Response to In Vitro Micropropagation of Plants with Different Degrees of Variegation of the Commercial Gymnocalycium cv. Fancy (Cactaceae)
Abstract
1. Introduction
2. Results and Discussion
2.1. Explant Activation and Frequency of Response
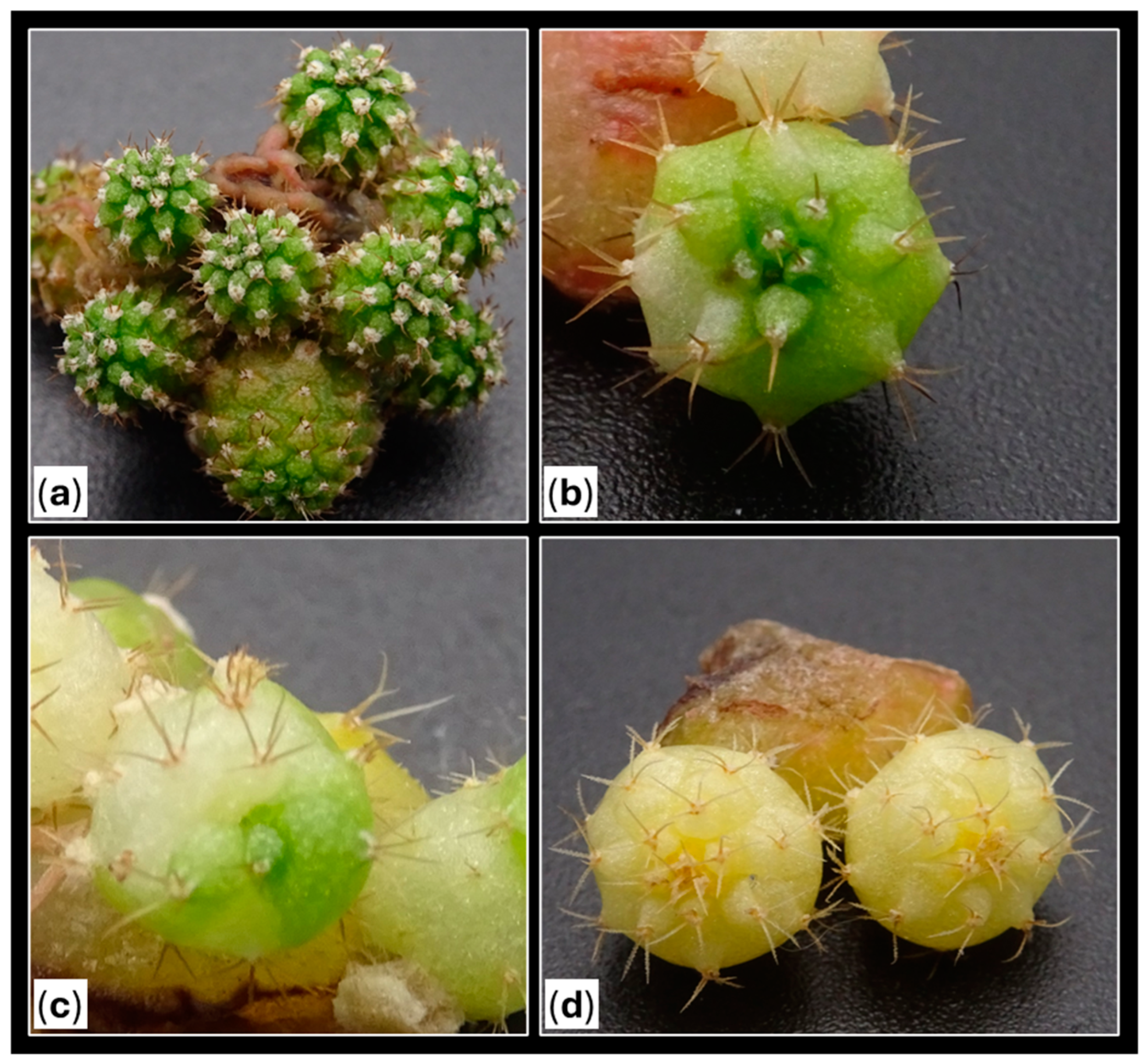
2.1.1. Calli Occurrence
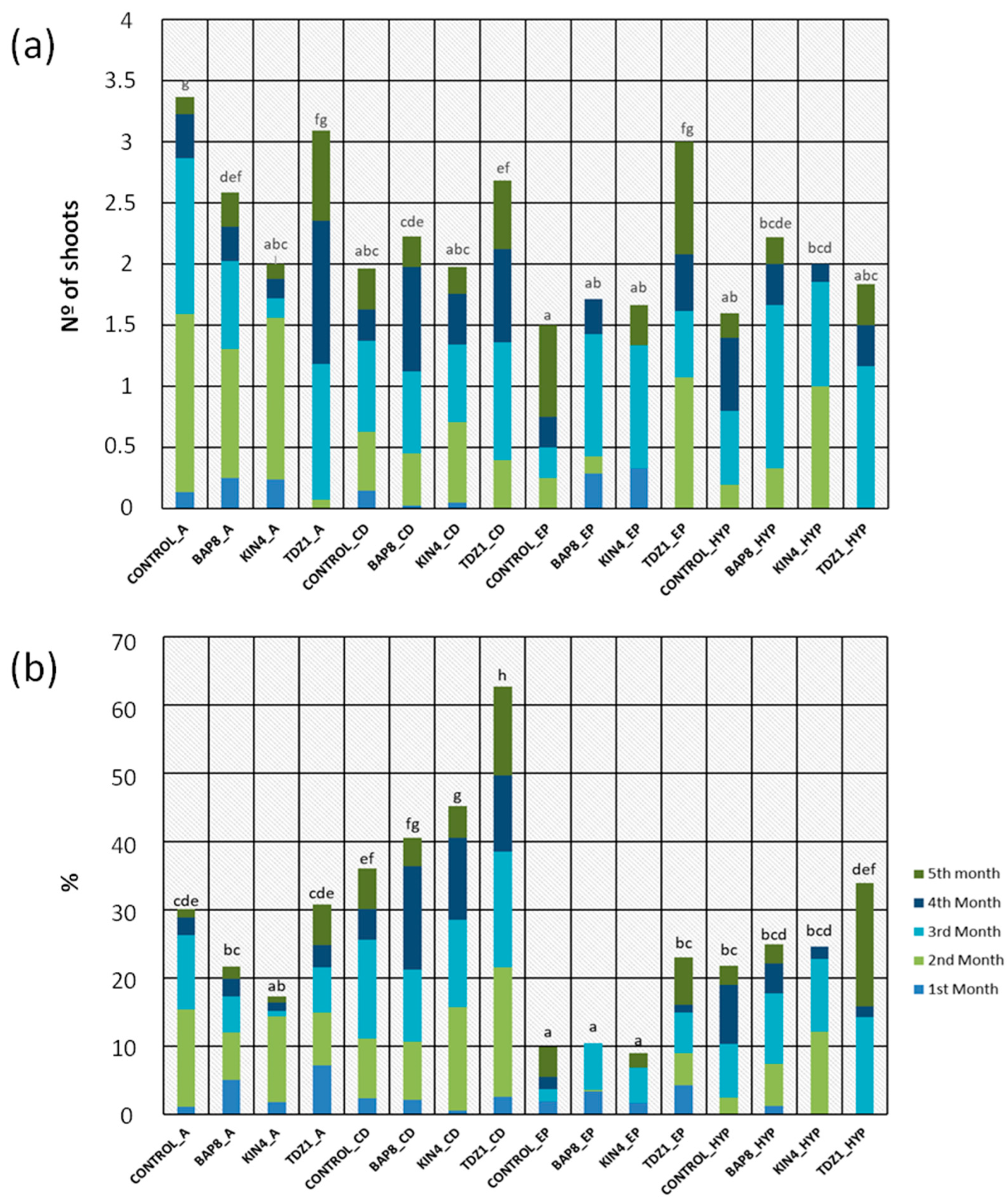
2.1.2. Shoot Production
2.1.3. Explant Efficiency
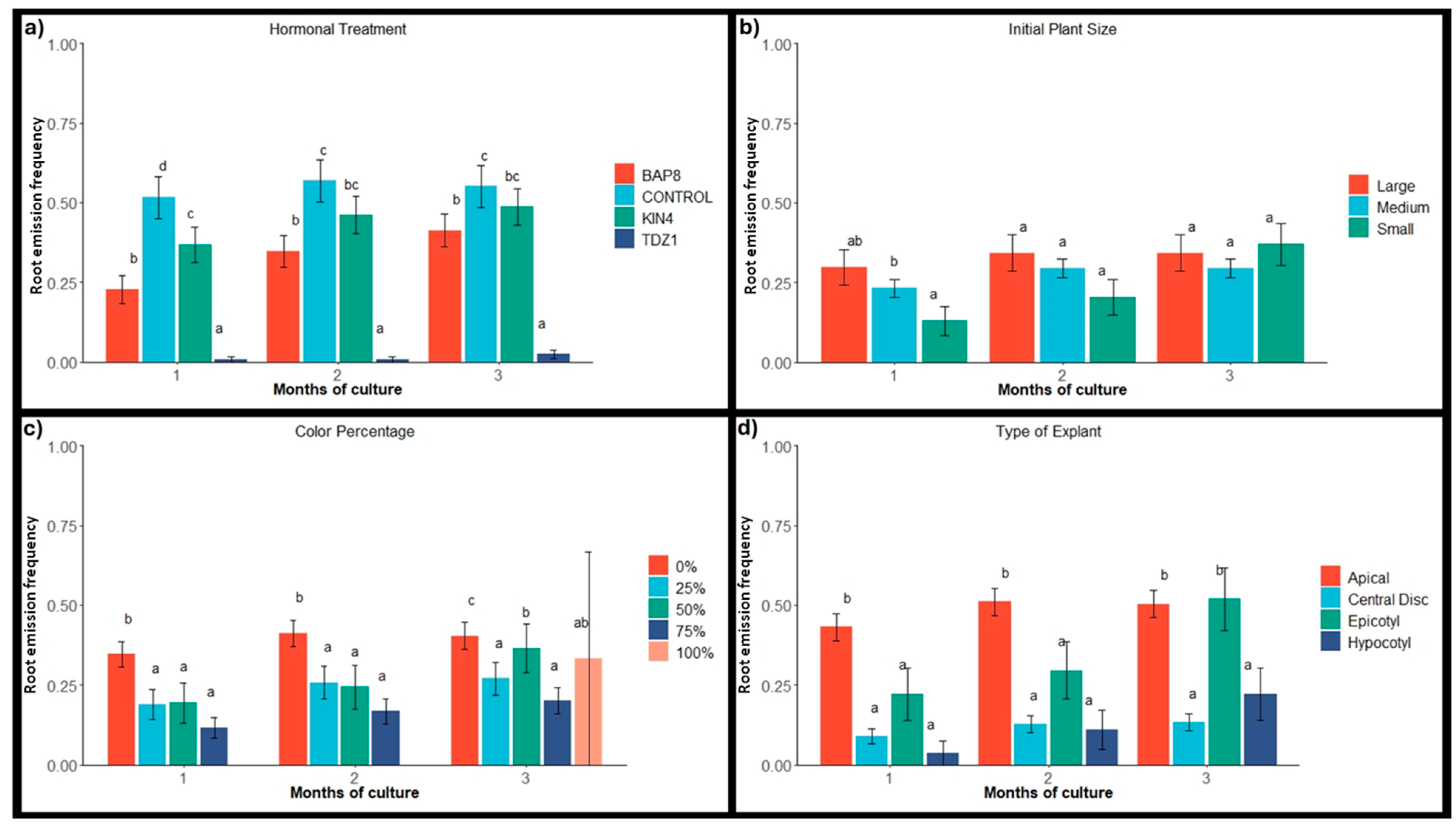
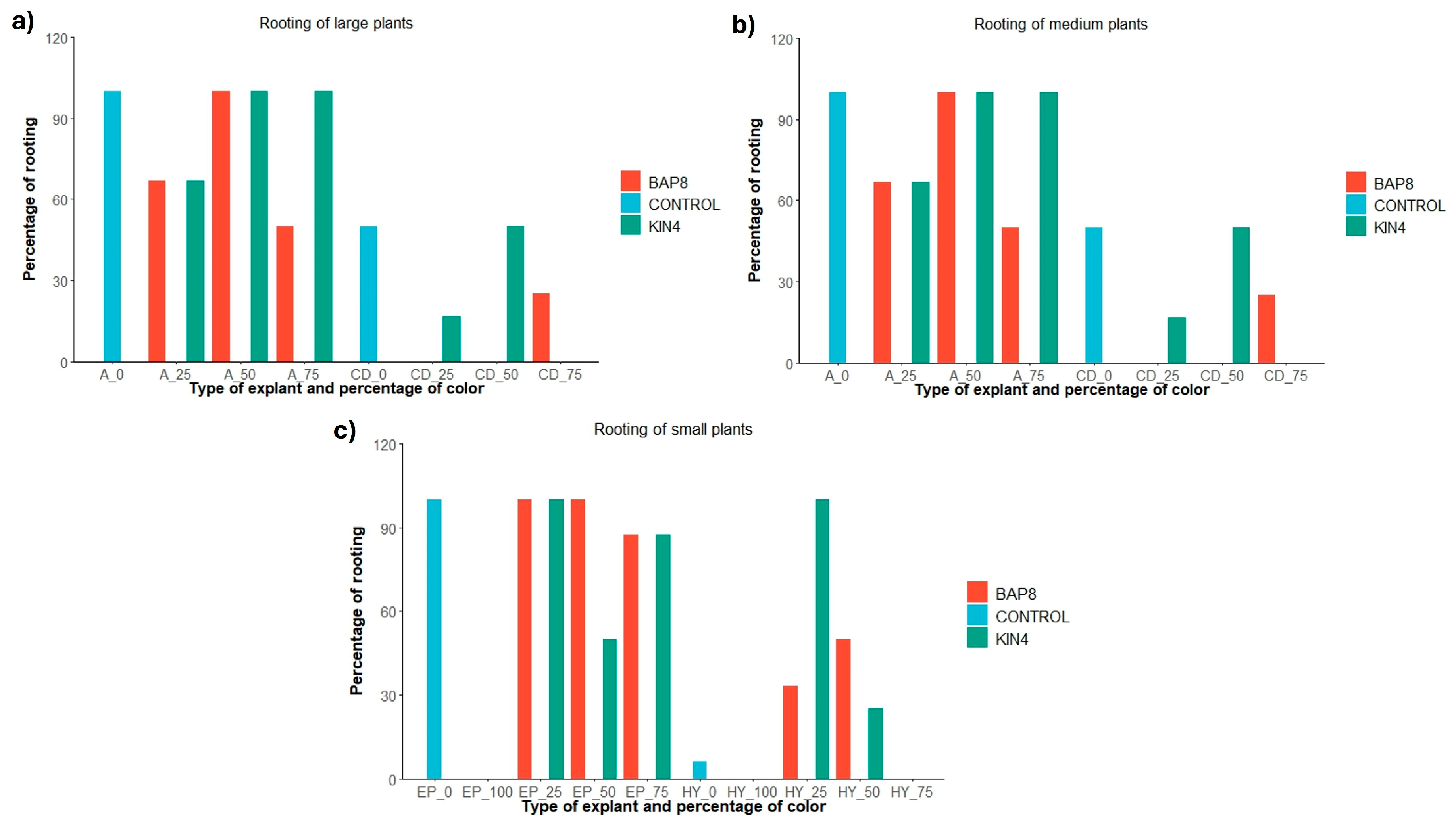
2.2. Root Emergence
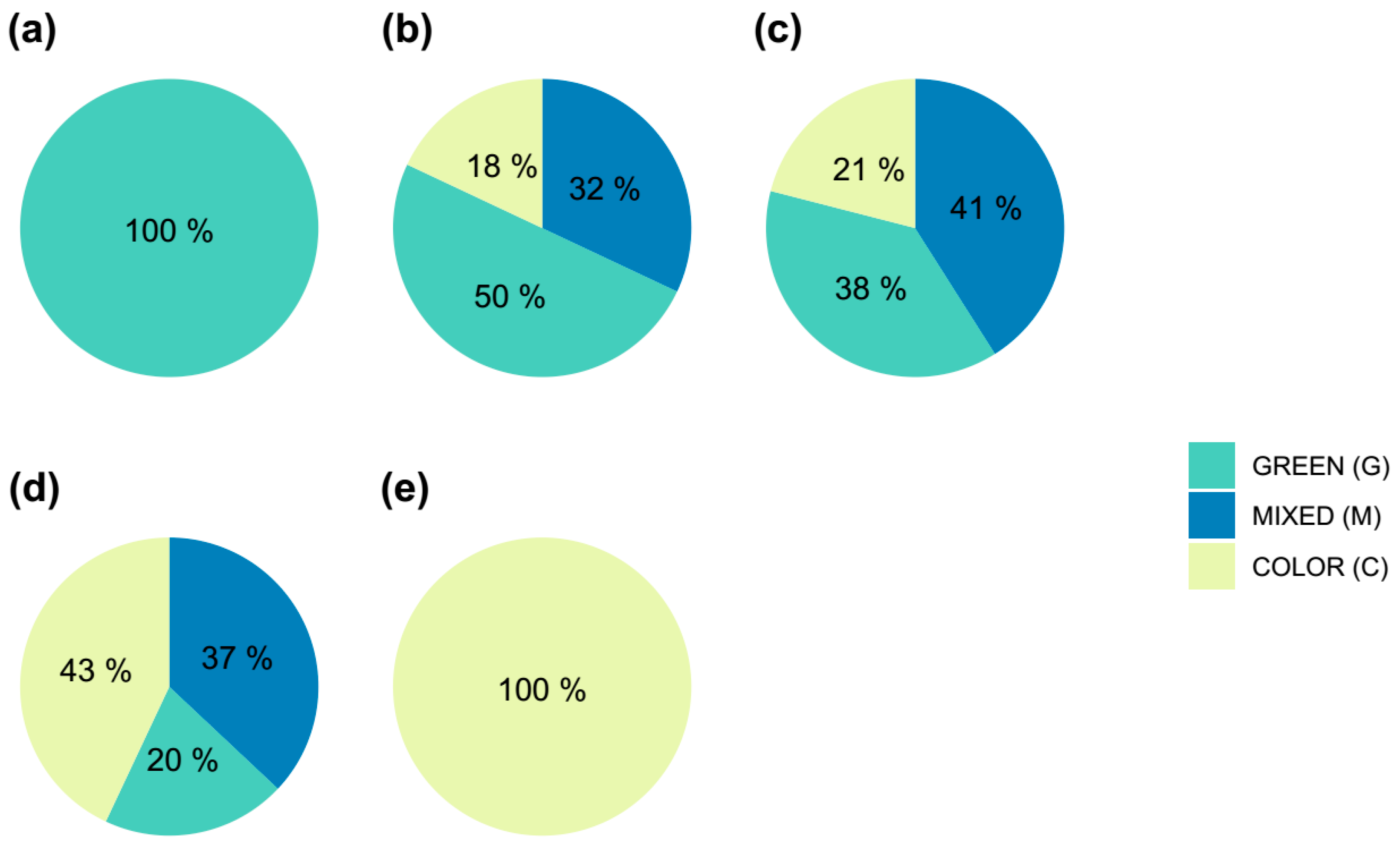
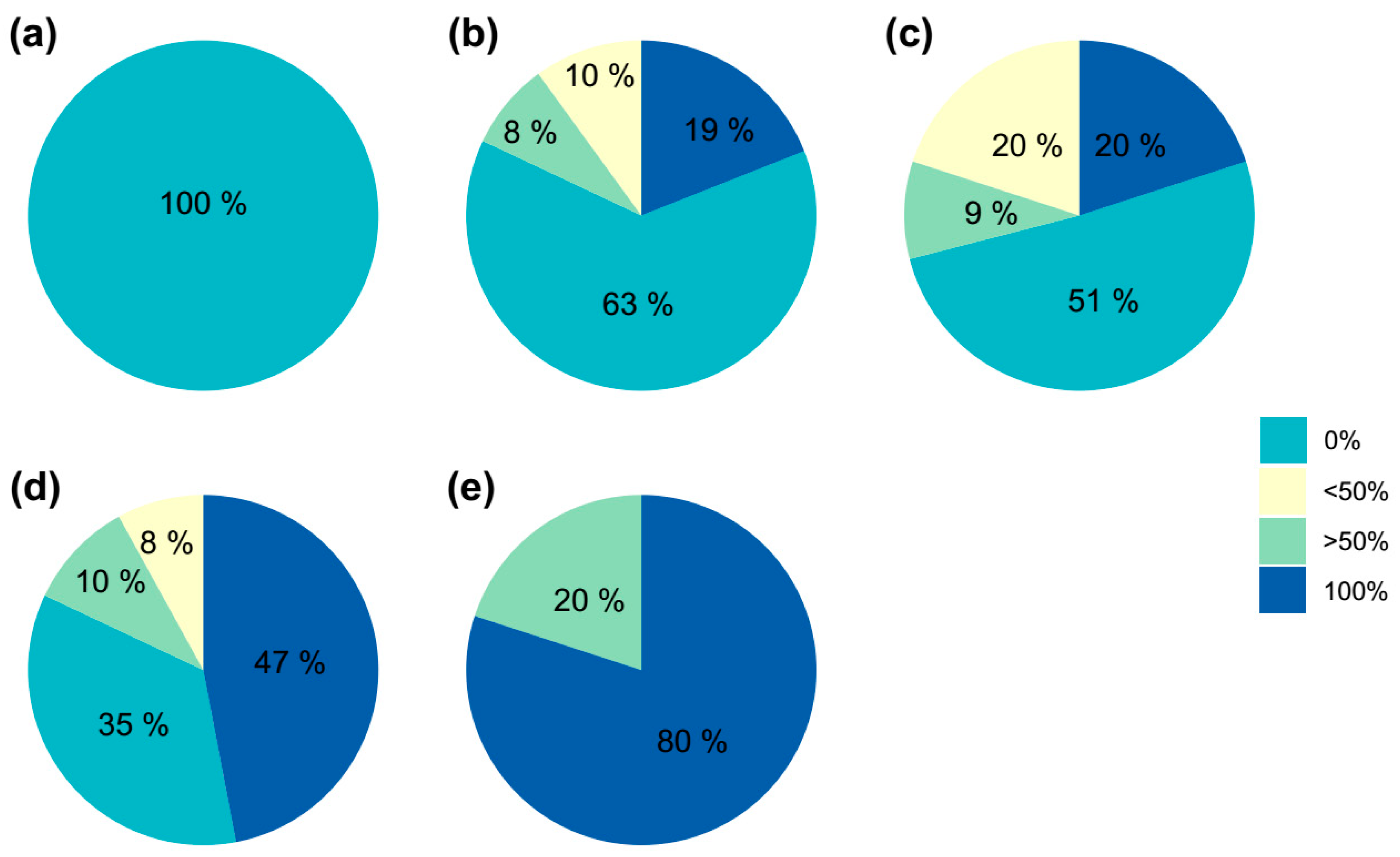
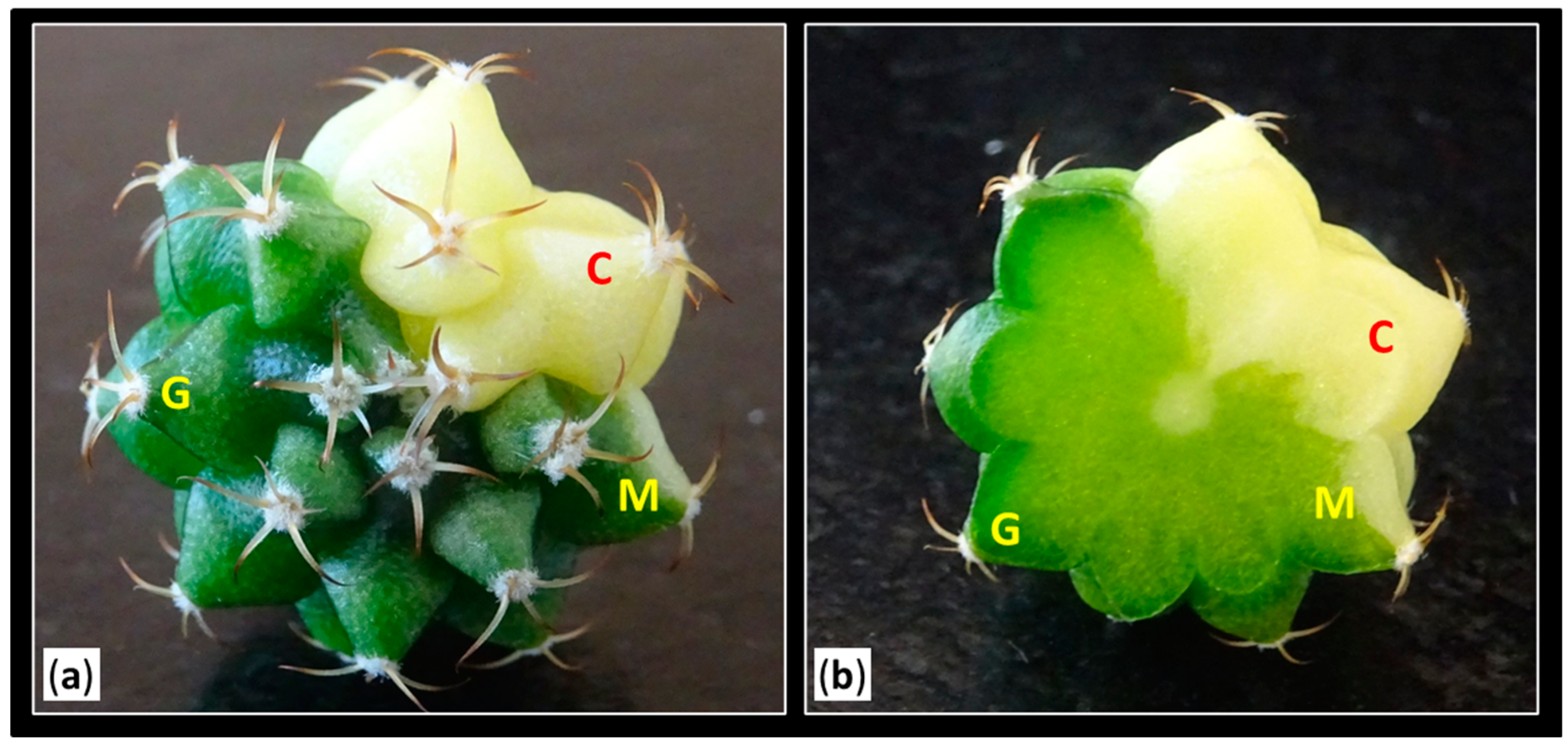
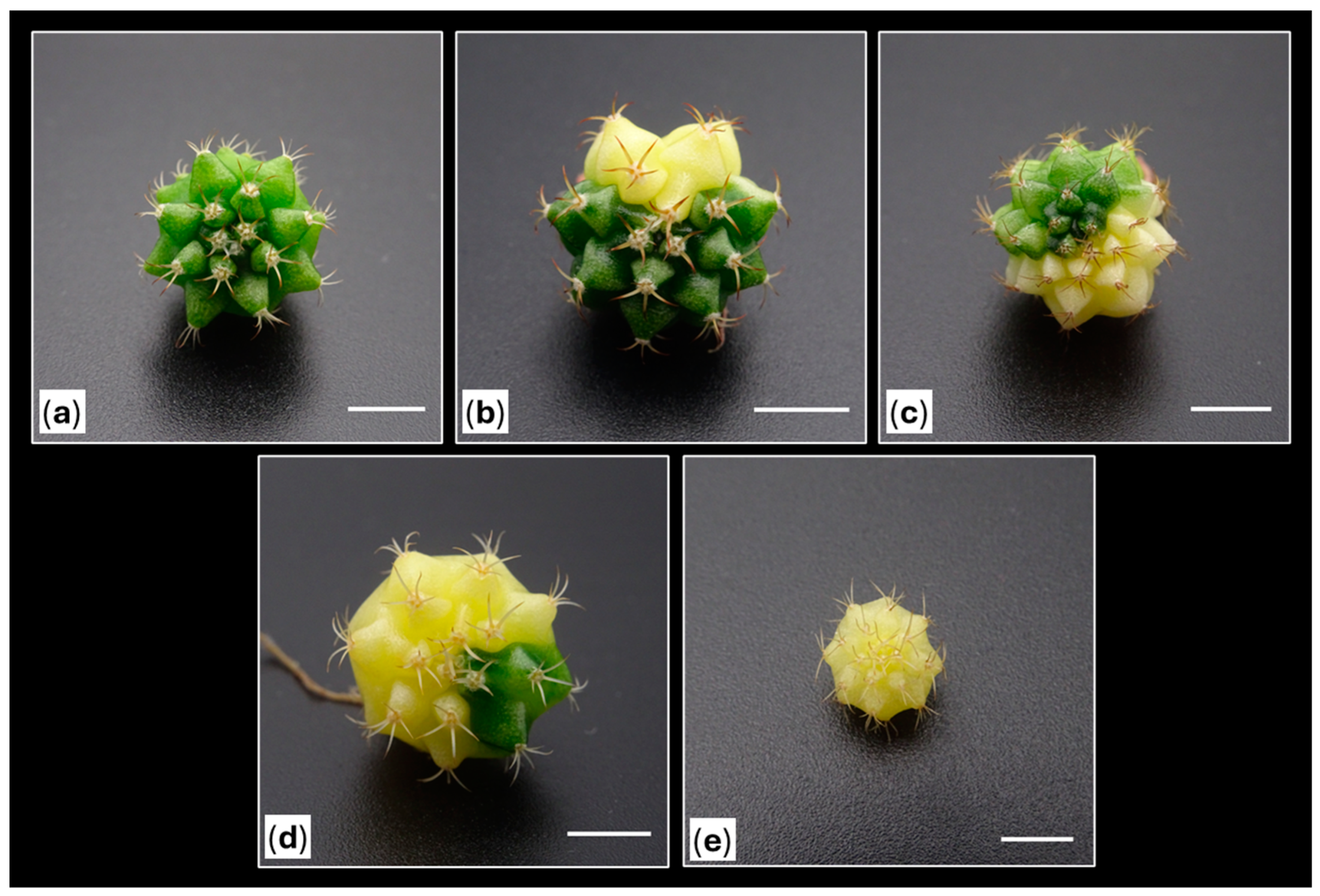
2.3. Color Evaluation of the Obtained Shoots
2.4. Acclimatization of the Obtained Shoots
3. Materials and Methods
3.1. Plant Material and Disinfection
3.2. In Vitro Establishment and Culture Conditions
3.3. Type of Explants
3.4. Shoot Induction and Tissue Culture Conditions
3.5. Experimental Design
Areole Evaluation
3.6. Statistical Analysis
- -
- For numerical and absolute data, including shoot emission, callus production, and averages.
- -
- For frequency and efficiency values, including rooting capacity,
3.7. Acclimatization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Henny, R.J. Somaclonal variation: An important source for cultivar development of floriculture crops. Floric. Ornam. Plant Biotechnol. 2006, 2, 244–253. [Google Scholar]
- Dole, J.; Wilkins, H. Floriculture: Principles and Species; Prentice Hall: Saddle River, NJ, USA, 1999; ISBN 0133747034. [Google Scholar]
- Chen, J.; McConnell, D.; Norman, D.; Henny, R. The foliage plant industry. Hortic. Rev. 2005, 31, 47–112. [Google Scholar]
- Pilbeam, J. Gymnocalycium a Collector’s Guide; A.A. Balkema Publishers: Rotterdam, The Netherlands, 1995. [Google Scholar]
- Kamalzade, N.; Miri, S.M.; Ghazijahani, N. Development of Graft Union Formation and Histological Observations in Cactus Influenced by Benzyladenine, Grafting Method and Rootstock. J. Ornam. Plants 2021, 1, 43–45. [Google Scholar]
- Giusti, P.; Vitti, D.; Fiocchetti, F.; Colla, G.; Saccardo, F.; Tucci, M. In vitro propagation of three endangered cactus species. Sci. Hortic. 2002, 95, 319–332. [Google Scholar]
- Torres-Silva, G.; Correia, L.N.F.; Koehler, A.D.; Batista, D.S.; Faria, D.V.; Resende, S.V.; Strickler, S.R.; Fouracre, J.; Romanel, E.; Specht, C.D.; et al. Expression of Melocactus glaucescens SERK1 sheds new light on the mechanism of areolar activation in cacti. Plant Cell Tissue Organ Cult. 2021, 147, 437–451. [Google Scholar]
- Pérez-Molphe-Balch, E.; Santos-Díaz, M.D.S.; Ramírez-Malagón, R.; Ochoa-Alejo, N. Tissue culture of ornamental cacti. Sci. Agric. 2015, 72, 540–561. [Google Scholar]
- Jeong, M.I.; Cho, C.-H.; Lee, J.-M. Production and Breeding of Cacti for Grafting in Korea. Chron. Horticult. 2004, 44, 7–10. [Google Scholar]
- Vidican, I.T.; Lazar, A.N.; Iancu, C.V.; Carbunar, M.M.; Vidican, O.M. Comparative Study on the Regenerative and Organogenic Capacity of Echinocactus (Pfiff.) Mihanovichii Explants, in the Presence in the Culture Medium of 2.5 mg/L of 3-Indolylbutyric Acid (AIB) and 2.5 mg/L of 2,4-Dichlorophenoxyacetic Acid (2, 4D). Annals of the University of Oradea, Fascicle: Environmental Protection. 2022. Available online: https://protmed.uoradea.ro/facultate/publicatii/protectia_mediului/2022B/hort/04.%20Vidican%20Teodora%202.pdf (accessed on 22 January 2025).
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar]
- Bhatia, S. Plant Tissue Culture. In Modern Applications of Plant Biotechnology in Pharmaceutical Sciences; Academic Press: Cambridge, MA, USA, 2015; pp. 31–107. [Google Scholar]
- Bouzroud, S.; El Maaiden, E.; Sobeh, M.; Devkota, K.P.; Boukcim, H.; Kouisni, L.; El Kharrassi, Y. Micropropagation of Opuntia and other cacti species through axillary shoot proliferation: A Comprehensive Review. Front. Plant Sci. 2022, 13, 926653. [Google Scholar]
- Lopez-Granero, M.; Arana, A.; Regalado, J.J.; Encina, C.L. Mass micropropagation and in vitro fowering of Mammillaria vetula ssp. gracilis var. Arizonica Snowcap. Plant Cell Tissue Organ Cult. 2023, 155, 759–771. [Google Scholar]
- Manokari, M.; Faisal, M.; Alatar, A.A.; Shekhawat, M.S. Optimization of in vitro regeneration of Ferocactus peninsulae (Barrel Cactus) through transverse thin cell layer (tTCL) culture: A strategy for large-scale propagation. Plant Cell Tissue Organ Cult. 2024, 159, 48. [Google Scholar]
- Mulas, M.; D’Hallewin, G.; Pellizaro, G.; Spano, D. Rooting of Opuntia ficus-indica Mill. young cladodes. Adv. Hortic. Sci. 1992, 6, 44–46. [Google Scholar]
- Estrada-Luna, A.A.; de Jesús Martínez-Hernández, J.; Torres-Torres, M.E.; Chablé-Moreno, F. In vitro micropropagation of the ornamental prickly pear cactus Opuntia lanigera Salm–Dyck and effects of sprayed GA3 after transplantation to ex vitro conditions. Sci. Hortic. 2008, 117, 378–385. [Google Scholar]
- Ghaffari, A.; Hasanloo, T.; Nekouei, M. Micropropagation of tuna (Opuntia ficus-indica) and effect of medium composition on proliferation and rooting. Int. J. Biosci. 2013, 3, 129–139. [Google Scholar]
- Radi, H.; Bouchiha, F.; El Maataoui, S.; Oubassou, E.Z.; Rham, I.; Alfeddy, M.N.; Aissam, S.; Mazri, M.A. Morphological and physio-biochemical responses of cactus pear (Opuntia ficus indica (L.) Mill.) organogenic cultures to salt and drought stresses induced in vitro. Plant Cell Tissue Organ Cult. 2023, 154, 337–350. [Google Scholar]
- Marhri, A.; Tikent, A.; Garros, L.; Merah, O.; Elamrani, A.; Hano, C.; Abid, M.; Addi, M. Rapid and Efficient In vitro Propagation Protocol of Endangered Wild Prickly Pear Growing in Eastern Morocco. Horticulturae 2023, 9, 491. [Google Scholar] [CrossRef]
- Fan, Q.J.; Zheng, S.C.; Yan, F.X.; Zhang, B.X.; Qiao, G.; Wen, X.P. Efficient regeneration of dragon fruit (Hylocereus undatus) and an assessment of the genetic fidelity of in vitro-derived plants using ISSR markers. J. Hortic. Sci. Biotechnol. 2015, 88, 631–637. [Google Scholar]
- Martínez-Arroyo, M.C.; Mancilla-Álvarez, E.; Spinoso-Castillo, J.L.; Bello-Bello, J.J. Evaluation of the effect of different culture systems on photomixotrophic capacity during in vitro multiplication of pitahaya (Hylocereus undatus). S. Afr. J. Bot. 2023, 159, 396–404. [Google Scholar]
- Oo, K.T.; Lynn, Z.M.; Oo, K.Z.; Htwe, M.Y.; Htet, W.T.; Soe, W.W.; Tun, W. In vitro Propagation of Three Pitaya Varieties (Hylocereus undatus, Hylocereus polyrhizus and Hylocereus megalanthus) with the Use of Different BAP Concentrations. J. Sci. Innov. Res. 2023, 12, 33–39. [Google Scholar]
- Everani, M. The History of Research on White-Green Variegated Plants. Bot. Rev. 1989, 55, 106–139. [Google Scholar]
- Cortés-Olmos, C.; Guerra-Sandoval, V.M.; Blanca-Giménez, V.; Rodríguez-Burruezo, A. Micropropagation and Acclimatization of Gymnocalycium cv. Fancy (Cactaceae): Developmental Responses to Different Explant Types and Hormone Conditions. Plants 2023, 12, 3932. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Zhang, X.S. The hormonal control of regeneration in plants. Curr. Top. Dev. Biol. 2014, 108, 35–69. [Google Scholar] [PubMed]
- Hu, W.; Fagundez, S.; Katin-Grazzini, L.; Li, Y.; Li, W.; Chen, Y.; Wang, X.; Deng, Z.; Xie, S.; McAvoy, R.J.; et al. Endogenous auxin and its manipulation influence in vitro shoot organogenesis of citrus epicotyl explants. Hortic. Res. 2017, 4, 17071. [Google Scholar] [CrossRef] [PubMed]
- Raspor, M.; Motyka, V.; Kaleri, A.R.; Ninković, S.; Tubić, L.; Cingel, A.; Ćosić, T. Integrating the roles for cytokinin and auxin in de Novo shoot organogenesis: From hormone uptake to signaling outputs. Int. J. Mol. Sci. 2021, 22, 8554. [Google Scholar] [CrossRef]
- Villavicencio Gutiérrez, E.E.; González Cortés, A.; Carranza Pérez, M.A. Micropropagation of Epithelantha micromeris (Engelm.) FAC Weber ex Britt. & Rose, an ornamental cactus and phytogenetic resource of the Chihuahuan Desert. Mex. J. For. Sci. 2012, 3, 83–102. [Google Scholar]
- Lin, R.-S. Studies on tissue culture of cactus (Gymnocalycium minansvichii Var.). Agric. Lab. 1982, 31, 220–224. [Google Scholar]
- Nitesh, K.; Vishal, S.; Satvaan, S.; Rohit, G.; Piyush, S.; Vinay, D.; Mohd, W.; Manoj, K.P. A Comprehensive Review on Role of Plant Tissue Culture in Ornamental Crops: Cultivation Factors, Applications and Future Aspects. Int. J. Environ. Clim. Change 2023, 13, 1802–1815. [Google Scholar]
- Shahab, S.; Seied, M.M.; Noushin, G. Callus induction from in vitro cultured leaf, hypocotyl and root of Hyssopus officinalis. In Proceedings of the 1st National Conference on the Application of Advancedchemical and Agricultural Research for Development of Medicinal Plants, Nahavand, Iran, 9–10 March 2021. [Google Scholar]
- Dar, S.A.; Nawchoo, I.A.; Tyub, S.; Kamili, A.N. Effect of plant growth regulators on in vitro induction and maintenance of callus from leaf and root explants of Atropa acuminata Royal ex Lindl. Biotechnol. Rep. 2021, 32, e00688. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; Nishikawa, T.; Shea, D.J.; Okazaki, K. Breeding of lilies and tulips-Interspecific hybridization and genetic background. Breed. Sci. 2018, 68, 35–52. [Google Scholar] [CrossRef]
- Alatar, A.A. Thidiazuron induced efficient in vitro multiplication and ex vitro conservation of Rauvolfia serpentina—A potent antihypertensive drug producing plant. Biotechnol. Biotechnol. Equip. 2015, 29, 489–497. [Google Scholar] [CrossRef]
- Vyskot, B.; JáRa, Z. Clonal propagation of cacti through axillary buds in vitro. J. Hortic. Sci. 1984, 59, 449–452. [Google Scholar]
- Martínez-Vázquez, O.; Rubluo, A. In-vitro mass propagation of the near-extinct Mammillaria san-angelensis Sánchez-Mejorada. J. Hortic. Sci. 1989, 64, 99–105. [Google Scholar]
- Lema-Rumińska, J.; Kulus, D. Micropropagation of Cacti—A Review. Haseltonia 2014, 2014, 46–63. [Google Scholar]
- Kaviani, B. Some Useful Information about Micropropagation. J. Ornam. Plants 2015, 5, 29–40. [Google Scholar]
- Mohamed-Yasseen, Y. Micropropagation of pitaya (Hylocereus undatus Britton et Rose). In Vitro Cell. Dev. Biol. Plant 2002, 38, 427–429. [Google Scholar]
- Rouinsard, A.; Hamama, L.; Hibrand-Saint Oyant, L.; Grapin, A. Effects of the in vitro behavior of micropropagated plants on the stability of variegation in Yucca gloriosa, Phormium tenax, and Cordyline australis cultivars. Sci. Hortic. 2021, 287, 110115. [Google Scholar]
- Viñas, M.; Fernández-Brenes, M.; Azofeifa, A.; Jiménez, V.M. In vitro propagation of purple pitahaya (Hylocereus costaricensis [F.A.C. Weber] Britton & Rose) cv. Cebra. In Vitro Cell. Dev. Biol. Plant 2012, 48, 469–477. [Google Scholar]
- Lázaro-Castellanos, J.O.; Mata-Rosas, M.; González, D.; Arias, S.; Reverchon, F. In vitro propagation of endangered Mammillaria genus (Cactaceae) species and genetic stability assessment using SSR markers. In Vitro Cell. Dev. Biol. Plant 2018, 54, 518–529. [Google Scholar] [CrossRef]
- Li, H.; Murch, S.J.; Saxena, P.K. Thidiazuron induced de novo shoot organogenesis on seedlings, etiolated hypocotyls and stem segments of Huang-qin. Plant Cell Tissue Organ Cult. 2000, 62, 169–173. [Google Scholar]
- Ahmad, N.; Siddique, I.; Anis, M. Improved plant regeneration in Capsicum annuum L. from nodal segments. Biol. Plant. 2006, 50, 701–704. [Google Scholar]
- Guo, B.; Abbasi, B.H.; Zeb, A.; Xu, L.L.; Wei, Y.H. Thidiazuron: A multi-dimensional plant growth regulator. Afr. J. Biotechnol. 2011, 10, 8984–9000. [Google Scholar]
- Zhou, J.; Ma, H.; Guo, F.; Luo, X. Effect of thidiazuron on somatic embryogenesis of Cayratia japonica. Plant Cell Tissue Organ Cult. 1994, 36, 73–79. [Google Scholar]
- Sankhla, D.; Davis, T.D.; Sankhla, N. Thidiazuron-induced in vitro shoot formation from roots of intact seedlings of Albizzia julibrissin. Plant Growth Regul. 1994, 14, 267–272. [Google Scholar]
- McClelland, M.T.; Smith, M.A.L.; Carothers, Z.B. The effects of in vitro and ex vitro root initiation on subsequent microcutting root quality in three woody plants. Plant Cell Tissue Organ Cult. 1990, 23, 115–123. [Google Scholar]
- Amghar, I.; Ibriz, M.; Ibrahimi, M.; Boudra, A.; Gaboun, F.; Meziani, R.; Iraqi, D.; Mazri, M.A.; Diria, G.; Abdelwahd, R. In vitro root induction from Argan (Argania spinosa (L.) Skeels) adventitious shoots: Influence of ammonium nitrate, auxins, silver nitrate and putrescine, and evaluation of plantlet acclimatization. Plants 2021, 10, 1062. [Google Scholar] [CrossRef]
- Fenning, T.; O’Donnell, M.; Preedy, K.; Bézanger, A.; Kenyon, D.; Lopez, G. The rooting ability of in vitro shoot cultures established from a UK collection of the common ash (Fraxinus excelsior L.) and their ex vitro survival. Ann. For. Sci. 2022, 79, 30. [Google Scholar]
- Pai, S.R.; Desai, N.S. Effect of TDZ on various plant cultures. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Springer: Singapore, 2018; pp. 439–454. [Google Scholar]
- Rubluo, A.; Marín-Hernández, T.; Duval, K.; Vargas, A.; Márquez-Guzmán, J. Auxin induced morphogenetic responses in long-term in vitro subcultured Mammillaria san-angelensis Sánchez-Mejorada (Cactaceae). Sci. Hortic. 2002, 95, 341–349. [Google Scholar]
- Retes-Pruneda, J.L.; de Lourdes Valadez-Aguilar, M.; Pérez-Reyes, M.E.; Pérez-Molphe-Balch, E. In vitro propagation of Echinocereus, Escontria, Mammillaria, Melocactus and Polaskia species (Cactaceae). Bot. Sci. 2007, 9–16. [Google Scholar]
- Su, Y.H.; Liu, Y.B.; Zhang, X.S. Auxin cytokinin interaction regulates meristem development. Mol. Plant 2011, 4, 616–625. [Google Scholar]
- Otiende, M.A.; Fricke, K.; Nyabundi, J.O.; Ngamau, K.; Hajirezaei, M.R.; Druege, U. Involvement of the auxin-cytokinin homeostasis in adventitious root formation of rose cuttings as affected by their nodal position in the stock plant. Planta 2021, 254, 65. [Google Scholar]
- Duarte-Aké, F.; De-la-Peña, C. High cytokinin concentration and nutrient starvation trigger DNA methylation changes in somaclonal variants of Agave angustifolia Haw. Ind. Crops Prod. 2021, 172, 114046. [Google Scholar]
- Duarte-Aké, F.; Castillo-Castro, E.; Pool, F.B.; Espadas, F.; Santamaría, J.M.; Robert, M.L.; De-La-peña, C. Physiological differences and changes in global DNA methylation levels in agave angustifolia haw. Albino variant somaclones during the micropropagation process. Plant Cell Rep. 2016, 35, 2489–2502. [Google Scholar] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar]
- Teixeira, S.L.; Ribeiro, J.M.; Teixeira, M.T. Influence of NaClO on nutrient medium sterilization and on pineapple (Ananas comosus cv Smooth cayenne) behavior. Plant Cell Tissue Organ Cult. 2006, 86, 375–378. [Google Scholar]


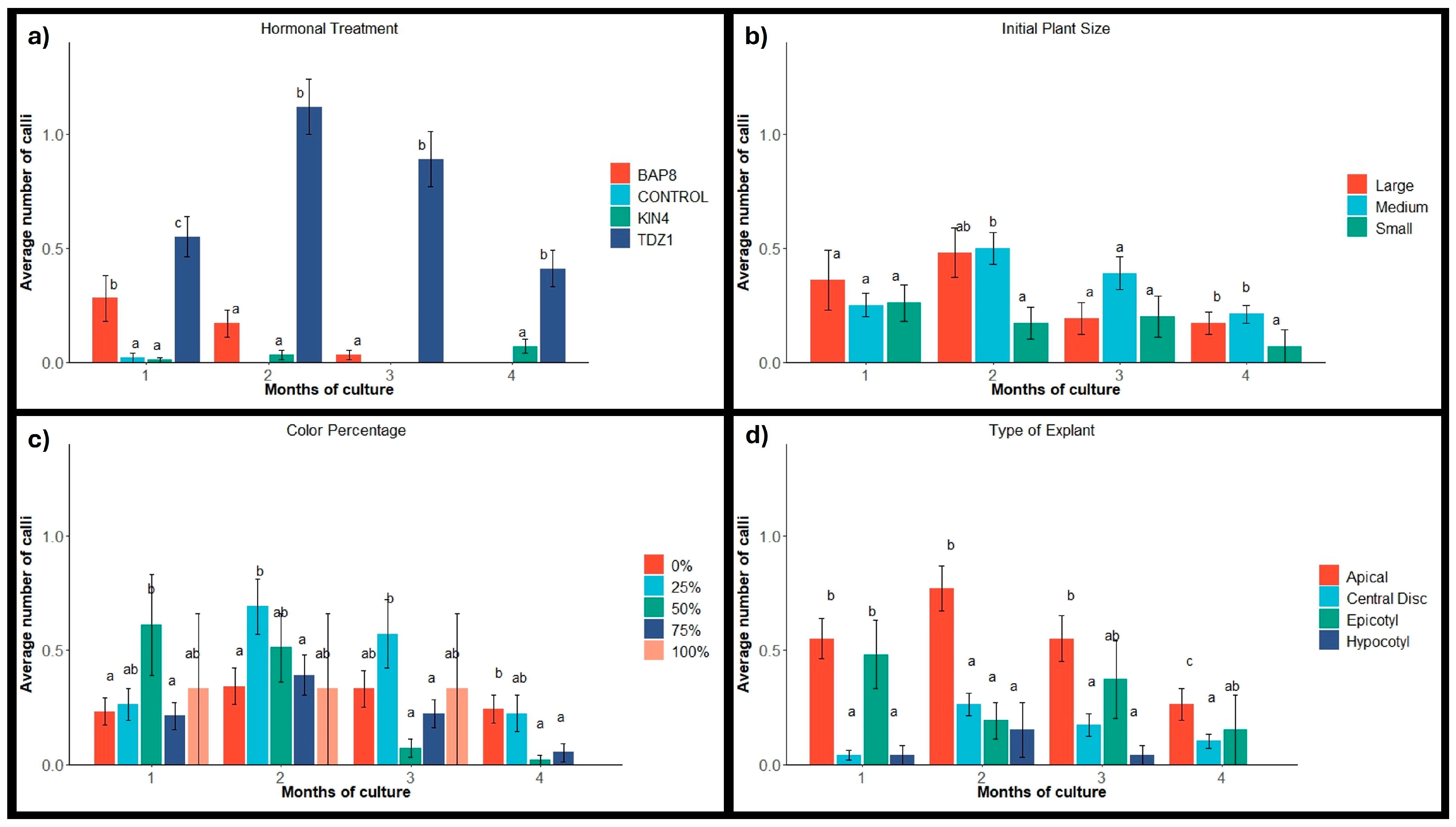
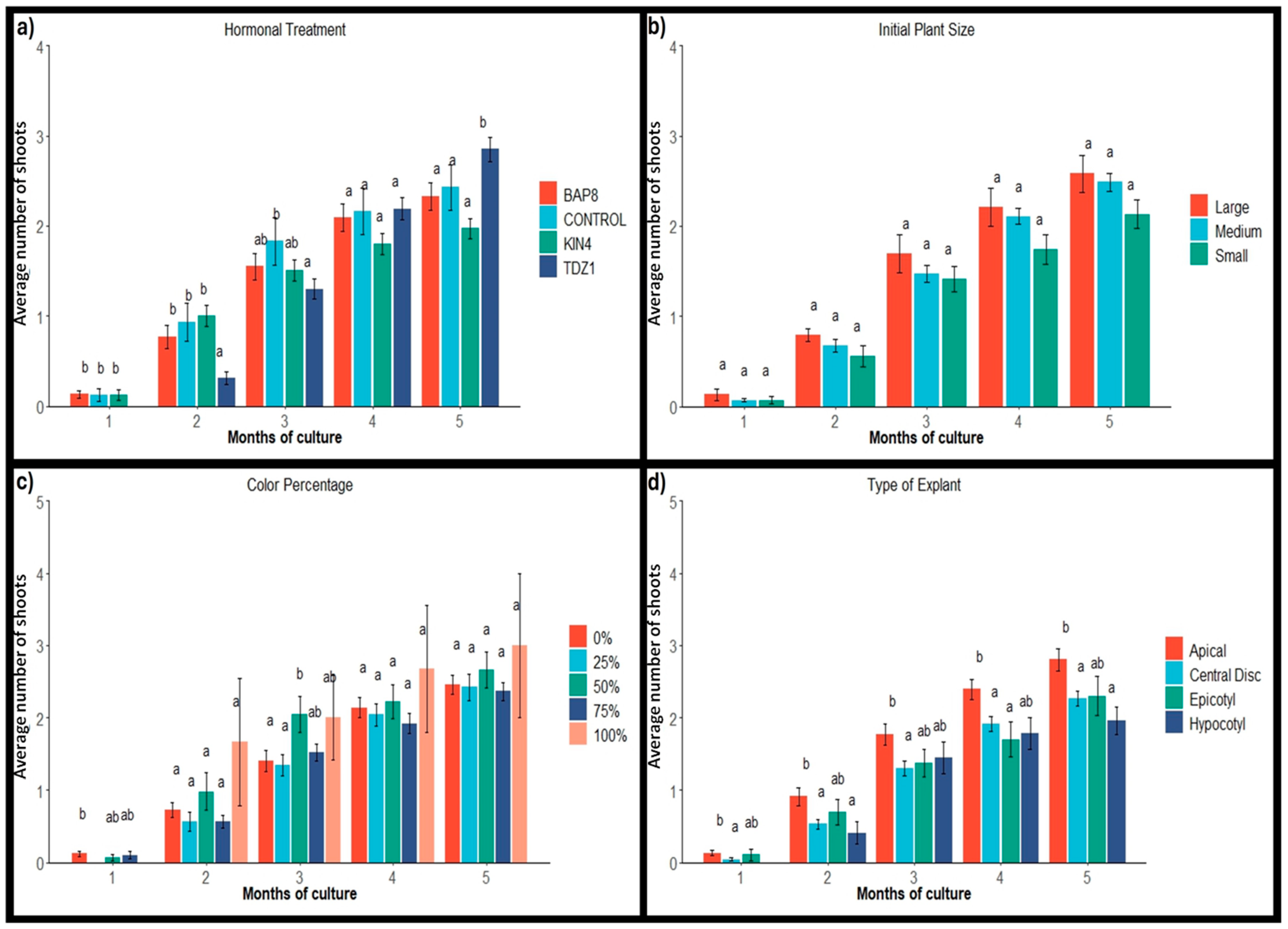
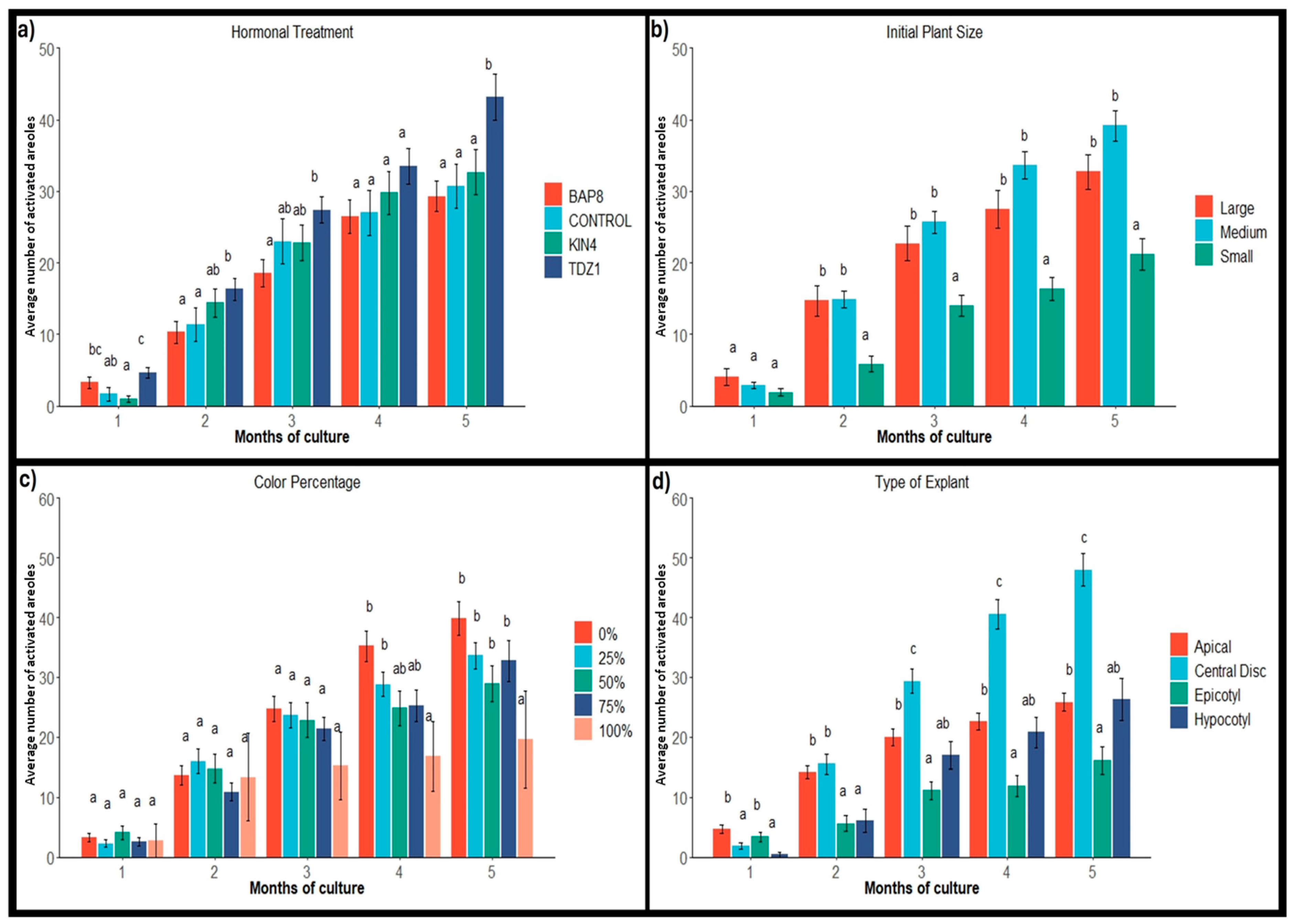


| Factor | Total Number of Explants | Activated Explants | % of Response (1) |
|---|---|---|---|
| Treatment (2) | |||
| BAP8 | 152 | 92 | 60.53 a |
| KIN4 | 150 | 76 | 50.67 a |
| TDZ1 | 148 | 123 | 83.11 b |
| CONTROL | 124 | 58 | 46.77 a |
| Plant Size | |||
| Large | 96 | 67 | 69.79 b |
| Medium | 332 | 228 | 68.67 b |
| Small | 146 | 54 | 36.99 a |
| % of Color | |||
| 0 | 220 | 136 | 61.82 b |
| 25 | 110 | 74 | 67.27 b |
| 50 | 60 | 41 | 68.33 b |
| 75 | 156 | 95 | 60.90 b |
| 100 | 28 | 3 | 10.71 a |
| Type of Explant | |||
| Apical | 214 | 137 | 64.02 b |
| Central Disc | 214 | 158 | 73.83 c |
| Epicotyl | 73 | 27 | 36.99 a |
| Hypocotyl | 73 | 27 | 36.99 a |
| Total | 574 | 349 | 60.8 |
| Factor | Cases | Average (1) |
|---|---|---|
| Plant Size | ||
| Large | 96 | 9.30 ± 0.43 a |
| Medium | 332 | 9.37 ± 0.27 a |
| Small | 146 | 10.21 ± 0.54 a |
| Type of Explant | ||
| Apical | 214 | 13.15 ± 0.25 b |
| Central Disc | 214 | 5.51 ± 0.13 a |
| Epicotyl | 73 | 15.30 ± 0.51 b |
| Hypocotyl | 73 | 5.12 ± 0.43 a |
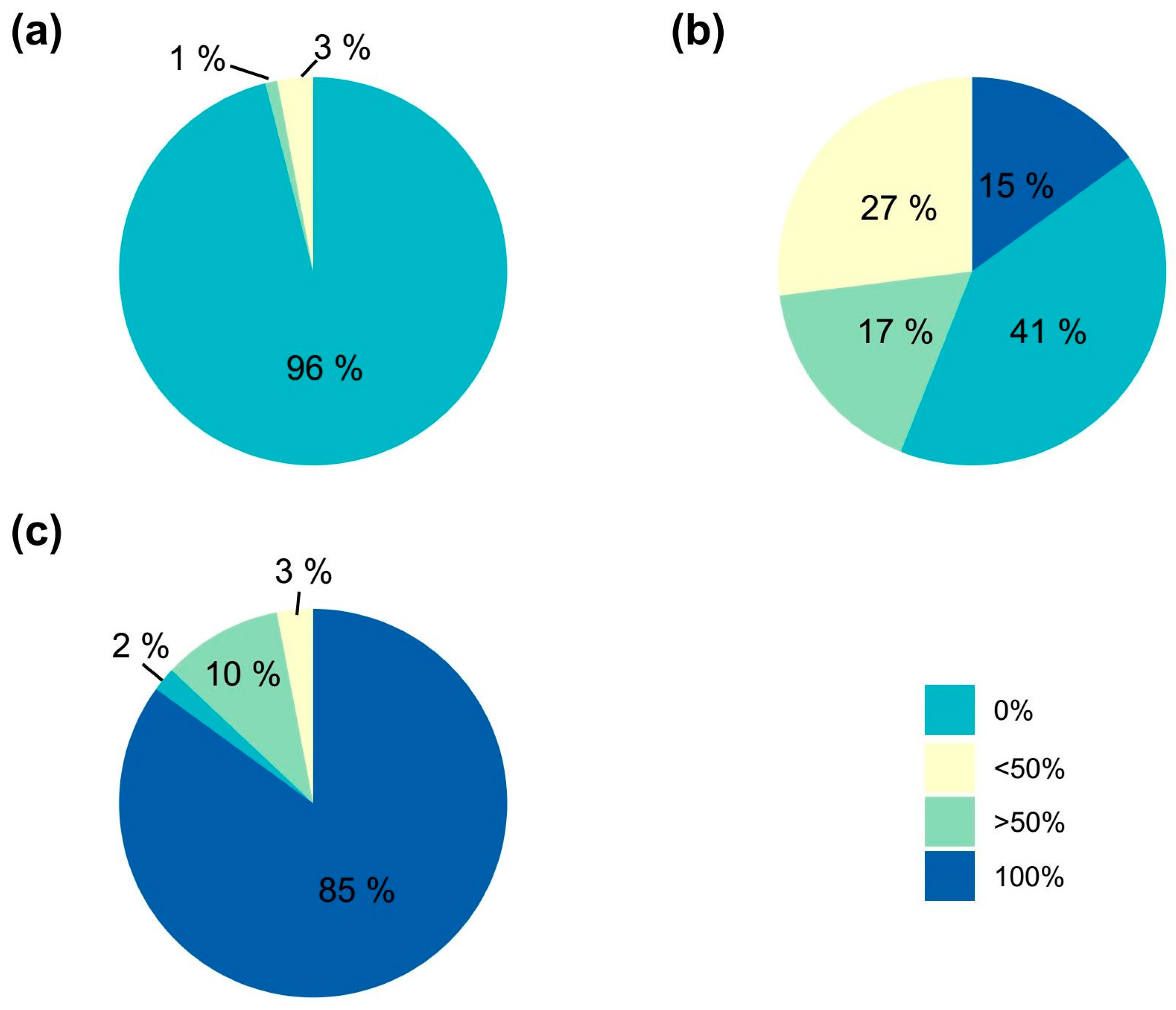
| % Color of the Original Plant | No. of Activated Areolas | Color of Activated Areola (1) | Percentage of Shoot Coloration | |||||
|---|---|---|---|---|---|---|---|---|
| C | M | G | 0% | <50% | >50% | 100% | ||
| 0 | 368 | 0 | 0 | 368 | 368 | 0 | 0 | 0 |
| 25 | 177 | 31 | 57 | 89 | 112 | 18 | 14 | 33 |
| 50 | 107 | 22 | 44 | 41 | 55 | 21 | 10 | 21 |
| 75 | 217 | 94 | 79 | 44 | 77 | 18 | 21 | 101 |
| 100 | 5 | 3 | 2 | 0 | 0 | 2 | 1 | 2 |
| Total | 874 | 150 | 182 | 542 | 612 | 59 | 46 | 157 |
| Interactions | p-Value |
|---|---|
| Color percentage of the initial plant × Color of the activated areolas | 0.000 |
| Color percentage of the initial plant × Color percentage of the obtained shoots | 0.000 |
| Color of the activated areolas × Color percentage of the obtained shoots | 0.000 |
| Hormonal treatment × Color of the activated areolas | 0.133 |
| Hormonal treatment × Color percentage of the obtained shoots | 0.766 |
| Type of Areola | Coloration of Shoots (1) | |||
|---|---|---|---|---|
| S0 | S1 | S2 | S3 | |
| Color (C) | 3 | 4 | 15 | 129 |
| Mixed (M) | 74 | 48 | 31 | 28 |
| Green (G) | 166 | 5 | 1 | 0 |
| Total | 243 | 57 | 47 | 157 |
| Total of shoots | 504 | |||
| Plant Size | % Color | No. Plants | No. of Explants Evaluated (1) | Treatment (2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | CD | EP | HYP | BAP8 | KIN4 | TDZ1 | CONTROL | |||
| Large (12–16 mm) | 0 | 7 | 14 | 14 | - | - | - | - | - | 28 |
| 25 | 9 | 18 | 18 | - | - | 12 | 12 | 12 | - | |
| 50 | 2 | 4 | 4 | - | - | 4 | 4 | - | - | |
| 75 | 6 | 12 | 12 | - | - | 8 | 8 | 8 | - | |
| Medium (8–12 mm) | 0 | 40 | 80 | 80 | - | - | 32 | 32 | 32 | 64 |
| 25 | 15 | 30 | 30 | - | - | 20 | 20 | 20 | - | |
| 50 | 7 | 14 | 14 | - | - | 8 | 8 | 12 | - | |
| 75 | 21 | 42 | 42 | - | - | 28 | 28 | 28 | - | |
| Small (4–8 mm) | 0 | 16 | - | - | 16 | 16 | - | - | - | 32 |
| 25 | 7 | - | - | 7 | 7 | 6 | 4 | 4 | - | |
| 50 | 12 | - | - | 12 | 12 | 8 | 8 | 8 | - | |
| 75 | 24 | - | - | 24 | 24 | 16 | 16 | 16 | - | |
| 100 | 14 | - | - | 14 | 14 | 10 | 10 | 8 | - | |
| Total for condition | 214 | 214 | 73 | 73 | 152 | 150 | 148 | 124 | ||
| Total trial | 180 | 574 | 574 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Olmos, C.; Guerra-Sandoval, V.M.; Guijarro-Real, C.; Pineda, B.; Fita, A.; Rodríguez-Burruezo, A. Response to In Vitro Micropropagation of Plants with Different Degrees of Variegation of the Commercial Gymnocalycium cv. Fancy (Cactaceae). Plants 2025, 14, 1091. https://doi.org/10.3390/plants14071091
Cortés-Olmos C, Guerra-Sandoval VM, Guijarro-Real C, Pineda B, Fita A, Rodríguez-Burruezo A. Response to In Vitro Micropropagation of Plants with Different Degrees of Variegation of the Commercial Gymnocalycium cv. Fancy (Cactaceae). Plants. 2025; 14(7):1091. https://doi.org/10.3390/plants14071091
Chicago/Turabian StyleCortés-Olmos, Carles, Vladimir Marín Guerra-Sandoval, Carla Guijarro-Real, Benito Pineda, Ana Fita, and Adrián Rodríguez-Burruezo. 2025. "Response to In Vitro Micropropagation of Plants with Different Degrees of Variegation of the Commercial Gymnocalycium cv. Fancy (Cactaceae)" Plants 14, no. 7: 1091. https://doi.org/10.3390/plants14071091
APA StyleCortés-Olmos, C., Guerra-Sandoval, V. M., Guijarro-Real, C., Pineda, B., Fita, A., & Rodríguez-Burruezo, A. (2025). Response to In Vitro Micropropagation of Plants with Different Degrees of Variegation of the Commercial Gymnocalycium cv. Fancy (Cactaceae). Plants, 14(7), 1091. https://doi.org/10.3390/plants14071091








