Suppressing Symptomless Nonhost Resistance of Barley to Tobacco mosaic virus by Short-Term Heat Stress—Role of Superoxide in Resistance
Abstract
1. Introduction
2. Results
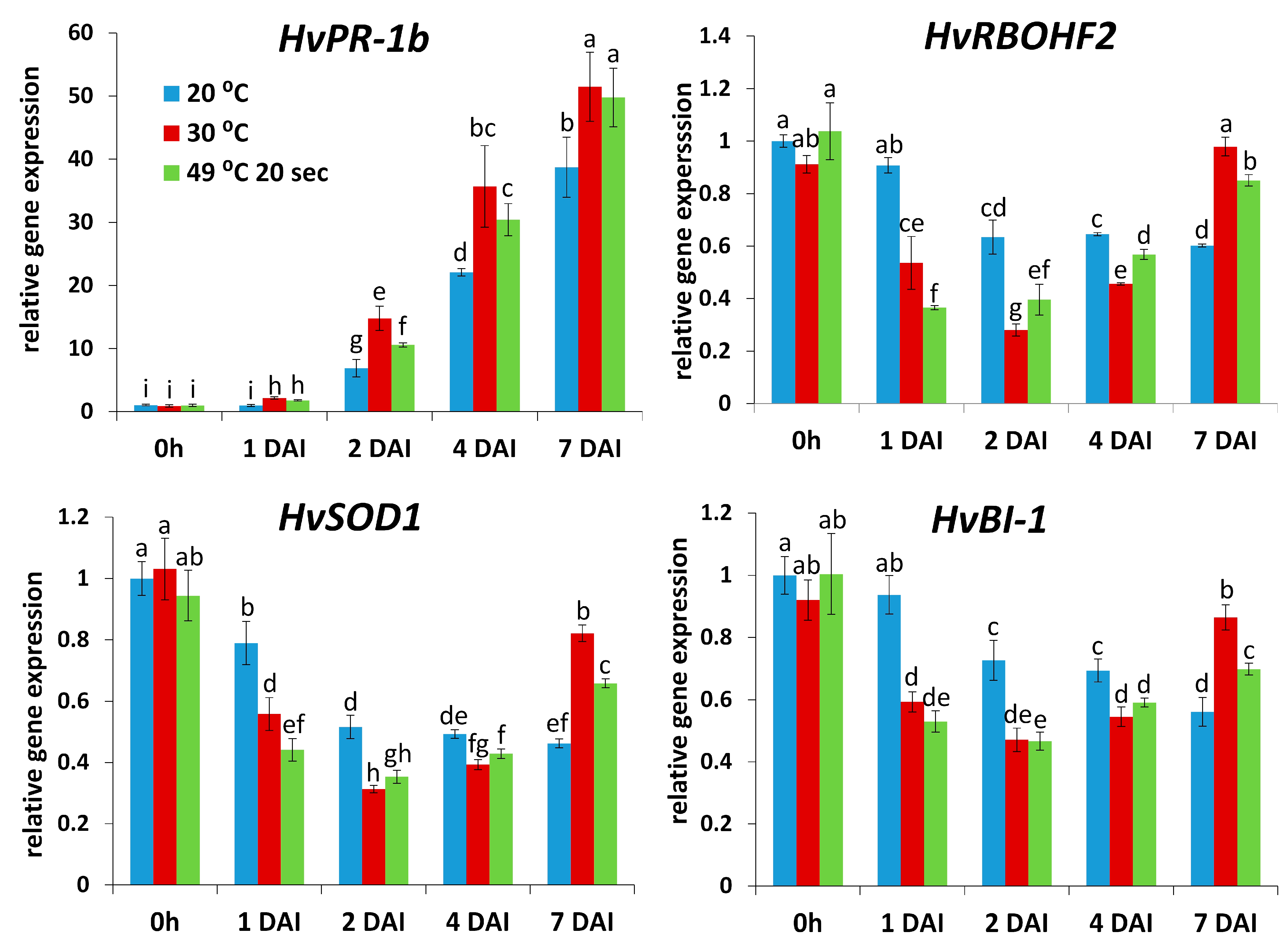
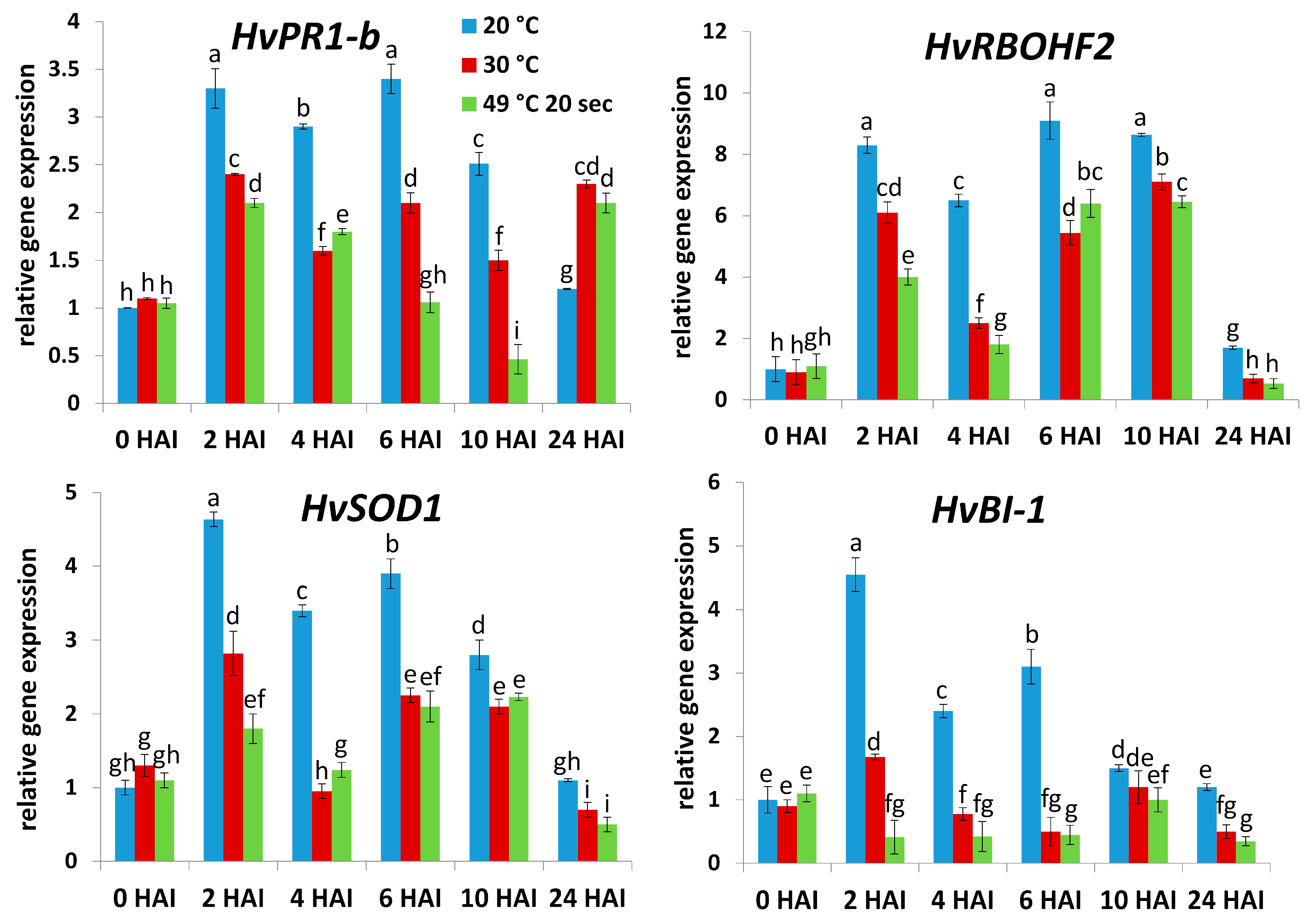
2.1. Roles of Heat Exposure and Defense Gene Expression in Symptomless (Type I) Nonhost Resistance of Barley cv. Ingrid to TMV
2.2. Roles of ROS (O2.−) in Symptomless (Type I) Nonhost Resistance of Barley cv. Ingrid to TMV
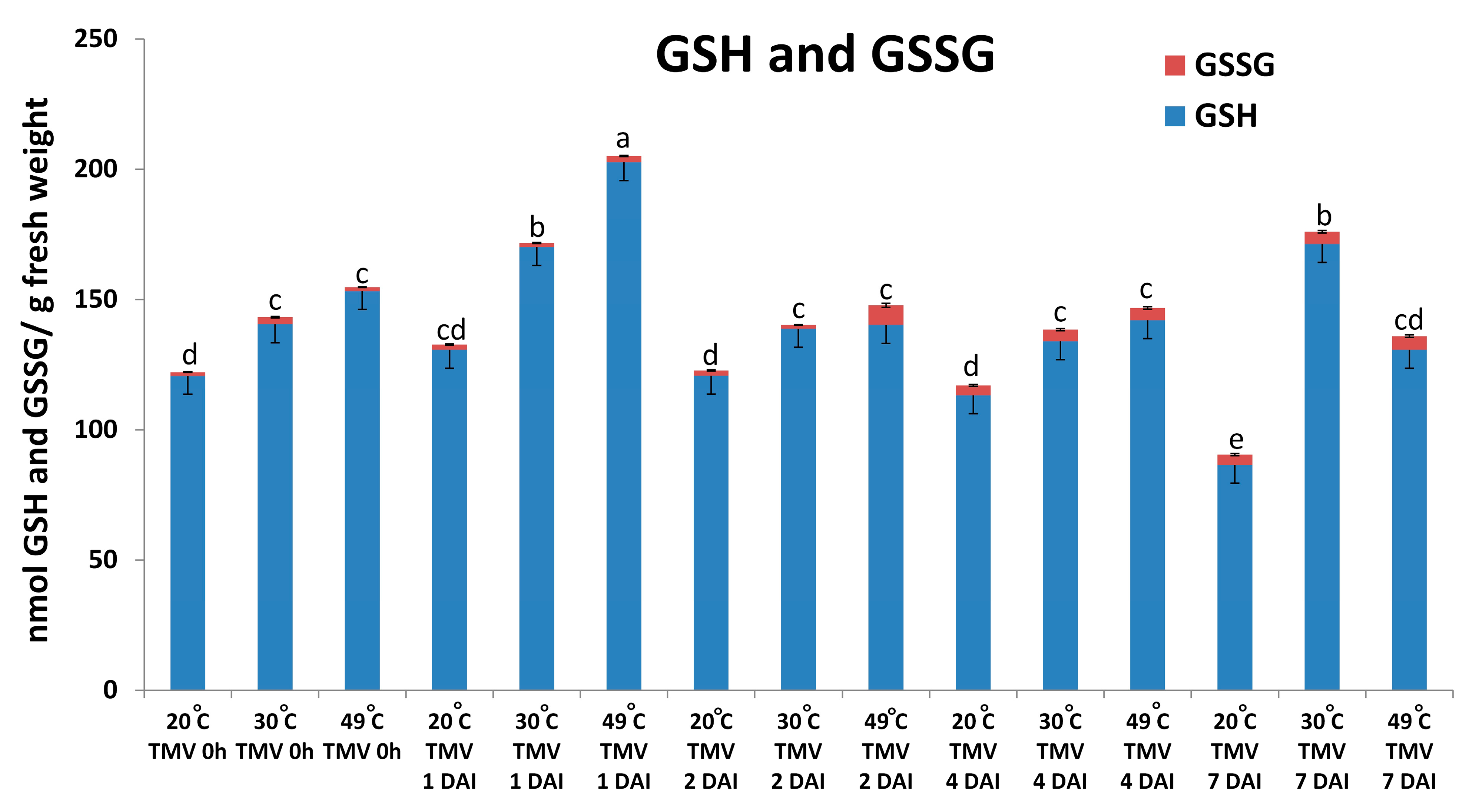
2.3. Glutathione May Take Part in Suppressing Symptomless (Type I) Nonhost Resistance of Barley cv. Ingrid to TMV at Later Stages of Infection
3. Discussion
4. Materials and Methods
4.1. Plants and Pathogen, Virus Inoculation
4.2. Short-Term Heat Stress Pre-Treatments of Barley
4.3. Virus Accumulation and Gene Expression Analyses
4.4. Antioxidant Treatments and Detection of Superoxide (O2.−) and Cell Death in Barley Leaves
4.5. Glutathione Assays
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zipfel, C. Early molecular events in PAMP-triggered immunity. Curr. Opin. Plant Biol. 2009, 12, 414–420. [Google Scholar] [CrossRef]
- Lacombe, S.; Rougon-Cardoso, A.; Sherwood, E.; Peeters, N.; Dahlbeck, D.; van Esse, H.P.; Smoker, M.; Rallapalli, G.; Thomma, B.P.H.J.; Staskawicz, B.; et al. Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance. Nat. Biotechnol. 2010, 28, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.H. Current status of the gene for gene concept. Annu. Rev. Phytopathol. 1971, 9, 275–296. [Google Scholar] [CrossRef]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef]
- Bentham, A.R.; De la Concepcion, J.C.; Mukhi, N.; Zdrzałek, R.; Draeger, M.; Gorenkin, D.; Hughes, R.K.; Banfield, M.J. A molecular roadmap to the plant immune system. J. Biol. Chem. 2020, 295, 14916–14935. [Google Scholar] [CrossRef]
- Pruitt, R.N.; Gust, A.A.; Nürnberger, T. Plant immunity unified. Nat. Plants 2021, 7, 382–383. [Google Scholar] [CrossRef]
- Goodman, R.N.; Novacky, A.J. The Hypersensitive Reaction in Plants to Pathogens; APS Press: St. Paul, MN, USA, 1994; 244p. [Google Scholar]
- Künstler, A.; Bacsó, R.; Gullner, G.; Hafez, Y.M.; Király, L. Staying alive—Is cell death dispensable for plant disease resistance during the hypersensitive response? Physiol. Mol. Plant Pathol. 2016, 93, 75–84. [Google Scholar] [CrossRef]
- Balint-Kurti, P. The plant hypersensitive response: Concepts, control and consequences. Mol. Plant Pathol. 2019, 20, 1163–1178. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.C. Nonhost resistance and nonspecific plant defenses. Curr. Opin. Plant Biol. 2000, 3, 315–319. [Google Scholar] [CrossRef]
- Mysore, K.S.; Ryu, C.-M. Nonhost resistance: How much do we know? Trends Plant Sci. 2004, 9, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Senthil-Kumar, M.; Mysore, K.S. Nonhost resistance against bacterial pathogens: Retrospectives and prospects. Annu. Rev. Phytopathol. 2013, 51, 407–427. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Lefert, P.; Panstruga, R. A molecular evolutionary concept connecting nonhost resistance, pathogen host range, and pathogen speciation. Trends Plant Sci. 2011, 16, 117–125. [Google Scholar] [CrossRef] [PubMed]
- McLellan, H.; Harvey, S.E.; Steinbrenner, J.; Armstrong, M.R.; He, Q.; Clewes, R.; Pritchard, L.; Wang, W.; Wang, S.; Nussbaumer, T.; et al. Exploiting breakdown in nonhost effector-target interactions to boost host disease resistance. Proc. Natl. Acad. Sci. USA 2022, 119, e2114064119. [Google Scholar] [CrossRef] [PubMed]
- Niks, R.E.; Marcel, T.C. Nonhost and basal resistance: How to explain specificity? New Phytol. 2009, 182, 817–828. [Google Scholar] [CrossRef]
- Gill, U.S.; Lee, S.; Mysore, K.S. Host versus nonhost resistance: Distinct wars with similar arsenals. Phytopathology 2015, 105, 580–587. [Google Scholar] [CrossRef]
- Lee, H.A.; Lee, H.Y.; Seo, E.; Lee, J.; Kim, S.B.; Oh, S.; Choi, E.; Choi, E.; Lee, S.E.; Choi, D. Current understandings of plant nonhost resistance. Mol. Plant-Microbe Interact. 2017, 30, 5–15. [Google Scholar] [CrossRef]
- Panstruga, R.; Moscou, M.J. What is the molecular basis of nonhost resistance? Mol. Plant-Microbe Inreract. 2020, 33, 1253–1264. [Google Scholar] [CrossRef]
- Ishibashi, K.; Naito, S.; Meshi, T.; Ishikawa, M. An inhibitory interaction between viral and cellular proteins underlies the resistance of tomato to nonadapted tobamoviruses. Proc. Natl. Acad. Sci. USA 2009, 106, 8778–8783. [Google Scholar] [CrossRef]
- Fujisaki, K.; Iwahashi, F.; Kaido, M.; Okuno, T.; Mise, K. Genetic analysis of a host determination mechanism of bromoviruses in Arabidopsis thaliana. Virus Res. 2009, 140, 103–111. [Google Scholar] [CrossRef]
- Sardaru, P.; Sinausía, L.; López-González, S.; Zindovic, J.; Sánchez, F.; Ponz, F. The apparent non-host resistance of Ethiopian mustard to a radish-infecting strain of Turnip mosaic virus is largely determined by the C-terminal region of the P3 viral protein. Mol. Plant Pathol. 2018, 19, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Pasin, F.; Tzanetakis, I.E.; Simón-Mateo, C.; García, J.A.; Rodamilans, B. Truncation of a P1 leader proteinase facilitates potyvirus replication in a non-permissive host. Mol. Plant Pathol. 2018, 19, 1504–1510. [Google Scholar] [CrossRef]
- Jaubert, M.; Bhattacharjee, S.; Mello, A.F.S.; Perry, K.L.; Moffett, P. ARGONAUTE2 mediates RNA-silencing antiviral defenses against Potato virus X in Arabidopsis. Plant Physiol. 2011, 156, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.; Rodriguez-Moreno, L.; Rodriguez-Hernandez, A.M.; Aranda, M.A.; Truniger, V. Nicotiana benthamiana resistance to non-adapted Melon necrotic spot virus results from an incompatible interaction between virus RNA and translation initiation factor 4E. Plant J. 2011, 66, 492–501. [Google Scholar] [CrossRef]
- Baruah, A.; Sivalingam, P.N.; Fatima, U.; Senthil-Kumar, M. Non-host resistance to plant viruses: What do we know? Physiol. Mol. Plant Pathol. 2020, 111, 101506. [Google Scholar] [CrossRef]
- Rai, A.; Sivalingam, P.N.; Senthil-Kumar, M. A spotlight on non-host resistance to plant viruses. PeerJ 2022, 10, e12996. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press Inc.: New York, NY, USA, 2015; 944p. [Google Scholar]
- Hernández, J.A.; Gullner, G.; Clemente-Moreno, M.J.; Künstler, A.; Juhász, C.; Díaz-Vivancos, P.; Király, L. Oxidative stress and antioxidative responses in plant-virus interactions. Physiol. Mol. Plant Pathol. 2016, 94, 134–148. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Doke, N.; Ohashi, Y. Involvement of an O2·− generating system in the induction of necrotic lesions on tobacco leaves infected with tobacco mosaic virus. Physiol. Mol. Plant Pathol. 1988, 32, 163–175. [Google Scholar] [CrossRef]
- Rossetti, S.; Bonatti, P.M. In situ histochemical monitoring of ozone- and TMV-induced reactive oxygen species in tobacco leaves. Plant Physiol. Biochem. 2001, 39, 433–442. [Google Scholar] [CrossRef]
- Moeder, W.; Yoshioka, K.; Klessig, D.F. Involvement of the small GTPase Rac in the defense responses of tobacco to pathogens. Mol. Plant-Microbe Interact. 2005, 18, 116–124. [Google Scholar] [CrossRef]
- Király, L.; Hafez, Y.M.; Fodor, J.; Király, Z. Suppression of Tobacco mosaic virus-induced hypersensitive-type necrotization in tobacco at high temperature is associated with downregulation of NADPH oxidase and superoxide and stimulation of dehydroascorbate reductase. J. Gen. Virol. 2008, 89, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Bacsó, R.; Hafez, Y.M.; Király, Z.; Király, L. Inhibition of virus replication and symptom expression by reactive oxygen species in tobacco infected with Tobacco mosaic virus. Acta Phytopathol. Entomol. Hung. 2011, 46, 1–10. [Google Scholar] [CrossRef]
- Knip, M.; Richard, M.M.S.; Oskam, L.; van Engelen, H.T.D.; Aalders, T.; Takken, F.L.W. Activation of immune receptor Rx1 triggers distinct immune responses culminating in cell death after 4 hours. Mol. Plant Pathol. 2019, 20, 575–588. [Google Scholar] [CrossRef]
- Király, L.; Albert, R.; Zsemberi, O.; Schwarczinger, I.; Hafez, Y.M.; Künstler, A. Reactive oxygen species contribute to symptomless, extreme resistance to Potato virus X in tobacco. Phytopathology 2021, 111, 1870–1884. [Google Scholar] [CrossRef]
- Desaint, H.; Aoun, N.; Deslandes, L.; Vailleau, F.; Roux, F.; Berthomé, R. Fight hard or die trying: When plants face pathogens under heat stress. New Phytol. 2021, 229, 712–734. [Google Scholar] [CrossRef]
- Shirdelmoghanloo, H.; Chen, K.; Paynter, B.H.; Angessa, T.T.; Westcott, S.; Khan, H.A.; Hill, C.B.; Li, C. Grain-filling rate improves physical grain quality in barley under heat stress conditions during the grain-filling period. Front. Plant Sci. 2022, 13, 858652. [Google Scholar] [CrossRef] [PubMed]
- Kolozsváriné Nagy, J.; Schwarczinger, I.; Király, L.; Bacsó, R.; Ádám, A.L.; Künstler, A. Near-Isogenic Barley Lines showenhanced Susceptibility to Powdery Mildew Infection Following High-Temperature Stress. Plants 2022, 11, 903. [Google Scholar] [CrossRef]
- Schwarzbach, E. Heat induced susceptibility of mlo-barley to powdery mildew (Blumeria graminis D.C. f. sp. hordei Marchal). Czech J. Genet. Plant Breed. 2001, 37, 82–87. [Google Scholar]
- Barna, B.; Harrach, B.D.; Viczián, O.; Fodor, J. Heat induced susceptibility of barley lines with various types of resistance genes to powdery mildew. Acta Phytopathol. Entomol. Hung. 2014, 49, 177–188. [Google Scholar] [CrossRef]
- Fodor, J.; Nagy, J.K.; Király, L.; Mészáros, K.; Bányai, J.; Cséplő, M.K.; Schwarczinger, I.; Künstler, A. Heat treatments at varying ambient temperatures and durations differentially affect plant defense to Blumeria hordei in a resistant and a susceptible Hordeum vulgare line. Phytopathology 2024, 114, 418–426. [Google Scholar] [CrossRef]
- Künstler, A.; Bacsó, R.; Albert, R.; Barna, B.; Király, Z.; Hafez, Y.M.; Fodor, J.; Schwarczinger, I.; Király, L. Superoxide (O2.−) accumulation contributes to symptomless (type I) nonhost resistance of plants to biotrophic pathogens. Plant Physiol. Biochem. 2018, 128, 115–125. [Google Scholar] [CrossRef]
- Hamilton, R.I.; Dodds, J.A. Infection of barley by Tobacco mosaic virus in single and mixed infection. Virology 1970, 42, 266–268. [Google Scholar] [CrossRef]
- Dodds, J.A.; Hamilton, R.I. The influence of Barley Stripe Mosaic Virus on the replication of Tobacco Mosaic Virus in Hordeum vulgare L. Virology 1972, 50, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Király, L.; Künstler, A.; Bacsó, R.; Hafez, Y.M.; Király, Z. Similarities and differences in plant and animal immune systems—What is inhibiting pathogens? Acta Phytopathol. Entomol. Hung. 2013, 48, 187–205. [Google Scholar] [CrossRef]
- Liu, P.-P.; Bhattacharjee, S.; Klessig, D.F.; Moffett, P. Systemic acquired resistance is induced by R gene-mediated responses independent of cell death. Mol. Plant Pathol. 2010, 11, 155–160. [Google Scholar] [CrossRef]
- Gullner, G.; Juhász, C.; Németh, A.; Barna, B. Reactions of tobacco genotypes with different antioxidant capacities to powdery mildew and Tobacco mosaic virus infections. Plant Physiol. Biochem. 2017, 119, 232–239. [Google Scholar] [CrossRef]
- Künstler, A.; Király, L.; Kátay, G.; Enyedi, A.J.; Gullner, G. Glutathione can compensate for salicylic acid deficiency in tobacco to maintain resistance to Tobacco mosaic virus. Front. Plant Sci. 2019, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Lam, E. Bax inhibitor-1, a conserved cell death suppressor, is a key molecular switch downstream from a variety of biotic and abiotic stress signals in plants. Int. J. Mol. Sci. 2009, 10, 3149–3167. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Huang, X.; Li, F.; Chen, X.; Zhang, G.; Sun, Y.; Han, D.; Kang, Z. Wheat BAX inhibitor-1 contributes to wheat resistance to Puccinia striiformis. J. Exp. Bot. 2012, 63, 4571–4584. [Google Scholar] [CrossRef] [PubMed]
- Gaguancela, O.A.; Zuñiga, L.P.; Arias, A.V.; Halterman, D.; Flores, F.J.; Johansen, I.E.; Wang, A.; Yamaji, Y.; Verchot, J. The IRE1/bZIP60 pathway and Bax inhibitor 1 suppress systemic accumulation of potyviruses and potexviruses in Arabidopsis and Nicotiana benthamiana plants. Mol. Plant-Microbe Interact. 2016, 29, 750–766. [Google Scholar] [CrossRef]
- Lu, P.-P.; Yu, T.-F.; Zheng, W.-J.; Chen, M.; Zhou, Y.-B.; Chen, J.; Ma, Y.-Z.; Xi, Y.-J.; Xu, Z.-S. The wheat Bax Inhibitor-1 protein interacts with an aquaporin TaPIP1 and enhances disease resistance in Arabidopsis. Front. Plant Sci. 2018, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Talarczyk, A.; Krzymowska, M.; Borucki, W.; Hennig, J. Effect of yeast CTA1 gene expression on response of tobacco plants to tobacco mosaic virus infection. Plant Physiol. 2002, 129, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Bendahmane, A.; Kanyuka, K.; Baulcombe, D.C. The Rx gene from potato controls separate virus resistance and cell death responses. Plant Cell 1999, 11, 781–791. [Google Scholar] [CrossRef][Green Version]
- Baebler, Š.; Coll, A.; Gruden, K. Plant molecular responses to Potato virus Y: A continuum of outcomes from sensitivity and tolerance to resistance. Viruses 2020, 12, 217. [Google Scholar] [CrossRef]
- Zurbriggen, M.D.; Carrillo, N.; Hajirezaei, M.-R. ROS signaling in the hypersensitive response: When, where and what for? Plant Signal. Behav. 2010, 4, 393–396. [Google Scholar] [CrossRef]
- Lukan, T.; Županič, A.; Mahkovec Povalej, T.; Brunkard, J.O.; Kmetič, M.; Juteršek, M.; Baebler, Š.; Gruden, K. Chloroplast redox state changes mark cell-to-cell signaling in the hypersensitive response. New Phytol. 2023, 237, 548–562. [Google Scholar] [CrossRef]
- Farkas, G.; Király, Z.; Solymosi, F. Role of oxidative metabolism in the localization of plant viruses. Virology 1960, 12, 408–421. [Google Scholar] [CrossRef]
- Fodor, J.; Gullner, G.; Ádám, A.L.; Barna, B.; Kőmíves, T.; Király, Z. Local and systemic responses of antioxidants to tobacco mosaic virus infection and to salicylic acid. Plant Physiol. 1997, 114, 1443–1451. [Google Scholar] [CrossRef]
- Harrach, B.D.; Fodor, J.; Pogány, M.; Preuss, J.; Barna, B. Antioxidant, ethylene and membrane leakage responses to powdery mildew infection of near-isogenic barley lines with various types of resistance. Eur. J. Plant Pathol. 2008, 121, 21–33. [Google Scholar] [CrossRef]
- Gullner, G.; Tóbiás, I.; Fodor, J.; Kőmíves, T. Elevation of glutathione level and activation of glutathione-related enzymes affect virus infection in tobacco. Free Rad. Res. 1999, 31, S155–S161. [Google Scholar] [CrossRef] [PubMed]
- Höller, K.; Király, L.; Künstler, A.; Müller, M.; Gullner, G.; Fattinger, M.; Zechmann, B. Enhanced glutathione metabolism is correlated with sulfur-induced resistance in Tobacco mosaic virus-infected genetically susceptible Nicotiana tabacum plants. Mol. Plant-Microbe Interact. 2010, 23, 1448–1459. [Google Scholar] [CrossRef] [PubMed]
- Király, L.; Künstler, A.; Höller, K.; Fattinger, M.; Juhász, C.; Müller, M.; Gullner, G.; Zechmann, B. Sulfate supply influences compartment specific glutathione metabolism and confers enhanced resistance to Tobacco mosaic virus during a hypersensitive response. Plant Physiol. Biochem. 2012, 59, 44–54. [Google Scholar] [CrossRef]
- Zechmann, B.; Zellnig, G.; Urbanek-Krajnc, A.; Müller, M. Artificial elevation of glutathione affects symptom development in ZYMV-infected Cucurbita pepo L. plants. Arch. Virol. 2007, 152, 747–762. [Google Scholar] [CrossRef]
- De, S.; Chavez-Calvillo, G.; Wahlsten, M.; Makkinen, K. Disruption of the methionine cycle and reduced cellular glutathione levels underlie potex–potyvirus synergism in Nicotiana benthamiana. Mol. Plant Pathol. 2018, 19, 1820–1835. [Google Scholar] [CrossRef]
- Gaffney, T.; Friedrich, L.; Vernooij, B.; Negrotto, D.; Nye, G.; Uknes, S.; Ward, E.; Kessman, H.; Ryals, J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 1993, 261, 754–756. [Google Scholar] [CrossRef]
- Künstler, A.; Füzék, K.; Schwarczinger, I.; Nagy, J.K.; Fodor, J.; Király, L. Heat shock confers enhanced susceptibility of barley to a necrotrophic pathogen, Pyrenophora teres f. teres, leading to a more pronounced redox imbalance. Plant Biol. 2025; ahead of print. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Rovenich, H.; Jeena, G.; Nizam, S.; Tissier, A.; Balcke, G.U.; Mahdi, L.K.; Bonkowski, M.; Langen, G.; Zuccaro, A. The inconspicuous gatekeeper: Endophytic Serendipita vermifera acts as extended plant protection barrier in the rhizosphere. New Phytol. 2019, 224, 886–901. [Google Scholar] [CrossRef]
- Trujillo, M.; Altschmied, L.; Schweizer, P.; Kogel, K.H.; Hückelhoven, R. Respiratory burst oxidase homologue A of barley contributes to penetration by the powdery mildew fungus Blumeria graminis f. sp. hordei. J. Exp. Bot. 2006, 57, 3781–3791. [Google Scholar] [CrossRef]
- Hafez, Y.M.; Király, Z. Role of hydrogen peroxide in symptom expression of barley susceptible and resistant to powdery mildew. Acta Phytopathol. Entomol. Hung. 2003, 38, 227–236. [Google Scholar] [CrossRef]
- Ádám, A.L.; Farkas, T.; Somlyai, G.; Hevesi, M.; Király, Z. Consequence of O2.− generation during a bacterially induced hypersensitive reaction in tobacco: Deterioration of membrane lipids. Physiol. Mol. Plant Pathol. 1989, 34, 13–26. [Google Scholar] [CrossRef]
- Hückelhoven, R.; Kogel, K.-H. Tissue-specific superoxide generation at interaction sites in resistant and susceptible near-isogenic barley lines attacked by the powdery mildew fungus (Erysiphe graminis fsp. hordei). Mol. Plant-Microbe Interact. 1998, 11, 292–300. [Google Scholar] [CrossRef]
- Pogány, M.; von Rad, U.; Grün, S.; Dongó, A.; Pintye, A.; Simoneau, P.; Bahnweg, G.; Kiss, L.; Barna, B.; Durner, J. Dual roles of reactive oxygen species and NADPH oxidase RBOHD in an Arabidopsis-Alternaria pathosystem. Plant Physiol. 2009, 151, 1459–1475. [Google Scholar] [CrossRef] [PubMed]

| Accession Number | Gene | Sequence 5′-3′ | Amplicon Length | Primer Efficiency | |
|---|---|---|---|---|---|
| AF165190 and AJ429078 | Tobacco mosaic virus coat protein (TMV-CP) | Fw | CTTGTCATCAGCGTGGGC | 165 bp | 101% |
| Rev | AAGTCACTGTCAGGGAAC | ||||
| X69885 | Tobacco actin (NtAct) | Fw | CGGAATCCACGAGACTACATAC | 230 bp | 100% |
| Rev | GGGAAGCCAAGATAGAGC | ||||
| M60175 | Barley ubiquitin (HvUbi) | Fw | ACCCTCGCCGACTACAACAT | 240 bp | 102% |
| Rev | CAGTAGTGGCGGTCGAAGTG | ||||
| KU179438 | Barley cytosolic copper zinc superoxide dismutase (HvSOD1) | Fw | TCAAGGGCACCATCTTCTTC | 214 bp | 102% |
| Rev | TTTCCGAGGTCACCAGCAT | ||||
| AJ290421 | Barley BAX inhibitor-1 (HvBI-1) | Fw | ATGTTCTCGGTGCCAGTCT | 409 bp | 101% |
| Rev | GGGCGTGCTTGATGTAGTC | ||||
| X74940 | Barley pathogenesis-related-1 b (HvPR1-b) | Fw | GGACTACGACTACGGCTCCA | 150 bp | 104% |
| Rev | GGCTCGTAGTTGCAGGTGAT | ||||
| EU566856 | Barley respiratory burst oxidase homolog F2 (HvRBOHF2) | Fw | TGCTCGGTCAGCACTC | 175 bp | 104% |
| Rev | TCCGCAATAGAACACTCC | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Király, L.; Bacsó, R.; Albert, R.; Schwarczinger, I.; Nagy, J.K.; Künstler, A. Suppressing Symptomless Nonhost Resistance of Barley to Tobacco mosaic virus by Short-Term Heat Stress—Role of Superoxide in Resistance. Plants 2025, 14, 2736. https://doi.org/10.3390/plants14172736
Király L, Bacsó R, Albert R, Schwarczinger I, Nagy JK, Künstler A. Suppressing Symptomless Nonhost Resistance of Barley to Tobacco mosaic virus by Short-Term Heat Stress—Role of Superoxide in Resistance. Plants. 2025; 14(17):2736. https://doi.org/10.3390/plants14172736
Chicago/Turabian StyleKirály, Lóránt, Renáta Bacsó, Réka Albert, Ildikó Schwarczinger, Judit Kolozsváriné Nagy, and András Künstler. 2025. "Suppressing Symptomless Nonhost Resistance of Barley to Tobacco mosaic virus by Short-Term Heat Stress—Role of Superoxide in Resistance" Plants 14, no. 17: 2736. https://doi.org/10.3390/plants14172736
APA StyleKirály, L., Bacsó, R., Albert, R., Schwarczinger, I., Nagy, J. K., & Künstler, A. (2025). Suppressing Symptomless Nonhost Resistance of Barley to Tobacco mosaic virus by Short-Term Heat Stress—Role of Superoxide in Resistance. Plants, 14(17), 2736. https://doi.org/10.3390/plants14172736







