Modulation of Endoplasmic Reticulum Stress by Selected Polyphenols from Sambucus ebulus L. Fruit
Abstract
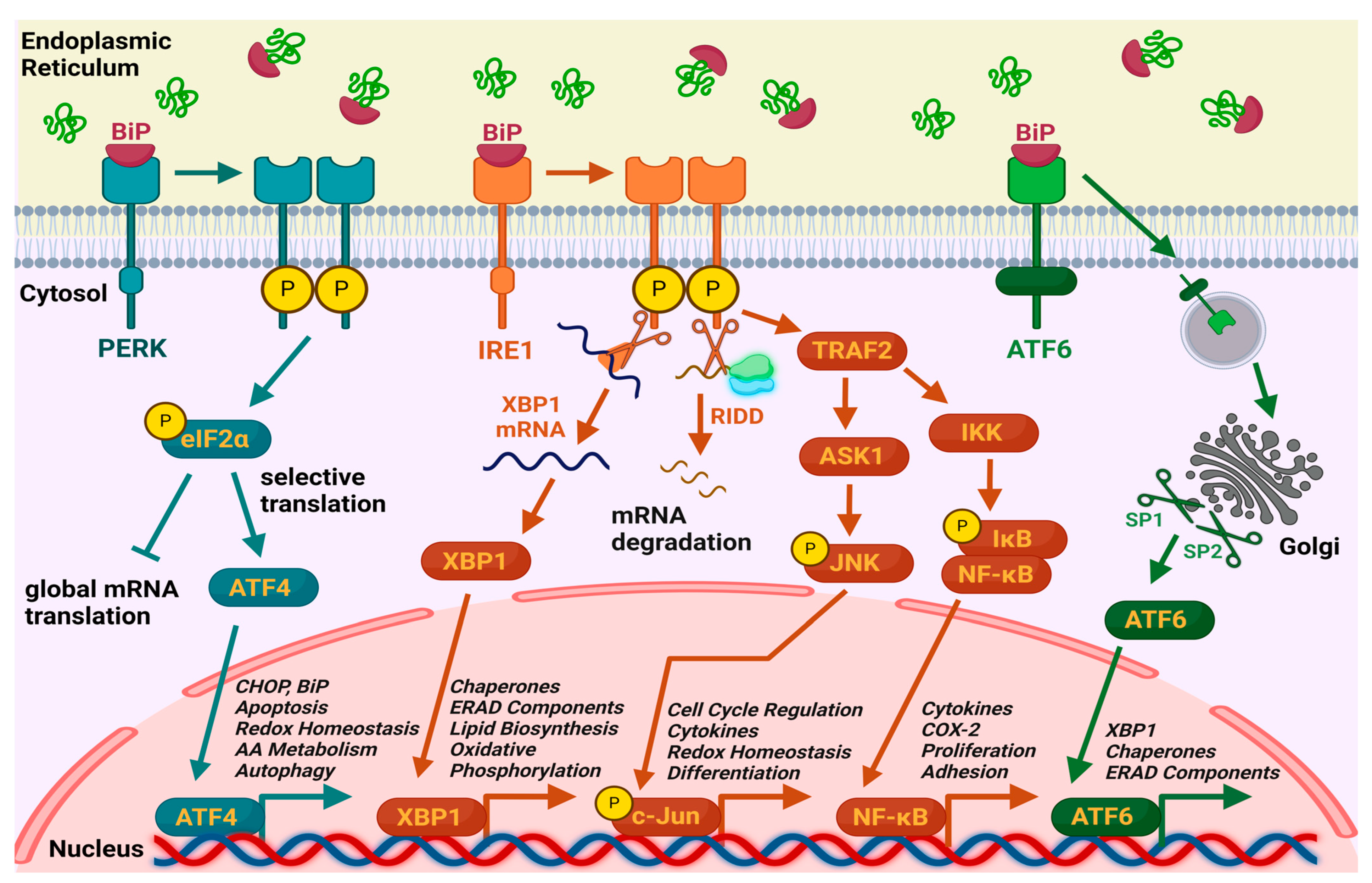
1. Introduction
2. Materials and Methods
2.1. Search Strategy
- Text availability—free full texts
- Publication date—from 2000/1/1 to 2025/5/1
- Article type—research articles
- Subject area—Biochemistry, Genetics, and Molecular Biology
- Access type—open access and open archive
2.2. Inclusion and Exclusion Criteria
2.3. Statistical Analyses and Presentation
3. Results
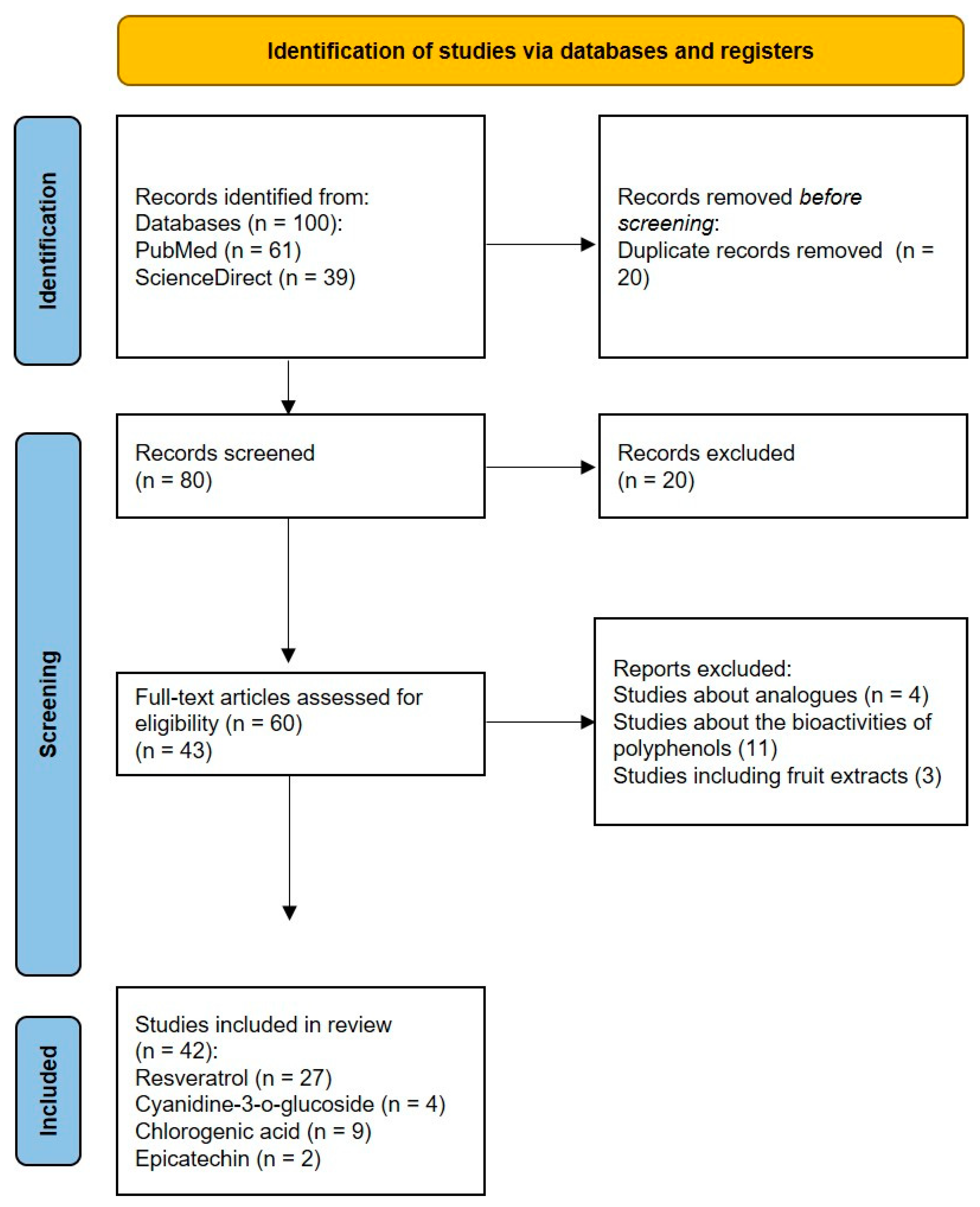
3.1. Study Selection
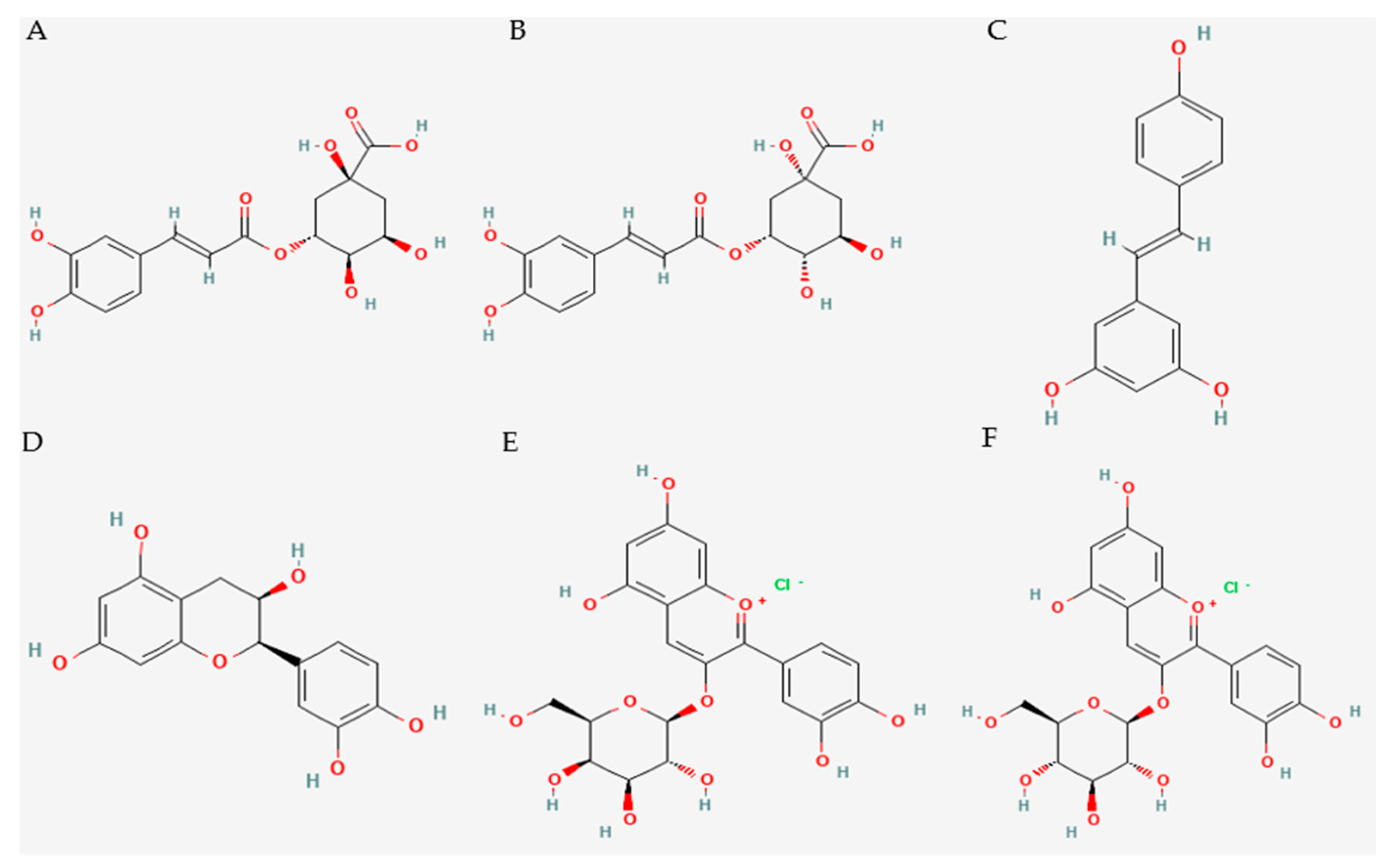
3.2. Presentation of the Results
3.2.1. Modulation of ER Stress by Resveratrol
3.2.2. Modulation of ERS by Cyanidin-3-o-Glucoside, Chlorogenic Acid, and Epicatechin
4. Discussion
- Insufficient information on the effects of some polyphenols across all UPR branches.
- Heterogeneity in experimental models limits direct comparisons and generalization.
- Dose variation and dual effect of resveratrol on ER stress—determining the precise therapeutic dose and anticipating potential side effects is challenging.
- Lack of clinical trials and data on long-term effects of polyphenol intake—it is difficult to determine the therapeutic potential of polyphenols, as well as to assess the long-term safety and efficacy of these compounds.
- Although the results highlight the significant contribution of the polyphenols found at the highest concentrations in the aqueous dwarf elder extract, the possible biological activity of other present phytochemicals in lower amounts cannot be excluded.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schwarz, D.S.; Blower, M.D. The Endoplasmic Reticulum: Structure, Function and Response to Cellular Signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef]
- Ozcan, L.; Tabas, I. Role of Endoplasmic Reticulum Stress in Metabolic Disease and Other Disorders. Annu. Rev. Med. 2012, 63, 317–328. [Google Scholar] [CrossRef]
- Lin, J.H.; Walter, P.; Yen, T.S.B. Endoplasmic Reticulum Stress in Disease Pathogenesis. Annu. Rev. Pathol. 2008, 3, 399–425. [Google Scholar] [CrossRef]
- Wu, J.; Kaufman, R.J. From Acute ER Stress to Physiological Roles of the Unfolded Protein Response. Cell Death Differ. 2006, 13, 374–384. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal Integration in the Endoplasmic Reticulum Unfolded Protein Response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Siwecka, N.; Rozpędek-Kamińska, W.; Wawrzynkiewicz, A.; Pytel, D.; Diehl, J.A.; Majsterek, I. The Structure, Activation and Signaling of IRE1 and Its Role in Determining Cell Fate. Biomedicines 2021, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Kaufman, R.J. A Trip to the ER: Coping with Stress. Trends Cell Biol. 2004, 14, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Ron, D. Integrating the Mechanisms of Apoptosis Induced by Endoplasmic Reticulum Stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, S.Z.; Lourie, R.; Das, I.; Chen, A.C.; McGuckin, M.A. The Interplay between Endoplasmic Reticulum Stress and Inflammation. Immunol. Cell Biol. 2012, 90, 260–270. [Google Scholar] [CrossRef]
- Sprenkle, N.T.; Sims, S.G.; Sánchez, C.L.; Meares, G.P. Endoplasmic Reticulum Stress and Inflammation in the Central Nervous System. Mol. Neurodegener. 2017, 12, 42. [Google Scholar] [CrossRef]
- So, A.Y.-L.; de la Fuente, E.; Walter, P.; Shuman, M.; Bernales, S. The Unfolded Protein Response during Prostate Cancer Development. Cancer Metastasis Rev. 2009, 28, 219–223. [Google Scholar] [CrossRef]
- Lee, A.S. GRP78 Induction in Cancer: Therapeutic and Prognostic Implications. Cancer Res. 2007, 67, 3496–3499. [Google Scholar] [CrossRef]
- Fernandez, P.M.; Tabbara, S.O.; Jacobs, L.K.; Manning, F.C.R.; Tsangaris, T.N.; Schwartz, A.M.; Kennedy, K.A.; Patierno, S.R. Overexpression of the Glucose-Regulated Stress Gene GRP78 in Malignant but Not Benign Human Breast Lesions. Breast Cancer Res. Treat. 2000, 59, 15–26. [Google Scholar] [CrossRef]
- Carrasco, D.R.; Sukhdeo, K.; Protopopova, M.; Sinha, R.; Enos, M.; Carrasco, D.E.; Zheng, M.; Mani, M.; Henderson, J.; Pinkus, G.S.; et al. The Differentiation and Stress Response Factor XBP-1 Drives Multiple Myeloma Pathogenesis. Cancer Cell 2007, 11, 349–360. [Google Scholar] [CrossRef]
- Tardif, K.D.; Mori, K.; Kaufman, R.J.; Siddiqui, A. Hepatitis C Virus Suppresses the IRE1-XBP1 Pathway of the Unfolded Protein Response. J. Biol. Chem. 2004, 279, 17158–17164. [Google Scholar] [CrossRef]
- Xu, Z.; Jensen, G.; Yen, T.S. Activation of Hepatitis B Virus S Promoter by the Viral Large Surface Protein via Induction of Stress in the Endoplasmic Reticulum. J. Virol. 1997, 71, 7387–7392. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Mehrian-Shai, R.; Chan, C.; Hsu, Y.-H.; Kaplowitz, N. Role of CHOP in Hepatic Apoptosis in the Murine Model of Intragastric Ethanol Feeding. Alcohol. Clin. Exp. Res. 2005, 29, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, H.L.; Chabanon, H.; Hainault, I.; Luquet, S.; Magnan, C.; Koike, T.; Ferré, P.; Foufelle, F. GRP78 Expression Inhibits Insulin and ER Stress–Induced SREBP-1c Activation and Reduces Hepatic Steatosis in Mice. J. Clin. Investig. 2009, 119, 1201–1215. [Google Scholar] [CrossRef]
- Özcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.-H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Tsukano, H.; Gotoh, T.; Endo, M.; Miyata, K.; Tazume, H.; Kadomatsu, T.; Yano, M.; Iwawaki, T.; Kohno, K.; Araki, K.; et al. The Endoplasmic Reticulum Stress-C/EBP Homologous Protein Pathway-Mediated Apoptosis in Macrophages Contributes to the Instability of Atherosclerotic Plaques. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1925–1932. [Google Scholar] [CrossRef]
- Katayama, T.; Imaizumi, K.; Sato, N.; Miyoshi, K.; Kudo, T.; Hitomi, J.; Morihara, T.; Yoneda, T.; Gomi, F.; Mori, Y.; et al. Presenilin-1 Mutations Downregulate the Signalling Pathway of the Unfolded-Protein Response. Nat. Cell Biol. 1999, 1, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Soda, M.; Takahashi, R. Parkin Suppresses Unfolded Protein Stress-Induced Cell Death through Its E3 Ubiquitin-Protein Ligase Activity. J. Biol. Chem. 2000, 275, 35661–35664. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Waswa, E.N.; Li, J.; Mkala, E.M.; Wanga, V.O.; Mutinda, E.S.; Nanjala, C.; Odago, W.O.; Katumo, D.M.; Gichua, M.K.; Gituru, R.W.; et al. Ethnobotany, Phytochemistry, Pharmacology, and Toxicology of the Genus Sambucus L. (Viburnaceae). J. Ethnopharmacol. 2022, 292, 115102. [Google Scholar] [CrossRef]
- Tasinov, O.; Kiselova-Kaneva, Y.; Ivanova, D. Sambucus ebulus—From Traditional Medicine to Recent Studies. Scr. Sci. Medica 2013, 45, 36–42. [Google Scholar] [CrossRef][Green Version]
- Pasa, C. The Use of Sambucus ebulus L. in Folk Medicine and Chemical Composition. GSC Adv. Res. Rev. 2023, 17, 081–085. [Google Scholar] [CrossRef]
- Kültür, S. Medicinal Plants Used in Kirklareli Province (Turkey). J. Ethnopharmacol. 2007, 111, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, M.; Daneshfard, B.; Emtiazy, M.; Khiveh, A.; Hashempur, M.H. Biological Effects and Clinical Applications of Dwarf Elder (Sambucus ebulus L): A Review. J. Evid. Based Complement. Altern. Med. 2017, 22, 996–1001. [Google Scholar] [CrossRef]
- Seymenska, D.; Shishkova, K.; Hinkov, A.; Benbassat, N.; Teneva, D.; Denev, P. Comparative Study on Phytochemical Composition, Antioxidant, and Anti-HSV-2 Activities of Sambucus nigra L. and Sambucus ebulus L. Extracts. Appl. Sci. 2023, 13, 12593. [Google Scholar] [CrossRef]
- Schwaiger, S.; Zeller, I.; Pölzelbauer, P.; Frotschnig, S.; Laufer, G.; Messner, B.; Pieri, V.; Stuppner, H.; Bernhard, D. Identification and Pharmacological Characterization of the Anti-Inflammatory Principal of the Leaves of Dwarf Elder (Sambucus ebulus L.). J. Ethnopharmacol. 2011, 133, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J.; Jiménez, P.; Quinto, E.J.; Cordoba-Diaz, D.; Garrosa, M.; Cordoba-Diaz, M.; Gayoso, M.J.; Girbés, T. Elderberries: A Source of Ribosome-Inactivating Proteins with Lectin Activity. Molecules 2015, 20, 2364–2387. [Google Scholar] [CrossRef] [PubMed]
- Zahmanov, G.; Alipieva, K.; Simova, S.; Georgiev, M.I. Metabolic Differentiations of Dwarf Elder by NMR-Based Metabolomics. Phytochem. Lett. 2015, 11, 404–409. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In Vitro Antioxidant Properties and Anthocyanin Compositions of Elderberry Extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Bubulica, M.-V.; Chirigiu, L.; Popescu, M.; Simionescu, A.; Anoaica, G.; Popescu, A. Analysis of Sterol Compounds from Sambucus ebulus. Chem. Nat. Compd. 2012, 48, 520–521. [Google Scholar] [CrossRef]
- Pieri, V.; Schwaiger, S.; Ellmerer, E.P.; Stuppner, H. Iridoid Glycosides from the Leaves of Sambucus ebulus. J. Nat. Prod. 2009, 72, 1798–1803. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Ivancic, A.; Todorovic, B.; Veberic, R.; Stampar, F. Fruit Phenolic Composition of Different Elderberry Species and Hybrids. J. Food Sci. 2015, 80, C2180–C2190. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Bekhradnia, A.R. Iron Chelating Activity, Phenol and Flavonoid Content of Some Medicinal Plants from Iran. Afr. J. Biotechnol. 2008, 7, 3188–3192. [Google Scholar]
- Ebrahimzadeh, M.A.; Nabavi, S.F.; Nabavi, S.M.; Pourmorad, F. Nitric Oxide Radical Scavenging Potential of Some Elburz Medicinal Plants. Afr. J. Biotechnol. 2010, 9, 5212–5217. [Google Scholar]
- Tasinov, O.; Kiselova-Kaneva, Y.; Ivanova, D. Antioxidant Activity, Total Polyphenol Content, Anthocyanins Content of Sambucus ebulus Aqueous and Aqueous-Ethanolic Extracts Depend on the Type and Concentration of Extragent. Sci. Technol. 2012, 2, 37–41. [Google Scholar] [CrossRef]
- Ivanova, D.; Tasinov, O.; Kiselova-Kaneva, Y. Improved Lipid Profile and Increased Serum Antioxidant Capacity in Healthy Volunteers after Sambucus ebulus L. Fruit Infusion Consumption. Int. J. Food Sci. Nutr. 2014, 65, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Ahmadiani, A.; Fereidoni, M.; Semnanian, S.; Kamalinejad, M.; Saremi, S. Antinociceptive and Anti-Inflammatory Effects of Sambucus ebulus Rhizome Extract in Rats. J. Ethnopharmacol. 1998, 61, 229–235. [Google Scholar] [CrossRef]
- Yesilada, E. Evaluation of the Anti-Inflammatory Activity of the Turkish Medicinal Plant Sambucus ebulus. Chem. Nat. Compd. 1997, 33, 539–540. [Google Scholar] [CrossRef]
- Tasinov, O.; Kiselova-Kaneva, Y.; Nazifova-Tasinova, N.; Todorova, M.; Trendafilova, A.; Ivanova, D. Chemical Composition and Cytoprotective and Anti-Inflammatory Potential of Sambucus ebulus Fruit Ethyl Acetate Fraction. Bulg. Chem. Commun. 2020, 52, 100–106. [Google Scholar]
- Tasinov, O.; Dincheva, I.; Badjakov, I.; Kiselova-Kaneva, Y.; Galunska, B.; Nogueiras, R.; Ivanova, D. Phytochemical Composition, Anti-Inflammatory and ER Stress-Reducing Potential of Sambucus ebulus L. Fruit Extract. Plants 2021, 10, 2446. [Google Scholar] [CrossRef]
- Aghajanzadeh, H.; Abdolmaleki, M.; Ebrahimzadeh, M.A.; Mojtabavi, N.; Mousavi, T.; Izad, M. Methanolic Extract of Sambucus ebulus Ameliorates Clinical Symptoms in Experimental Type 1 Diabetes through Anti-Inflammatory and Immunomodulatory Actions. Cell J. 2021, 23, 465–473. [Google Scholar] [CrossRef]
- Hou, D.-X.; Yanagita, T.; Uto, T.; Masuzaki, S.; Fujii, M. Anthocyanidins Inhibit Cyclooxygenase-2 Expression in LPS-Evoked Macrophages: Structure–Activity Relationship and Molecular Mechanisms Involved. Biochem. Pharmacol. 2005, 70, 417–425. [Google Scholar] [CrossRef]
- Chung, J.Y.; Park, J.O.; Phyu, H.; Dong, Z.; Yang, C.S. Mechanisms of Inhibition of the Ras-MAP Kinase Signaling Pathway in 30.7b Ras 12 Cells by Tea Polyphenols (-)-Epigallocatechin-3-Gallate and Theaflavin-3,3′-Digallate. FASEB J. 2001, 15, 2022–2024. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Immunomodulatory Activity of Resveratrol: Suppression of Lymphocyte Proliferation, Development of Cell-Mediated Cytotoxicity, and Cytokine Production. Biochem. Pharmacol. 2001, 62, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Choi, H.; Jo, A.; Kang, H.; Yun, H.; Im, S.; Choi, C. Anti-Inflammatory Effects of a Stauntonia hexaphylla Fruit Extract in Lipopolysaccharide-Activated RAW-264.7 Macrophages and Rats by Carrageenan-Induced Hind Paw Swelling. Nutrients 2018, 10, 110. [Google Scholar] [CrossRef]
- Liu, Y.H.; Weng, Y.P.; Lin, H.Y.; Tang, S.W.; Chen, C.J.; Liang, C.J.; Ku, C.Y.; Lin, J.Y. Aqueous Extract of Polygonum bistorta Modulates Proteostasis by ROS-Induced ER Stress in Human Hepatoma Cells. Sci. Rep. 2017, 7, 41437. [Google Scholar] [CrossRef]
- Hsieh, P.C.; Peng, C.K.; Liu, G.T.; Kuo, C.Y.; Tzeng, I.S.; Wang, M.C.; Lan, C.C.; Huang, K.L. Aqueous Extract of Descuraniae Semen Attenuates Lipopolysaccharide-Induced Inflammation and Apoptosis by Regulating the Proteasomal Degradation and IRE1α-Dependent Unfolded Protein Response in A549 Cells. Front. Immunol. 2022, 13, 916102. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiao, D.; Zhang, Y.; Chen, Q.; Chen, Y.; Tang, Y.; Que, R.; Chen, Y.; Zheng, L.; Dai, Y.; et al. Portulaca oleracea L. Extract Ameliorates Intestinal Inflammation by Regulating Endoplasmic Reticulum Stress and Autophagy. Mol. Nutr. Food Res. 2022, 66, 2100791. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Lin, H.H.; Chyau, C.C.; Wang, Z.H.; Chen, J.H. Aqueous Extract of Pepino Leaves Ameliorates Palmitic Acid-Induced Hepatocellular Lipotoxicity via Inhibition of Endoplasmic Reticulum Stress and Apoptosis. Antioxidants 2021, 10, 903. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Giles, A.; Nakamura, K.; Lee, J.W.; Hou, X.; Donmez, G.; Li, J.; Luo, Z.; Walsh, K.; et al. Hepatic Overexpression of SIRT1 in Mice Attenuates Endoplasmic Reticulum Stress and Insulin Resistance in the Liver. FASEB J. 2011, 25, 1664–1679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, X.; Xie, W.; Li, Y.; Ma, M.; Yuan, T.; Luo, B. Resveratrol Exerts an Anti-Apoptotic Effect on Human Bronchial Epithelial Cells Undergoing Cigarette Smoke Exposure. Mol. Med. Rep. 2015, 11, 1752–1758. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, J.; Zhang, X.; Wang, J.; Xiao, W.; Li, B.; Jin, L.; Lian, J.; Zhou, L.; Liu, J. Inhibition of Cardiomyocytes Hypertrophy by Resveratrol Is Associated with Amelioration of Endoplasmic Reticulum Stress. Cell. Physiol. Biochem. 2016, 39, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Xia, X.; Rui, Y.; Zhang, Z.; Qin, L.; Han, S.; Wan, Z. The Combination of 1α,25dihydroxyvitaminD3 with Resveratrol Improves Neuronal Degeneration by Regulating Endoplasmic Reticulum Stress, Insulin Signaling and Inhibiting Tau Hyperphosphorylation in SH-SY5Y Cells. Food Chem. Toxicol. 2016, 93, 32–40. [Google Scholar] [CrossRef]
- Yan, W.-J.; Liu, R.-B.; Wang, L.-K.; Ma, Y.-B.; Ding, S.-L.; Deng, F.; Hu, Z.-Y.; Wang, D.-B. Sirt3-Mediated Autophagy Contributes to Resveratrol-Induced Protection against ER Stress in HT22 Cells. Front. Neurosci. 2018, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, S.-W.; Kwon, H.; Park, S.E.; Rhee, E.-J.; Park, C.-Y.; Oh, K.-W.; Park, S.-W.; Lee, W.-Y. Resveratrol, an Activator of SIRT1, Improves ER Stress by Increasing Clusterin Expression in HepG2 Cells. Cell Stress Chaperones 2019, 24, 825–833. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Shu, L.; Song, G.; Ma, H. Resveratrol Reduces Liver Endoplasmic Reticulum Stress and Improves Insulin Sensitivity in Vivo and in Vitro. Drug Des. Devel. Ther. 2019, 13, 1473–1485. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, X.; Ding, M.; You, C.; Lin, X.; Wang, Y.; Wu, M.; Xu, G.; Wang, G. Resveratrol Decreases High Glucose-induced Apoptosis in Renal Tubular Cells via Suppressing Endoplasmic Reticulum Stress. Mol. Med. Rep. 2020, 22, 4367–4375. [Google Scholar] [CrossRef]
- Neal, S.E.; Buehne, K.L.; Besley, N.A.; Yang, P.; Silinski, P.; Hong, J.; Ryde, I.T.; Meyer, J.N.; Jaffe, G.J. Resveratrol Protects Against Hydroquinone-Induced Oxidative Threat in Retinal Pigment Epithelial Cells. Investig. Opthalmology Vis. Sci. 2020, 61, 32. [Google Scholar] [CrossRef]
- Yu, X.; Xu, X.; Dong, W.; Yang, C.; Luo, Y.; He, Y.; Jiang, C.; Wu, Y.; Wang, J. DDIT3/CHOP Mediates the Inhibitory Effect of ER Stress on Chondrocyte Differentiation by AMPKα-SIRT1 Pathway. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 2022, 1869, 119265. [Google Scholar] [CrossRef]
- Luo, Y.; Hu, N.; Zhao, Y.; Lai, J.; Luo, X.; Liu, J. Resveratrol-mediated Activation of SIRT1 Inhibits the PERK-eIF2α-ATF4 Pathway and Mitigates Bupivacaine-induced Neurotoxicity in PC12 Cells. Exp. Ther. Med. 2023, 26, 433. [Google Scholar] [CrossRef]
- Chinta, S.J.; Poksay, K.S.; Kaundinya, G.; Hart, M.; Bredesen, D.E.; Andersen, J.K.; Rao, R.V. Endoplasmic Reticulum Stress–Induced Cell Death in Dopaminergic Cells: Effect of Resveratrol. J. Mol. Neurosci. 2009, 39, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-M.; Galson, D.L.; Roodman, G.D.; Ouyang, H. Resveratrol Triggers the Pro-Apoptotic Endoplasmic Reticulum Stress Response and Represses pro-Survival XBP1 Signaling in Human Multiple Myeloma Cells. Exp. Hematol. 2011, 39, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Rojas, C.; Pan-Castillo, B.; Valls, C.; Pujadas, G.; Garcia-Vallve, S.; Arola, L.; Mulero, M. Resveratrol Enhances Palmitate-Induced ER Stress and Apoptosis in Cancer Cells. PLoS ONE 2014, 9, e113929. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Sun, Y.; Sukumaran, P.; Singh, B.B. Resveratrol Activates Autophagic Cell Death in Prostate Cancer Cells via Downregulation of STIM1 and the MTOR Pathway. Mol. Carcinog. 2016, 55, 818–831. [Google Scholar] [CrossRef]
- Heo, J.; Kim, S.; Hwang, K.; Kang, J.; Choi, K. Resveratrol Induced Reactive Oxygen Species and Endoplasmic Reticulum Stress-mediated Apoptosis, and Cell Cycle Arrest in the A375SM Malignant Melanoma Cell Line. Int. J. Mol. Med. 2018, 42, 1427–1435. [Google Scholar] [CrossRef]
- Ren, M.; Zhou, X.; Gu, M.; Jiao, W.; Yu, M.; Wang, Y.; Liu, S.; Yang, J.; Ji, F. Resveratrol Synergizes with Cisplatin in Antineoplastic Effects against AGS Gastric Cancer Cells by Inducing Endoplasmic Reticulum Stress-mediated Apoptosis and G2/M Phase Arrest. Oncol. Rep. 2020, 44, 1605–1615. [Google Scholar] [CrossRef]
- Pan, P.; Lin, H.; Chuang, C.; Wang, P.; Wan, H.; Lee, M.; Kao, M. Resveratrol Alleviates Nuclear Factor-κB-mediated Neuroinflammation in Vasculitic Peripheral Neuropathy Induced by Ischaemia–Reperfusion via Suppressing Endoplasmic Reticulum Stress. Clin. Exp. Pharmacol. Physiol. 2019, 46, 770–779. [Google Scholar] [CrossRef]
- Wang, B.; Ge, S.; Xiong, W.; Xue, Z. Effects of Resveratrol Pretreatment on Endoplasmic Reticulum Stress and Cognitive Function after Surgery in Aged Mice. BMC Anesthesiol. 2018, 18, 141. [Google Scholar] [CrossRef]
- Pan, Q.-R.; Ren, Y.-L.; Liu, W.-X.; Hu, Y.-J.; Zheng, J.-S.; Xu, Y.; Wang, G. Resveratrol Prevents Hepatic Steatosis and Endoplasmic Reticulum Stress and Regulates the Expression of Genes Involved in Lipid Metabolism, Insulin Resistance, and Inflammation in Rats. Nutr. Res. 2015, 35, 576–584. [Google Scholar] [CrossRef]
- Graham, R.M.; Hernandez, F.; Puerta, N.; De Angulo, G.; Webster, K.A.; Vanni, S. Resveratrol Augments ER Stress and the Cytotoxic Effects of Glycolytic Inhibition in Neuroblastoma by Downregulating Akt in a Mechanism Independent of SIRT1. Exp. Mol. Med. 2016, 48, e210. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiang, J.; Zhang, G.; Bu, Y.; Zhang, G.; Zhao, X. Resveratrol and Caloric Restriction Prevent Hepatic Steatosis by Regulating SIRT1-Autophagy Pathway and Alleviating Endoplasmic Reticulum Stress in High-Fat Diet-Fed Rats. PLoS ONE 2017, 12, e0183541. [Google Scholar] [CrossRef] [PubMed]
- Ardid-Ruiz, A.; Ibars, M.; Mena, P.; Del Rio, D.; Muguerza, B.; Bladé, C.; Arola, L.; Aragonès, G.; Suárez, M. Potential Involvement of Peripheral Leptin/STAT3 Signaling in the Effects of Resveratrol and Its Metabolites on Reducing Body Fat Accumulation. Nutrients 2018, 10, 1757. [Google Scholar] [CrossRef]
- Lu, J.; Zheng, Y.; Yang, J.; Zhang, J.; Cao, W.; Chen, X.; Fang, S. Resveratrol Alleviates Inflammatory Injury and Enhances the Apoptosis of Fibroblast-like Synoviocytes via Mitochondrial Dysfunction and ER Stress in Rats with Adjuvant Arthritis. Mol. Med. Rep. 2019, 20, 463–472. [Google Scholar] [CrossRef]
- Arena, A.; Romeo, M.A.; Benedetti, R.; Masuelli, L.; Bei, R.; Gilardini Montani, M.S.; Cirone, M. New Insights into Curcumin- and Resveratrol-Mediated Anti-Cancer Effects. Pharmaceuticals 2021, 14, 1068. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.T.; Veerisetty, A.C.; Wu, J.; Coustry, F.; Hossain, M.G.; Chiu, F.; Gannon, F.H.; Posey, K.L. Primary Osteoarthritis Early Joint Degeneration Induced by Endoplasmic Reticulum Stress Is Mitigated by Resveratrol. Am. J. Pathol. 2021, 191, 1624–1637. [Google Scholar] [CrossRef]
- Totonchi, H.; Mokarram, P.; Karima, S.; Rezaei, R.; Dastghaib, S.; Koohpeyma, F.; Noori, S.; Azarpira, N. Resveratrol Promotes Liver Cell Survival in Mice Liver-Induced Ischemia-Reperfusion through Unfolded Protein Response: A Possible Approach in Liver Transplantation. BMC Pharmacol. Toxicol. 2022, 23, 74. [Google Scholar] [CrossRef]
- Thummayot, S.; Tocharus, C.; Suksamrarn, A.; Tocharus, J. Neuroprotective Effects of Cyanidin against Aβ-Induced Oxidative and ER Stress in SK-N-SH Cells. Neurochem Int 2016, 101, 15–21. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Su, L.; Hu, Q.; Li, W.; He, J.; Zhao, L. Cyanidin-3-O-Glucoside Ameliorates Palmitic-Acid-Induced Pancreatic Beta Cell Dysfunction by Modulating CHOP-Mediated Endoplasmic Reticulum Stress Pathways. Nutrients 2022, 14, 1835. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.-P.; Kuo, C.-Y.; Fu, M.M.-J.; Chin, Y.-T.; Chiang, C.-Y.; Chiu, H.-C.; Hsia, Y.-J.; Fu, E. Cyanidin-3-O-Glucoside Downregulates Ligation-Activated Endoplasmic Reticulum Stress and Alleviates Induced Periodontal Destruction in Rats. Arch Oral Biol 2022, 134, 105313. [Google Scholar] [CrossRef]
- Peng, W.; Wu, Y.; Peng, Z.; Qi, W.; Liu, T.; Yang, B.; He, D.; Liu, Y.; Wang, Y. Cyanidin-3-Glucoside Improves the Barrier Function of Retinal Pigment Epithelium Cells by Attenuating Endoplasmic Reticulum Stress-Induced Apoptosis. Food Research International 2022, 157, 111313. [Google Scholar] [CrossRef]
- Bettaieb, A.; Vazquez Prieto, M.A.; Rodriguez Lanzi, C.; Miatello, R.M.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (−)-Epicatechin Mitigates High-Fructose-Associated Insulin Resistance by Modulating Redox Signaling and Endoplasmic Reticulum Stress. Free Radic. Biol. Med. 2014, 72, 247–256. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, J.-H.; Seo, Y.H.; Jang, J.-H.; Jeong, C.-H.; Lee, S.; Jeong, G.-S.; Park, B. Epicatechin Prevents Methamphetamine-Induced Neuronal Cell Death via Inhibition of ER Stress. Biomol. Ther. 2019, 27, 145–151. [Google Scholar] [CrossRef]
- Ye, H.-Y.; Li, Z.-Y.; Zheng, Y.; Chen, Y.; Zhou, Z.-H.; Jin, J. The Attenuation of Chlorogenic Acid on Oxidative Stress for Renal Injury in Streptozotocin-Induced Diabetic Nephropathy Rats. Arch. Pharm. Res. 2016, 39, 989–997. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Dong, J.; Nie, J.; Zhu, J.-X.; Wang, H.; Chen, Q.; Chen, J.-Y.; Xia, J.-M.; Shuai, W. Amelioration of Bleomycin-Induced Pulmonary Fibrosis by Chlorogenic Acid through Endoplasmic Reticulum Stress Inhibition. Apoptosis 2017, 22, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Miao, L.; Zhang, H.; Wu, G.; Zhang, Z.; Lv, J. Chlorogenic Acid against Palmitic Acid in Endoplasmic Reticulum Stress-Mediated Apoptosis Resulting in Protective Effect of Primary Rat Hepatocytes. Lipids Health Dis. 2018, 17, 270. [Google Scholar] [CrossRef]
- Kazaz, I.O.; Demir, S.; Kerimoglu, G.; Colak, F.; Turkmen Alemdar, N.; Yilmaz Dogan, S.; Bostan, S.; Mentese, A. Chlorogenic Acid Ameliorates Torsion/Detorsion-Induced Testicular Injury via Decreasing Endoplasmic Reticulum Stress. J. Pediatr. Urol. 2022, 18, 289.e1–289.e7. [Google Scholar] [CrossRef] [PubMed]
- Preetha Rani, M.R.; Salin Raj, P.; Nair, A.; Ranjith, S.; Rajankutty, K.; Raghu, K.G. In Vitro and in Vivo Studies Reveal the Beneficial Effects of Chlorogenic Acid against ER Stress Mediated ER-Phagy and Associated Apoptosis in the Heart of Diabetic Rat. Chem. Biol. Interact. 2022, 351, 109755. [Google Scholar] [CrossRef] [PubMed]
- Sari, I.; Moch Rizal, D.; Afiyah Syarif, R. The Effect of Chlorogenic Acid on Endoplasmic Reticulum Stress and Steroidogenesis in the Testes of Diabetic Rats: Study of MRNA Expressions of GRP78, XBP1s, 3β-HSD, and 17β-HSD. BIO Web Conf. 2022, 49, 01001. [Google Scholar] [CrossRef]
- Moslehi, A.; Komeili-Movahhed, T.; Ahmadian, M.; Ghoddoosi, M.; Heidari, F. Chlorogenic Acid Attenuates Liver Apoptosis and Inflammation in Endoplasmic Reticulum Stress-Induced Mice. Iran. J. Basic Med. Sci. 2023, 26, 478–485. [Google Scholar] [CrossRef]
- Boonyong, C.; Angkhasirisap, W.; Kengkoom, K.; Jianmongkol, S. Different Protective Capability of Chlorogenic Acid and Quercetin against Indomethacin-Induced Gastrointestinal Ulceration. J. Pharm. Pharmacol. 2023, 75, 427–436. [Google Scholar] [CrossRef]
- Ping, P.; Yang, T.; Ning, C.; Zhao, Q.; Zhao, Y.; Yang, T.; Gao, Z.; Fu, S. Chlorogenic Acid Attenuates Cardiac Hypertrophy via Up-regulating Sphingosine-1-phosphate Receptor1 to Inhibit Endoplasmic Reticulum Stress. ESC Heart Fail. 2024, 11, 1580–1593. [Google Scholar] [CrossRef]
- Kiselova-Kaneva, Y.; Galunska, B.; Nikolova, M.; Dincheva, I.; Badjakov, I. High Resolution LC-MS/MS Characterization of Polyphenolic Composition and Evaluation of Antioxidant Activity of Sambucus ebulus Fruit Tea Traditionally Used in Bulgaria as a Functional Food. Food Chem. 2022, 367, 130759. [Google Scholar] [CrossRef]
- Wang, S.; Huang, Y.; Luo, G.; Yang, X.; Huang, W. Cyanidin-3-O-Glucoside Attenuates High Glucose–Induced Podocyte Dysfunction by Inhibiting Apoptosis and Promoting Autophagy via Activation of SIRT1/AMPK Pathway. Can. J. Physiol. Pharmacol. 2021, 99, 589–598. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]
- Soleas, G.J.; Diamandis, E.P.; Goldberg, D.M. Resveratrol: A Molecule Whose Time Has Come? And Gone? Clin. Biochem. 1997, 30, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Frountzas, M.; Karanikki, E.; Toutouza, O.; Sotirakis, D.; Schizas, D.; Theofilis, P.; Tousoulis, D.; Toutouzas, K.G. Exploring the Impact of Cyanidin-3-Glucoside on Inflammatory Bowel Diseases: Investigating New Mechanisms for Emerging Interventions. Int. J. Mol. Sci. 2023, 24, 9399. [Google Scholar] [CrossRef]
- Cheng, Z.; Si, X.; Tan, H.; Zang, Z.; Tian, J.; Shu, C.; Sun, X.; Li, Z.; Jiang, Q.; Meng, X.; et al. Cyanidin-3-O-Glucoside and Its Phenolic Metabolites Ameliorate Intestinal Diseases via Modulating Intestinal Mucosal Immune System: Potential Mechanisms and Therapeutic Strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 1629–1647. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, M.H.; Saravanan, T.S.; Monteclaro, C.C.; Presser, N.; Ye, X.; Selvan, S.R.; Brosman, S. Epicatechins Purified from Green Tea (Camellia sinensis) Differentially Suppress Growth of Gender-Dependent Human Cancer Cell Lines. Evid. Based Complement. Altern. Med. 2006, 3, 237–247. [Google Scholar] [CrossRef]
- Shimoyama, A.T.; Santin, J.R.; Machado, I.D.; de Oliveira e Silva, A.M.; de Melo, I.L.P.; Mancini-Filho, J.; Farsky, S.H.P. Antiulcerogenic Activity of Chlorogenic Acid in Different Models of Gastric Ulcer. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Patel, K.R.; Andreadi, C.; Britton, R.G.; Horner-Glister, E.; Karmokar, A.; Sale, S.; Brown, V.A.; Brenner, D.E.; Singh, R.; Steward, W.P.; et al. Sulfate Metabolites Provide an Intracellular Pool for Resveratrol Generation and Induce Autophagy with Senescence. Sci. Transl. Med. 2013, 5, 205ra133. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, J.; Park, E.J.; Kondratyuk, T.P.; Marler, L.; Pezzuto, J.M.; Van Breemen, R.B.; Mo, S.; Li, Y.; Cushman, M. Selective Synthesis and Biological Evaluation of Sulfate-Conjugated Resveratrol Metabolites. J. Med. Chem. 2010, 53, 5033–5043. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite Profiling of Hydroxycinnamate Derivatives in Plasma and Urine after the Ingestion of Coffee by Humans: Identification of Biomarkers of Coffee Consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef]
- Williamson, G. Bioavailability of Food Polyphenols: Current State of Knowledge. Annu. Rev. Food Sci. Technol. 2025, 16, 315–332. [Google Scholar] [CrossRef]
- Rahman, S.; Mathew, S.; Nair, P.; Ramadan, W.S.; Vazhappilly, C.G. Health Benefits of Cyanidin-3-Glucoside as a Potent Modulator of Nrf2-Mediated Oxidative Stress. Inflammopharmacology 2021, 29, 907–923. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, Y.; Zhao, L.; Lu, F.; Wang, O.; Yang, X.; Ji, B.; Zhou, F. Cyanidin-3-Glucoside and Its Phenolic Acid Metabolites Attenuate Visible Light-Induced Retinal Degeneration in Vivo via Activation of Nrf2/HO-1 Pathway and NF-ΚB Suppression. Mol. Nutr. Food Res. 2016, 60, 1564–1577. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Osakabe, N.; Yasuda, A.; Natsume, M.; Takizawa, T.; Nakamura, T.; Terao, J. Bioavailability of (-)-Epicatechin upon Intake of Chocolate and Cocoa in Human Volunteers. Free Radic. Res. 2000, 33, 635–641. [Google Scholar] [CrossRef]
- Blount, J.W.; Ferruzzi, M.; Raftery, D.; Pasinetti, G.M.; Dixon, R.A. Enzymatic Synthesis of Substituted Epicatechins for Bioactivity Studies in Neurological Disorders. Biochem. Biophys. Res. Commun. 2012, 417, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Actis-Goretta, L.; Lévèques, A.; Giuffrida, F.; Romanov-Michailidis, F.; Viton, F.; Barron, D.; Duenas-Paton, M.; Gonzalez-Manzano, S.; Santos-Buelga, C.; Williamson, G.; et al. Elucidation of (-)-Epicatechin Metabolites after Ingestion of Chocolate by Healthy Humans. Free Radic. Biol. Med. 2012, 53, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Vesely, O.; Baldovska, S.; Kolesarova, A. Enhancing Bioavailability of Nutraceutically Used Resveratrol and Other Stilbenoids. Nutrients 2021, 13, 3095. [Google Scholar] [CrossRef]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef]
- Latifkar, A.; Ling, L.; Hingorani, A.; Johansen, E.; Clement, A.; Zhang, X.; Hartman, J.; Fischbach, C.; Lin, H.; Cerione, R.A.; et al. Loss of Sirtuin 1 Alters the Secretome of Breast Cancer Cells by Impairing Lysosomal Integrity. Dev. Cell 2019, 49, 393. [Google Scholar] [CrossRef]
- Jang, K.Y.; Kim, K.S.; Hwang, S.H.; Kwon, K.S.; Kim, K.R.; Park, H.S.; Park, B.H.; Chung, M.J.; Kang, M.J.; Lee, D.G.; et al. Expression and Prognostic Significance of SIRT1 in Ovarian Epithelial Tumours. Pathology 2009, 41, 366–371. [Google Scholar] [CrossRef]
- Di Sante, G.; Pestell, T.G.; Casimiro, M.C.; Bisetto, S.; Powell, M.J.; Lisanti, M.P.; Cordon-Cardo, C.; Castillo-Martin, M.; Bonal, D.M.; Debattisti, V.; et al. Loss of Sirt1 Promotes Prostatic Intraepithelial Neoplasia, Reduces Mitophagy, and Delays Park2 Translocation to Mitochondria. Am. J. Pathol. 2015, 185, 266–279. [Google Scholar] [CrossRef]
- Chanaday, N.L.; Nosyreva, E.; Shin, O.-H.; Zhang, H.; Aklan, I.; Atasoy, D.; Bezprozvanny, I.; Kavalali, E.T. Presynaptic Store-Operated Ca2+ Entry Drives Excitatory Spontaneous Neurotransmission and Augments Endoplasmic Reticulum Stress. Neuron 2021, 109, 1314–1332.e5. [Google Scholar] [CrossRef]
- Hecht, J.T.; Coustry, F.; Veerisetty, A.C.; Hossain, M.G.; Posey, K.L. Resveratrol Reduces COMPopathy in Mice Through Activation of Autophagy. JBMR Plus 2021, 5, e10456. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Ramírez, N.; Alquisiras-Burgos, I.; Ortiz-Plata, A.; Ruiz-Tachiquín, M.E.; Espinoza-Rojo, M.; Aguilera, P. Resveratrol Activates Neuronal Autophagy Through AMPK in the Ischemic Brain. Mol. Neurobiol. 2020, 57, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhu, C.; Wang, W.; Li, M.; Ma, C.; Gao, B. SIRT1 Is a Regulator of Autophagy: Implications for the Progression and Treatment of Myocardial Ischemia-Reperfusion. Pharmacol. Res. 2024, 199, 106957. [Google Scholar] [CrossRef] [PubMed]
| Author(s) | Year | Experimental Model | Altered Expression or Activity of ER Stress Markers | Dosage |
|---|---|---|---|---|
| Chinta et al. [65] | 2009 | In vitro (dopaminergic N27 cells) | ↑cleavage of caspases 7 and 3 ↑GRP78 expression ↑GRP94 expression ↑CHOP expression ↑p-eIF2α expression | 50–250 μM |
| Wang et al. [66] | 2011 | In vitro (multiple myeloma cell lines ANBL-6, OPM2, MM.15) | ↑JNK phosphorylation ↑CHOP expression ↑XBP1 mRNA splicing ↓transcription of XBP1s | 100 μM |
| Li et al. [54] | 2011 | In vitro (tunicamycin-induced ER stress in HepG2 cells) | ↓XBP1 mRNA splicing ↓GRP78 expression ↓CHOP expression | 10 μM |
| Rojas et al. [67] | 2014 | In vitro (palmitate-induced ER stress in HepG2 cells) | ↑XBP1 mRNA splicing ↑CHOP expression | 100 μM |
| Zhang et al. [55] | 2015 | In vitro (cigarette smoke extract-induced apoptosis in cultured human bronchial epithelial cells) | ↓CHOP expression ↓caspases 3 and 4 expression | 20 µmol/L |
| Pan et al. [73] | 2015 | In vivo (high-fat diet-fed rats) | ↓ATF4 expression ↓GRP78 expression ↓CHOP expression ↓GRP78 expression ↓p-PERK expression | 100 mg/kg |
| Graham et al. [74] | 2016 | In vitro (2-deoxy-D-glucose inhibition of glycolysis in neuroblastoma cells) | ↑CHOP expression ↓GRP78 expression ↓GRP94 expression | 10 μM |
| Lin et al. [56] | 2016 | In vitro (neonatal rat cardiomyocytes) | ↓GRP78 expression ↓GRP94 expression ↓CHOP expression | 50 μM |
| Cheng et al. [57] | 2016 | In vitro (tunicamycin and Aβ25-35 induced ER stress in SH-SYSY cells) | ↓GRP78 expression ↓CHOP expression ↓p-eIF2α expression | 25 μM |
| Selvaraj et al. [68] | 2016 | In vitro (PC3 and DU145 prostate cancer cell lines) | ↑CHOP expression | 100 μM |
| Ding et al. [75] | 2017 | In vivo (high-fat diet-fed rats) | ↓GRP78 expression ↓CHOP expression | 200 mg/kg |
| Yan et al. [58] | 2018 | In vitro (tunicamycin-induced ER stress in neuronal HT22 cells) | ↓GRP78 expression ↓CHOP expression ↓caspase 12 expression | 50 μM |
| Heo et al. [69] | 2018 | In vitro (A375SM melanoma cells) | ↑p-eIF2α expression ↑CHOP expression | 10 μM |
| Ardid-Ruiz et al. [76] | 2018 | In vivo (diet induced obesity in rats) | ↓XBP1s expression | 200 mg/kg |
| Wang et al. [72] | 2018 | In vivo (surgical mice model) | ↓GRP78 expression ↓XBP1 expression ↓PERK expression ↓IRE1 expression | 100 mg/kg |
| Lee et al. [59] | 2019 | In vitro (tunicamycin-induced ER stress in HepG2 cells) | ↓PERK expression ↓IRE1 expression ↓CHOP expression ↑ERAD factors expression | 10, 50, and 100 μM |
| Zhao et al. [60] | 2019 | In vivo (high-fat diet-fed mice) In vitro (palmitic acid-induced insulin-resistant HepG2 cells) | in vivo model—↓p-PERK expression and ↓ATF4 expression in vitro model—↑p-PERK expression, ↑ATF4 expression, ↓ATF6 expression (at 50 and 100 μM) and ↓p-PERK expression, ↓ATF4 expression, ↑ATF6 expression (at 20 μM) | 60 mg/kg (in vivo model) 20, 50, and 100 μM (in vitro model) |
| Lu et al. [77] | 2019 | In vitro (fibroblast-like synoviocytes treated with H2O2) | ↑CHOP expression ↑caspase 12 and caspase 3 expression | 50, 100, 200, and 400 μM |
| Pan et al. [71] | 2019 | In vivo (induced vasculitic peripheral neuropathy by ischemia–reperfusion in rats) | ↓p-PERK expression ↓p-IRE1 expression ↓ATF6 expression | 20 and 40 mg/kg |
| Zhang et al. [61] | 2020 | In vivo (db/db mice) In vitro (high glucose induced ER stress in NRK-52E cells) | ↓GRP78 expression ↓CHOP expression ↓caspase 12 expression | 20 μM (in vitro model) 40 mg/kg (in vivo model) |
| Ren et al. [70] | 2020 | In vitro (AGS stomach cancer cell line) | ↑GRP78 expression ↑p-eIF2α expression ↑CHOP expression | 20 μM |
| Neal et al. [62] | 2020 | In vitro (retinal pigment cells treated with hydroquinone) | ↑XBP1 expression ↑CHOP expression | 15 and 30 μM |
| Arena et al. [78] | 2021 | In vitro (Her-2 positive breast cancer and salivary gland cancer cell lines) | ↑CHOP expression | 15 μM |
| Hecht et al. [79] | 2021 | In vivo (model of primary osteoarthritis in mice) | ↓CHOP expression | 0.25 g/L |
| Yu et al. [63] | 2022 | In vitro (tunicamycin-induced ER stress in chondrocytes) | ↓CHOP expression | 50 μM |
| Totonchi et al. [80] | 2022 | In vivo (mice liver-induced ischemia-reperfusion) | ↓GRP78 expression ↓PERK expression ↓IRE1α expression ↓CHOP expression ↓XBP1 expression | 0.02 and 0.2 mg/kg |
| Luo et al. [64] | 2023 | In vitro (bupivacaine-induced cytotoxicity in PC12 rat adrenal pheochromocytoma cells) | ↓p-PERK expression ↓p-eIF2α expression ↓ATF4 expression | 20 µM |
| Author(s) | Year | Experimental Model | ER Stress Modulating Substance | Altered Expression or Activity of ER stress Markers | Dosage |
|---|---|---|---|---|---|
| Thummayot et al. [81] | 2016 | In vitro (Aβ 25-35 induced neuronal cell death in SK-N-SH cells) | Cyanidin-3-o-glucoside | ↓GRP78 expression ↓p-PERK expression ↓p-eIF2α expression ↓IRE1 expression ↓XBP1 expression ↓ATF6 expression ↓CHOP expression | 0.2; 2; 18; and 20 µM |
| Chen et al. [82] | 2022 | In vitro (treated with palmitate isolated mouse pancreatic islets and INS-1E cells) | Cyanidin-3-o-glucoside | ↓CHOP expression | 12,5; 25; and 50 µM |
| Tu et al. [83] | 2022 | In vivo (induced periodontitis in rats) | Cyanidin-3-o-glucoside | ↓CHOP expression ↓JNK and p-JNK expression | 3 or 9 mg/kg |
| Peng et al. [84] | 2022 | In vitro (blue light-irradiated retinal pigment epithelial cells) | Cyanidin-3-o-glucoside | ↓ATF4 expression ↓CHOP expression | 10 and 25 μM |
| Bettaieb et al. [85] | 2014 | In vivo (high-fructose diet-fed rats) | Epicatechin | ↓p-PERK expression ↓p-IRE1 expression ↓XBP1 splicing | 20 mg/kg |
| Kang et al. [86] | 2019 | In vitro (methamphetamine-induced neurotoxicity in HT22 hippocampal neuronal cells) | Epicatechin | ↓CHOP expression | 10 and 20 μM |
| Ye et al. [87] | 2016 | In vivo (streptozotocin-induced diabetic nephropathy in rats) | Chlorogenic acid | ↓CHOP expression ↓ATF6 expression ↓p-eIF2α expression ↓p-PERK expression | 5 mg/kg, 10 mg/kg, 20 mg/kg |
| Wang et al. [88] | 2017 | In vivo (bleomycin-induced pulmonary fibrosis in mice), in vitro (pulmonary fibroblasts and RLE-6TN cells) | Chlorogenic acid | ↓GRP78 expression ↓CHOP expression ↓p-PERK expression ↓ATF6 expression ↓caspases 9, 3, and 12 expression | 15 mg/kg, 30 mg/kg, 60 mg/kg |
| Zhang et al. [89] | 2018 | In vitro (thapsigargin and palmitic acid-induced ER stress in rat hepatocytes) | Chlorogenic acid | ↓GRP78 expression ↓GRP94 expression ↓CHOP expression | 5 μmol/L |
| Kazaz et al. [90] | 2022 | In vivo (torsion/detorsion-induced testicular injury in rats) | Chlorogenic acid | ↓GRP78 expression ↓ATF6 expression ↓CHOP expression | 100 mg/kg |
| Rani et al. [91] | 2022 | In vitro (model of hyperglycemia in H9c2 embryonic rat heart cells) | Chlorogenic acid | ↓p-PERK expression ↓p-eIF2α expression ↓ATF4 expression ↓p-IRE1 expression ↓TRAF2 expression ↓p-JNK expression ↓XBP1 expression ↓ATF6 expression | 10 and 30 μM |
| Sari et al. [92] | 2022 | In vivo (diabetic model in rats) | Chlorogenic acid | ↓GRP78 expression ↓XBP1 expression | 12.5 mg/kg, 25 mg/kg, and 50 mg/kg |
| Moslehi et al. [93] | 2023 | In vivo (tunicamycin-induced ER stress in mice) | Chlorogenic acid | ↓GRP78 expression ↓PERK expression ↓IRE1 expression ↓caspase 3 expression | 20 and 50 mg/kg |
| Boonyong et al. [94] | 2023 | In vivo (indomethacin-induced gastrointestinal ulcer in rats) | Chlorogenic acid | ↓p-PERK expression ↓p-eIF2α expression ↓ATF-4 expression ↓CHOP expression | 100 mg/kg |
| Ping et al. [95] | 2024 | In vitro (isoproterenol stimulated H9c2 myocardial cells) In vivo (isoproterenol stimulated rats) | Chlorogenic acid | ↓GRP78 expression ↓p-PERK expression ↓CHOP expression ↓caspases 12, 3, and 9 expression | 90 mg/kg (in vivo model) 50 μM (in vitro model) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanov, S.; Barbolov, M.; Yaneva, G.; Tasinov, O. Modulation of Endoplasmic Reticulum Stress by Selected Polyphenols from Sambucus ebulus L. Fruit. Plants 2025, 14, 2748. https://doi.org/10.3390/plants14172748
Stoyanov S, Barbolov M, Yaneva G, Tasinov O. Modulation of Endoplasmic Reticulum Stress by Selected Polyphenols from Sambucus ebulus L. Fruit. Plants. 2025; 14(17):2748. https://doi.org/10.3390/plants14172748
Chicago/Turabian StyleStoyanov, Stoyan, Momchil Barbolov, Galina Yaneva, and Oskan Tasinov. 2025. "Modulation of Endoplasmic Reticulum Stress by Selected Polyphenols from Sambucus ebulus L. Fruit" Plants 14, no. 17: 2748. https://doi.org/10.3390/plants14172748
APA StyleStoyanov, S., Barbolov, M., Yaneva, G., & Tasinov, O. (2025). Modulation of Endoplasmic Reticulum Stress by Selected Polyphenols from Sambucus ebulus L. Fruit. Plants, 14(17), 2748. https://doi.org/10.3390/plants14172748










