholar] [CrossRef]
- Yan, J.; Chen, X.; Zhu, T.; Zhang, Z.; Fan, J. Effects of selenium fertilizer application on yield and selenium accumulation characteristics of different japonica rice varieties. Sustainability 2021, 13, 10284. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, W.; Wang, M.; Miao, Y.; Cui, Z.; Li, Z.; Liang, D. Effects of selenium application on Se content and speciation in Lentinula edodes. Food Chem. 2018, 265, 182–188. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Pilon-Smits, E.A.H.; Zhao, F.-J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 14, 436–442. [Google Scholar] [CrossRef]
- Chen, L.; Yang, F.; Xu, J.; Hu, Y.; Hu, Q.; Zhang, Y.; Pan, G. Determination of selenium concentration of rice in china and effect of fertilization of selenite and selenate on selenium content of rice. J. Agric. Food Chem. 2002, 50, 5128–5130. [Google Scholar] [CrossRef]
- Shengnan, L.; Shizhong, Y.; Huafen, L.; Yuhui, Q. Effect of Se-enriched organic fertilizers on selenium accumulation in corn and soil. J. Agric. Resour. Environ. 2015, 32, 571–576. [Google Scholar]
- Kikkert, J.; Hale, B.; Berkelaar, E. Selenium accumulation in durum wheat and spring canola as a function of amending soils with selenite, selenate and or sulphate. Plant Soil 2013, 372, 629–641. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, W.; Wang, X. Difference in selenite absorption between high- and low-selenium rice cultivars and its mechanism. Plant Soil 2006, 282, 183–193. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.; De Souza, M.; Tarun, A. Selenium in higher plants. Annu. Rev. Plant Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shi, W.; Wang, X. Selenium speciation and distribution characteristics in the rhizosphere soil of rice (Oryza sativa L.) seedlings. Commun. Soil Sci. Plant Anal. 2010, 41, 1411–1425. [Google Scholar] [CrossRef]
- Liu, K.; Gu, Z. Selenium accumulation in different brown rice cultivars and its distribution in fractions. J. Agric. Food Chem. 2009, 57, 695–700. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, S.; Fan, L. Mechanisms of differences in selenium absorption and transport between rice plants different in cultivar. Acta Pedol. Sin. 2014, 51, 594–599. [Google Scholar]
- Zhang, X.; Li, W.; Gong, Z.; Ludlow, R.A.; Xiao, M.; Zhao, B.; Li, X.; Jia, C.; Li, P.; Liu, W. Increased nutritional quality of rice grains and migration mechanisms of selenium by spraying a foliar selenium-rich nutrient solution. J. Plant Nutr. 2024, 47, 1347–1363. [Google Scholar] [CrossRef]
- Luo, H.; He, L.; Du, B.; Pan, S.; Mo, Z.; Duan, M.; Tian, H.; Tang, X. Biofortification with chelating selenium in fragrant rice: Effects on photosynthetic rates, aroma, grain quality and yield formation. Field Crops Res. 2020, 255, 107909. [Google Scholar] [CrossRef]
- Lei, H.; Zhou, M.; Li, B.; Fu, Y.; Shi, Z.; Ji, W.; Zhang, R.; Wang, Z. Humic acid chelated selenium is suitable for wheat biofortification. J. Sci. Food Agric. 2023, 103, 4887–4898. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Bai, L.Y.; Huang, M.L.; Geng, C.Z.; Yan, D.Y. Review on application of EDTA and its structural isomers in the environment. Environ. Eng. 2019, 37, 159–163+147. [Google Scholar]
- Kumari, R.; Kumar, S.; Kumar, R.; Das, A.; Kumari, R.; Choudhary, C.; Sharma, R. Effect of long-term integrated nutrient management on crop yield, nutrition and soil fertility under rice-wheat system. J. Appl. Nat. Sci. 2017, 9, 1801–1807. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Grintzalis, K.; Georgiou, C.D.; Schneider, Y.-J. An accurate and sensitive Coomassie Brilliant Blue G-250-based assay for protein determination. Anal. Biochem. 2015, 480, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.; Cremer, K.; Van Hulle, M.; Chéry, C.; Vanhaecke, F.; Cornelis, R. Identification of the major selenium compound, Se-Methionine, in three yeast (Saccharomyces cerevisiae) dietary supplements by on-line narrowbore liquid chromatography electrospray tandem mass spectrometry. J. chromatography. A 2005, 1071, 191–196. [Google Scholar] [CrossRef]
- Fang, Y.; Catron, B.; Zhang, Y.; Zhao, L.; Caruso, J.A.; Hu, Q. Distribution and in vitro availability of selenium in selenium-containing storage protein from selenium-enriched rice utilizing optimized extraction. J. Agric. Food Chem. 2010, 58, 9731–9738. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, T.; Hintelmann, H. Selenium speciation by HPLC with tandem mass spectrometric detection. Anal. Bioanal. Chem. 2002, 372, 486–490. [Google Scholar] [CrossRef]
- Yuan, Z.; Long, W.; Liang, T.; Zhu, M.; Zhu, A.; Luo, X.; Fu, L.; Hu, Z.; Zhu, R.; Wu, X. Effect of foliar spraying of organic and inorganic selenium fertilizers during different growth stages on selenium accumulation and speciation in rice. Plant Soil 2023, 486, 87–101. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef]
- Yin, H.; Qi, Z.; Li, M.; Ahammed, G.J.; Chu, X.; Zhou, J. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol. Environ. Saf. 2019, 169, 911–917. [Google Scholar] [CrossRef]
- Marques, A.C.; Lidon, F.C.; Coelho, A.R.F.; Pessoa, C.C.; Luís, I.C.; Campos, P.S.; Simões, M.; Almeida, A.S.; Pessoa, M.F.; Galhano, C. Effect of rice grain (Oryza sativa L.) enrichment with selenium on foliar leaf gas exchanges and accumulation of nutrients. Plants 2021, 10, 288. [Google Scholar] [CrossRef]
- Farooq, M.U.; Tang, Z.; Zeng, R.; Liang, Y.; Zhang, Y.; Zheng, T.; Ei, H.H.; Ye, X.; Jia, X.; Zhu, J. Accumulation, mobilization, and transformation of selenium in rice grain provided with foliar sodium selenite. J. Sci. Food Agric. 2019, 99, 2892–2900. [Google Scholar] [CrossRef]
- Zhou, B.; Cao, H.; Wu, Q.; Mao, K.; Yang, X.; Su, J.; Zhang, H. Agronomic and genetic strategies to enhance sSelenium accumulation in crops and theirinfluence on quality. Foods 2023, 12, 4442. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Lytle, C.M.; Terry, N. Accumulation and volatilization of different chemical species of selenium by plants. Planta 1998, 206, 284–292. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Li, W.; Gong, Z.; Jia, C.; Li, P. Effect of foliar application of the selenium-rich nutrient solution on the selenium accumulation in grains of Foxtail millet (Zhangzagu 10). Environ. Sci. Pollut. Res. 2022, 29, 5569–5576. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Liu, P.; Qin, J.; Song, L.; Yang, W.; Feng, M.; Zhang, M.; Wang, C.; Song, X. Effects of applying different doses of selenite to soil and foliar at different growth stage on selenium content and yield of different oat varieties. Plants 2022, 11, 1810. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Stoddard, F.L.; Hartikainen, H.; Seppänen, M.M. Plant species and growing season weather influence the efficiency of selenium biofortification. Nutr. Cycl. Agroecosystems 2019, 114, 111–124. [Google Scholar] [CrossRef]
- Wang, Q.; Kong, L.; Huang, Q.; Li, H.; Wan, Y. Uptake and translocation mechanisms of different forms of organic selenium in rice (Oryza sativa L.). Front. Plant Sci. 2022, 13, 970480. [Google Scholar] [CrossRef]
- Wu, W.; Qi, D.; Chen, Y.; Wang, J.; Wang, Q.; Yang, Y.; Niu, H.; Zhao, Q.; Peng, T. Enhancement of nutrient, trace element, and organic selenium contents of ratooning rice grains and straw through foliar application of selenite. Foods 2024, 13, 3637. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, H.; Li, F.; Yang, T.; Shi, Y.; Gu, X.; Chen, M.; Jiang, S. Optimized tillage method increased rice yield in rice ratooning system. Agriculture 2024, 14, 1768. [Google Scholar] [CrossRef]
- Luo, H.; He, L.; Du, B.; Wang, Z.; Zheng, A.; Lai, R.; Tang, X. Foliar application of selenium (Se) at heading stage induces regulation of photosynthesis, yield formation, and quality characteristics in fragrant rice. Photosynthetica 2019, 57, 1007–1014. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Luo, H.; Xing, P.; Liu, J.; Pan, S.; Tang, X.; Duan, M. Selenium improved antioxidant response and photosynthesis in fragrant rice (Oryza sativa L.) seedlings during drought stress. Physiol. Mol. Biol. Plants 2021, 27, 2849–2858. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Luo, Z.; Peng, Z. Research on the influence of selenium provided at different levels upon the growth of rice and its accumulation of selenium. J. Hunan Agric. Univ. (Nat. Sci.) 1998, 24, 176–179. [Google Scholar]
- Feng, R.; Liao, G.; Guo, J.; Wang, R.; Xu, Y.; Ding, Y.; Mo, L.; Fan, Z.; Li, N. Responses of root growth and antioxidative systems of paddy rice exposed to antimony and selenium. Environ. Exp. Bot. 2016, 122, 29–38. [Google Scholar] [CrossRef]
- Zhang, M.; Xing, G.; Tang, S.; Pang, Y.; Yi, Q.; Huang, Q.; Huang, X.; Huang, J.; Li, P.; Fu, H. Improving soil selenium availability as a strategy to promote selenium uptake by high-Se rice cultivar. Environ. Exp. Bot. 2019, 163, 45–54. [Google Scholar] [CrossRef]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.M.; Mahdi, A.H.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of nano silicon and nano selenium on root characters, growth, ion selectivity, yield, and yield components of rice (Oryza sativa L.) under salinity conditions. Plants 2021, 10, 1657. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Liu, X.; Wang, R.; Zhang, H.; Yang, Y. Enhancing rice (Oryza sativa L.) yield and quality by improving photosynthesis with zinc oxide nanoparticles foliar application. Environ. Sci. Nano 2025, 12, 2331–2342. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, M.; Xu, J.; Xu, F.; Zhang, W. The application of organic selenium (SeMet) improve the photosynthetic characteristics, yield and quality of hybrid rice. Plant Physiol. Biochem. 2024, 208, 108457. [Google Scholar] [CrossRef]
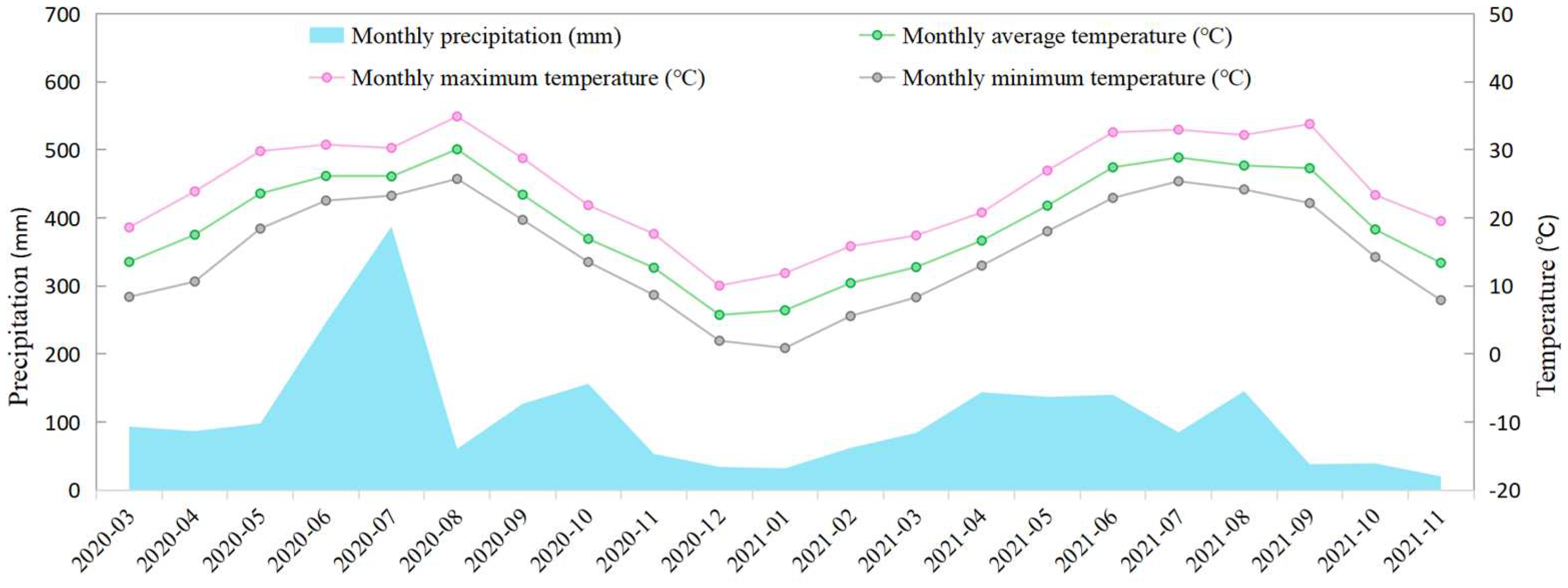


| pH | Organic Matter (g/kg) | Total Nitrogen (g/kg) | Total Phosphorus (g/kg) | Total Potassium (g/kg) | Alkaline Nitrogen (mg/kg) | Available Phosphorus (mg/kg) | Available Potassium (mg/kg) | Total Selenium (mg/kg) | Available Selenium (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| 5.79 | 21.00 | 1.69 | 0.61 | 3.49 | 81.49 | 45.01 | 113.98 | 0.17 | 0.01 |
| Year | Variety | Treatment | Panicle Number (PN) | Spikelet Per Panicle (SP) | Grain Filling Rate (GF) | 1000 Grain Weight (GW) |
|---|---|---|---|---|---|---|
| (%) | (g) | |||||
| 2020 | FLYX1 | CK | 380.95 a | 77.35 c | 68.78 c | 28.82 a |
| T1 | 368.20 a | 80.40 b | 75.62 a | 28.15 b | ||
| T2 | 377.95 a | 78.9 bc | 71.11 b | 28.40 b | ||
| T3 | 374.33 a | 83.39 a | 75.56 a | 28.23 b | ||
| Mean | 375.36 | 80.01 | 72.62 | 28.40 | ||
| JLYHZ | CK | 373.60 ab | 78.33 b | 69.09 d | 28.78 b | |
| T1 | 383.25 a | 79.61 a | 74.07 a | 28.16 c | ||
| T2 | 368.43 b | 78.64 ab | 71.98 c | 28.64 b | ||
| T3 | 365.09 b | 79.49 a | 73.10 b | 30.61 a | ||
| Mean | 372.59 | 79.02 | 72.06 | 29.05 | ||
| 2021 | FLYX1 | CK | 376.39 a | 77.24 c | 68.52 d | 28.66 a |
| T1 | 365.98 a | 79.93 b | 76.12 a | 28.20 bc | ||
| T2 | 378.30 a | 79.55 b | 70.83 c | 28.44 ab | ||
| T3 | 361.57 a | 83.12 a | 75.10 b | 27.99 c | ||
| Mean | 370.56 | 79.96 | 72.64 | 28.32 | ||
| JLYHZ | CK | 371.80 a | 78.23 a | 68.86 c | 28.71 b | |
| T1 | 374.66 a | 78.81 a | 74.08 a | 28.06 c | ||
| T2 | 376.08 a | 78.92 a | 72.24 b | 29.06 b | ||
| T3 | 372.73 a | 79.42 a | 72.31 b | 30.58 a | ||
| Mean | 373.81 | 78.84 | 71.87 | 29.10 |
| ANOVA | Panicle Number (PN) | Spikelet Per Panicle (SP) | Grain Filling Rate (GF) | 1000 Grain Weight (GW) |
|---|---|---|---|---|
| (%) | (g) | |||
| Year (Y) | ns | ns | ns | ns |
| Variety (V) | ns | 14.43 *** | 28.26 *** | 148.56 *** |
| Treatment (T) | ns | 28.76 *** | 466.17 *** | 72.00 *** |
| Y × V | ns | ns | ns | ns |
| Y × T | ns | ns | ns | ns |
| V × T | ns | 13.04 *** | 55.90 *** | 107.42 *** |
| Y × V × T | ns | ns | ns | ns |
| Year | Variety | Treatment | Brown Rice Rate (BR) | Milled Rice Rate (MR) | Chalk Grain Rate (CGR) | Chalkiness Degree (CD) |
|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | |||
| 2020 | FLYX1 | CK | 74.85 c | 55.52 c | 12.18 a | 2.67 c |
| T1 | 79.61 a | 57.64 b | 10.42 c | 3.12 a | ||
| T2 | 75.60 c | 55.67 c | 11.25 b | 2.76 b | ||
| T3 | 77.86 b | 58.63 a | 9.39 d | 2.53 d | ||
| Mean | 76.98 | 56.87 | 10.81 | 2.77 | ||
| JLYHZ | CK | 75.48 b | 55.71 c | 11.71 a | 2.47 b | |
| T1 | 77.49 a | 57.66 a | 10.89 c | 2.44 bc | ||
| T2 | 76.15 ab | 56.60 b | 11.39 b | 2.98 a | ||
| T3 | 76.59 ab | 57.67 a | 10.01 d | 2.41 c | ||
| Mean | 76.43 | 56.91 | 11.00 | 2.57 | ||
| 2021 | FLYX1 | CK | 74.50 c | 55.50 d | 11.98 a | 2.65 c |
| T1 | 79.26 a | 58.22 b | 10.37 c | 3.11 a | ||
| T2 | 75.06 c | 56.20 c | 11.35 b | 2.77 b | ||
| T3 | 78.26 b | 58.97 a | 9.40 d | 2.54 d | ||
| Mean | 76.77 | 57.22 | 10.78 | 2.77 | ||
| JLYHZ | CK | 75.80 b | 55.80 c | 11.65 a | 2.50 b | |
| T1 | 77.57 a | 57.45 a | 10.81 c | 2.48 b | ||
| T2 | 75.81 b | 56.70 b | 11.27 b | 2.97 a | ||
| T3 | 77.22 a | 57.69 a | 9.96 d | 2.40 c | ||
| Mean | 76.60 | 56.91 | 10.93 | 2.59 |
| ANOVA | Brown Rice Rate (BR) | Milled Rice Rate (MR) | Chalk Grain Rate (CGR) | Chalkiness Degree (CD) |
|---|---|---|---|---|
| (%) | (%) | (%) | (%) | |
| Year (Y) | ns | 4.46 * | ns | ns |
| Variety(V) | ns | ns | 12.42 ** | 354.44 *** |
| Treatment (T) | 73.56 *** | 206.00 *** | 388.05 *** | 337.81 *** |
| Y × V | ns | 4.41 * | ns | ns |
| Y × T | ns | ns | ns | ns |
| V × T | 14.67 *** | 21.77 *** | 21.92 *** | 309.98 *** |
| Y × V × T | ns | ns | ns | ns |
| Year | Variety | Treatment | Amylose Content (AC1) | Glutenin Content (GC1) | Albumin Content (AC2) | Globulin Content (GC2) | Alcoholic Protein Content (APC) | Total Protein Content (TPC) |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | |||
| 2020 | FLYX1 | CK | 19.51 b | 7.23 c | 0.33 d | 0.33 c | 0.11 d | 7.90 c |
| T1 | 20.58 a | 7.89 a | 0.46 b | 0.37 a | 0.14 b | 8.86 a | ||
| T2 | 20.30 a | 7.34 b | 0.37 c | 0.34 b | 0.13 c | 8.19 b | ||
| T3 | 20.32 a | 7.91 a | 0.47 a | 0.37 a | 0.18 a | 8.93 a | ||
| Mean | 20.18 | 7.59 | 0.41 | 0.35 | 0.14 | 8.47 | ||
| JLYHZ | CK | 20.36 b | 7.19 d | 0.33 c | 0.33 b | 0.11 d | 8.02 c | |
| T1 | 20.90 b | 7.50 b | 0.38 b | 0.35 a | 0.16 b | 8.44 a | ||
| T2 | 22.34 a | 7.34 c | 0.37 c | 0.34 b | 0.17 a | 8.20 b | ||
| T3 | 19.61 c | 7.60 a | 0.39 a | 0.35 a | 0.15 c | 8.49 a | ||
| Mean | 20.80 | 7.41 | 0.37 | 0.34 | 0.15 | 8.29 | ||
| 2021 | FLYX1 | CK | 19.67 b | 7.17 c | 0.33 d | 0.33 c | 0.11 d | 7.89 d |
| T1 | 20.66 a | 7.93 a | 0.46 b | 0.37 a | 0.14 b | 8.89 b | ||
| T2 | 20.62 a | 7.35 b | 0.37 c | 0.34 b | 0.13 c | 8.12 c | ||
| T3 | 20.48 a | 7.95 a | 0.47 a | 0.37 a | 0.18 a | 8.95 a | ||
| Mean | 20.36 | 7.60 | 0.41 | 0.35 | 0.14 | 8.46 | ||
| JLYHZ | CK | 20.56 b | 7.16 c | 0.33 c | 0.33 b | 0.11 d | 7.97 c | |
| T1 | 21.01 b | 7.59 a | 0.38 b | 0.35 a | 0.16 b | 8.42 a | ||
| T2 | 21.97 a | 7.32 b | 0.37 c | 0.34 b | 0.17 a | 8.24 b | ||
| T3 | 19.89 c | 7.60 a | 0.39 a | 0.35 a | 0.15 c | 8.45 a | ||
| Mean | 20.86 | 7.42 | 0.37 | 0.34 | 0.15 | 8.27 |
| ANOVA | Amylose Content (AC1) | Glutenin Content (GC1) | Albumin Content (AC2) | Globulin Content (GC2) | Alcoholic Protein Content (APC) | Total Protein Content (TPC) |
|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | |
| Year (Y) | ns | ns | 4.93 * | ns | ns | ns |
| Variety (V) | 50.23 ** | 266.10 *** | 2971.35 *** | 409.61 *** | 756.00 *** | 206.79 *** |
| Treatment (T) | 59.66 ** | 651.89 *** | 4350.78 *** | 843.65 *** | 7778.00 *** | 797.34 *** |
| Y × V | ns | ns | ns | ns | ns | ns |
| Y × T | ns | 5.66 ** | ns | ns | ns | ns |
| V × T | 38.23 ** | 71.83 *** | 1220.14 *** | 139.06 *** | 3213.49 *** | 146.81 *** |
| Y × V × T | ns | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Feng, D.; Tang, Z.; Ya, C.; Lin, X.; Zhang, K.; Yao, X. Effects of Foliar Organic Selenium Application During the Main Season on Ratoon Rice Yield, Grain Quality, and Selenium Accumulation. Plants 2025, 14, 2758. https://doi.org/10.3390/plants14172758
Hu J, Feng D, Tang Z, Ya C, Lin X, Zhang K, Yao X. Effects of Foliar Organic Selenium Application During the Main Season on Ratoon Rice Yield, Grain Quality, and Selenium Accumulation. Plants. 2025; 14(17):2758. https://doi.org/10.3390/plants14172758
Chicago/Turabian StyleHu, Jinfu, Dehao Feng, Ziran Tang, Caise Ya, Xueer Lin, Kai Zhang, and Xiong Yao. 2025. "Effects of Foliar Organic Selenium Application During the Main Season on Ratoon Rice Yield, Grain Quality, and Selenium Accumulation" Plants 14, no. 17: 2758. https://doi.org/10.3390/plants14172758
APA StyleHu, J., Feng, D., Tang, Z., Ya, C., Lin, X., Zhang, K., & Yao, X. (2025). Effects of Foliar Organic Selenium Application During the Main Season on Ratoon Rice Yield, Grain Quality, and Selenium Accumulation. Plants, 14(17), 2758. https://doi.org/10.3390/plants14172758






