Water Residues from Rosemary Essential Oil Production: Transforming Waste into a Potential Bioherbicide
Abstract
1. Introduction
2. Results
2.1. Weed Germination
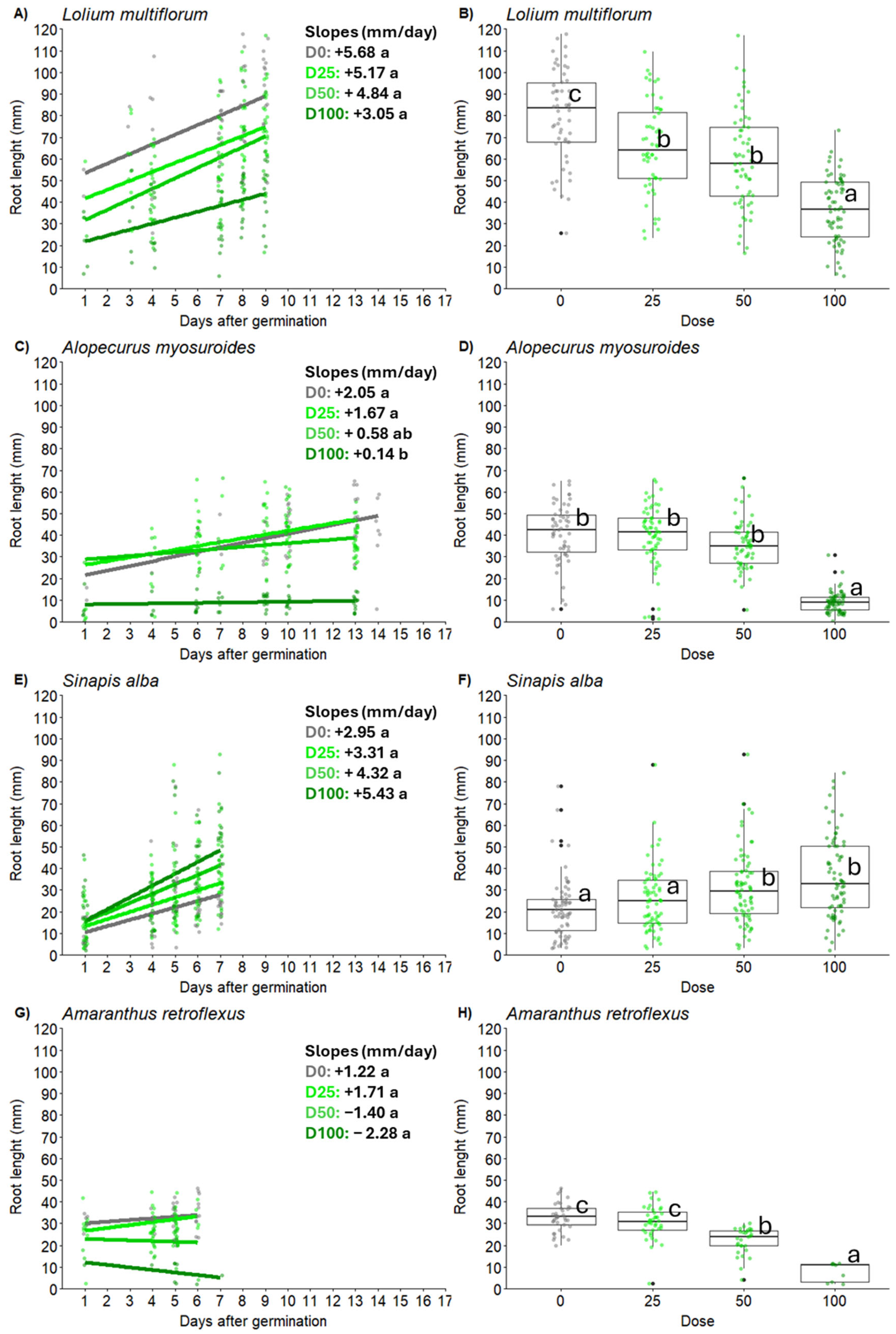
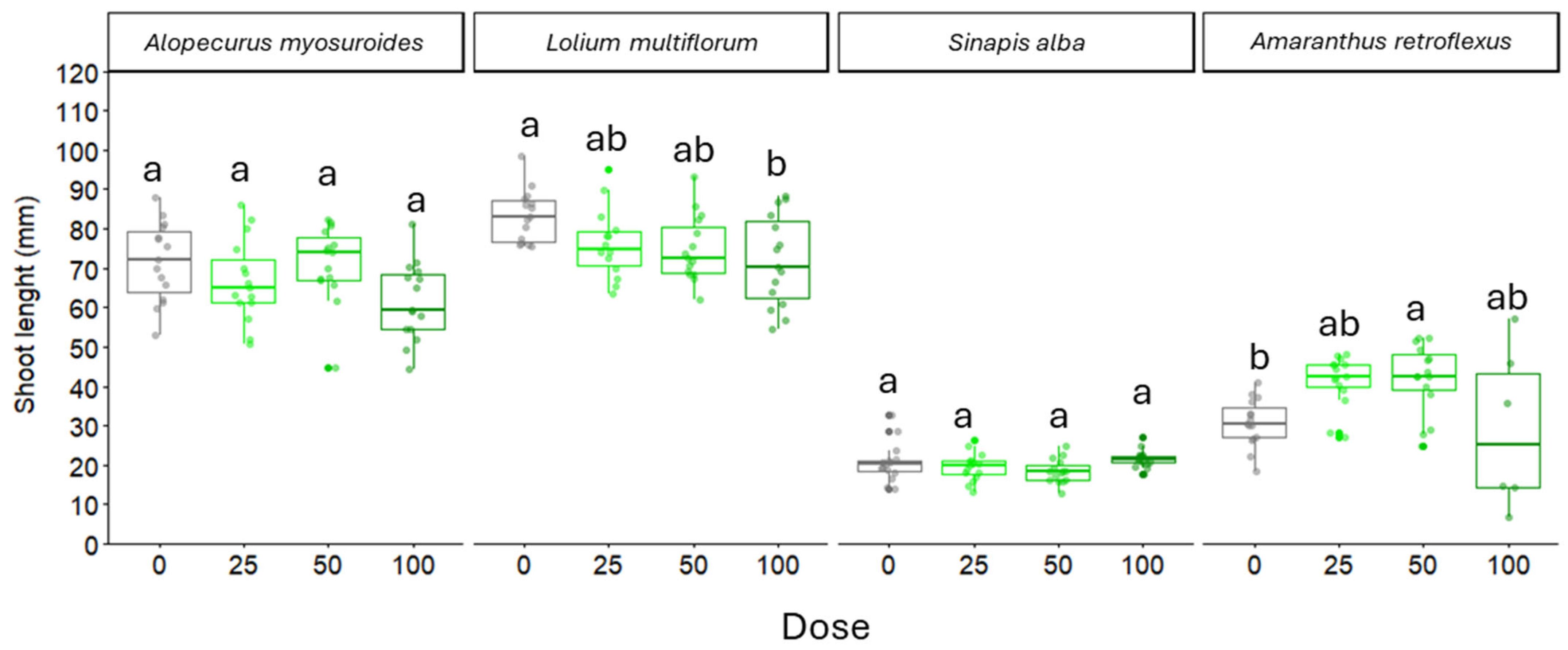
2.2. Root and Shoot Length of Weed Seedlings
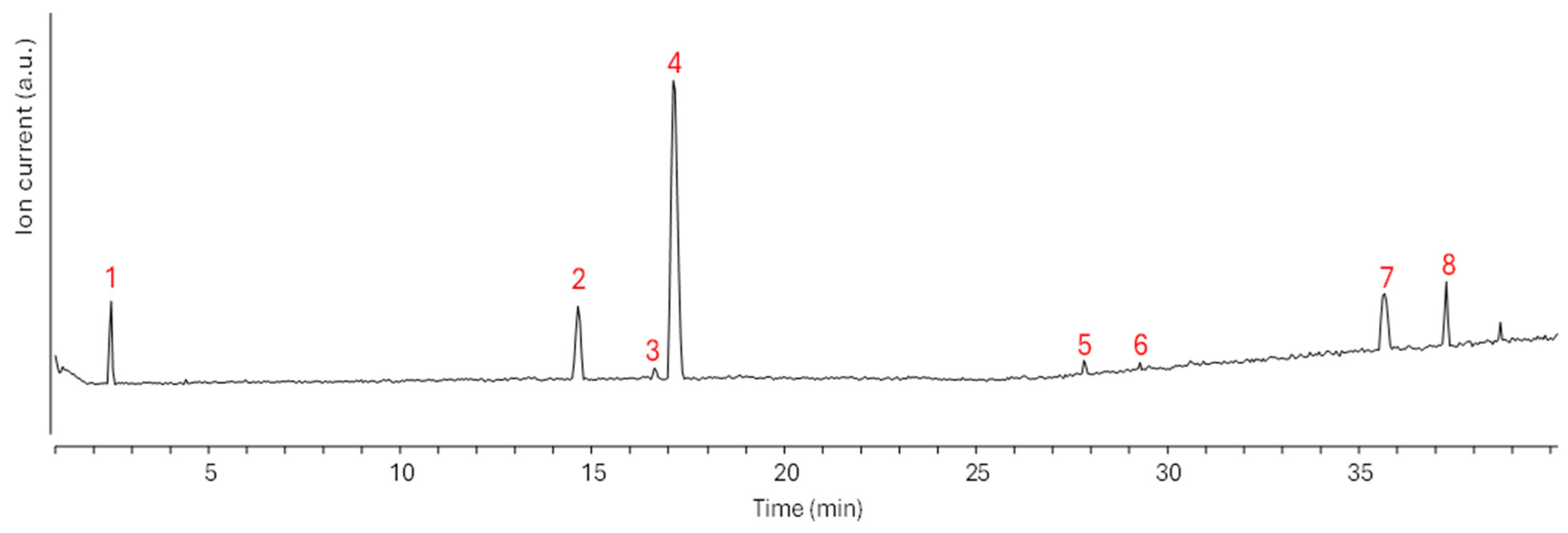
2.3. Chemical Composition of the WR Fraction
3. Discussion
4. Materials and Methods
4.1. Plant Material, Extraction Procedure, and Collection of Water Residues (WRs)
4.2. Germination Experiment
4.3. WR Chemical Characterization
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WRs | Water Residues |
References
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A Novel Insight on an Ancient Aromatic Plant: The Rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Formica, V.; Leoni, F.; Duce, C.; González-Rivera, J.; Onor, M.; Guarnaccia, P.; Carlesi, S.; Bàrberi, P. Controlled Drought Stress Affects Rosemary Essential Oil Composition with Minimal Impact on Biomass Yield. Ind. Crops Prod. 2024, 221, 119315. [Google Scholar] [CrossRef]
- Bousbia, N.; Abert Vian, M.; Ferhat, M.A.; Petitcolas, E.; Meklati, B.Y.; Chemat, F. Comparison of Two Isolation Methods for Essential Oil from Rosemary Leaves: Hydrodistillation and Microwave Hydrodiffusion and Gravity. Food Chem. 2009, 114, 355–362. [Google Scholar] [CrossRef]
- Da Silva Bomfim, N.; Nakassugi, L.P.; Faggion Pinheiro Oliveira, J.; Kohiyama, C.Y.; Mossini, S.A.G.; Grespan, R.; Nerilo, S.B.; Mallmann, C.A.; Abreu Filho, B.A.; Machinski, M. Antifungal Activity and Inhibition of Fumonisin Production by Rosmarinus officinalis L. Essential Oil in Fusarium verticillioides (Sacc.) Nirenberg. Food Chem. 2015, 166, 330–336. [Google Scholar] [CrossRef]
- Karakaya, S.; El, S.N.; Karagozlu, N.; Sahin, S.; Sumnu, G.; Bayramoglu, B. Microwave-Assisted Hydrodistillation of Essential Oil from Rosemary. J. Food Sci. Technol. 2014, 51, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- González-Rivera, J.; Duce, C.; Falconieri, D.; Ferrari, C.; Ghezzi, L.; Piras, A.; Tine, M.R. Coaxial Microwave Assisted Hydrodistillation of Essential Oils from Five Different Herbs (Lavender, Rosemary, Sage, Fennel Seeds and Clove Buds): Chemical Composition and Thermal Analysis. Innov. Food Sci. Emerg. Technol. 2016, 33, 308–318. [Google Scholar] [CrossRef]
- Mwithiga, G.; Maina, S.; Gitari, J.; Muturi, P. Rosemary (Rosmarinus officinalis L.) Growth Rate, Oil Yield and Oil Quality under Differing Soil Amendments. Heliyon 2022, 8, e09277. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, M.; Ragusa, S.; Trozzi, A.; Dugo, P.; Visinoni, F.; Fazio, A.; Dugo, G.; Mondello, L. A Comparison between Different Techniques for the Isolation of Rosemary Essential Oil. J. Sep. Sci. 2005, 28, 273–280. [Google Scholar] [CrossRef]
- Aziz, E.; Batool, R.; Akhtar, W.; Shahzad, T.; Malik, A.; Shah, M.A.; Iqbal, S.; Rauf, A.; Zengin, G.; Bouyahya, A.; et al. Rosemary Species: A Review of Phytochemicals, Bioactivities and Industrial Applications. S. Afr. J. Bot. 2022, 151, 3–18. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial Opportunities for Pesticides Based on Plant Essential Oils in Agriculture, Industry and Consumer Products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Bou, M.; Ginies, C.; Chevret, D.; Navarro, D.; Drula, E.; Bonnin, E.; Del Río, J.C.; Odinot, E.; Bisotto, A.; et al. Lavender- and Lavandin-Distilled Straws: An Untapped Feedstock with Great Potential for the Production of High-Added Value Compounds and Fungal Enzymes. Biotechnol. Biofuels 2018, 11, 217. [Google Scholar] [CrossRef]
- Gonzalez-Rivera, J.; Duce, C.; Campanella, B.; Bernazzani, L.; Ferrari, C.; Tanzini, E.; Onor, M.; Longo, I.; Ruiz, J.C.; Tinè, M.R.; et al. In Situ Microwave Assisted Extraction of Clove Buds to Isolate Essential Oil, Polyphenols, and Lignocellulosic Compounds. Ind. Crops Prod. 2021, 161, 113203. [Google Scholar] [CrossRef]
- Pulidori, E.; Micalizzi, S.; Koutsomarkos, N.; Bramanti, E.; Tinè, M.R.; Vozzi, G.; De Maria, C.; Chatzinikolaidou, M.; Duce, C. Analysis of Gelatin Secondary Structure in Gelatin/Keratin-Based Biomaterials. J. Mol. Struct. 2023, 1279, 134984. [Google Scholar] [CrossRef]
- Stashenko, E.; Martínez, J.R. Essential Oils and the Circular Bioeconomy. In Biochemistry; Jonas, V., Ed.; IntechOpen: London, UK, 2024; Volume 49, ISBN 978-0-85014-204-4. [Google Scholar]
- De Elguea-Culebras, G.O.; Panamá-Tapia, L.A.; Melero-Bravo, E.; Cerro-Ibáñez, N.; Calvo-Martínez, A.; Sánchez-Vioque, R. Comparison of the Phenolic Composition and Biological Capacities of Wastewater from Origanum vulgare L., Rosmarinus officinalis L., Salvia lavandulifolia Vahl. and Thymus mastichina L. Resulting from Two Hydrodistillation Systems: Clevenger and MAE. J. Appl. Res. Med. Aromat. Plants 2023, 34, 100480. [Google Scholar] [CrossRef]
- Ziani, I.; Bouakline, H.; Bouknana, S.; Bentouhami, N.E.; Sher, F.; Ansar, S.; Fauconnier, M.-L.; Bnouham, M.; El Bachiri, A. Sustainable Management of Rosemary Wastewater and Essential Oil in Agri-Environmental Bioprocessing. Food Biosci. 2024, 62, 105263. [Google Scholar] [CrossRef]
- Rao, B.R. Hydrosols and Water-Soluble Essential Oils: Medicinal and Biological Properties. In Recent Progress in Medicinal Plants, Essential Oils I; Govil, J.N., Bhattacharya, S., Eds.; StudiumPress LLC.: Houston, TX, USA, 2013. [Google Scholar]
- Vella, F.M.; Laratta, B. Rosemary Essential Oil Extraction and Residue Valorization by Means of Polyphenol Recovery. Biol. Life Sci. Forum 2023, 26, 8. [Google Scholar] [CrossRef]
- Appiah, K.S.; Mardani, H.K.; Omari, R.A.; Eziah, V.Y.; Ofosu-Anim, J.; Onwona-Agyeman, S.; Amoatey, C.A.; Kawada, K.; Katsura, K.; Oikawa, Y.; et al. Involvement of Carnosic Acid in the Phytotoxicity of Rosmarinus Officinalis Leaves. Toxins 2018, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Appiah, K.S.; Omari, R.A.; Onwona-Agyeman, S.; Amoatey, C.A.; Ofosu-Anim, J.; Smaoui, A.; Arfa, A.B.; Suzuki, Y.; Oikawa, Y.; Okazaki, S.; et al. Seasonal Changes in the Plant Growth-Inhibitory Effects of Rosemary Leaves on Lettuce Seedlings. Plants 2022, 11, 673. [Google Scholar] [CrossRef]
- Araniti, F.; Costas-Gil, A.; Cabeiras-Freijanes, L.; Lupini, A.; Sunseri, F.; Reigosa, M.J.; Abenavoli, M.R.; Sánchez-Moreiras, A.M. Rosmarinic Acid Induces Programmed Cell Death in Arabidopsis Seedlings through Reactive Oxygen Species and Mitochondrial Dysfunction. PLoS ONE 2018, 13, e0208802. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, J.; Zhu, Y.; Guo, L.; Ji, R.; Miao, Y.; Guo, L.; Du, H.; Liu, D. Caffeic Acid, an Allelochemical in Artemisia argyi, Inhibits Weed Growth via Suppression of Mitogen-Activated Protein Kinase Signaling Pathway and the Biosynthesis of Gibberellin and Phytoalexin. Front. Plant Sci. 2022, 12, 802198. [Google Scholar] [CrossRef] [PubMed]
- Khamare, Y.; Chen, J.; Marble, S.C. Allelopathy and Its Application as a Weed Management Tool: A Review. Front. Plant Sci. 2022, 13, 1034649. [Google Scholar] [CrossRef] [PubMed]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.-P. Bioherbicides: Dead in the Water? A Review of the Existing Products for Integrated Weed Management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- Synowiec, A.; Kalemba, D.; Drozdek, E.; Bocianowski, J. Phytotoxic Potential of Essential Oils from Temperate Climate Plants against the Germination of Selected Weeds and Crops. J. Pest Sci. 2017, 90, 407–419. [Google Scholar] [CrossRef]
- Frabboni, L.; Tarantino, A.; Petruzzi, F.; Disciglio, G. Bio-Herbicidal Effects of Oregano and Rosemary Essential Oils on Chamomile (Matricaria chamomilla L.) Crop in Organic Farming System. Agronomy 2019, 9, 475. [Google Scholar] [CrossRef]
- Haliniarz, M.; Chojnacka, S.; Różańska-Boczula, M.; Gawęda, D.; Woźniak, A. Galinsoga parviflora Response to Different Application Rates of the Herbicide Chwastox Turbo 340 SL in Spring Barley. Acta Agrobot. 2020, 73, 7335. [Google Scholar] [CrossRef]
- Habtemariam, S. Introduction to Plant Secondary Metabolites—From Biosynthesis to Chemistry and Antidiabetic Action. In Medicinal Foods as Potential Therapies for Type-2 Diabetes and Associated Diseases; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–132. ISBN 978-0-08-102922-0. [Google Scholar]
- Christopoulou, S.D.; Androutsopoulou, C.; Hahalis, P.; Kotsalou, C.; Vantarakis, A.; Lamari, F.N. Rosemary Extract and Essential Oil as Drink Ingredients: An Evaluation of Their Chemical Composition, Genotoxicity, Antimicrobial, Antiviral, and Antioxidant Properties. Foods 2021, 10, 3143. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.M.G.; Gouda, N.A.A.; Abdelgaleil, S.A.M. Bioherbicidal Activity of Terpenes and Phenylpropenes against Echinochloa crus-galli. J. Environ. Sci. Health Part B 2019, 54, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Reigosa, M.J.; Souto, X.C.; Gonz´lez, L. Effect of Phenolic Compounds on the Germination of Six Weeds Species. Plant Growth Regul. 1999, 28, 83–88. [Google Scholar] [CrossRef]
- Peng, C.; Wu, Y.; Shi, F.; Shen, Y. Review of the Current Research Progress of Seed Germination Inhibitors. Horticulturae 2023, 9, 462. [Google Scholar] [CrossRef]
- Hoang Anh, L.; Van Quan, N.; Tuan Nghia, L.; Dang Xuan, T. Phenolic Allelochemicals: Achievements, Limitations, and Prospective Approaches in Weed Management. Weed Biol. Manag. 2021, 21, 37–67. [Google Scholar] [CrossRef]
- Wu, Y.; Lv, Y.; Li, X.; Gao, H.; Zhou, M.; Ma, S.; Lu, G.; Sang, S.; Song, Y.; Zhang, J.; et al. The Effect of Epigallocatechin-3-Gallate (EGCG), a Main Active Ingredient in Tea Residues, on Improving Fruit Quality and Prolonging Postharvest Storage in Apple. Sci. Hortic. 2024, 326, 112782. [Google Scholar] [CrossRef]
- Weidner, S.; Powałka, A.; Karamać, M.; Amarowicz, R. Extracts of Phenolic Compounds from Seeds of Three Wild Grapevines—Comparison of Their Antioxidant Activities and the Content of Phenolic Compounds. Int. J. Mol. Sci. 2012, 13, 3444–3457. [Google Scholar] [CrossRef] [PubMed]
- Saludes-Zanfaño, M.I.; Vivar-Quintana, A.M.; Morales-Corts, M.R. Pistacia Root and Leaf Extracts as Potential Bioherbicides. Plants 2022, 11, 916. [Google Scholar] [CrossRef] [PubMed]
- De Martino, L.; Mencherini, T.; Mancini, E.; Aquino, R.P.; De Almeida, L.F.R.; De Feo, V. In Vitro Phytotoxicity and Antioxidant Activity of Selected Flavonoids. Int. J. Mol. Sci. 2012, 13, 5406–5419. [Google Scholar] [CrossRef]
- Veronesi, C.; Billard, E.; Delavault, P.; Simier, P. (±)-Catechins Inhibit Prehaustorium Formation in the Parasitic Weed Phelipanche ramosa and Reduce Tomato Infestation. Pest Manag. Sci. 2025, 81, 720–726. [Google Scholar] [CrossRef]
- Đorđević, T.; Đurović-Pejčev, R.; Stevanović, M.; Sarić-Krsmanović, M.; Radivojević, L.; Šantrić, L.; Gajić-Umiljendić, J. Phytotoxicity and Allelopathic Potential of Juglans regia L. Leaf Extract. Front. Plant Sci. 2022, 13, 986740. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Wright, L.P.; Gershenzon, J.; Wasternack, C.; Hause, B.; Schaller, A.; Stintzi, A. Jasmonic Acid and Its Precursor 12-Oxophytodienoic Acid Control Different Aspects of Constitutive and Induced Herbivore Defenses in Tomato. Plant Physiol. 2014, 166, 396–410. [Google Scholar] [CrossRef]
- Pan, J.; Hu, Y.; Wang, H.; Guo, Q.; Chen, Y.; Howe, G.A.; Yu, D. Molecular Mechanism Underlying the Synergetic Effect of Jasmonate on Abscisic Acid Signaling during Seed Germination in Arabidopsis. Plant Cell 2020, 32, 3846–3865. [Google Scholar] [CrossRef]
- Cutler, H.G. Biologically Active Natural Products: Potential Use in Agriculture; Cutler, H.G., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1988; Volume 380, ISBN 978-0-8412-1556-6. [Google Scholar]
- Tawaha, A.M.; Turk, M.A. Allelopathic Effects of Black Mustard (Brassica nigra) on Germination and Growth of Wild Barley (Hordeum spontaneum). J. Agron. Crop Sci. 2003, 189, 298–303. [Google Scholar] [CrossRef]
- Elghobashy, R.M.; El-Darier, S.M.; Atia, A.M.; Zakaria, M. Allelopathic Potential of Aqueous Extracts and Essential Oils of Rosmarinus officinalis L. and Thymus vulgaris L. J. Soil Sci. Plant Nutr. 2024, 24, 700–715. [Google Scholar] [CrossRef]
- Alipour, M.; Saharkhiz, M.J.; Niakousari, M.; Seidi Damyeh, M. Phytotoxicity of Encapsulated Essential Oil of Rosemary on Germination and Morphophysiological Features of Amaranth and Radish Seedlings. Sci. Hortic. 2019, 243, 131–139. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic Effects of Volatile Monoterpenoids Produced by Salvia leucophylla: Inhibition of Cell Proliferation and DNA Synthesis in the Root Apical Meristem of Brassica campestris Seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Islam, A.; Kato-Noguchi, H. Plant Growth Inhibitory Activity of Medicinal Plant Hyptis Suaveolens: Couldallelopathy Be a Cause? Emir. J. Food Agric. 2013, 25, 692. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Thimann, K.V. Interactions of Phenolic Acids, Metallic Ions and Chelating Agents on Auxin-Induced Growth. Plant Physiol. 1966, 41, 1443–1454. [Google Scholar] [CrossRef]
- Einhellig, F.A. Interactions Involving Allelopathy in Cropping Systems. Agron. J. 1996, 88, 886–893. [Google Scholar] [CrossRef]
- Souri, M.K.; Bakhtiarizade, M. Biostimulation Effects of Rosemary Essential Oil on Growth and Nutrient Uptake of Tomato Seedlings. Sci. Hortic. 2019, 243, 472–476. [Google Scholar] [CrossRef]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots Withstanding Their Environment: Exploiting Root System Architecture Responses to Abiotic Stress to Improve Crop Tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef]
- Rehman, S.; Shahzad, B.; Bajwa, A.A.; Hussain, S.; Rehman, A.; Cheema, S.A.; Abbas, T.; Ali, A.; Shah, L.; Adkins, S.; et al. Utilizing the Allelopathic Potential of Brassica Species for Sustainable Crop Production: A Review. J. Plant Growth Regul. 2019, 38, 343–356. [Google Scholar] [CrossRef]
- Golisz, A.; Gawronska, H.; Gawronski, S. Influence of Buckwheat Allelochemicals on Crops and Weeds. Allelopath. J. 2007, 19, 337–350. [Google Scholar]
- Liebman, M.; Sundberg, D.N. Seed Mass Affects the Susceptibility of Weed and Crop Species to Phytotoxins Extracted from Red Clover Shoots. Weed Sci. 2006, 54, 340–345. [Google Scholar] [CrossRef]
- Bàrberi, P. Weed Management in Organic Agriculture: Are We Addressing the Right Issues? Weed Res. 2002, 42, 177–193. [Google Scholar] [CrossRef]
- Deguine, J.-P.; Aubertot, J.-N.; Bellon, S.; Côte, F.; Lauri, P.-E.; Lescourret, F.; Ratnadass, A.; Scopel, E.; Andrieu, N.; Bàrberi, P.; et al. Agroecological Crop Protection for Sustainable Agriculture. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2023; Volume 178, pp. 1–59. ISBN 978-0-443-19260-9. [Google Scholar]
- Sauer, D.B.; Burroughs, R. Disinfection of Seed Surfaces with Sodium Hypochlorite. Phytopathology 1986, 76, 745–749. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-based Metabolite Profiling of Methanolic Extracts from the Medicinal and Aromatic Species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.G. LC-MS Identification and Quantification of Phenolic Compounds in Solid Residues from the Essential Oil Industry. Antioxidants 2021, 10, 2016. [Google Scholar] [CrossRef]
- Ritz, C.; Strebig, J.C. Drc: Analysis of Dose-Response Curves; Version 3.0-1; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Zeileis, A.; Köll, S.; Graham, N. Various Versatile Variances: An Object-Oriented Implementation of Clustered Covariances in R. J. Stat. Softw. 2020, 95, 1–36. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; Version 1.11.2; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models; Version 0.4.7; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
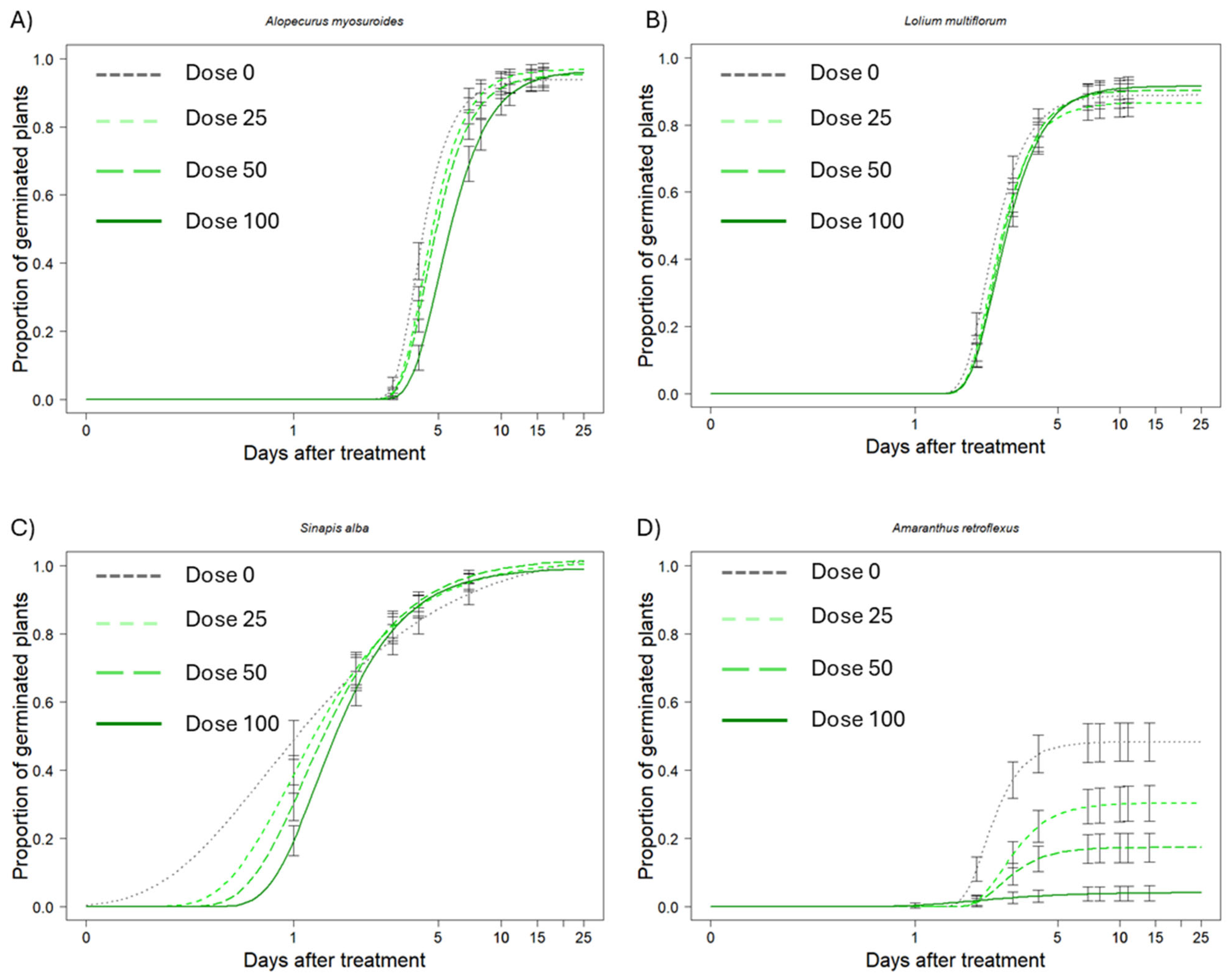
| Weed Species | Dose | Slope | Upper Limit | ET50 (DAT) |
|---|---|---|---|---|
| Alopecurus myosuroides | 0 | −4.53 (0.28) c | 0.93 (0.01) a | 3.84 (0.06) d |
| 25 | −3.94 (0.24) bc | 0.96 (0.01) a | 4.21 (0.07) bc | |
| 50 | −3.73 (0.23) ab | 0.95 (0.01) a | 4.34 (0.08) b | |
| 100 | −3.24 (0.20) a | 0.96 (0.01) a | 4.99 (0.11) a | |
| Lolium multiflorum | 0 | −3.97 (0.25) a | 0.88 (0.01) a | 2.21 (0.04) c |
| 25 | −3.90 (0.24) a | 0.86 (0.01) a | 2.34 (0.04) b | |
| 50 | −3.77 (0.22) a | 0.90 (0.01) a | 2.41 (0.04) ab | |
| 100 | −3.46 (0.20) a | 0.91 (0.01) a | 2.47 (0.05) a | |
| Sinapis alba | 0 | −0.84 (0.16) c | 1.00 (0.06) a | 0.74 (0.07) d |
| 25 | −1.35 (0.15) b | 1.01 (0.02) a | 0.97 (0.05) c | |
| 50 | −1.58 (0.15) ab | 1.02 (0.01) a | 1.12 (0.05) b | |
| 100 | −1.89 (0.14) a | 0.99 (0.01) a | 1.29 (0.04) a | |
| Amaranthus retroflexus | 0 | −4.25 (0.37) b | 0.48 (0.02) a | 1.79 (0.05) cd |
| 25 | −3.45 (0.33) ab | 0.30 (0.02) b | 2.69 (0.09) a | |
| 50 | −3.60 (0.46) ab | 0.17 (0.02) c | 2.60 (0.11) ab | |
| 100 | −1.51 (0.46) a | 0.04 (0.01) d | 2.19 (0.37) abcd |
| # | Compound | Formula | MW (amu) | PMI | MSMS Signals | C (ppm) |
|---|---|---|---|---|---|---|
| 1 | 3,4-Dihydroxyphenyllactic acid | C9H10O5 | 198.2 | 197.05 | 179, 135, 123 | 7.3 |
| 2 | Tuberonic acid glucoside | C18H28O9 | 388.4 | 387.17 | 207, 163, 119 | 33.4 |
| 3 | Gallocatechin 1 | C15H14O7 | 306.3 | 305.07 | 225, 97 | 2.4 |
| 4 | Gallocatechin 2 | C15H14O7 | 306.3 | 305.07 | 225, 97 | 12.1 |
| 5 | Isorhamnetin 3-O-glucoside | C22H22O11 | 478.4 | 477.10 | 315, 299 | 0.2 |
| 6 | Hispidulin 7-O-glucoside | C22H22O11 | 462.4 | 461.11 | 283 | 2.7 |
| 7 | Carinol | C20H26O7 | 378.4 | 377.16 | 359, 315, 289, 211, 165 | 11.5 |
| 8 | Triptolidenol | C20H24O7 | 376.4 | 375.16 | 331, 313, 303, 287, 275, 259 | 10.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leoni, F.; Nigro, F.; Duce, C.; González-Rivera, J.; Mattonai, M.; Ribechini, E.; Bàrberi, P.; Carlesi, S. Water Residues from Rosemary Essential Oil Production: Transforming Waste into a Potential Bioherbicide. Plants 2025, 14, 2717. https://doi.org/10.3390/plants14172717
Leoni F, Nigro F, Duce C, González-Rivera J, Mattonai M, Ribechini E, Bàrberi P, Carlesi S. Water Residues from Rosemary Essential Oil Production: Transforming Waste into a Potential Bioherbicide. Plants. 2025; 14(17):2717. https://doi.org/10.3390/plants14172717
Chicago/Turabian StyleLeoni, Federico, Francesco Nigro, Celia Duce, José González-Rivera, Marco Mattonai, Erika Ribechini, Paolo Bàrberi, and Stefano Carlesi. 2025. "Water Residues from Rosemary Essential Oil Production: Transforming Waste into a Potential Bioherbicide" Plants 14, no. 17: 2717. https://doi.org/10.3390/plants14172717
APA StyleLeoni, F., Nigro, F., Duce, C., González-Rivera, J., Mattonai, M., Ribechini, E., Bàrberi, P., & Carlesi, S. (2025). Water Residues from Rosemary Essential Oil Production: Transforming Waste into a Potential Bioherbicide. Plants, 14(17), 2717. https://doi.org/10.3390/plants14172717








