Micropropagation of Apple Cultivars ‘Golden Delicious’ and ‘Royal Gala’ in Bioreactors
Abstract
1. Introduction
2. Results
2.1. First Experiments with Golden Delicious and Royal Gala in Bioreactors: Effect of Support
2.2. Optimization of Multiplication of ‘Golden Delicious’ in Bioreactors
2.2.1. Effect of Cytokinin Concentration, Subculture Duration and Support
2.2.2. Strategies to Reduce Hyperhydricity in ‘Golden Delicious’: Using Silver Nitrate and Activated Charcoal
2.3. Optimization of Multiplication of ‘Royal Gala’ in Bioreactors
2.3.1. Effect of Support and Subculture Duration
2.3.2. Strategies to Reduce Hyperhydricity in ‘Royal Gala’: Using Silver Nitrate and Activated Charcoal
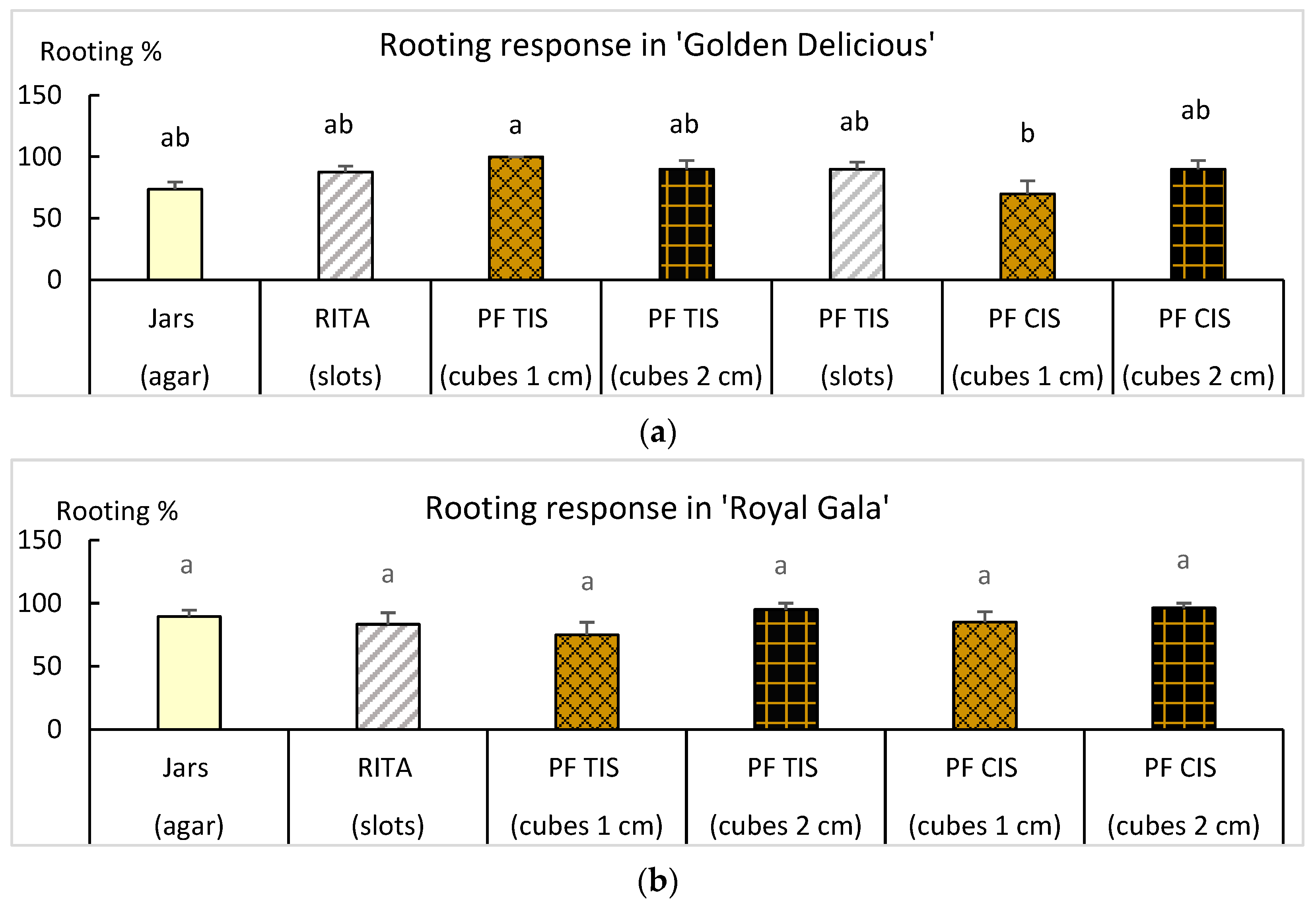
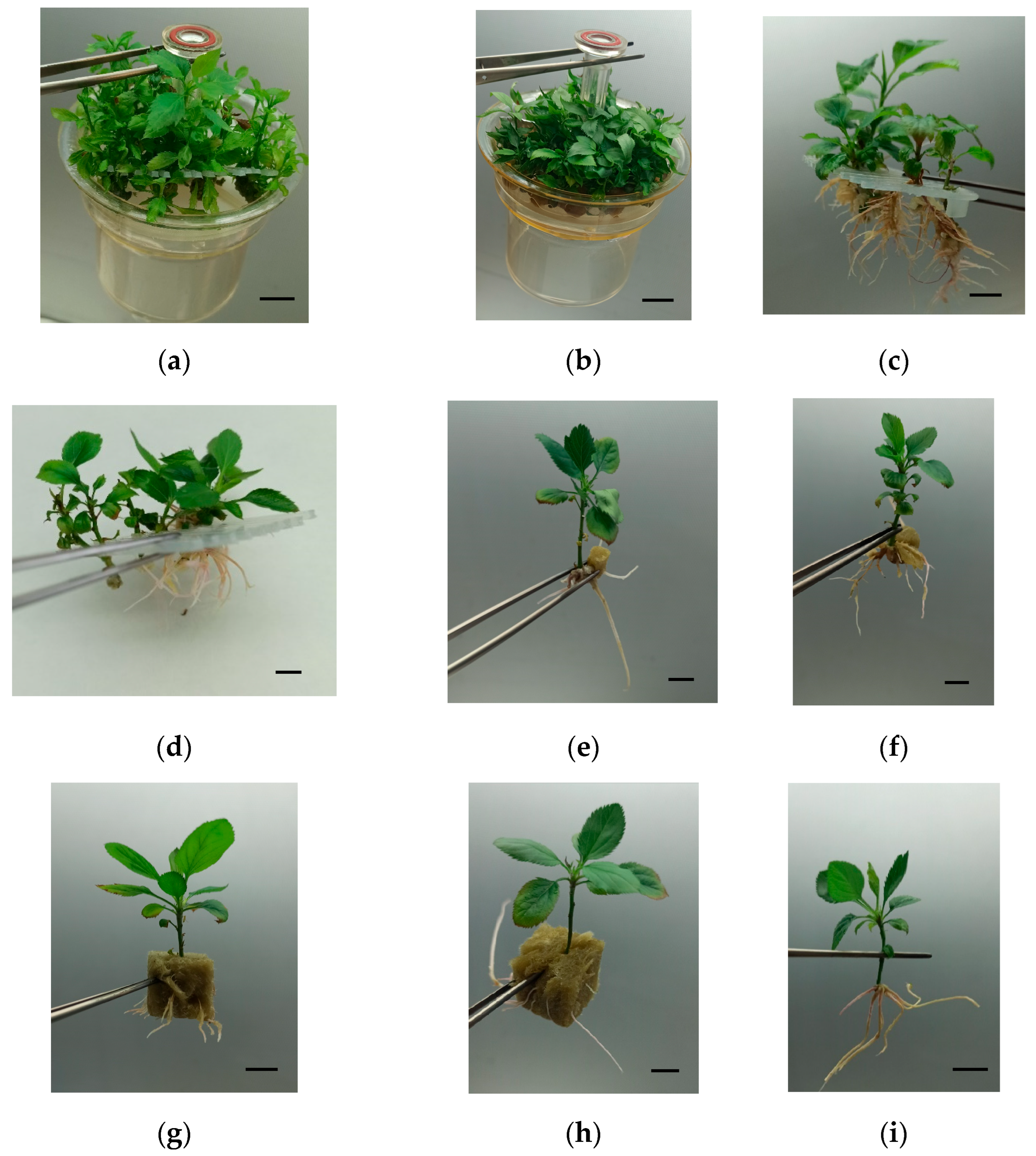
2.4. Rooting and Acclimation of Golden Delicious and Royal Gala
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Growth Conditions in Bioreactors
4.2.1. Physical Support
4.2.2. Immersion Frequency
4.2.3. Media Composition
4.2.4. Root Induction
4.3. Rooting Assessment and Acclimation
4.4. Data Recording and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPF | Photosynthetic photon flux |
| TIS | Temporary immersion system |
| CIS | Continuous immersion system |
| AC | Activated charcoal |
| SN | Silver nitrate |
| BA | N6-benzyladenine |
| IBA | indole 3-butyric acid |
| SSM | Semisolid medium |
| MS | Murashige and Skoog |
| MS ½ N | Murashige and Skoog medium with half-strength nitrates |
| HH | Hyperhydricity |
| MC | Multiplication coefficient |
| SL | Shoot Length |
| TS No. | Total Shoot number |
| NS | Normal Shoot |
| LL | Length of the largest leaf per explant |
| LW | Width of the largest leaf per explant |
| RS No | Number of rootable shoots per explant |
| SRS No | Spontaneously rooted shoot per explant |
References
- Li, M.; Xiao, Y.; Mount, S.; Liu, Z. An Atlas of Genomic Resources for Studying Rosaceae Fruits and Ornamentals. Front. Plant Sci. 2021, 12, 644881. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef] [PubMed]
- Fruit Production by Variety Worldwide 2023. Available online: https://www.statista.com/statistics/264001/worldwide-production-of-fruit-by-variety/ (accessed on 28 July 2025).
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QV/visualize (accessed on 28 July 2025).
- OECD. Unclassified Environment Directorate Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology. Consensus Document on the Biology of Apple (Malus domestica Borkh.); Series on Harmonisation of Regulatory Oversight in Biotechnology; OECD: Paris, France, 2019; Volume 66, pp. 1–51. Available online: https://one.oecd.org/document/ENV/JM/MONO(2019)30/En/pdf (accessed on 11 August 2025).
- Sun, X.; Jiao, C.; Schwaninger, H.; Chao, C.T.; Ma, Y.; Duan, N.; Khan, A.; Ban, S.; Xu, K.; Cheng, L.; et al. Phased Diploid Genome Assemblies and Pan-Genomes Provide Insights into the Genetic History of Apple Domestication. Nat. Genet. 2020, 52, 1423–1432. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The Genome of the Domesticated Apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Daccord, N.; Celton, J.-M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; Van De Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R.; et al. High-Quality de Novo Assembly of the Apple Genome and Methylome Dynamics of Early Fruit Development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar] [CrossRef]
- Teixeira Da Silva, J.A.; Gulyás, A.; Magyar-Tábori, K.; Wang, M.-R.; Wang, Q.-C.; Dobránszki, J. In Vitro Tissue Culture of Apple and Other Malus Species: Recent Advances and Applications. Planta 2019, 249, 975–1006. [Google Scholar] [CrossRef]
- Aldwinckle, H.; Malnoy, M. Plant Regeneration and Transformation in the Rosaceae. Transgenic Plant J. 2009, 3, 1–39. [Google Scholar]
- Bettoni, J.C.; Wang, M.R.; Li, J.W.; Fan, X.; Fazio, G.; Hurtado-Gonzales, O.P.; Volk, G.M.; Wang, Q.C. Application of Biotechniques for in Vitro Virus and Viroid Elimination in Pome Fruit crops. Phytopathology 2024, 114, 930–954. [Google Scholar] [CrossRef]
- Pérez-Caselles, C.; Burgos, L.; Yelo, E.; Faize, L.; Alburquerque, N. Production of HSVd- and PPV-free apricot cultivars by in vitro thermotherapy followed by meristem culture. Plant Methods 2025, 21, 23. [Google Scholar] [CrossRef]
- Lizárraga, A.; Fraga, M.; Ascasíbar, J.; González, M. In Vitro Propagation and Recovery of Eight Apple and Two Pear Cultivars Held in a Germplasm Bank. Am. J. Plant Sci. 2017, 8, 2238–2254. [Google Scholar] [CrossRef]
- Nurtaza, A.; Magzumova, G.; Yessimseitova, A.; Karimova, V.; Shevtsov, A.; Silayev, D.; Lutsay, V.; Ramankulov, Y.; Kakimzhanova, A. Micropropagation of the endangered species Malus niedzwetzkyana for conservation biodiversity in Kazakhstan. In Vitro Cell. Dev. Biol.-Plant 2021, 57, 965–976. [Google Scholar] [CrossRef]
- Komárková, M.; Cvrčková, H.; Dostál, J.; Buriánek, V.; Máchová, P. Long-Term Observation of In Vitro-Derived Malus sylvestris (L.) Mill., the Path from the Bud to the Tree. In Apple Cultivation-Recent Advances; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Abdalla, N.; Dobránszki, J. Meta-Topolin as an effective Benzyladenine derivative to improve the multiplication rate and quality of In Vitro axillary shoots of Húsvéti Rozmaring apple scion. Plants 2024, 13, 1568. [Google Scholar] [CrossRef] [PubMed]
- Pompili, V.; Dalla Costa, L.; Piazza, S.; Pindo, M.; Malnoy, M. Reduced Fire Blight Susceptibility in Apple Cultivars Using a High-efficiency CRISPR/Cas9-FLP/FRT-based Gene Editing System. Plant Biotechnol. J. 2020, 18, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Piazza, S.; Nuzzo, F.; Li, M.; Lagrèze, J.; Mithöfer, A.; Cestaro, A.; Tarkowska, D.; Espley, R.; Dare, A.; et al. Cas9 Genome-editing Applied to MdPGT1 in Apple Results in Reduced Foliar Phloridzin without Impacting Plant Growth. Plant J. 2023, 113, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Vidal, N.; Sánchez, C. Use of Bioreactor Systems in the Propagation of Forest Trees. Eng. Life Sci. 2019, 19, 896–915. [Google Scholar] [CrossRef]
- Gupta, N.; Upadhyaya, C.P.; Singh, A.; Abd-Elsalam, K.A.; Prasad, R. Applications of Silver Nanoparticles in Plant Protection. In Nanobiotechnology Applications in Plant Protection; Abd-Elsalam, K.A., Prasad, R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 247–265. ISBN 978-3-319-91161-8. [Google Scholar] [CrossRef]
- Pérez-Caselles, C.; Burgos, L.; Sánchez-Balibrea, I.; Egea, J.A.; Faize, L.; Martín-Valmaseda, M.; Bogdanchikova, N.; Pestryakov, A.; Alburquerque, N. The effect of silver nanoparticle addition on micropropagation of apricot cultivars (Prunus armeniaca L.) in semisolid and liquid media. Plants 2023, 12, 1547. [Google Scholar] [CrossRef]
- Cantabella, D.; Mendoza, C.R.; Teixidó, N.; Vilaró, F.; Torres, R.; Dolcet-Sanjuan, R. GreenTray® TIS bioreactor as an effective in vitro culture system for the micropropagation of Prunus spp. rootstocks and analysis of the plant-PGPMs interactions. Sci. Hortic. 2022, 291, 110622. [Google Scholar] [CrossRef]
- Godoy, S.; Tapia, E.; Seit, P.; Andrade, D.; Sánchez, E.; Andrade, P.; Almeida, A.M.; Prieto, H. Temporary Immersion Systems for the Mass Propagation of Sweet Cherry Cultivars and Cherry Rootstocks: Development of a Micropropagation Procedure and Effect of Culture Conditions on Plant Quality. In Vitro Cell. Dev. Biol.-Plant 2017, 53, 494–504. [Google Scholar] [CrossRef]
- Dolcet-Sanjuan, R.; Casanovas, M.; Franquesa, S.; Alsina, E.; Carrasco-Cuello, F.; Torres, E.; Rufat, J.; Solsona, C.; Teixido, N. GreenTray®, a TIS Bioreactor for Plant Micropropagation and Abiotic or Biotic Stress Bioassays. Appl. Sci. 2024, 14, 4051. [Google Scholar] [CrossRef]
- Gago, D.; Sánchez, C.; Aldrey, A.; Christie, C.B.; Bernal, M.Á.; Vidal, N. Micropropagation of Plum (Prunus domestica L.) in Bioreactors Using Photomixotrophic and Photoautotrophic Conditions. Horticulturae 2022, 8, 286. [Google Scholar] [CrossRef]
- Gago, D.; Bernal, M.Á.; Sánchez, C.; Aldrey, A.; Cuenca, B.; Christie, C.B.; Vidal, N. Effect of Sucrose on Growth and Stress Status of Castanea sativa x C. crenata Shoots Cultured in Liquid Medium. Plants 2022, 11, 965. [Google Scholar] [CrossRef]
- Dobránszki, J.; da Silva, J.A.T. Micropropagation of apple—A review. Biotechnol. Adv. 2010, 28, 462–488. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Hahn, E.J.; Yoon, Y.J.; Paek, K.Y. Micropropagation of Apple Rootstock M.9 EMLA Using Bioreactor. J. Hortic. Sci. Biotechnol. 2003, 78, 605–609. [Google Scholar] [CrossRef]
- Zhu, L.-H.; Li, X.-Y.; Welander, M. Optimisation of Growing Conditions for the Apple Rootstock M26 Grown in RITA Containers Using Temporary Immersion Principle. Plant Cell Tiss. Organ Cult. 2005, 81, 313–318. [Google Scholar] [CrossRef]
- Damiano, C.; La Starza, S.R.; Monticelli, S.; Gentile, A.; Caboni, E.; Frattarelli, A. Propagation of Prunus and Malus by temporary immersion. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Berlin, Germany, 2005; pp. 243–251. [Google Scholar]
- Kim, N.Y.; Hwang, H.D.; Kim, J.H.; Kwon, B.-M.; Kim, D.; Park, S.-Y. Efficient production of virus-free apple plantlets using the temporary immersion bioreactor system. Hortic. Environ. Biotechnol. 2020, 61, 779–785. [Google Scholar] [CrossRef]
- Fuljahn, S.; Tantau, H.-J. Process engineering as a means of regulating the microclimate in a photoautotrophic in vitro culture. Acta Hortic. 2009, 817, 143–150. [Google Scholar] [CrossRef]
- Sota, V.; Benelli, C.; Çuko, B.; Papakosta, E.; Depaoli, C.; Lambardi, M.; Kongjika, E. Evaluation of ElecTIS Bioreactor for the Micropropagation of Malus sylvestris (L.) Mill., an Important Autochthonous Species of Albania. Hortic. Sci. 2021, 48, 12–21. [Google Scholar] [CrossRef]
- Lotfi, M.; Bayoudh, C.; Werbrouck, S.; Mars, M. Effects of meta–topolin derivatives and temporary immersion on hyperhydricity and in vitro shoot proliferation in Pyrus communis. Plant Cell Tiss. Organ Cult. 2020, 143, 499–505. [Google Scholar] [CrossRef]
- Regueira, M.; Rial, E.; Blanco, B.; Bogo, B.; Aldrey, A.; Correa, B.; Varas, E.; Sánchez, C.; Vidal, N. Micropropagation of axillary shoots of Salix viminalis using a temporary immersion system. Trees 2018, 32, 61–71. [Google Scholar] [CrossRef]
- Lane, W.D.; McDougald, J.M. Shoot tissue culture of apple: Comparative response of five cultivars to cytokinin and auxin. Can. J. Plant Sci. 1982, 62, 689–694. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Welander, M.; Persson, J.; Asp, H.; Zhu, L.H. Evaluation of a new vessel system based on temporary immersion system for micropropagation. Sci. Hortic. 2014, 179, 227–232. [Google Scholar] [CrossRef]
- Cuenca, B.; Sánchez, C.; Aldrey, A.; Bogo, B.; Blanco, B.; Correa, B.; Vidal, N. Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa x C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tiss. Organ Cult. 2017, 131, 307–320. [Google Scholar] [CrossRef]
- New South Wales Government. Available online: https://www.dpi.nsw.gov.au/agriculture/horticulture/pomes/apples/varieties/gala (accessed on 30 July 2025).
- Yepes, L.M.; Aldwinckle, H.S. Micropropagation of thirteen Malus cultivars and rootstocks, and effect of antibiotics on proliferation. Plant Growth Reg. 1994, 15, 55–67. [Google Scholar] [CrossRef]
- Saher, S.; Piqueras, A.; Hellin, E.; Olmos, E. Prevention of hyperhydricity in micropropagated carnation shoots by bottom cooling: Implications of oxidative stress. Plant Cell Tiss. Org. Cult. 2005, 81, 149–158. [Google Scholar] [CrossRef]
- Polivanova, O.B.; Bedarev, V.A. Hyperhydricity in plant tissue culture. Plants 2022, 11, 3313. [Google Scholar] [CrossRef]
- Pasqualetto, P.L.; Zimmerman, R.H.; Fordham, I. The influence of cation and gelling agent concentrations on vitrification of apple cultivars in vitro. Plant Cell Tiss. Org. Cult. 1988, 14, 31–40. [Google Scholar] [CrossRef]
- Tabart, J.; Franck, T.; Kevers, C.; Dommes, J. Effect of polyamines and polyamine precursors on hyperhydricity in micropropagated apple shoots. Plant Cell Tiss. Organ Cult. 2015, 120, 11–18. [Google Scholar] [CrossRef]
- Bethge, H.; Mohammadi Nakhjiri, Z.; Rath, T.; Winkelmann, T. Towards automated detection of hyperhydricity in plant in vitro culture. Plant Cell Tiss. Organ Cult. 2023, 154, 551–573. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Park, S.Y.; Ali, M.B.; Shin, K.S.; Paek, K.Y. Hyperhydricity in apple: Ultrastuctural and physiological aspects. Tree Physiol. 2008, 26, 377–388. [Google Scholar] [CrossRef]
- Vidal, N.; Blanco, B.; Cuenca, B. A Temporary Immersion System for Micropropagation of Axillary Shoots of Hybrid Chestnut. Plant Cell Tiss. Organ Cult. 2015, 123, 229–243. [Google Scholar] [CrossRef]
- Nezami-Alanagh, E.; Garoosi, G.A.; Landín, M.; Gallego, P.P. Computer-based tools provide new insight into the key factors that cause physiological disorders of pistachio rootstocks cultured in vitro. Sci. Rep. 2019, 9, 9740. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Ballester, A.; San Jose, M.C.; Vieitez, E. Anatomical and chemical studies on vitrified shoots of chestnut regenerated in vitro. Physiol. Plant. 1985, 65, 177–184. [Google Scholar] [CrossRef]
- Brand, M.H. Agar and ammonium nitrate influence hyperhydricity, tissue nitrate and total nitrogen content of serviceberry (Amelanchier arborea) shoots in vitro. Plant Cell Tissue Organ Cult. 1993, 35, 203–209. [Google Scholar] [CrossRef]
- Daguin, F.; Letouze, R. Ammonium-induced vitrification in cultured tissues. Physiol. Plant. 1986, 66, 94–98. [Google Scholar] [CrossRef]
- Kevers, C.; Franck, T.; Strasser, R.J.; Dommes, S.; Gaspar, T. Hyperhydricity of micropropagated shoots: A typically stress-induced change of physiological state. Plant Cell Tissue Organ Cult. 2004, 77, 181–191. [Google Scholar] [CrossRef]
- Barker, A.V. Foliar ammonium accumulation as an index of stress in plants. Commun. Soil Sci. Plant Anal. 1999, 30, 167–174. [Google Scholar] [CrossRef]
- Li, G.; Li, B.; Dong, G.; Feng, X.; Kronzucker, H.J.; Shi, W. Ammonium-induced shoot ethylene production is associated with the inhibition of lateral root formation in Arabidopsis. J. Exp. Bot. 2013, 64, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Zobayed, S.M.A.; Armstrong, J.; Amstrong, W. Micropropagation of potato; evaluation of closed, diffusive and forced ventilation on growth and tuberization. Ann. Bot. 2001, 87, 53–59. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tiss. Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Jackson, M.B. Aeration stress in plant tissue cultures. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 459–473. [Google Scholar]
- Žd’árská, M.; Zatloukalová, P.; Benítez, M.; Šedo, O.; Potěšil, D.; Novák, O.; Svačinová, J.; Pešek, B.; Malbeck, J.; Vašíčková, J.; et al. Proteome analysis in arabidopsis reveals shoot- and root-specific targets of cytokinin action and differential regulation of hormonal homeostasis. Plant Physiol. 2013, 161, 918–930. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, F.; Kong, X.; Tian, J.; Wu, Z.; Wu, Z. Effects of multiple factors on hyperhydricity of Allium sativum L. Sci. Hortic. 2017, 217, 285–296. [Google Scholar] [CrossRef]
- Feito, I.; González, A.; Centeno, M.L.; Fernández, B.; Rodríguez, A. Transport and distribution of benzyladenine in Actinidia deliciosa explants cultured in liquid and solid media. Plant Physiol. Biochem. 2001, 39, 909–916. [Google Scholar] [CrossRef]
- Quiala, E.; Cañal, M.J.; Meijón, M.; Rodriguez, R.; Chávez, M.; Valledon, L.; Feria, M.; Barbón, R. Morphological and physiological responses of proliferating shoots of teak to temporary immersion and BA treatments. Plant Cell Tissue Organ Cult. 2012, 109, 223–234. [Google Scholar] [CrossRef]
- Sreelekshmi, R.; Siril, E.A. Effective reversal of hyperhydricity leading to efficient micropropagation of Dianthus chinensis L. 3Biotech 2021, 11, 95. [Google Scholar] [CrossRef]
- San José, M.C.; Blázquez, N.; Cernadas, M.J.; Janeiro, L.V.; Cuenca, B.; Sánchez, C.; Vidal, N. Temporary immersion systems to improve alder micropropagation. Plant Cell Tissue Organ Cult. 2020, 143, 265–275. [Google Scholar] [CrossRef]
- Chmielarz, P.; Sánchez, C.; Martins, J.R.; Ley-López, J.M.; Covelo, P.; Cernadas, M.J.; Aldrey, A.; Rico, S.; Vielba, J.M.; Christie, B.; et al. Multiplication of axillary shoots of adult Quercus robur L. trees in RITA® bioreactors. Forests 2025, 16, 1285. [Google Scholar] [CrossRef]
- Gatti, E.; Sgarbi, E.; Ozudogru, E.A.; Lambardi, M. The effect of Plantform™ bioreactor on micropropagation of Quercus robur in comparison to a conventional in vitro culture system on gelled medium, and assessment of the microenvironment influence on leaf structure. Plant Biosyst. 2017, 151, 1129–1136. [Google Scholar] [CrossRef]
- Gao, H.; Xia, X.; An, L.; Xin, X.; Liang, Y. Reversion of hyperhydricity in pink (Dianthus chinensis L.) plantlets by AgNO3 and its associated mechanism during in vitro culture. Plant Sci. 2017, 254, 1–11. [Google Scholar] [CrossRef]
- Lee, J.; Naing, A.H.; Park, K.I.; Kim, C.K. Silver nitrate reduces hyperhydricity in shoots regenerated from the hypocotyl of snapdragon cv. Maryland Apple Blossom. Sci. Hortic. 2023, 308, 111593. [Google Scholar] [CrossRef]
- Drisya Ravi, R.S.; Siril, E.A.; Nair, B.R. The effect of silver nitrate on micropropagation of Moringa oleifera Lam. an important vegetable crop of tropics with substantial nutritional value. Physiol. Mol. Biol. Plants 2019, 25, 1311–1322. [Google Scholar] [CrossRef]
- Kumar, V.; Parvatam, G.; Ravishankar, G.A. AgNO3—A potential regulator of ethylene activity and plant growth modulator. Electron. J. Biotech. 2009, 12, 8–9. [Google Scholar] [CrossRef]
- Ticona, S.A.; Oropeza, M. Effect of culture medium consistence and silver nitrate on micropropagation of two potato (Solanum tuberosum) cultivars. Rev. Colomb. Biotecnol. 2013, 15, 55–62. [Google Scholar] [CrossRef]
- Nour, K.A.; Thorpe, T.A. The effect of the gaseous state on bud induction and shoot multiplication in vitro in eastern white cedar. Physiol. Plant. 1994, 90, 163–172. [Google Scholar] [CrossRef]
- González, A.; Arigita, L.; Majada, J.; Sánchez Tamés, R. Ethylene involvement in in vitro organogenesis and plant growth of Populus tremula L. Plant Growth Regul. 1997, 22, 1–6. [Google Scholar] [CrossRef]
- Dimasi-Theriou, K.; Economou, A.S. Ethylene enhances shoot formation in cultures of the peach rootstock GF-677 (Prunus persica × P. amygdalus). Plant Cell Rep. 1995, 15, 87–90. [Google Scholar] [CrossRef]
- Strader, L.C.; Beisner, E.R.; Bartel, B. Silver ions increase auxin efflux independently of effects on ethylene response. Plant Cell 2009, 21, 3585–3590. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.J.; van Staden, J. The use of charcoal in in vitro culture—A review. Plant Growth Reg. 1998, 26, 155–163. [Google Scholar] [CrossRef]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef]
- Elmaataoui, S.; Mazri, M.A.; Meziani, R.; Bouchiha, F. Effects of culture medium strength and antioxidants on adventitious bud multiplication, hyperhydricity and tissue browning of date palm cv. Aziza Bouzid. World J. Adv. Res. Rev. 2020, 6, 103–109. [Google Scholar] [CrossRef]
- Eymar, E.; Alegre, J.; Toribio, M.; Lopez-Vela, D. Effect of activated charcoal and 6-benzyladenine on in vitro nitrogen uptake by Lagerstroemia indica. Plant Cell Tissue Organ Cult. 2000, 63, 57–65. [Google Scholar] [CrossRef]
- Mensuali-Sodi, A.; Panizza, M.; Serra, G.; Tognoni, F. Involvement of activated charcoal in the modulation of abiotic and biotic ethylene levels in tissue cultures. Sci. Hort. 1993, 54, 49–57. [Google Scholar] [CrossRef]
- Magyar-Tábori, K.; Dobránszki, J.; Jámbor-Benczúr, E.; Lazányi, J.; Szalai, J. Effects of activated charcoal on rooting of in vitro apple (Malus domestica Borkh.) shoots. Int. J. Hortic. Sci. 2001, 7, 98–101. [Google Scholar]
- Druart, P. Optimization of culture media for in vitro rooting of Malus domestica Borkh. cv. Compact Spartan. Biol. Plant. 1997, 39, 67–77. [Google Scholar] [CrossRef]
- Modgil, M.; Sharma, D.R.; Bhardwaj, S.V. Micropropagation of apple cv. Tydeman Early Worcester. Sci. Hortic. 1999, 81, 179–188. [Google Scholar] [CrossRef]
- Modgil, M.; Sharma, T.; Thakur, M. Commercially feasible protocol for rooting and acclimatization of micropropagated apple rootstocks. Acta Hortic. 2009, 839, 209–214. [Google Scholar] [CrossRef]
- Magyar-Tábori, K.; Dobránszki, J.; Jámbor-Benczúr, E.; Bubán, T.; Lazányi, J.; Szalai, J.; Ferenczy, A. Posteffects of cytokinins and auxin levels of proliferation media on rooting ability of in vitro apple shoots (Malus domestica Borkh.) ‘Red Fuji’. Int. J. Hortic. Sci. 2001, 7, 26–29. [Google Scholar] [CrossRef][Green Version]
- Newel, C.; Growns, D.; McComb, J. The influence of medium aeration on in vitro rooting of Australian plant microcuttings. Plant Cell Tissue Organ Cult. 2003, 75, 131–142. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Prasad, V.S.S. Matrix-Supported Liquid Culture Systems for Efficient Micropropagation of Floricultural Plants. In Floriculture, Ornamental and Plant Biotechnology; Teixeira da Silva, J., Ed.; Global Science Books: Kagawa, Japan, 2006; pp. 487–495. [Google Scholar]
- Maner, L.; Merkle, S. Polymerized peat plugs improve American chestnut somatic embryo germination in vitro. J. Am. Chestnut Found. 2010, 24, 16. [Google Scholar]
- Liu, Z.; Bi, W.-L.; Shukla, M.R.; Saxena, P.K. In Vitro Technologies for American Chestnut (Castanea dentata (Marshall) Borkh) Conservation. Plants 2022, 11, 464. [Google Scholar] [CrossRef]
- Adelberg, J.; Naylor-Adelberg, J.; Miller, S.; Gasic, K.; Schnabel, G.; Bryson, P.; Saski, C.; Parris, S.; Reighard, G. In vitro co-culture system for Prunus spp. and Armillaria mellea in phenolic foam rooting matric. In Vitro Cell. Dev. Biol.-Plant 2021, 57, 387–397. [Google Scholar] [CrossRef]
- Teisson, C.; Alvard, D. A new concept of plant in vitro cultivation liquid medium: Temporary immersion. In Current Issues in Plant Molecular and Cellular Biology. In Current Plant Science and Biotechnology in Agriculture; Terzi, M., Cella, R., Falavigna, A., Eds.; Kluver Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 105–110. [Google Scholar]
- Vidal, N.; Sánchez, C.; Cuenca, B. Proliferation of Axillary Shoots of Chestnut in Temporary Immersion Systems. In Micropropagation Methods in Temporary Immersion Systems; Methods in Molecular, Biology; Ramírez-Mosqueda, M.A., Cruz-Cruz, C.A., Eds.; Springer: Humana, NY, USA, 2024; Volume 2759, pp. 167–181. [Google Scholar] [CrossRef]







| Cultivar | [BA] | Support | HH % | MC 1 |
|---|---|---|---|---|
| Golden Delicious | 4.4 µM | none | 26.3 | 3.7 ± 0.8 b |
| slots | 11.0 | 6.6 ± 1.4 a | ||
| cubes | 9.4 | 6.1 ± 0.9 a | ||
| Royal Gala | 3.1 µM | none | 100.0 | 0.0 |
| slots | 100.0 | 0.0 | ||
| cubes | 100.0 | 0.0 |
| Medium | [BA] | Immersions | HH (%) | MC |
|---|---|---|---|---|
| MS | 3.10 µM | 6 | 100 c | 0.00 c |
| MS | 3.10 µM | 3 | 100 c | 0.00 c |
| MS | 3.10 µM | 2 | 90 c | 0.50 bc |
| MS | 1.55 µM | 6 | 97 c | 0.13 c |
| MS | 1.55 µM | 3 | 91 c | 0.37 bc |
| MS | 1.55 µM | 2 | 86 c | 0.65 b |
| MS ½ N | 3.10 µM | 6 | 92 c | 0.37 bc |
| MS ½ N | 3.10 µM | 3 | 75 bc | 1.12 b |
| MS ½ N | 3.10 µM | 2 | 67 b | 1.13 b |
| MS ½ N | 1.55 µM | 6 | 40 a | 3.50 a |
| MS ½ N | 1.55 µM | 3 | 26 a | 2.50 a |
| MS ½ N | 1.55 µM | 2 | 20 a | 2.00 a |
| Variable | Control | Silver Nitrate | Activated Charcoal | |||
|---|---|---|---|---|---|---|
| No Support | Cubes | No Support | Cubes | No Support | Cubes | |
| Total Shoot No. | 5.5 ± 0.49 Aa 1,2 | 5.9 ± 0.52 Aa | 3.1 ± 0.42 Ba | 3.6 ± 0.56 Ba | 1.0 ± 0.04 Ca | 1.0 ± 0.00 Ca |
| Normal Shoot No. | 2.7 ± 0.55 Ab | 4.8 ± 0.37 Aa | 2.9 ± 0.44 Ba | 2.9 ± 0.51 Ba | 1.0 ± 0.04 Ca | 1.0 ± 0.00 Ca |
| HH Shoots (%) | 47 ± 9.1 Aa | 13 ± 4.0 Ab | 7 ± 4.6 Bb | 14 ± 6.6 Ba | 0 ± 0.0 Bb | 0 ± 0.0 Bb |
| Shoot Length (mm) | 32.3 ± 1.76 Aa | 37.6 ± 1.73 Aa | 27.3 ± 3.04 Aa | 35.7 ± 3.11 Aa | 23.8 ± 2.97 Ba | 21.0 ± 0.83 Ba |
| Multiplication Coefficient | 4.1 ± 0.78 Ab | 8.8 ± 0.75 Aa | 4.4 ± 0.78 Ba | 4.9 ± 0.96 Ba | 1.6 ± 0.16 Ca | 1.7 ± 0.09 Ca |
| Leaf Length (mm) | 8.2 ± 0.77 Ca | 9.7 ± 0.52 Ca | 15.5 ± 1.56 Ba | 15.4 ± 1.49 Ba | 23.9 ± 1.45 Aa | 20.4 ± 1.01 Aa |
| Leaf Width (mm) | 4.6 ± 0.51 Ca | 5.3 ± 0.41 Ca | 9.1 ± 0.88 Ba | 8.7 ± 1.08 Ba | 13.4 ± 0.65 Aa | 12.3 ± 0.54 Aa |
| Rootable Shoot No. | 1.1 ± 0.24 Ab | 3.2 ± 0.26 Aa | 1.3 ± 0.33 Ab | 2.1 ± 0.37 Aa | 0.5 ± 0.10 Ba | 0.9 ± 0.06 Ba |
| Variable 1 | Control | Silver Nitrate (SN) | Activated Charcoal (AC) | SN + AC | ||||
|---|---|---|---|---|---|---|---|---|
| No Support | Cubes | No Support | Cubes | No Support | Cubes | No Support | Cubes | |
| TS No. | 5.6 ± 0.69 Aa 2,3 | 6.1 ± 0.69 Aa | 4.4 ± 0.40 Ba | 3.9 ± 0.47 Ba | 1.3 ± 0.11 Ca | 1.3 ± 0.14 Ca | 1.5 ± 0.15 Ca | 1.3 ± 0.09 Ca |
| NS No. | 3.4 ± 0.41 Ab | 4.2 ± 0.49 Aa | 1.5 ± 0.35 Bb | 2.3 ± 0.36 Ba | 0.8 ± 0.12 Cb | 1.3 ± 0.15 Ca | 0.9 ± 0.14 Cb | 1.0 ± 0.13 Ca |
| HH S (%) | 23 ± 7.2 Ba | 24 ± 5.6 Ba | 62 ± 8.5 Aa | 36 ± 8.1 Ab | 33 ± 9.8 Ba | 4 ± 4.2 Bb | 33 ± 9.1 Ba | 17 ± 8.1 Bb |
| SL (mm) | 21.2 ± 1.68 Ab | 33.0 ± 2.10 Aa | 30.3 ± 2.73 Aa | 30.7 ± 2.92 Aa | 21.0 ± 1.70 Ba | 22.9 ± 1.00 Ba | 13.4 ± 0.64 Bb | 19.3 ± 0.78 Ba |
| MC | 4.3 ± 0.72 Ab | 7.3 ± 0.80 Aa | 1.96 ± 0.48 Bb | 3.2 ± 0.45 Ba | 0.8 ± 0.16 Cb | 1.8 ± 0.23 Ca | 0.8 ± 0.14 Cb | 1.2 ± 0.15 Ca |
| LL (mm) | 7.2 ± 0.48 Cb | 8.1 ± 0.38 Ca | 10.6 ± 1.94 Bb | 11.2 ± 1.21 Ba | 18.3 ± 1.52 Ab | 21.5 ± 1.12 Aa | 16.3 ± 1.21 Ab | 22.3 ± 1.84 Aa |
| LW (mm) | 3.6 ± 0.27 Ba | 4.3 ± 0.18 Ba | 4.2 ± 0.58 Ba | 4.8 ± 0.66 Ba | 9.4 ± 0.64 Aa | 9.9 ± 0.53 Aa | 8.2 ± 0.63 Aa | 8.5 ± 0.81 Aa |
| RS No. | 1.0 ± 0.35 Ab | 2.8 ± 0.40 Aa | 0.6 ± 0.21 Bb | 1.0 ± 0.18 Ba | 0.4 ± 0.10 Bb | 0.9 ± 0.06 Ba | 0.1 ± 0.06 Bb | 0.7 ± 0.10 Ba |
| SRS No. | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.04 ± 0.04 Ba | 0.04 ± 0.04 Ba | 0.58 ± 0.10 Ab | 0.92 ± 0.06 Aa | 0.65 ± 0.10 Ab | 0.74 ± 0.09 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, S.; Malnoy, M.; Aldrey, A.; Cernadas, M.J.; Sánchez, C.; Christie, B.; Vidal, N. Micropropagation of Apple Cultivars ‘Golden Delicious’ and ‘Royal Gala’ in Bioreactors. Plants 2025, 14, 2740. https://doi.org/10.3390/plants14172740
Miranda S, Malnoy M, Aldrey A, Cernadas MJ, Sánchez C, Christie B, Vidal N. Micropropagation of Apple Cultivars ‘Golden Delicious’ and ‘Royal Gala’ in Bioreactors. Plants. 2025; 14(17):2740. https://doi.org/10.3390/plants14172740
Chicago/Turabian StyleMiranda, Simón, Mickael Malnoy, Anxela Aldrey, María José Cernadas, Conchi Sánchez, Bruce Christie, and Nieves Vidal. 2025. "Micropropagation of Apple Cultivars ‘Golden Delicious’ and ‘Royal Gala’ in Bioreactors" Plants 14, no. 17: 2740. https://doi.org/10.3390/plants14172740
APA StyleMiranda, S., Malnoy, M., Aldrey, A., Cernadas, M. J., Sánchez, C., Christie, B., & Vidal, N. (2025). Micropropagation of Apple Cultivars ‘Golden Delicious’ and ‘Royal Gala’ in Bioreactors. Plants, 14(17), 2740. https://doi.org/10.3390/plants14172740








