Biochar Enhances Nutrient Uptake, Yield, and NHX Gene Expression in Chinese Cabbage Under Salinity Stress
Abstract
1. Introduction
2. Results
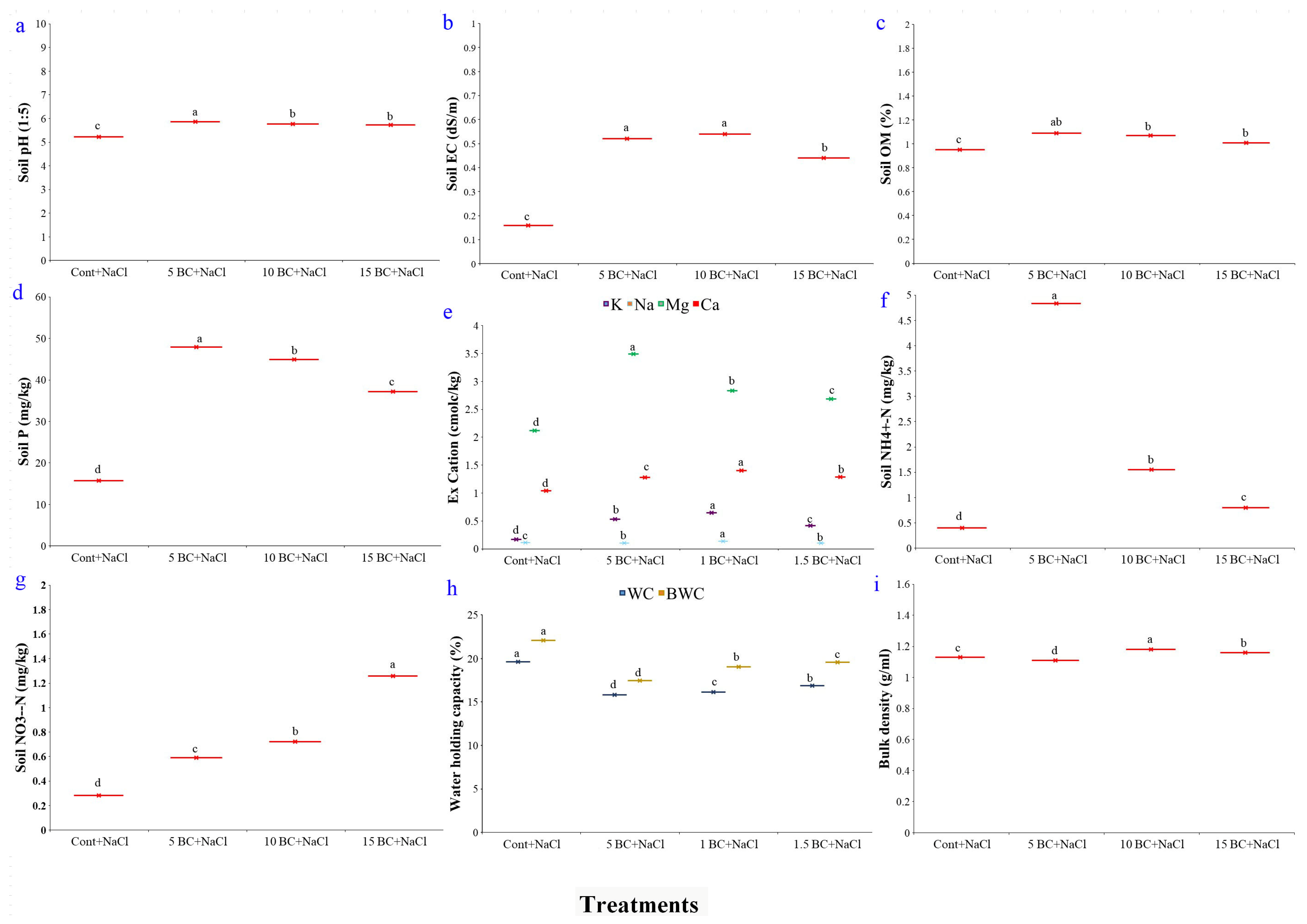
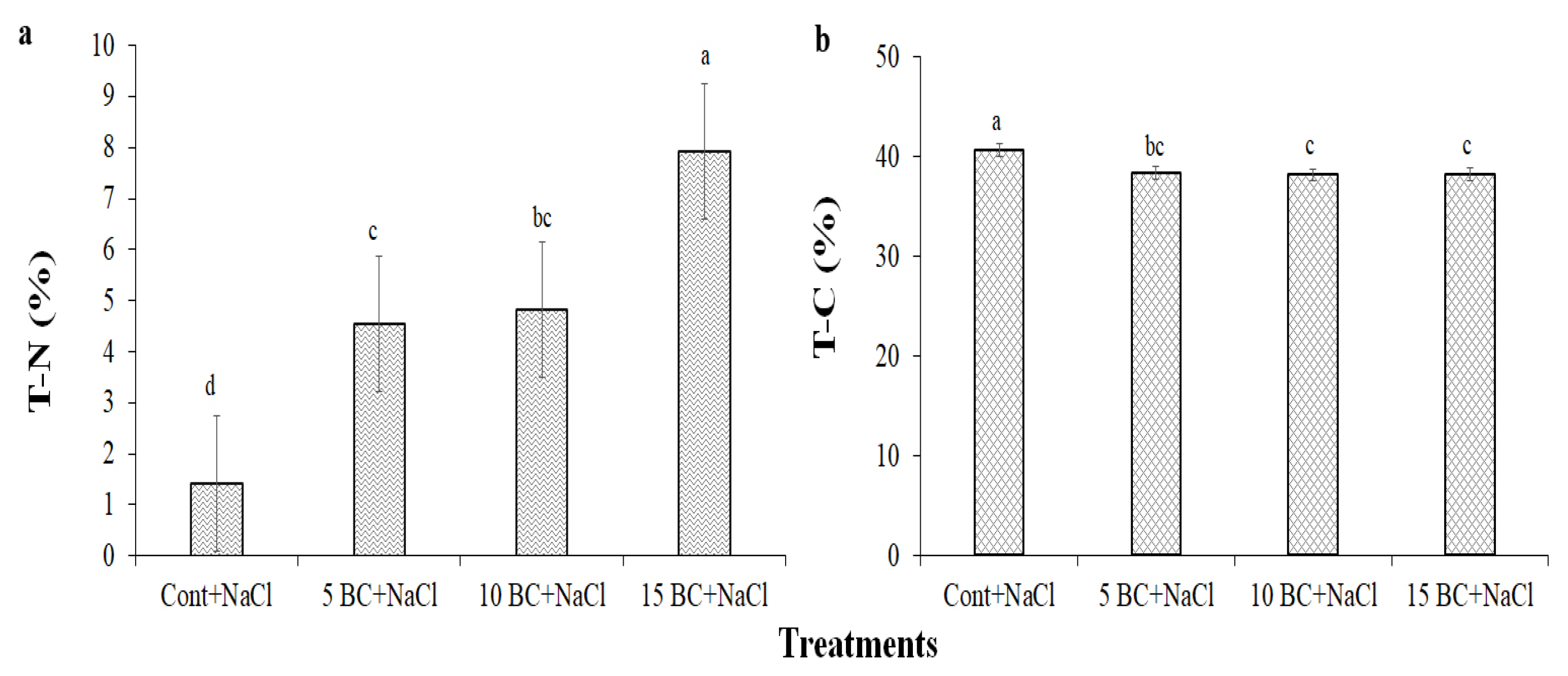
2.1. Effects of BC on Soil Parameters Under Salinity Stress
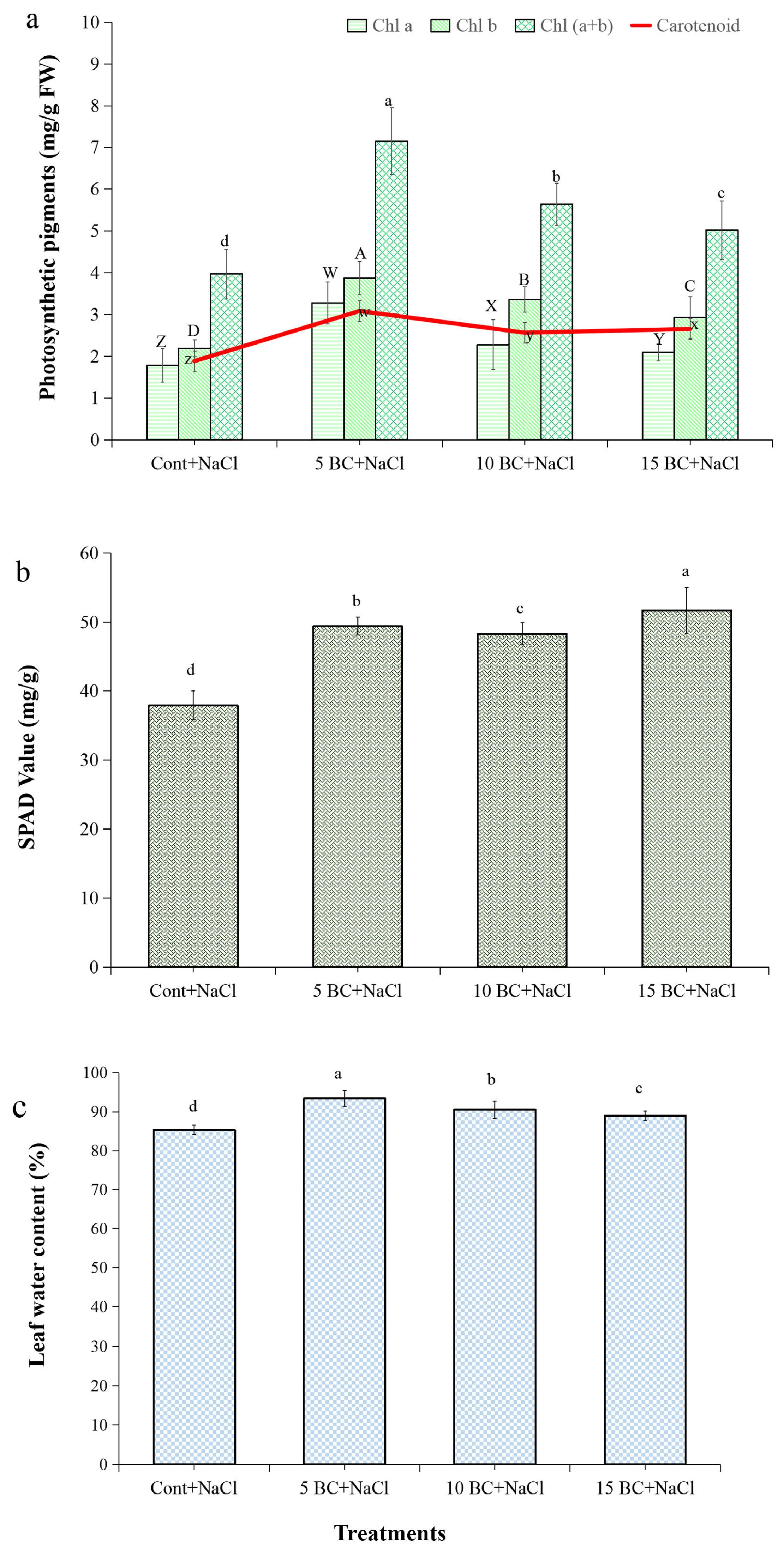
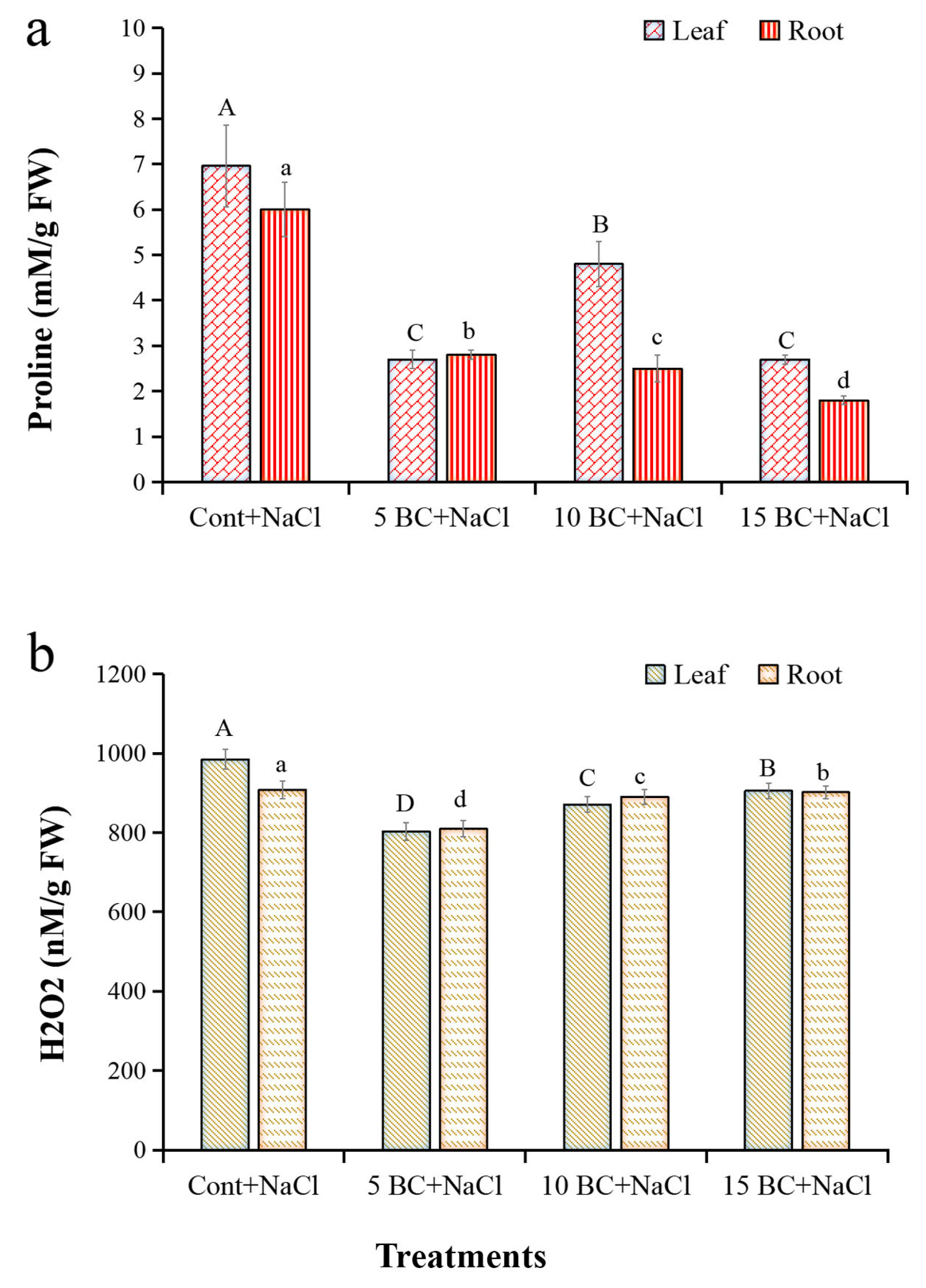
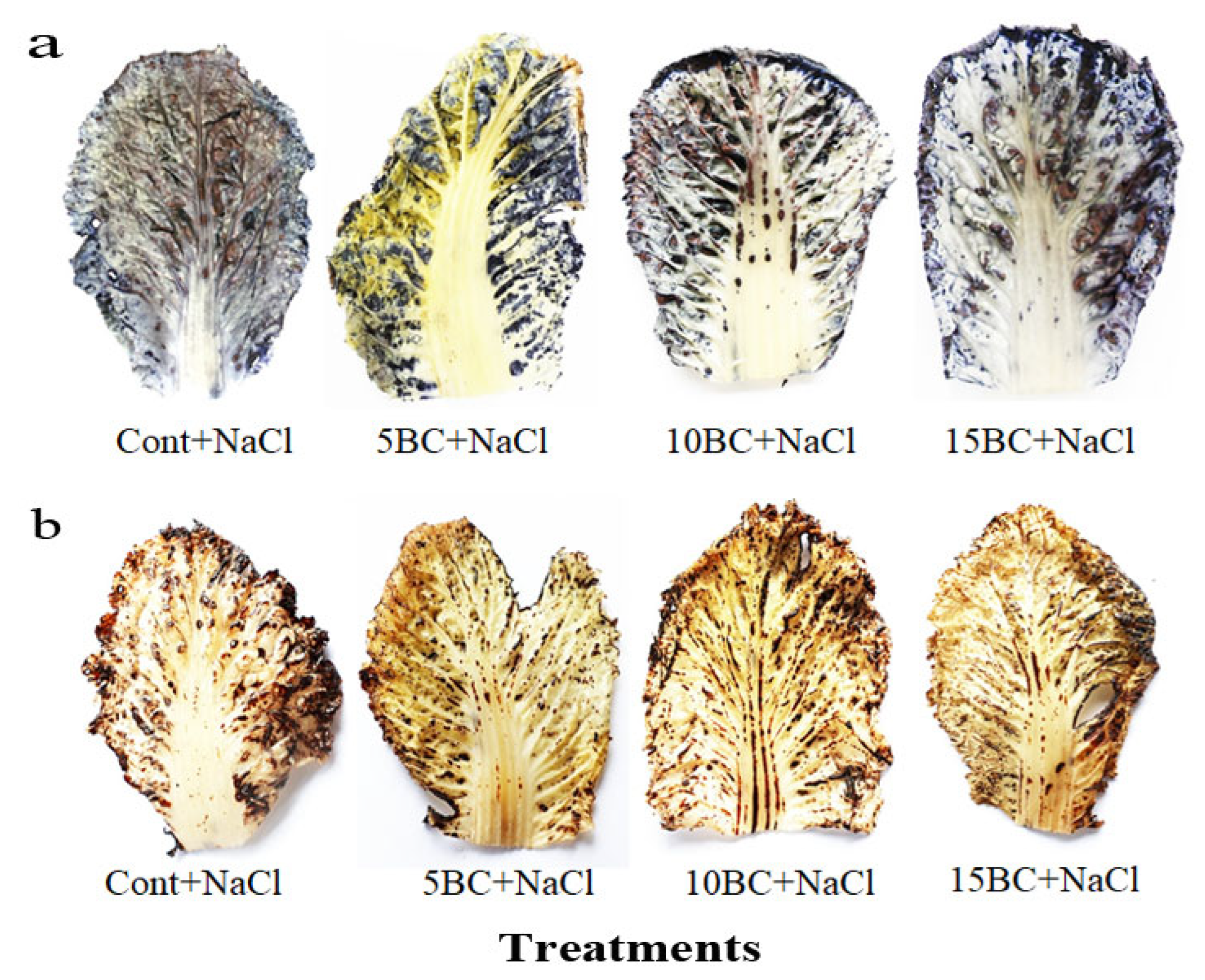
2.2. Effects of BC on Biochemical Traits of Chinese Cabbage Under Salinity Stress
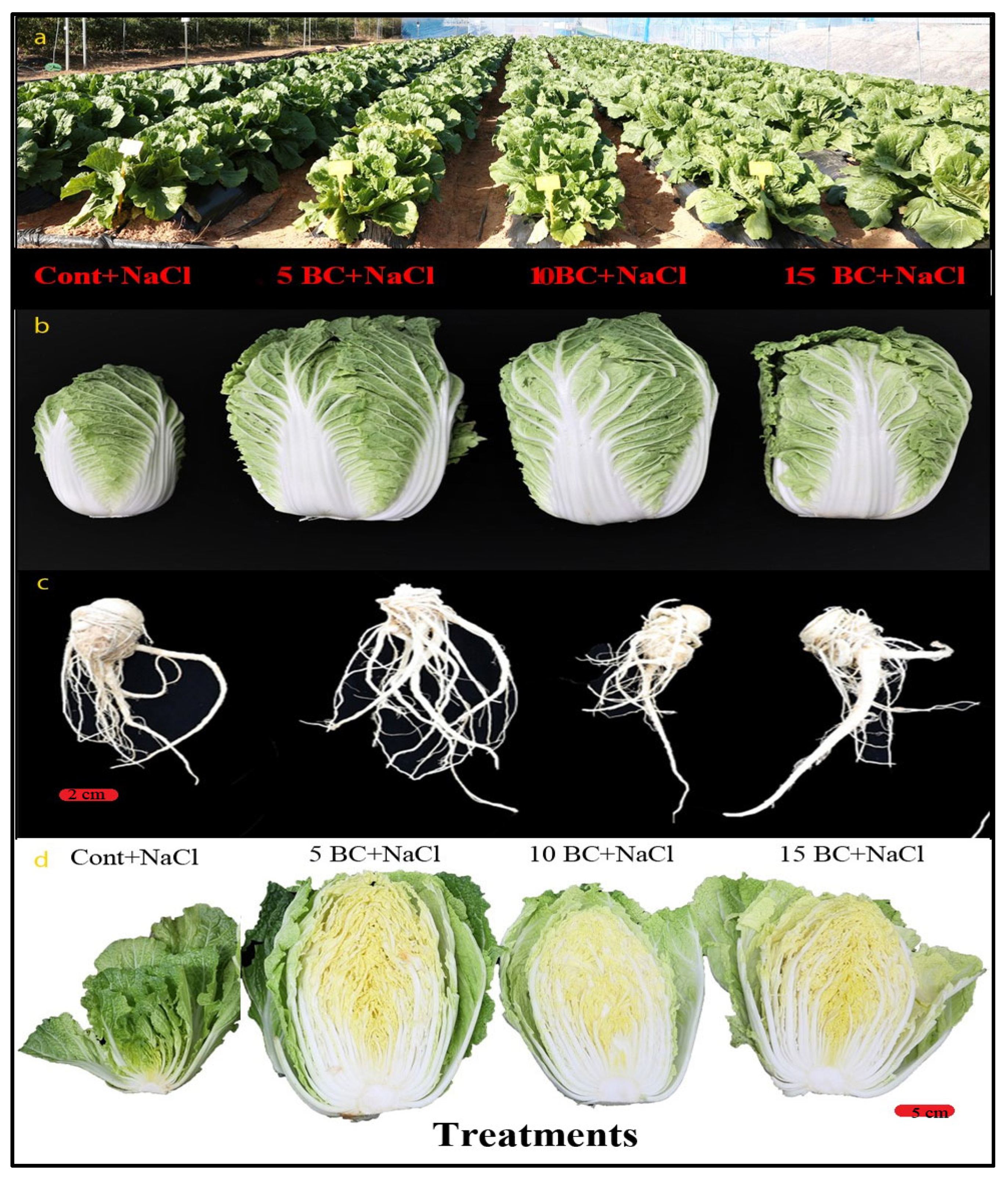
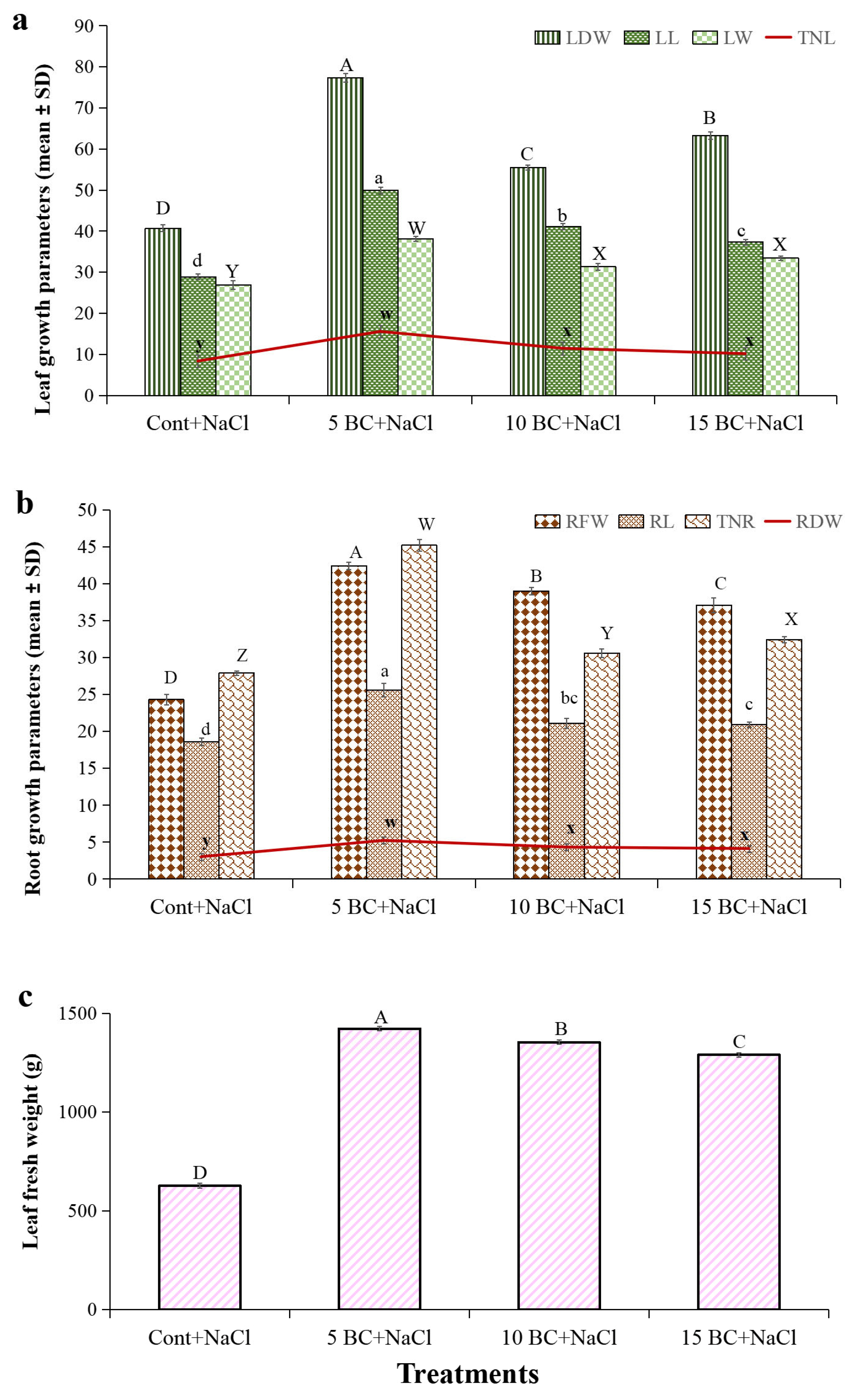
2.3. Effects of BC on Chinese Cabbage Phenotypic Traits
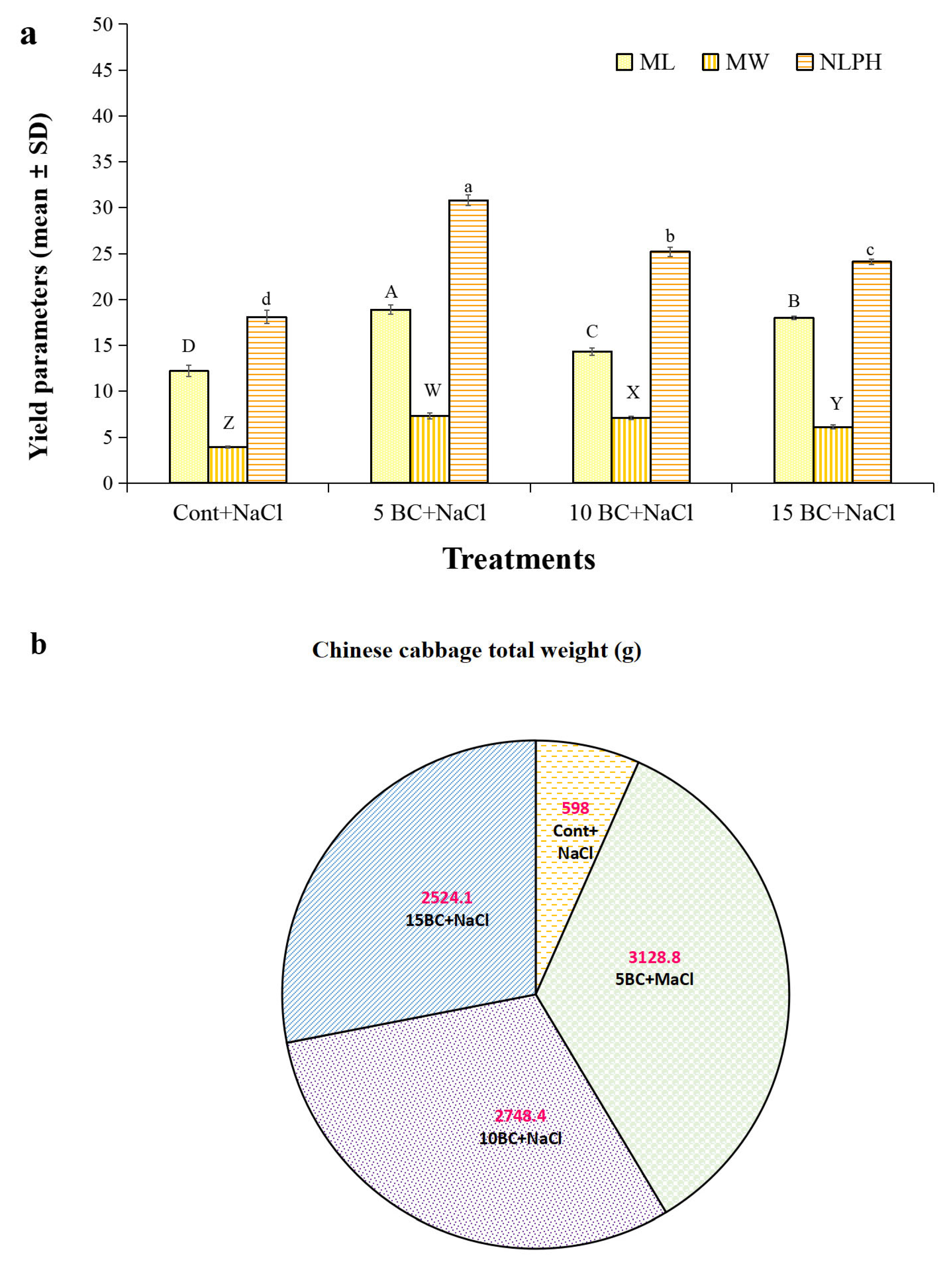
2.4. Influence of BC upon Salinity Stress in Yield Parameters of Chinese Cabbage
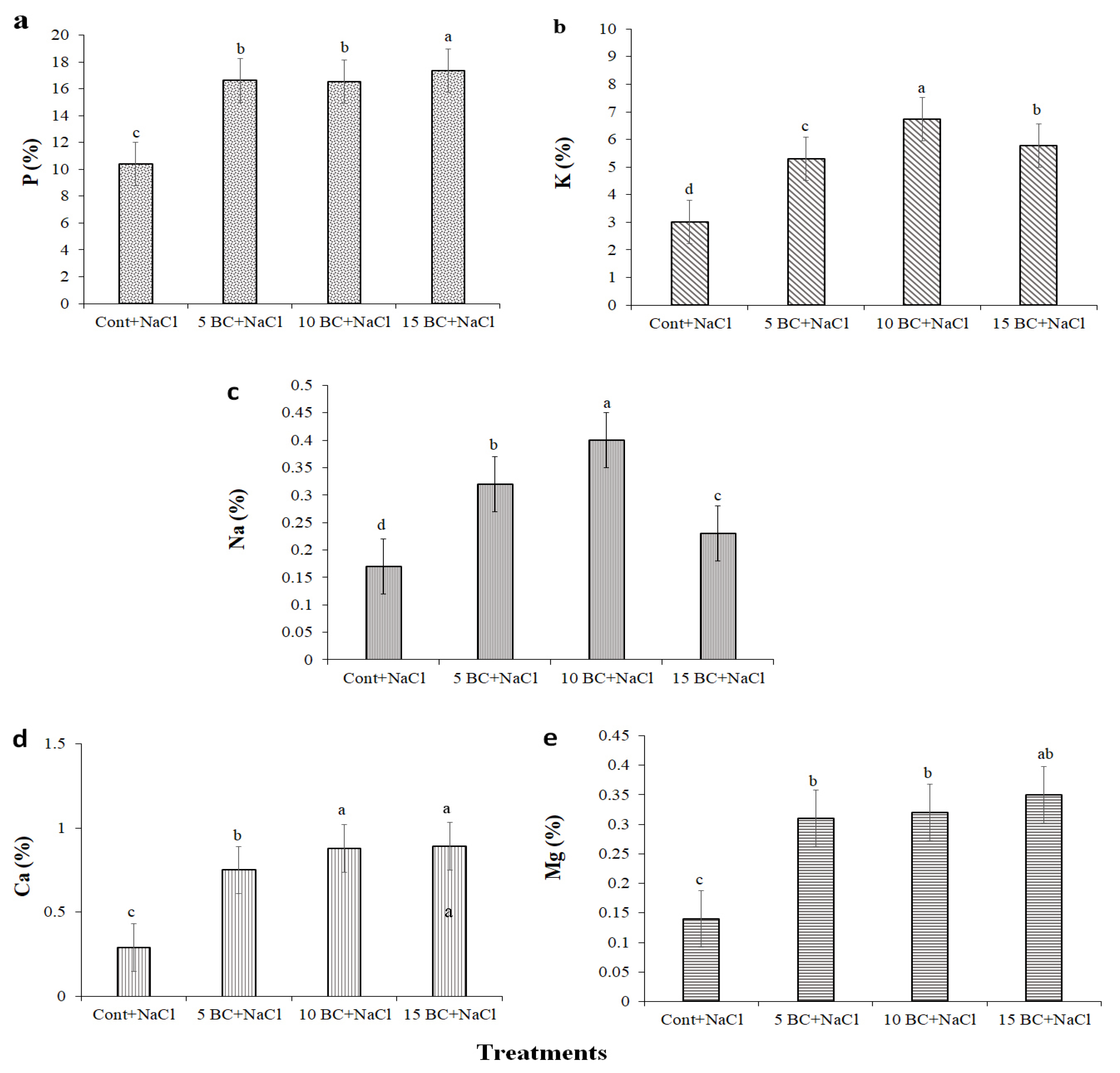
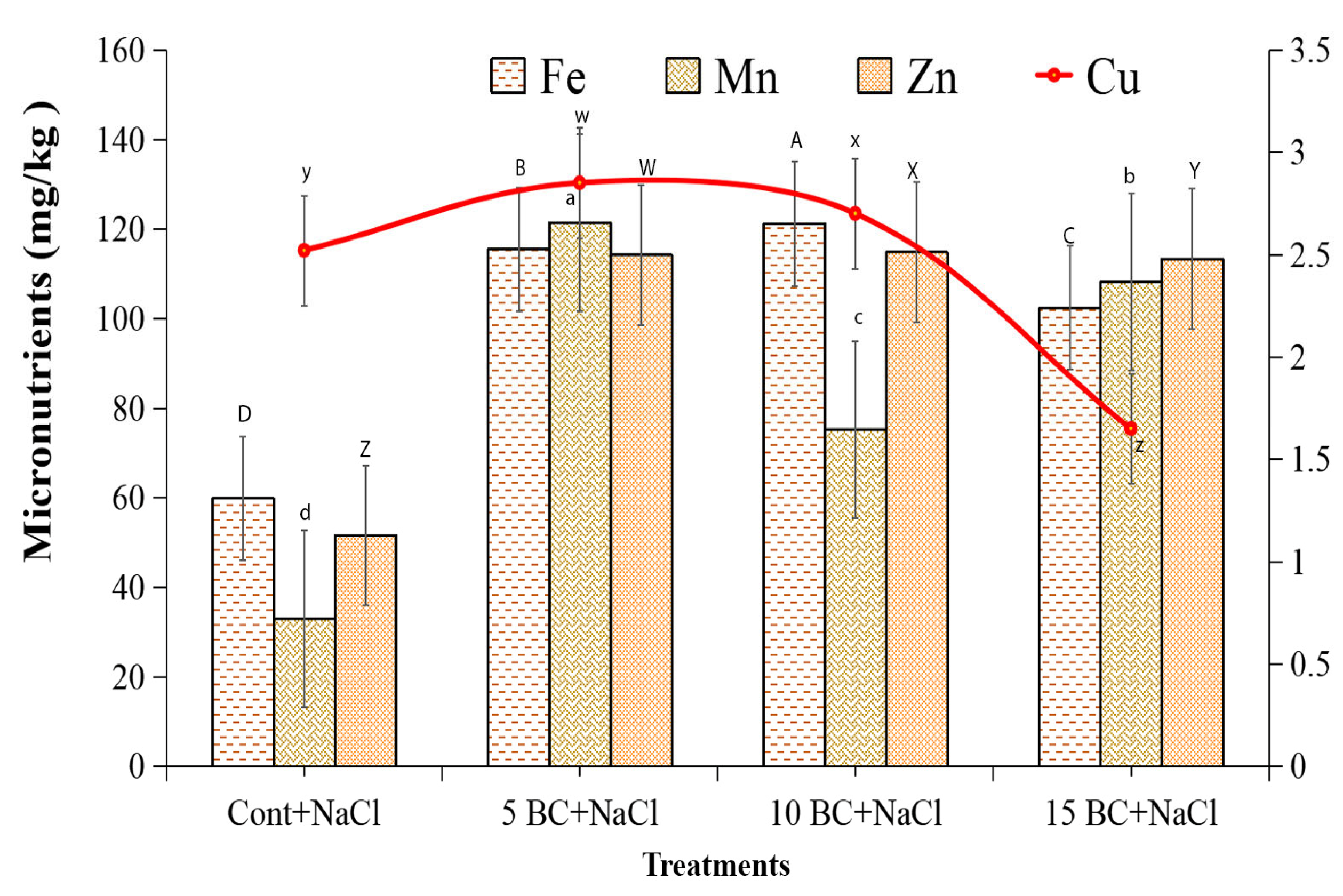
2.5. Analysis of Essential Macro- and Micronutrient Contents of Chinese Cabbage Grown Under Salinity Stress with a Supply of BC
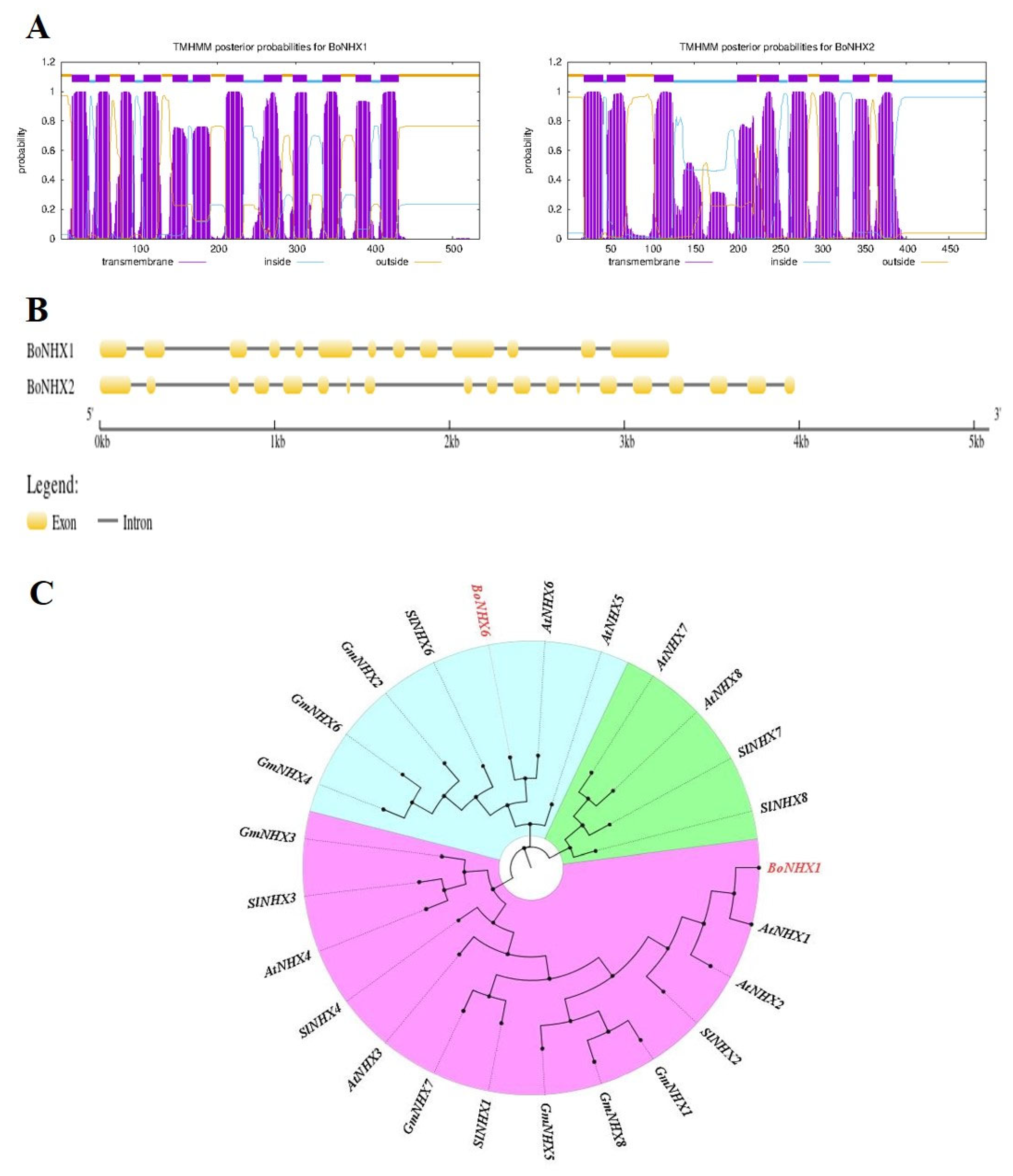
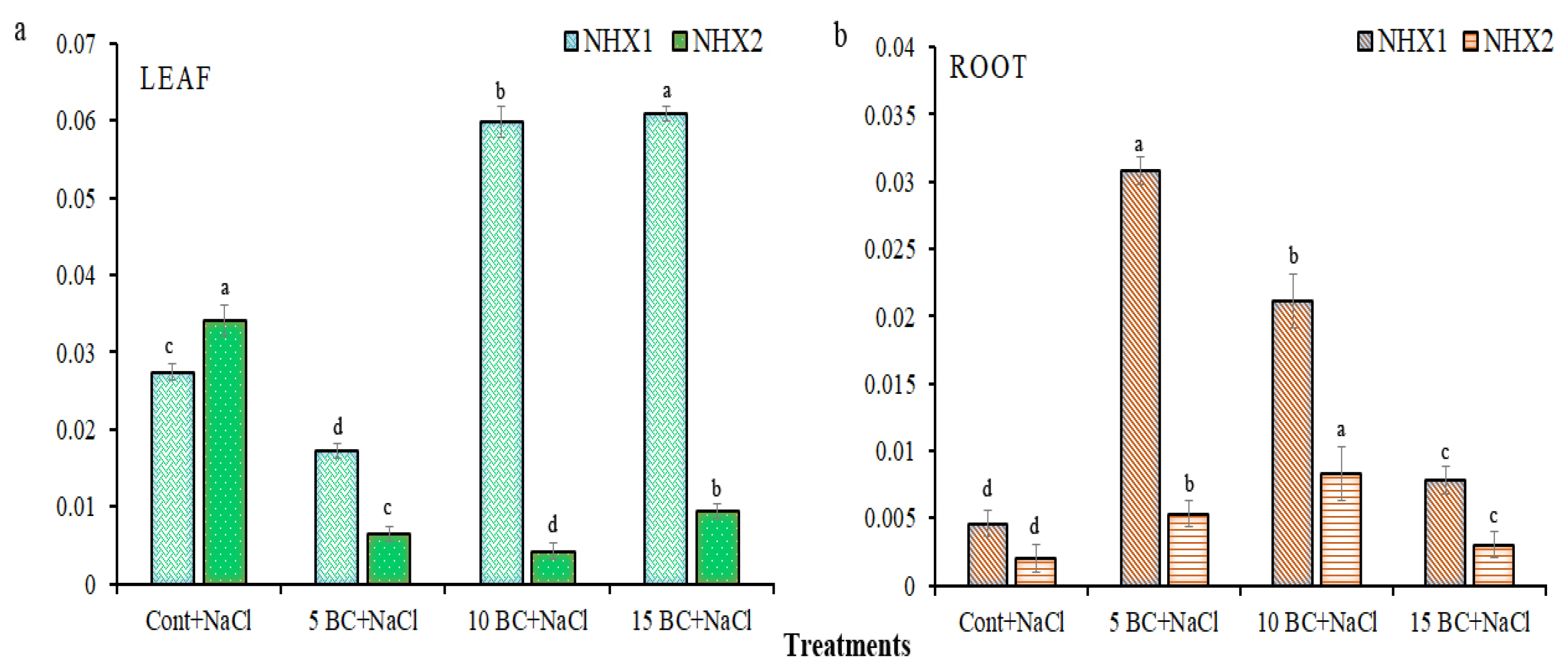
2.6. Expression Pattern of NHX Family Genes in Leaf and Root Tissues of Chinese Cabbage Grown Under Salinity Stress with Different Combinations of BC
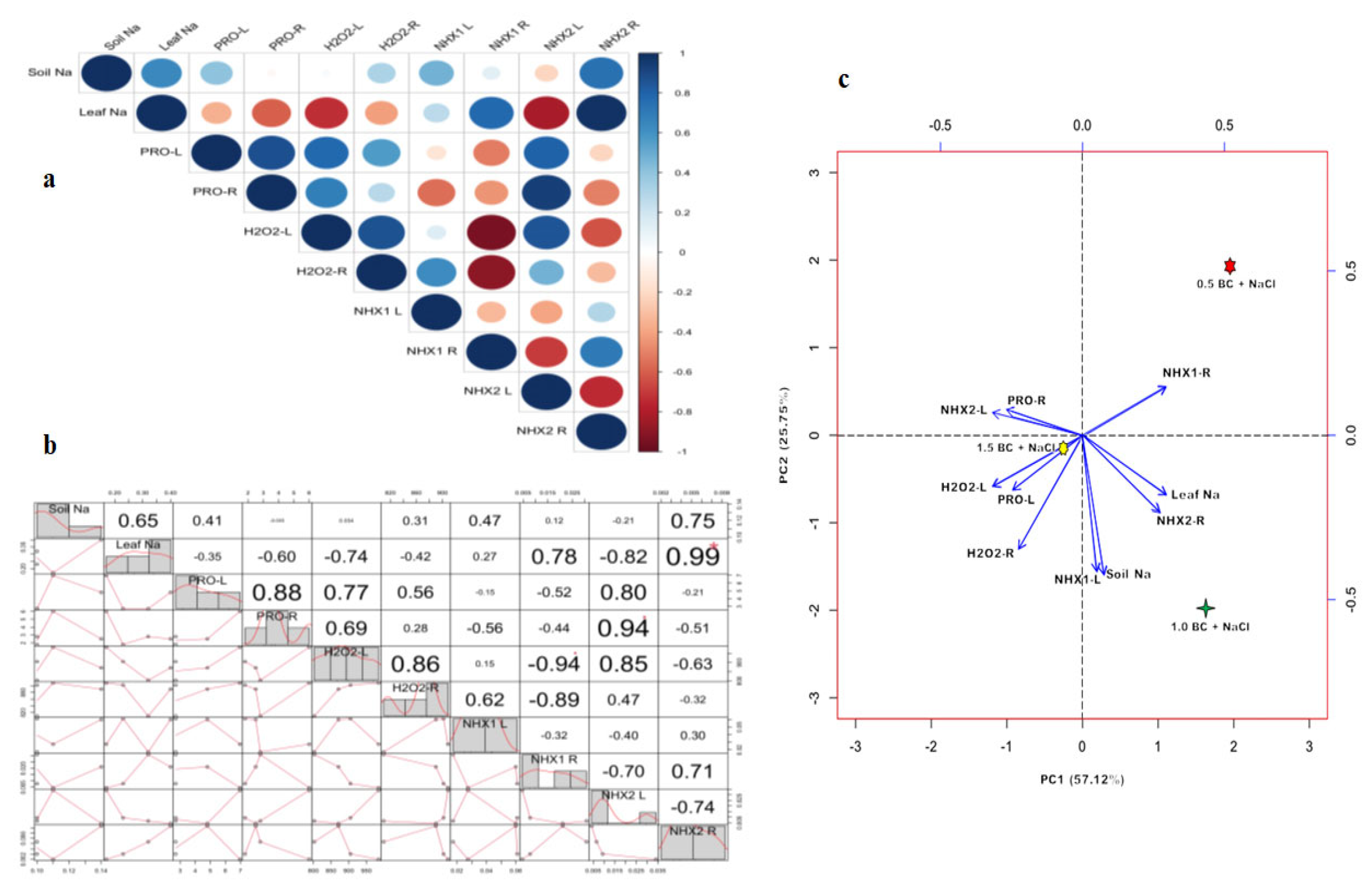
2.7. Principal Component Analysis (PCA) and Correlation Analysis Between Key Parameters
3. Discussion
4. Materials and Methods
4.1. Experimental Conditions
4.2. Effects of BC on Soil Traits
4.2.1. pH and Electrical Conductivity (EC)
4.2.2. Organic Matter (OM)
4.2.3. Exchangeable Cations
4.2.4. Nitrogen (N) and Phosphorus (P) Content
4.2.5. Water Holding Capacity
4.3. Effects of BC on Biochemical Traits of Chinese Cabbage
4.3.1. Photosynthetic Pigment Analysis
4.3.2. Leaf Water Content (LWC)
4.3.3. Proline and Hydrogen Peroxide (H2O2) Analysis
4.4. In Situ Analysis of Superoxide Anion (O2−) and H2O2
4.5. Effects of BC on Phenotypic Traits of Chinese Cabbage
4.6. Analysis of Essential Macro- and Micronutrient Contents in Leaf Tissue of Chinese Cabbage
4.7. Molecular Studies
4.7.1. Collection of Nucleotide and Protein Sequences of NHX Family Genes for Chinese Cabbage
4.7.2. Analysis of Protein Features of BoNHX and Gene Structure of BoNHX
4.7.3. RNA Isolation and cDNA Synthesis
4.7.4. Quantitative Real-Time RT-PCR (qRT-PCR)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAB | 3,3-diaminobenzidine |
| BC | biochar |
| BD | bulk density |
| BWC | bulk water content |
| Ca | calcium |
| C | carbon |
| CAR | carotenoid |
| CHL | chlorophyll |
| EC | electrical conductivity |
| H2O2 | hydrogen peroxide |
| Fe | iron |
| LL | leaf length |
| LWC | leaf water content |
| LDW | leaves dry weight |
| LFW | leaves fresh weight |
| Mg | magnesium |
| Mn | manganese |
| ML | midrib length |
| MW | midrib width |
| N | nitrogen |
| NBT | nitrotetrazolium blue chloride |
| NLPH | number of leaves per head |
| OM | organic matter |
| P | phosphorus |
| K | potassium |
| PCA | principal component analysis |
| PRO-L | leaf proline |
| PRO-R | root proline |
| ROS | reactive oxygen species |
| RT | room temperature |
| RL | root length |
| NaCl | sodium chloride |
| Na+ | sodium ions |
| O2− | superoxide anion |
| T-C | total carbon |
| T-N | total nitrogen |
| TNL | total number of leaves |
| TNR | total number of roots per plant |
| TW | total weight |
| WC | water content |
| Zn | zinc |
References
- United States Department of Agriculture (USDA). Food Data Central: Chinese Cabbage, Raw. 2023. Available online: https://fdc.nal.usda.gov/ (accessed on 4 November 2024).
- Stefan, I.M.A.; Ona, A.D. Cabbage (Brassica oleracea L.): Overview of the Health Benefits and Therapeutical Uses. 2020, pp. 150–169. Available online: https://odontoanamaria.com/artigos/repolho01.pdf (accessed on 4 November 2024).
- Sarkar, D.; Rakshit, A.; Parewa, H.P.; Danish, S.; Alfarraj, S.; Datta, R. Bio-priming with compatible rhizospheric microbes enhances growth and micronutrient uptake of red cabbage. Land 2022, 11, 536. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Jang, Y.; Lee, S.; Lee, W.M.; Wi, S.; Yoon, H.I. Elucidating Genetic Mechanisms of Summer Stress Tolerance in Chinese Cabbage through GWAS and Phenotypic Analysis. Agronomy 2024, 14, 1960. [Google Scholar] [CrossRef]
- Hongu, N.; Kim, A.S.; Suzuki, A.; Wilson, H.; Tsui, K.C.; Park, S. Korean kimchi: Promoting healthy meals through cultural tradition. J. Ethn. Foods 2017, 4, 172–180. [Google Scholar] [CrossRef]
- Kim, S.; Rho, H.Y.; Kim, S. The Effects of Climate Change on Heading Type Chinese Cabbage (Brassica rapa L. ssp. Pekinensis) Economic Production in South Korea. Agronomy 2022, 12, 3172. [Google Scholar] [CrossRef]
- Kayum, M.A.; Kim, H.T.; Nath, U.K.; Park, J.I.; Kho, K.H.; Cho, Y.G.; Nou, I.S. Research on biotic and abiotic stress related genes exploration and prediction in Brassica rapa and B. oleracea: A review. Plant Breed. Biotechnol. 2016, 4, 135–144. [Google Scholar] [CrossRef]
- Jabeen, A.; Mir, J.I.; Malik, G.; Yasmeen, S.; Ganie, S.A.; Rasool, R.; Hakeem, K.R. Biotechnological interventions of improvement in cabbage (Brassica oleracea var. capitata L.). Sci. Hortic. 2024, 329, 112966. [Google Scholar] [CrossRef]
- Li, X.; Ayub, M.A.; Fox, J.P.; Shen, S.; Rossi, L. Nutrient uptake, growth, and physiology of Chinese cabbage (Brassica rapa L. ssp. pekinensis) varieties under NaCl stress. Soil Environ. 2024, 43, 1–13. [Google Scholar] [CrossRef]
- Sande, T.J.; Tindwa, H.J.; Alovisi, A.M.T.; Shitindi, M.J.; Semoka, J.M. Enhancing sustainable crop production through integrated nutrient management: A focus on vermicompost, bio-enriched rock phosphate, and inorganic fertilisers—A systematic review. Front. Agron. 2024, 6, 1422876. [Google Scholar] [CrossRef]
- Al-Shammary, A.A.G.; Al-Shihmani, L.S.S.; Fernández-Gálvez, J.; Caballero-Calvo, A. Optimizing sustainable agriculture: A comprehensive review of agronomic practices and their impacts on soil attributes. J. Environ. Manag. 2024, 364, 121487. [Google Scholar] [CrossRef]
- Yasmeen, A.R.; Maharajan, T.; Rameshkumar, R.; Sindhamani, S.; Banumathi, B.; Prabakaran, M.; Atchaya, S.; Rathinapriya, P. Role of Seaweeds for Improving Soil Fertility and Crop Development to Address Global Food Insecurity. Crops 2025, 5, 29. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Mishra, A.K.; Das, R.; George Kerry, R.; Biswal, B.; Sinha, T.; Sharma, S.; Kumar, M. Promising management strategies to improve crop sustainability and to amend soil salinity. Front. Environ. Sci. 2023, 10, 962581. [Google Scholar] [CrossRef]
- Okorogbona, A.O.M.; Managa, L.R.; Adebola, P.O.; Ngobeni, H.M.; Khosa, T.B. Salinity and crop productivity. Sustain. Agric. Rev. 2015, 17, 89–120. [Google Scholar] [CrossRef]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar] [CrossRef]
- Nielsen, S.; Joseph, S.; Ye, J.; Chia, C.; Munroe, P.; van Zwieten, L.; Thomas, T. Crop-season and residual effects of sequentially applied mineral enhanced biochar and N fertiliser on crop yield, soil chemistry and microbial communities. Agric. Ecosyst. Environ. 2018, 255, 52–61. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Gao, S.; DeLuca, T.H. Influence of biochar on soil nutrient transformations, nutrient leaching, and crop yield. Adv. Plants Agric. Res. 2016, 4, 348–362. [Google Scholar] [CrossRef]
- De Vasconcelos, A.C.F. Biochar effects on amelioration of adverse salinity effects in soils. In Applications of Biochar for Environmental Safety; IntechOpen: London, UK, 2020; p. 12. [Google Scholar]
- Xiao, Q.; Zhu, L.X.; Zhang, H.P.; Li, X.Y.; Shen, Y.F.; Li, S.Q. Soil amendment with biochar increases maize yields in a semi-arid region by improving soil quality and root growth. Crop Pasture Sci. 2016, 67, 495–507. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Olayanju, A.; Ejue, W.S.; Adekanye, T.A.; Adenusi, T.T.; Ayeni, J.F. Effect of biochar on soil properties, soil loss, and cocoyam yield on a tropical sandy loam Alfisol. Sci. World J. 2020, 2020, 9391630. [Google Scholar] [CrossRef]
- Kumari, K.; Khalid, Z.; Alam, S.N.; Sweta; Singh, B.; Guldhe, A.; Shahi, D.K.; Bauddh, K. Biochar amendment in agricultural soil for mitigation of abiotic stress. In Ecological and Practical Applications for Sustainable Agriculture; Springer Singapore: Singapore, 2020; pp. 305–344. [Google Scholar] [CrossRef]
- Karimi, A.; Moezzi, A.; Chorom, M.; Enayatizamir, N. Application of biochar changed the status of nutrients and biological activity in a calcareous soil. J. Soil Sci. Plant Nutr. 2020, 20, 450–459. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of Northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Alkharabsheh, H.M.; Seleiman, M.F.; Shami, A.; Al-Gheraibah, H.M.; Ammar, K.A.; Alkahtani, J.; Battaglia, M.L. Biochar and its impact on soil fertility, nutrient leaching, and crop productivity: A review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar mitigates salinity stress in potato. J. Agron. Crop Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Biochar improved nodulation and nitrogen metabolism of soybean under salt stress. Symbiosis 2018, 74, 215–223. [Google Scholar] [CrossRef]
- She, D.; Sun, Y.; Liang, Z.; Jiang, S.; Zhang, L. Biochar increased photosynthesis, transpiration rate, yield, and fruit number in tomato under salinity stress. J. Plant Growth Regul. 2018, 37, 591–601. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abdel-Fattah, M.; Taha, M. Effects of biochar on plant growth and yield under salinity stress in sorghum. Environ. Exp. Bot. 2020, 176, 104087. [Google Scholar] [CrossRef]
- Nikpour-Rashidabad, R.; Baghizadeh, A.; Gholamin, R. The effect of biochar on physiological and anatomical characteristics of mung bean roots under salt stress. Arch. Biol. Sci. 2019, 71, 321–327. [Google Scholar] [CrossRef]
- Soothar, M.K.; Mounkaila Hamani, A.K.; Kumar Sootahar, M.; Sun, J.; Yang, G.; Bhatti, S.M.; Traore, A. Assessment of Acidic Biochar on the Growth, Physiology and Nutrients Uptake of Maize (Zea mays L.) Seedlings under Salinity Stress. Sustainability 2021, 13, 3150. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S. Potential of biochar application to mitigate salinity stress in eggplant. HortScience 2020, 55, 1946–1955. [Google Scholar] [CrossRef]
- Chen, G.; Wu, F.; Li, X.; Luo, C. Biochar application enhances growth and yield of cabbage under salinity stress. Agron. J. 2023, 115, 1–12. [Google Scholar]
- Huang, M.; Yang, L.; Qin, F.; Jiang, L.; Zou, Y. Effects of biochar on growth parameters and nutrient uptake in wheat under salinity stress. Soil Use Manag. 2019, 35, 282–293. [Google Scholar] [CrossRef]
- Kanwal, S.; Ilyas, N.; Shabir, S.; Mahmood, T.; Akhtar, N.; Hussain, T. Biochar application to mitigate negative effects of salinity stress on wheat. J. Plant Nutr. 2018, 41, 526–538. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Li, L.; Fu, Q.; Li, Q. Biochar mitigates combined salinity and drought stress in quinoa. Agronomy 2020, 10, 912. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef]
- Ketehouli, T.; Idrice Carther, K.F.; Noman, M.; Wang, F.W.; Li, X.W.; Li, H.Y. Adaptation of plants to salt stress: Characterization of Na+ and K+ transporters and role of CBL gene family in regulating salt stress response. Agronomy 2019, 9, 687. [Google Scholar] [CrossRef]
- Ji, H.; Pardo, J.M.; Batelli, G.; Van Oosten, M.J.; Bressan, R.A.; Li, X. The salt overly sensitive (SOS) pathway: Established and emerging roles. Mol. Plant 2013, 6, 275–286. [Google Scholar] [CrossRef]
- Venkataraman, G.; Shabala, S.; Véry, A.A.; Hariharan, G.N.; Somasundaram, S.; Pulipati, S.; Chen, Z.H. To exclude or to accumulate? Revealing the role of the sodium HKT1; 5 transporters in plant adaptive responses to varying soil salinity. Plant Physiol. Biochem. 2021, 169, 333–342. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Leidi, E.O.; Pardo, J.M. How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal. Behav. 2010, 5, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Akram, U.; Song, Y.; Liang, C.; Abid, M.A.; Askari, M.; Myat, A.A.; Meng, Z. Genome-wide characterization and expression analysis of NHX gene family under salinity stress in Gossypium barbadense and its comparison with Gossypium hirsutum. Genes 2020, 11, 803. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, K.; Mei, Q.; Wang, X.; Yang, J.; Ma, F.; Mao, K. Genome-wide analysis of the Actinidia chinensis NHX family and characterization of the roles of AcNHX3 and AcNHX7 in regulating salt tolerance in Arabidopsis. Environ. Exp. Bot. 2023, 214, 105477. [Google Scholar] [CrossRef]
- Ratner, A.; Jacoby, B. Effect of K+, its counter anion, and pH on sodium efflux from barley root tips. J. Exp. Bot. 1976, 27, 843–852. [Google Scholar] [CrossRef]
- Sharma, P.; Mishra, S.; Pandey, B.; Singh, G. Genome-wide identification and expression analysis of the NHX gene family under salt stress in wheat (Triticum aestivum L). Front. Plant Sci. 2023, 14, 1266699. [Google Scholar] [CrossRef]
- Joshi, S.; Kaur, K.; Khare, T.; Srivastava, A.K.; Suprasanna, P.; Kumar, V. Genome-wide identification, characterization and transcriptional profiling of NHX-type (Na+/H+) antiporters under salinity stress in soybean. 3 Biotech 2021, 11, 16. [Google Scholar] [CrossRef]
- Cavusoglu, E.; Sari, U.; Tiryaki, I. Genome-wide identification and expression analysis of Na+/H+ antiporter (NHX) genes in tomato under salt stress. Plant Direct 2023, 7, e543. [Google Scholar] [CrossRef]
- Wu, G.Q.; Wang, J.L.; Li, S.J. Genome-wide identification of Na+/H+ antiporter (NHX) genes in sugar beet (Beta vulgaris L.) and their regulated expression under salt stress. Genes 2019, 10, 401. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.; Paterson, A.H. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef]
- Bian, X.; Ren, Z.; Zeng, L.; Zhao, F.; Yao, Y.; Li, X. Effects of biochar on the compressibility of soil with high water content. J. Clean. Prod. 2024, 434, 140032. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Mandal, S.; Niazi, N.K.; Vithanage, M.; Parikh, S.J.; Mukome, F.N.; Ok, Y.S. Advances and future directions of biochar characterization methods and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2275–2330. [Google Scholar] [CrossRef]
- He, K.; Xu, Y.; He, G.; Zhao, X.; Wang, C.; Li, S.; Hu, R. Combined application of acidic biochar and fertilizer synergistically enhances Miscanthus productivity in coastal saline-alkaline soil. Sci. Total Environ. 2023, 893, 164811. [Google Scholar] [CrossRef] [PubMed]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Harris, E. Impact of Biochar Amendment on Nutrient Retention by Riparian Soils. Ph.D. Thesis, Lincoln University, Lincoln, UK, 2011. [Google Scholar]
- Chen, X.; Yang, S.H.; Jiang, Z.W.; Ding, J.; Sun, X. Biochar as a tool to reduce environmental impacts of nitrogen loss in water-saving irrigation paddy field. J. Clean. Prod. 2021, 290, 125811. [Google Scholar] [CrossRef]
- Hou, J.; Yi, G.; Hao, Y.; Li, L.; Shen, L.; Zhang, Q. The effect of combined application of biochar and phosphate fertilizers on phosphorus transformation in saline-alkali soil and its microbiological mechanism. Sci. Total Environ. 2024, 951, 175610. [Google Scholar] [CrossRef]
- Kang, M.W.; Yibeltal, M.; Kim, Y.H.; Oh, S.J.; Lee, J.C.; Kwon, E.E.; Lee, S.S. Enhancement of soil physical properties and soil water retention with biochar-based soil amendments. Sci. Total Environ. 2022, 836, 155746. [Google Scholar] [CrossRef]
- Xu, C.Y.; Hosseini-Bai, S.; Hao, Y.; Rachaputi, R.C.N.; Wang, H.; Xu, Z.; Wallace, H. Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environ. Sci. Pollut. Res. 2015, 22, 6112–6125. [Google Scholar] [CrossRef]
- Abbas, G.; Amjad, M.; Saqib, M.; Murtaza, B.; Naeem, M.A.; Shabbir, A.; Murtaza, G. Soil sodicity is more detrimental than salinity for quinoa (Chenopodium quinoa Willd.): A multivariate comparison of physiological, biochemical and nutritional quality attributes. J. Agron. Crop Sci. 2021, 207, 59–73. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Naveed, M.; Zahir, Z.A.; Liu, F. Interactive effect of biochar and plant growth promoting bacterial endophytes on ameliorating salinity stress in maize. Funct. Plant Biol. 2015, 42, 770–781. [Google Scholar] [CrossRef]
- Kul, R.; Arjumend, T.; Ekinci, M.; Yildirim, E.; Turan, M.; Argin, S. Biochar as an organic soil conditioner for mitigating salinity stress in tomato. Soil Sci. Plant Nutr. 2021, 67, 693–706. [Google Scholar] [CrossRef]
- Ullah, I.; Ali, N.; Durrani, S.; Shabaz, M.A.; Hafeez, A.; Ishfaq, M.; Fayyaz, M.R.; Rehman, A.; Waheed, A. Effect of different nitrogen levels on growth, yield and yield contributing attributes of wheat. Int. J. Sci. Eng. Res. 2018, 9, 595–602. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, M.S. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, M.; Turan, M.; Yildirim, E. Biochar mitigates salt stress by regulating nutrient uptake and antioxidant activity, alleviating the oxidative stress and abscisic acid content in cabbage seedlings. Turk. J. Agric. For. 2022, 46, 28–37. [Google Scholar] [CrossRef]
- Hassan, M.U.; Aamer, M.; Chattha, M.U.; Ullah, M.A.; Sulaman, S.; Nawaz, M.; Zhiqiang, W.; Yanqin, M.; Guoqin, H. The role of potassium in plants under drought stress: A mini review. J. Basic Appl. Sci. 2017, 13, 268–271. [Google Scholar] [CrossRef]
- Hualpa-Ramirez, E.; Carrasco-Lozano, E.C.; Madrid-Espinoza, J.; Tejos, R.; Ruiz-Lara, S.; Stange, C.; Norambuena, L. Stress salinity in plants: New strategies to cope with in the foreseeable scenario. Plant Physiol. Biochem. 2024, 208, 108507. [Google Scholar] [CrossRef]
- Rathinapriya, P.; Pandian, S.; Rakkammal, K.; Balasangeetha, M.; Alexpandi, R.; Satish, L.; Ramesh, M. The protective effects of polyamines on salinity stress tolerance in foxtail millet (Setaria italica L.), an important C4 model crop. Physiol. Mol. Biol. Plants 2020, 26, 1815–1829. [Google Scholar] [CrossRef]
- Qiu, N.; Liu, Q.; Li, J.; Zhang, Y.; Wang, F.; Gao, J. Physiological and transcriptomic responses of Chinese cabbage (Brassica rapa L. ssp. Pekinensis) to salt stress. Int. J. Mol. Sci. 2017, 18, 1953. [Google Scholar] [CrossRef]
- Pavlović, I.; Mlinarić, S.; Tarkowská, D.; Oklestkova, J.; Novák, O.; Lepeduš, H.; Salopek-Sondi, B. Early Brassica crops responses to salinity stress: A comparative analysis between Chinese cabbage, white cabbage, and kale. Front. Plant Sci. 2019, 10, 450. [Google Scholar] [CrossRef]
- Ud Din, M.M.; Khan, M.I.; Azam, M.; Ali, M.H.; Qadri, R.; Naveed, M.; Nasir, A. Effect of biochar and compost addition on mitigating salinity stress and improving fruit quality of tomato. Agronomy 2023, 13, 2197. [Google Scholar] [CrossRef]
- Duan, S.; Al-Huqail, A.A.; Alsudays, I.M.; Younas, M.; Aslam, A.; Shahzad, A.N.; Hong Yong, J.W. Effects of biochar types on seed germination, growth, chlorophyll contents, grain yield, sodium, and potassium uptake by wheat (Triticum aestivum L.) under salt stress. BMC Plant Biol. 2024, 24, 487. [Google Scholar] [CrossRef] [PubMed]
- Saifullah; Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.W.; Ding, J.; Ali, B.; Nawaz, M.; Hassan, M.U.; Ali, A.; Sabagh, A.E. Putting Biochar in Action: A Black Gold for Efficient Mitigation of Salinity Stress in Plants. ACS Omega 2024, 9, 31237–31253. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liu, C.; Wang, Y.; Zhao, Y.; Ge, D.; Yuan, Z. Genome-wide identification of the NHX gene family in Punica granatum L. and their expressional patterns under salt stress. Agronomy 2021, 11, 264. [Google Scholar] [CrossRef]
- Shen, C.; Yuan, J.; Li, X.; Chen, R.; Li, D.; Wang, F.; Li, X. Genome-wide identification of NHX (Na+/H+ antiporter) gene family in Cucurbita L. and functional analysis of CmoNHX1 under salt stress. Front. Plant Sci. 2023, 14, 1136810. [Google Scholar] [CrossRef]
- Parveen, K.; Saddique, M.A.B.; Rehman, S.U.; Ali, Z.; Aziz, I.; Shamsi, I.H.; Muneer, M.A. Identification and characterization of salt stress-responsive NHX gene family in chickpea. Plant Stress 2023, 10, 100266. [Google Scholar] [CrossRef]
- Yarra, R. The wheat NHX gene family: Potential role in improving salinity stress tolerance of plants. Plant Gene 2019, 18, 100178. [Google Scholar] [CrossRef]
- Kargar, F.; Niazi, A.; Fakhrfeshani, M.; Malekzadeh, K. Expression analysis of genes encoding NHX2 antiporter and subunit A of vacuolar H+-ATPase pump in salt-resistant and salt-sensitive barley (Hordeum vulgare L.) cultivars under salt stress. Russ. J. Plant Physiol. 2019, 66, 572–582. [Google Scholar] [CrossRef]
- NLAST. Soil and Plant Analysis Method; National Institute of Agricultural Science and Technology: Suwon, Republic of Korea, 2000. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Proto. Food Anal. Chem. 2001, 1, 3–4. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Del Pozo, O.; Lam, E. Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Curr. Biol. 1998, 8, 1129–1132. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71. [Google Scholar]
- Sheng, X.G.; Zhao, Z.Q.; Yu, H.F.; Wang, J.S.; Zheng, C.F.; Gu, H.H. In-depth analysis of internal control genes for quantitative real-time PCR in Brassica oleracea var. botrytis. Genet. Mol. Res. 2016, 15, 10–4238. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathinapriya, P.; Maharajan, T.; Lim, T.-J.; Kang, B.; Jeong, S.T. Biochar Enhances Nutrient Uptake, Yield, and NHX Gene Expression in Chinese Cabbage Under Salinity Stress. Plants 2025, 14, 2743. https://doi.org/10.3390/plants14172743
Rathinapriya P, Maharajan T, Lim T-J, Kang B, Jeong ST. Biochar Enhances Nutrient Uptake, Yield, and NHX Gene Expression in Chinese Cabbage Under Salinity Stress. Plants. 2025; 14(17):2743. https://doi.org/10.3390/plants14172743
Chicago/Turabian StyleRathinapriya, Periyasamy, Theivanayagam Maharajan, Tae-Jun Lim, Byeongeun Kang, and Seung Tak Jeong. 2025. "Biochar Enhances Nutrient Uptake, Yield, and NHX Gene Expression in Chinese Cabbage Under Salinity Stress" Plants 14, no. 17: 2743. https://doi.org/10.3390/plants14172743
APA StyleRathinapriya, P., Maharajan, T., Lim, T.-J., Kang, B., & Jeong, S. T. (2025). Biochar Enhances Nutrient Uptake, Yield, and NHX Gene Expression in Chinese Cabbage Under Salinity Stress. Plants, 14(17), 2743. https://doi.org/10.3390/plants14172743







