Agrobacterium rhizogenes-Mediated Transformation for Generation of Composite Sugar Beet with Transgenic Adventitious Roots
Abstract
1. Introduction
2. Results
2.1. Effect of A. rhizogenes on Adventitious Root Induction from Sugar Beet Shoots and Leaves
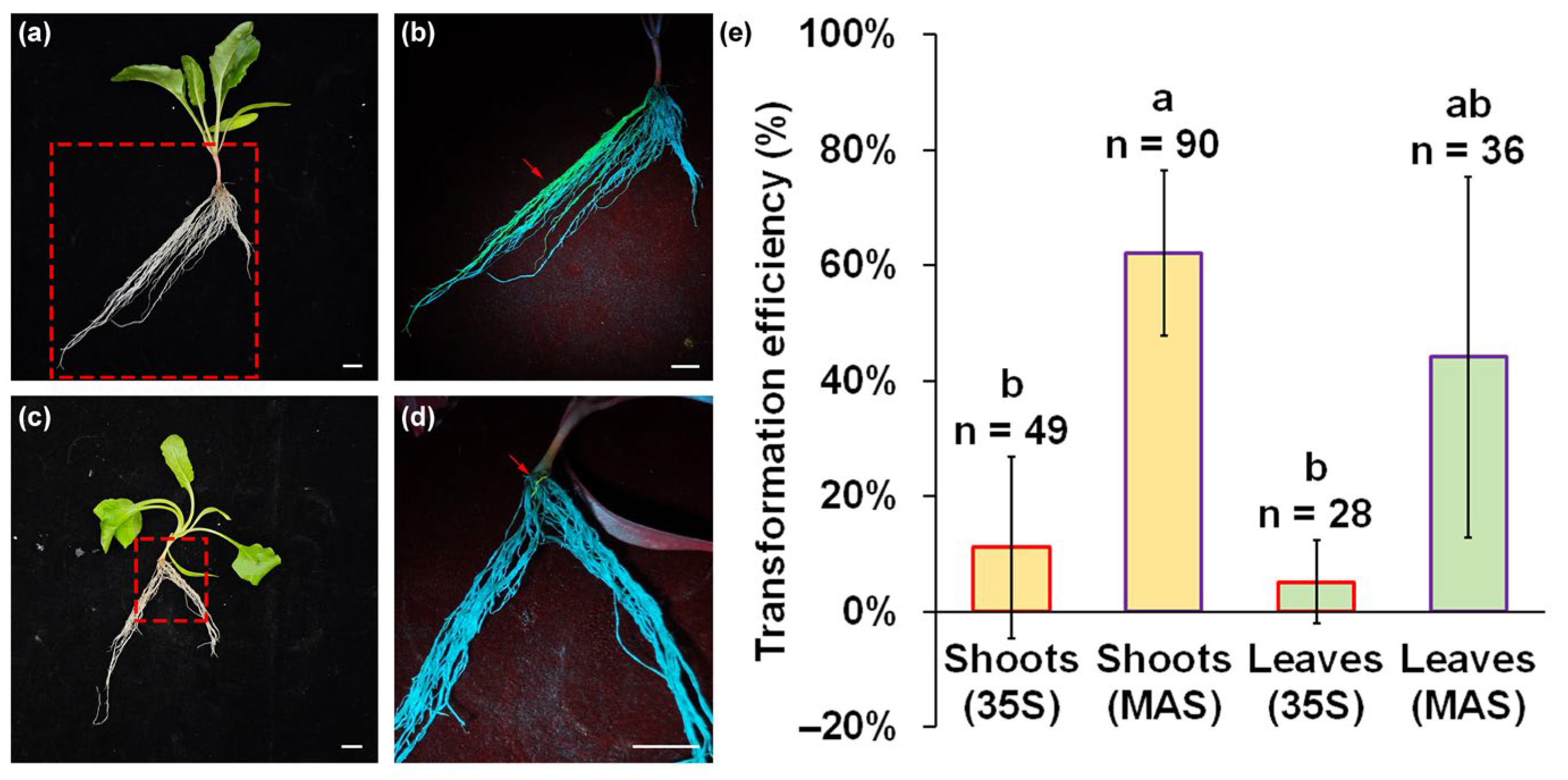
2.2. Transformation Efficiency of Adventitious Roots in Sugar Beet
2.3. The Long-Term Stability and Spatial Distribution of eGFP Expression in Adventitious Roots
2.4. The Validation of Transformation in Adventitious Roots at Molecular Level
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Cultivation
4.2. Induction of Adventitious Roots
4.3. Detection of Genetic Transformation Efficiency
4.4. The Validation of Transformation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| qRT-PCR | quantitative real-time polymerase chain reaction |
| CDB | cut–dip–budding delivery system |
| eGFP | enhanced green fluorescent protein |
| CaMV35S | cauliflower mosaic virus 35S |
| MAS | mannopine synthase |
| BSI | bacterial suspensions infection |
| BCI | bacterial colony infection |
| TSI | two-step infection |
| HPT | hygromycin resistance |
References
- Yan, Y.; Zhu, X.; Yu, Y.; Li, C.; Zhang, Z.; Wang, F. Nanotechnology strategies for plant genetic engineering. Adv. Mater. 2022, 34, 2106945. [Google Scholar] [CrossRef]
- Gelvin, S.B. Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. R. 2003, 67, 16–37. [Google Scholar] [CrossRef]
- Thomson, G.; Dickinson, L.; Jacob, Y. Genomic consequences associated with Agrobacterium-mediated transformation of plants. Plant J. 2024, 117, 342–363. [Google Scholar] [CrossRef]
- Tóth, M.; Tóth, Z.G.; Fekete, S.; Szabó, Z.; Tóth, Z. Improved and highly efficient Agrobacterium rhizogenes-mediated genetic transformation protocol: Efficient tools for functional analysis of root-specific resistance genes for Solanum lycopersicum cv. Micro-Tom. Sustainability 2022, 14, 6525. [Google Scholar] [CrossRef]
- Fan, Y.L.; Zhang, X.H.; Zhong, L.J.; Wang, X.Y.; Jin, L.S.; Lyu, S.H. One-step generation of composite soybean plants with transgenic roots by Agrobacterium rhizogenes-mediated transformation. BMC Plant Biol. 2020, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, F.; Zhou, H.; Zhou, H.; Liu, X.; Yang, X.; Weng, K.; Sun, X.; Lyu, S. A fast, simple, high efficient and one-step generation of composite cucumber plants with transgenic roots by Agrobacterium rhizogenes-mediated transformation. Plant Cell Tissue Organ Cult. 2020, 141, 207–216. [Google Scholar] [CrossRef]
- Cao, X.; Xie, H.; Song, M.; Lu, J.; Ma, P.; Huang, B.; Wang, M.; Tian, Y.; Chen, F.; Peng, J.; et al. Cut–dip–budding delivery system enables genetic modifications in plants without tissue culture. Innovation 2022, 4, 8. [Google Scholar] [CrossRef]
- Lu, J.; Li, S.; Deng, S.; Wang, M.; Wu, Y.; Li, M.; Dong, J.; Lu, S.; Su, C.; Li, G. A method of genetic transformation and gene editing of succulents without tissue culture. Plant Biotechnol. J. 2024, 22, 1981–1988. [Google Scholar] [CrossRef]
- Biłas, R.; Szafran, K.; Hnatuszko-Konka, K.; Kononowicz, A.K. Cis-regulatory elements used to control gene expression in plants. Plant Cell 2016, 127, 269–287. [Google Scholar] [CrossRef]
- Shah, S.; Jan, S.A.; Ahmad, N.; Khan, S. Use of different promoters in transgenic plant development: Current challenges and future perspectives. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 664. [Google Scholar]
- Gupta, P.; Raghuvanshi, S.; Tyagi, A.K. Assessment of the efficiency of various gene promoters via biolistics in leaf and regenerating seed callus of millets Eleusine coracana and Echinochloa crusgalli. Plant Biotechnol. 2001, 18, 275–282. [Google Scholar] [CrossRef]
- Shinoyama, H.; Shimizu, M.; Hosokawa, M.; Matsuda, K. Establishment of an efficient genetic transformation system for Tanacetum cinerariifolium. Plant Cell Tissue Organ Cult. 2024, 156, 97. [Google Scholar] [CrossRef]
- Hall, R.D.; Riksen-Bruinsma, T.; Weyens, G.J.; Rosquin, I.J.; Denys, P.N.; Evans, I.J.; Lathouwers, J.E.; Lefèbvre, M.P.; Dunwell, J.M.; Tunen, A.V.; et al. A high efficiency technique for the generation of transgenic sugar beets from stomatal guard cells. Nat. Biotechnol. 1996, 14, 1133–1138. [Google Scholar] [CrossRef]
- Kagami, H.; Kurata, M.; Matsuhira, H.; Taguchi, K.; Mikami, T.; Tamagake, H.; Kubo, T. Sugar beet (Beta vulgaris L.). In Agrobacterium Protocols: Volume 1, 3rd ed.; Springer: New York, NY, USA, 2015; pp. 335–347. [Google Scholar]
- Moazami, K.; Mortazavi, S.E.; Heidari, B.; Nouroozi, P. Agrobacterium-mediated transient assay of the gus gene expression in sugar beet. Annu. Res. Rev. Biol. 2018, 30, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Wang, L.; Zhang, B.; Fu, Z.; Zhao, S.; Yuanyuan, E.; Zheng, W.; Zhang, H.; Han, P.; et al. An efficient protocol for Agrobacterium-mediated transformation and regeneration of sugar beet (Beta vulgaris L.) based on blade–petiole transition zone explants. Sugar Tech 2023, 25, 154–159. [Google Scholar] [CrossRef]
- Mukherjee, E.; Gantait, S. Genetic transformation in sugar beet (Beta vulgaris L.): Technologies and applications. Sugar Tech 2023, 25, 269–281. [Google Scholar] [CrossRef]
- Kereszt, A.; Li, D.; Indrasumunar, A.; Nguyen, C.D.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef]
- Sun, S.; Liu, X.; Zhang, T.; Yang, H.; Yu, B. Functional characterisation of the transcription factor GsWRKY23 gene from Glycine soja in overexpressed soybean composite plants and Arabidopsis under salt stress. Plants 2023, 12, 3030. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Agostini, E.; Ludwig-Müller, J.; Xu, J. Genetically transformed roots: From plant disease to biotechnological resource. Trends Biotechnol. 2012, 30, 528–537. [Google Scholar] [CrossRef]
- Parks, T.; Yordanov, Y.S. Composite plants for a composite plant: An efficient protocol for root studies in the sunflower using composite plants approach. Plant Cell Tissue Organ Cult. 2020, 140, 647–659. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Wang, W.; Wang, Y.; Chen, X.; Wu, H.; Zhang, C. Efficient genetic transformation and gene editing of Chinese cabbage using Agrobacterium rhizogenes. Plant Physiol. 2025, 197, 543. [Google Scholar] [CrossRef]
- Youssef, A.B.; Rslan, W.M. Sugar beet improvement using Agrobacterium-mediated transformation technology. Highlights Biosci. 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Klimek-Chodacka, M.; Baranski, R. A protocol for sonication-assisted Agrobacterium rhizogenes-mediated transformation of haploid and diploid sugar beet (Beta vulgaris L.) explants. Acta Biochim. Pol. 2014, 61, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyeswari, T.; Gantait, S. Advancements and prospectives of sugar beet (Beta vulgaris L.) biotechnology. Appl. Microbiol. Biotechnol. 2022, 106, 7417–7430. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Cui, D.; Einstein, J.; Narasimhulu, S.; Vergara, C.E.; Gelvin, S.B. Strength and tissue specificity of chimeric promoters derived from the octopine and mannopine synthase genes. Plant J. 1995, 7, 661–676. [Google Scholar] [CrossRef]
- Lee, L.Y.; Kononov, M.E.; Bassuner, B.; Frame, B.R.; Wang, K.; Gelvin, S.B. Novel plant transformation vectors containing the superpromoter. Plant Physiol. 2007, 145, 1294–1300. [Google Scholar] [CrossRef]
- An, Y.; Geng, Y.; Yao, J.; Wang, C.; Du, J. An improved CRISPR/Cas9 system for genome editing in populus by using mannopine synthase (MAS) promoter. Front. Plant Sci. 2021, 12, 703546. [Google Scholar] [CrossRef]
- Vitha, S.; Phillips, J.P.; Gartland, J.S.; Gartland, K.M.A.; Beneš, K.; Elliott, M.C. Activity of β-glucuronidase in root tips of different types of transgenic sugar beet plants. Biol. Plantarum. 1997, 40, 531–541. [Google Scholar] [CrossRef]
- Meng, D.; Yang, Q.; Dong, B.; Song, Z.; Niu, L.; Wang, L.; Cao, H.; Li, H.; Fu, Y. Development of an efficient root transgenic system for pigeon pea and its application to other important economically plants. Plant Biotechnol. J. 2019, 17, 1804–1813. [Google Scholar] [CrossRef]
- Cao, D.; Hou, W.; Song, S.; Sun, H.; Wu, C.; Gao, Y.; Han, T. Assessment of conditions affecting Agrobacterium rhizogenes-mediated transformation of soybean. Plant Cell Tissue Organ Cult. 2009, 96, 45–52. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, B.; Wu, C.; Sun, S.; Hou, W.; Han, T. GmPRP2 promoter drives root-preferential expression in transgenic Arabidopsis and soybean hairy roots. BMC Plant Biol. 2014, 14, 245. [Google Scholar] [CrossRef]
- Dohm, J.C.; Minoche, A.E.; Holtgräwe, D.; Capella-Gutiérrez, S.; Zakrzewski, F.; Tafer, H.; Rupp, O.; Sörensen, T.R.; Stracke, R.; Reinhardt, R.; et al. The genome of the recently domesticated crop plant sugar beet (Beta vulgaris). Nature 2014, 505, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, S.; Pi, Z.; Wu, Z. Research progress in controlling root rot in sugar beet. Sugar Tech 2025, 27, 1003–1011. [Google Scholar] [CrossRef]
- Cui, R.; Geng, G.; Wang, G.; Stevanato, P.; Dong, Y.; Li, T.; Yu, L.; Wang, Y. The response of sugar beet rhizosphere micro-ecological environment to continuous cropping. Front. Microbiol. 2022, 13, 956785. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Y.; Li, N.; Li, G.; Sun, Y.; Zhang, S. BvCPD promotes parenchyma cell and vascular bundle development in sugar beet (Beta vulgaris L.) taproot. Front. Plant Sci. 2023, 14, 1271329. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Duplancic, R.; Kero, D. Novel approach for quantification of multiple immunofluorescent signals using histograms and 2D plot profiling of whole-section panoramic images. Sci. Rep. 2021, 11, 8619. [Google Scholar] [CrossRef]
- Long, J.; Xing, W.; Wang, Y.; Wu, Z.; Li, W.; Zou, Y.; Sun, J.; Zhang, F.; Zhi, P. Comparative proteomic analysis on chloroplast proteins provides new insights into the effects of low temperature in sugar beet. Bot. Stud. 2022, 63, 18. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhao, Y.; Jia, M.; Zhang, X.; Zhou, X.; Li, S.; Wu, Z.; Pi, Z. Agrobacterium rhizogenes-Mediated Transformation for Generation of Composite Sugar Beet with Transgenic Adventitious Roots. Plants 2025, 14, 2747. https://doi.org/10.3390/plants14172747
Sun Y, Zhao Y, Jia M, Zhang X, Zhou X, Li S, Wu Z, Pi Z. Agrobacterium rhizogenes-Mediated Transformation for Generation of Composite Sugar Beet with Transgenic Adventitious Roots. Plants. 2025; 14(17):2747. https://doi.org/10.3390/plants14172747
Chicago/Turabian StyleSun, Yue, Yiduo Zhao, Minshi Jia, Xudong Zhang, Xixuan Zhou, Shengnan Li, Zedong Wu, and Zhi Pi. 2025. "Agrobacterium rhizogenes-Mediated Transformation for Generation of Composite Sugar Beet with Transgenic Adventitious Roots" Plants 14, no. 17: 2747. https://doi.org/10.3390/plants14172747
APA StyleSun, Y., Zhao, Y., Jia, M., Zhang, X., Zhou, X., Li, S., Wu, Z., & Pi, Z. (2025). Agrobacterium rhizogenes-Mediated Transformation for Generation of Composite Sugar Beet with Transgenic Adventitious Roots. Plants, 14(17), 2747. https://doi.org/10.3390/plants14172747




