Geospatial Metabolomics Unravel Regional Disparities in Sedative Compounds and Volatile Profiles of Ziziphi Spinosae Semen Across Chinese Production Areas
Abstract
1. Introduction
2. Results
2.1. Metabolite Profiling and Classification of ZSS
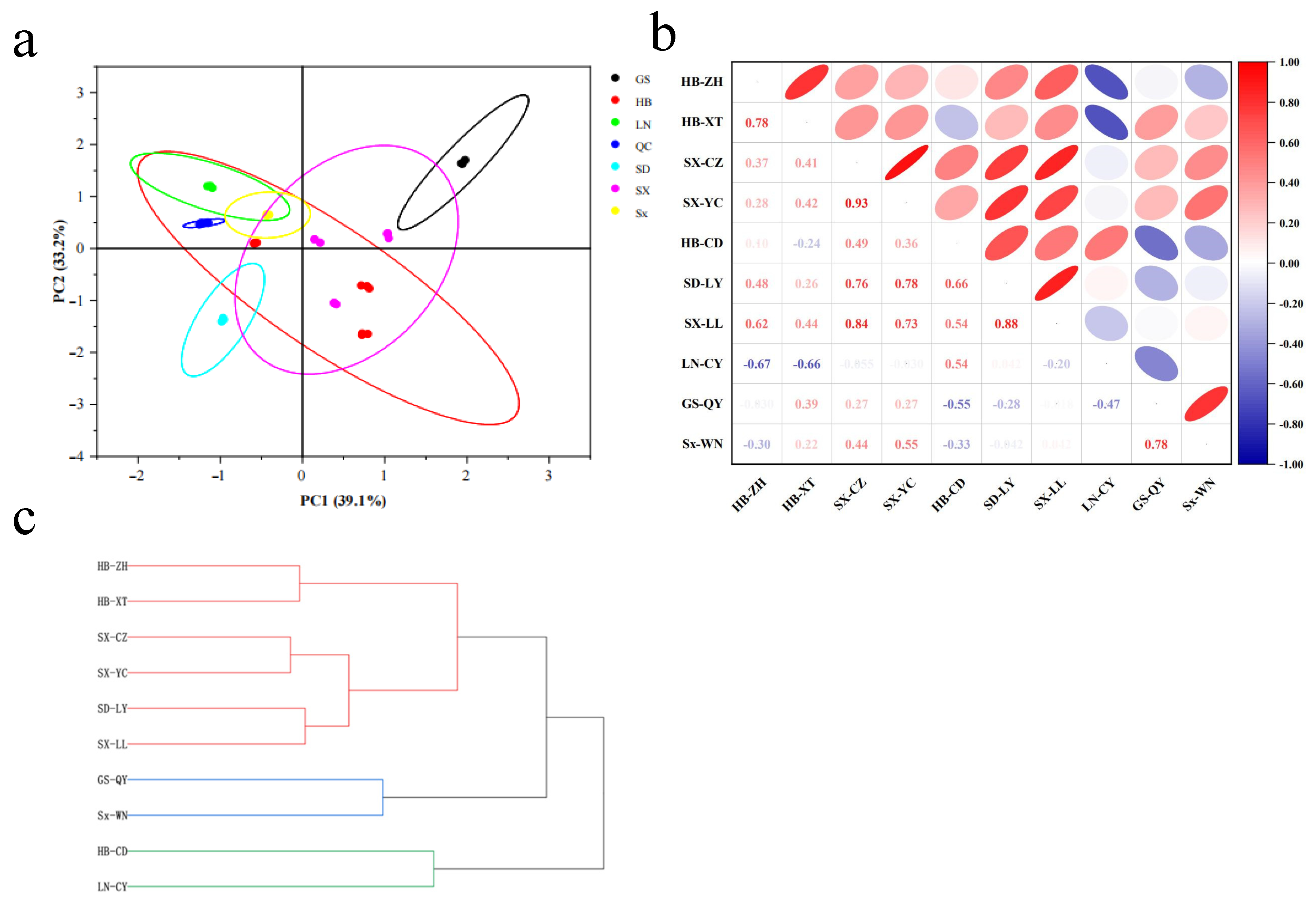
2.2. Geographic Variation in Metabolic Profiles
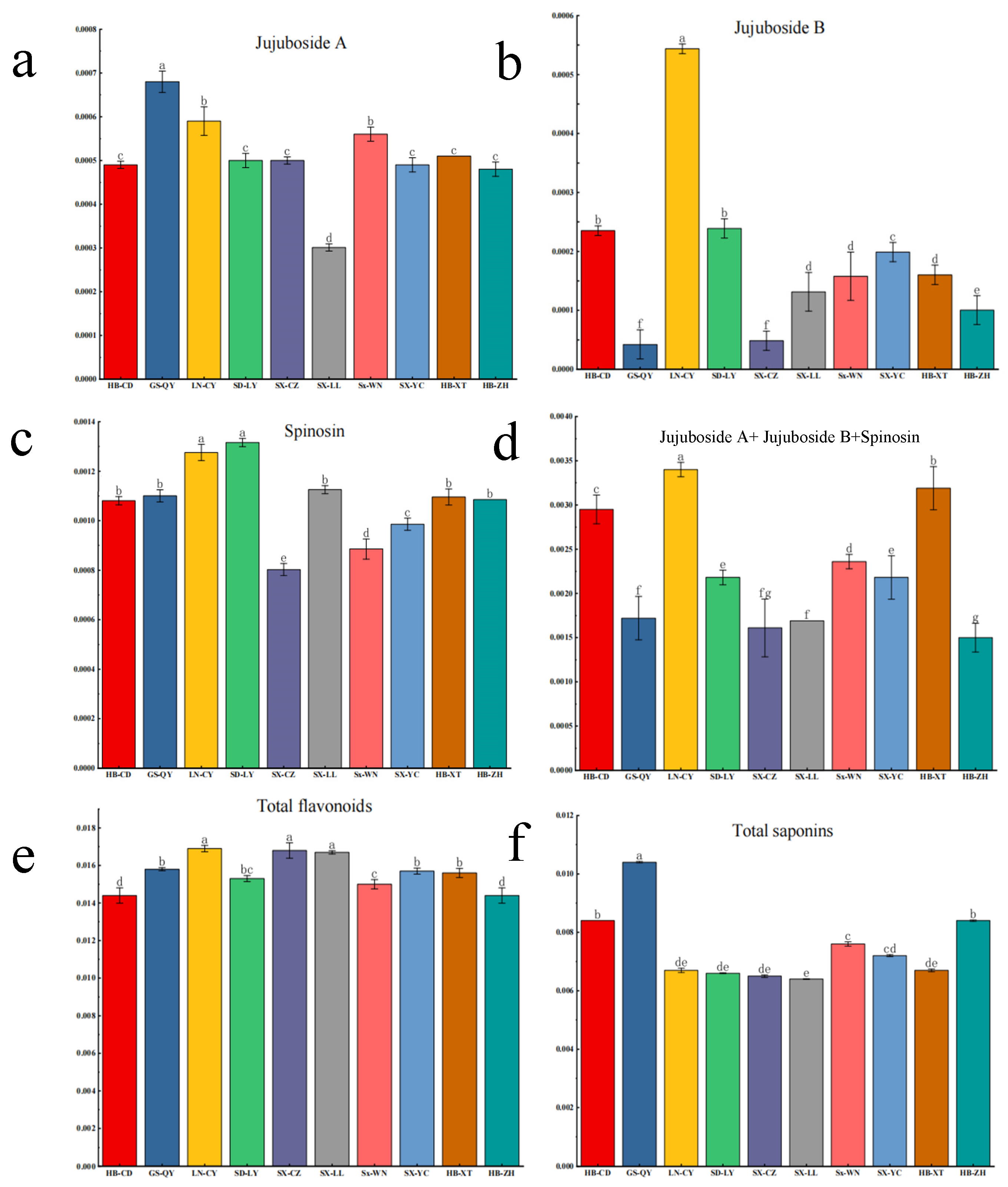
2.3. Quantification of Bioactive Compounds
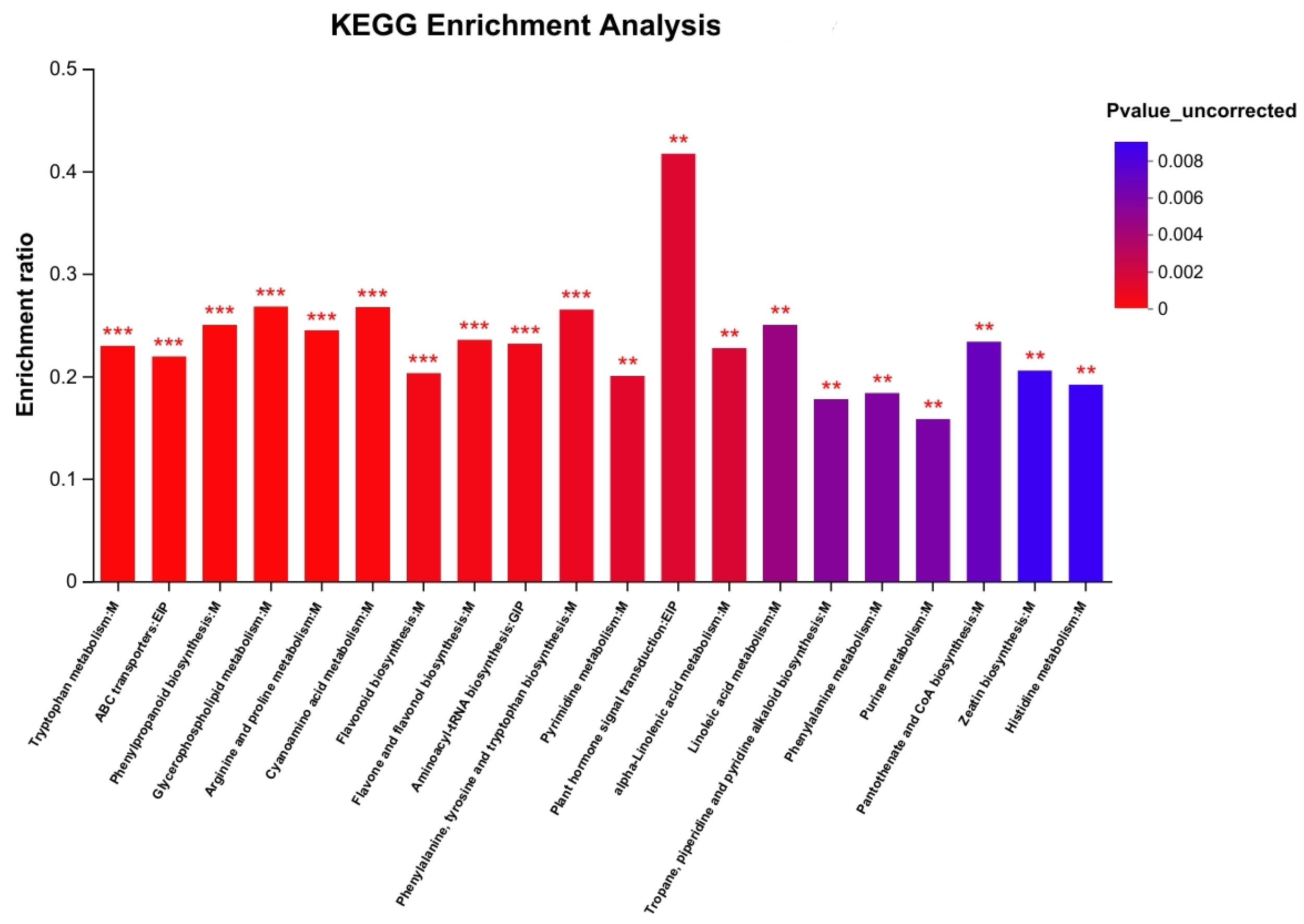
2.4. Metabolic Pathway Enrichment
2.5. Analysis of Volatile Organic Compounds in ZSS
3. Discussion
4. Materials and Methods
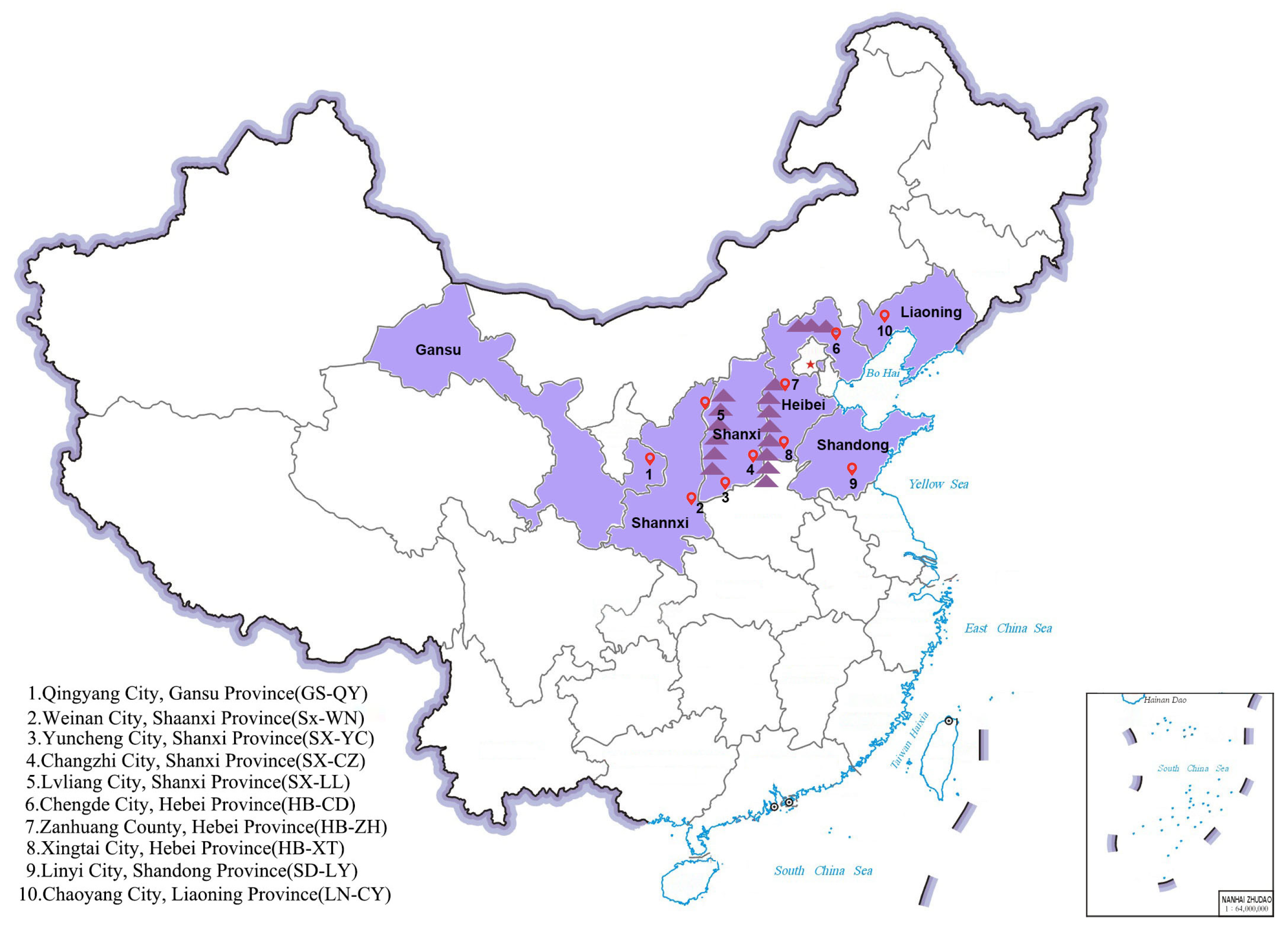
4.1. Experimental Materials
4.2. Sample Preparation
4.2.1. Sample Preparation for Metabolomics Analysis
4.2.2. Sample Preparation for Total Flavonoids, Saponins, Jujuboside A, Jujuboside B, and Spinosin Analysis
4.3. LC-MS Analysis Method
4.4. GC-IMS Analysis Method
4.5. HPLC-UV Analysis Method
4.6. UV-5200PC Spectrophotometer Analysis Method
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, Q.; Liu, P.; Liu, M.; Wang, J.; Zhao, J.; Zhao, Z.; Dai, L. In vivo fast induction of homogeneous autopolyploids via callus in sour jujube (Ziziphus acidojujuba Cheng et Liu). Hortic. Plant J. 2016, 2, 147–153. [Google Scholar] [CrossRef]
- Shergis, J.L.; Ni, X.; Sarris, J.; Zhang, A.L.; Guo, X.; Xue, C.C.; Lu, C.; Hugel, H. Ziziphus spinosa seeds for insomnia: A review of chemistry and psychopharmacology. Phytomedicine 2017, 34, 38–43. [Google Scholar] [CrossRef]
- Liu, J.; Chen, B.; Yao, S. Simultaneous analysis and identification of main bioactive constituents in extract of Zizyphus jujuba var. sapinosa (Zizyphi spinosi semen) by high-performance liquid chromatography–photodiode array detection–electrospray mass spectrometry. Talanta 2007, 71, 668–675. [Google Scholar] [CrossRef]
- Oh, M.; Houghton, P.; Whang, W.; Cho, J. Screening of Korean herbal medicines used to improve cognitive function for anti-cholinesterase activity. Phytomedicine 2004, 11, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, H.J.; Koh, S.B.; Ban, J.Y.; Seong, Y.H. Protection of NMDA-induced neuronal cell damage by methanol extract of Zizyphi Spinosi Semen in cultured rat cerebellar granule cells. J. Ethnopharmacol. 2004, 95, 39–45. [Google Scholar] [CrossRef]
- Su, B.-N.; Cuendet, M.; Farnsworth, N.R.; Fong, H.H.; Pezzuto, J.M.; Kinghorn, A.D. Activity-guided fractionation of the seeds of Ziziphus jujuba using a cyclooxygenase-2 inhibitory assay. Planta Medica 2002, 68, 1125–1128. [Google Scholar] [CrossRef]
- Jiang, J.-G.; Huang, X.-J.; Chen, J.; Lin, Q.-S. Comparison of the sedative and hypnotic effects of flavonoids, saponins, and polysaccharides extracted from Semen Ziziphus jujube. Nat. Prod. Res. 2007, 21, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; You, Z.-l.; Xia, Q.; Xiong, T.; Xia, Y.; Yao, D.z. Upregulation of Mark3 and Rpgrip1 mRNA expression by jujuboside A in mouse hippocampus 3. Acta Pharmacol. Sin. 2007, 28, 334–338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.-E.; Cui, X.-Y.; Cui, S.-Y.; Cao, J.-X.; Zhang, J.; Zhang, Y.-H.; Zhang, Q.-Y.; Bai, Y.-J.; Zhao, Y.-Y. Potentiating effect of spinosin, a C-glycoside flavonoid of Semen Ziziphi spinosae, on pentobarbital-induced sleep may be related to postsynaptic 5-HT1A receptors. Phytomedicine 2010, 17, 404–409. [Google Scholar] [CrossRef]
- Lee, Y.; Jeon, S.J.; Lee, H.E.; Jung, I.H.; Jo, Y.-W.; Lee, S.; Cheong, J.H.; Jang, D.S.; Ryu, J.H. Spinosin, a C-glycoside flavonoid, enhances cognitive performance and adult hippocampal neurogenesis in mice. Pharmacol. Biochem. Behav. 2016, 145, 9–16. [Google Scholar] [CrossRef]
- You, Z.-l.; Xia, Q.; Liang, F.-r.; Tang, Y.-j.; Xu, C.-l.; Huang, J.; Zhao, L.; Zhang, W.-z.; He, J.-j. Effects on the expression of GABAA receptor subunits by jujuboside A treatment in rat hippocampal neurons. J. Ethnopharmacol. 2010, 128, 419–423. [Google Scholar] [CrossRef]
- Ogwuegbu, M.C.; Chukwudi, P.; Okafor-Paul, C.; Edeh, H.O.; Ani, A.O.; Okpanachi, U.; Mthiyane, D.M.N. Effect of varying levels of benzoic acid on growth performance, haemato-biochemical indices, carcass traits, gut morphology and intestinal microbiota of broiler chickens. J. Appl. Poult. Res. 2025, 34, 100556. [Google Scholar] [CrossRef]
- Tian, Y.; Meng, J.; Zhang, D.; Zhai, B.; Cheng, J.; Zou, J.; Shi, Y.; Guo, D. Wendan Decoction exerts therapeutic effects on insomnia by regulating gut microbiota and tryptophan metabolism. Phytomedicine 2025, 145, 157028. [Google Scholar] [CrossRef]
- Liu, W.-C.; Song, R.-F.; Zheng, S.-Q.; Li, T.-T.; Zhang, B.-L.; Gao, X.; Lu, Y.-T. Coordination of plant growth and abiotic stress responses by tryptophan synthase β subunit 1 through modulation of tryptophan and ABA homeostasis in Arabidopsis. Mol. Plant 2022, 15, 973–990. [Google Scholar] [CrossRef]
- Zhou, F.; Gao, Y.; Wang, H.; Gou, S.; Zhao, Y.; Wei, Z.; Gong, C.; Bai, J. CsERF21-l-CsASA2 module positively regulates cold tolerance by promoting tryptophan biosynthesis in tea plants. Plant Physiol. Biochem. 2025, 221, 109610. [Google Scholar] [CrossRef]
- Huang, S.; Yao, B.; Guo, Y.; Zhang, Y.; Li, H.; Zhang, Y.; Liu, S.; Wang, X. Human trophoblast organoids for improved prediction of placental ABC transporter-mediated drug transport. Toxicol. Appl. Pharmacol. 2024, 492, 117112. [Google Scholar] [CrossRef]
- Xiao, X.; Lang, D.; Yong, J.; Zhang, X. Bacillus cereus G2 alleviate salt stress in Glycyrrhiza uralensis Fisch. by balancing the downstream branches of phenylpropanoids and activating flavonoid biosynthesis. Ecotoxicol. Environ. Saf. 2024, 273, 116129. [Google Scholar] [CrossRef]
- Das, A.; Ruhal, R. Potential of plants-based alkaloids, terpenoids and flavonoids as antibacterial agents: An update. Process Biochem. 2025, 150, 94–120. [Google Scholar] [CrossRef]
- Han, H.; Ma, Y.; Eun, J.S.; Li, R.; Hong, J.-T.; Lee, M.-K.; Oh, K.-W. Anxiolytic-like effects of sanjoinine A isolated from Zizyphi Spinosi Semen: Possible involvement of GABAergic transmission. Pharmacol. Biochem. Behav. 2009, 92, 206–213. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, M.; Lu, X.; Wei, R.; Xu, J. Ziziphi spinosae lily powder suspension in the treatment of depression-like behaviors in rats. BMC Complement. Altern. Med. 2017, 17, 238. [Google Scholar] [CrossRef]
- Fang, X.S.; Hao, J.; Zhou, H.; Zhu, L.; Wang, J.; Song, F. Pharmacological studies on the sedative-hypnotic effect of Semen Ziziphi spinosae (Suanzaoren) and Radix et Rhizoma Salviae miltiorrhizae (Danshen) extracts and the synergistic effect of their combinations. Phytomedicine 2010, 17, 75–80. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Wang, L.; Qi, W.; Zhang, M. Composition of fatty oils from semen Ziziphi spinosae and its cardiotonic effect on isolated toad hearts. Nat. Prod. Res. 2012, 26, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-B.; Wang, Y.-S.; Luo, Z.-F.; Lu, X.-Y. SZSJ protects against insomnia by a decrease in ADMA level and an improvement in DDAH production in sleep-deprived rats. Life Sci. 2018, 209, 97–102. [Google Scholar] [CrossRef]
- Hao, J.; Hou, D.; Yu, W.; Zhang, H.; Guo, Q.; Zhang, H.; Xiong, H.; Li, Y. Metabolomic and transcriptomic analysis of the synthesis process of unsaturated fatty acids in Korean pine seed kernels. Food Chem. 2025, 481, 143895. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, A.; Zhang, T.; Tu, M.; Du, Q.; Ling, N.; Wu, J.; Zeng, X.; Wu, Z.; Pan, D. Effects of Semen Ziziphi spinosae extract and binary probiotics co-fermentation on the quality of yogurt and their underlying molecular mechanisms. Food Chem. X 2024, 21, 101191. [Google Scholar] [CrossRef]
- Salanitro, M.; Wrigley, T.; Ghabra, H.; de Haan, E.; Hill, C.M.; Solmi, M.; Cortese, S. Efficacy on sleep parameters and tolerability of melatonin in individuals with sleep or mental disorders: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 139, 104723. [Google Scholar] [CrossRef]
- Battur, M.; Aaqil, M.; Zheng, J.; Lin, H.X.; Chuluunotgon, B.; Zorigtbaatar, T.; Zhao, C.; Tian, Y. Exploring the effects of milk-enriched walnut soy sauce: Insights from GC-IMS and metagenomics approach to flavor and microbial shifts. Food Chem. X 2025, 27, 102364. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, C.; Qiao, M.; Fan, W.; Chen, Z. Effect of stir-frying temperature on volatile aroma compounds in Pixian broad bean paste. Int. J. Gastron. Food Sci. 2025, 40, 101156. [Google Scholar] [CrossRef]
- Sun, H.; Duan, Y.; Xu, R.; Song, H.; Gao, H.; Huang, X.; Qiang, W.; Zhang, R. Characterization of key aroma-active compounds in Chinese steamed breads by sensory-directed aroma analysis. Int. J. Gastron. Food Sci. 2025, 40, 101154. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Wu, Y.; Tan, H.; Bai, J.; Li, J.; Li, A.; Ma, L.; Yuan, L.; Wei, C.; et al. Decoding the aroma characterization and influencing factor in the core brewing process of China’s northern Sauce-Flavored Baijiu. J. Food Compos. Anal. 2025, 144, 107666. [Google Scholar] [CrossRef]
- Zheng, Y.; Oellig, C.; Zhu, L.; Bauer, V.; Vetter, W.; Zhang, Y. Revealing the key aroma codes and (furan) fatty acids in fresh red goji berries and the impacts of the hot-air drying process. Food Chem. 2025, 484, 144336. [Google Scholar] [CrossRef]
- Xu, Y.; Yao, L.; Wang, Y.; Shen, J.; Chen, D.; Feng, T. Comparative analysis of the aromatic profiles of citri sarcodactylis fructus from various geographical regions using GC-IMS, GC-MS, and sensory evaluation. Food Biosci. 2024, 58, 103752. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Xu, W.; He, C.; Zhu, J.; Zhang, D.; Chen, Y.; Yu, Z.; Wan, X.; Ni, D. Key aroma components in Lu’an guapian green tea with different aroma types from five tea tree varieties decoded by sensomics. Food Biosci. 2024, 61, 104551. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Chen, R.; Huang, J.; Yu, P.; Wei, Q.; Wang, Y.; Jin, Q. Function analysis of essential oils as environmental scents for improving undergraduate students emotional state. Ind. Crops Prod. 2025, 232, 121200. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Hou, S.; Zhu, H.; Dao, R.; Ning, J.; Ren, P.; Pan, F.; Liu, M.; Zhao, Z. Geospatial Metabolomics Unravel Regional Disparities in Sedative Compounds and Volatile Profiles of Ziziphi Spinosae Semen Across Chinese Production Areas. Plants 2025, 14, 2739. https://doi.org/10.3390/plants14172739
Tian J, Hou S, Zhu H, Dao R, Ning J, Ren P, Pan F, Liu M, Zhao Z. Geospatial Metabolomics Unravel Regional Disparities in Sedative Compounds and Volatile Profiles of Ziziphi Spinosae Semen Across Chinese Production Areas. Plants. 2025; 14(17):2739. https://doi.org/10.3390/plants14172739
Chicago/Turabian StyleTian, Jia, Shujuan Hou, Hanbing Zhu, Ruirui Dao, Junguang Ning, Peixing Ren, Fuxu Pan, Mengjun Liu, and Zhihui Zhao. 2025. "Geospatial Metabolomics Unravel Regional Disparities in Sedative Compounds and Volatile Profiles of Ziziphi Spinosae Semen Across Chinese Production Areas" Plants 14, no. 17: 2739. https://doi.org/10.3390/plants14172739
APA StyleTian, J., Hou, S., Zhu, H., Dao, R., Ning, J., Ren, P., Pan, F., Liu, M., & Zhao, Z. (2025). Geospatial Metabolomics Unravel Regional Disparities in Sedative Compounds and Volatile Profiles of Ziziphi Spinosae Semen Across Chinese Production Areas. Plants, 14(17), 2739. https://doi.org/10.3390/plants14172739





