Optimization of Swertiamarin and Isogentisin Extraction from Gentiana lutea L. Leaves by Response Surface Methodology
Abstract
1. Introduction
2. Results
2.1. Model Fitting
2.2. Influence Analysis
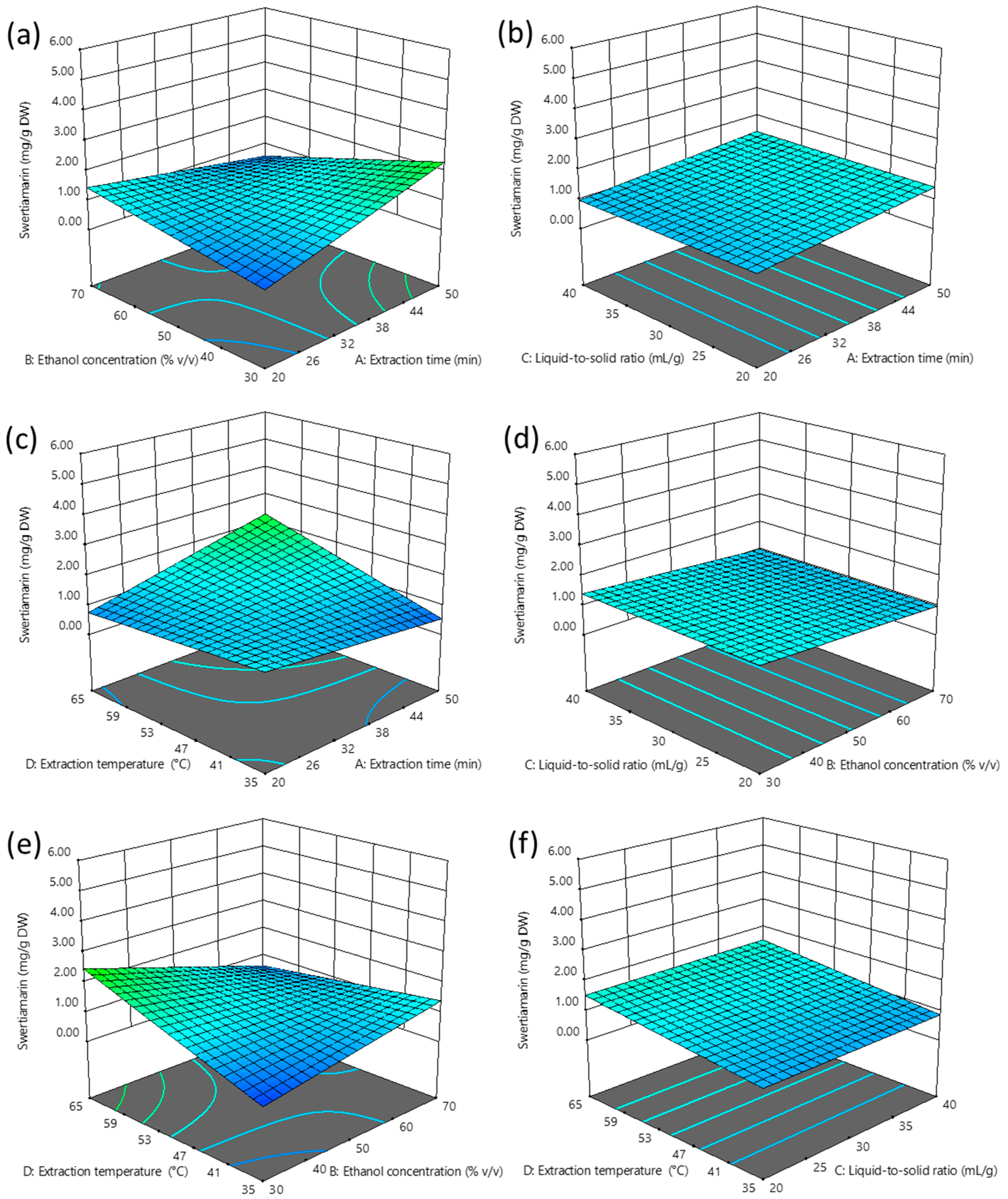
2.2.1. Influence of Independent Variables on the Extraction Efficiency of Swertiamarin
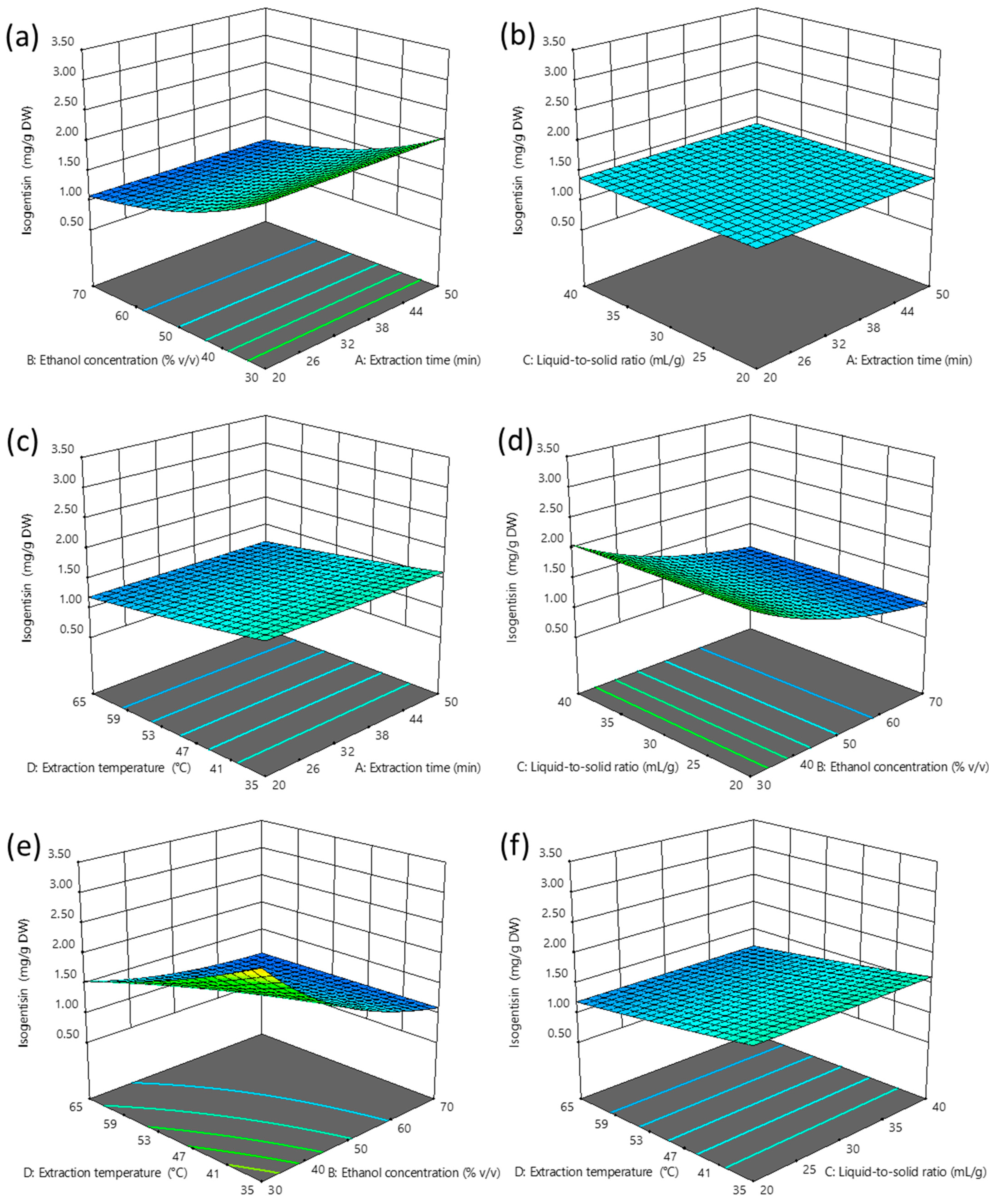
2.2.2. Influence of Independent Variables on the Extraction Efficiency of Isogentisin
2.3. Optimization of Process Parameters
2.4. Stability of Active Ingredients
3. Discussion
3.1. Extraction Optimization
3.1.1. Extraction of Swertiamarin
3.1.2. Extraction of Isogentisin
3.2. Stability Study
4. Materials and Methods
4.1. Plant Material
4.2. Modeling and Optimization
4.3. Ultrasound-Assisted Extraction
4.4. HPLC Analysis
4.5. Stability Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, M.; Cui, B.W.; Wu, Y.L.; Nan, J.X.; Lian, L.H. Genus Gentiana: A review on phytochemistry, pharmacology and molecular mechanism. J. Ethnopharmacol. 2021, 264, 113391. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhao, Y.L.; Zhang, J.; Li, W.Y.; Wang, Y.Z. Phytochemistry and Pharmacological Activities of the Genus Gentiana (Gentianaceae). Chem. Biodivers. 2016, 13, 107–150. [Google Scholar] [CrossRef]
- Pasdaran, A.; Butovska, D.; Kerr, P.; Naychov, Z.; Aneva, I.; Kozuharova, E. Gentians, natural remedies for future of visceral pain control: An ethnopharmacological review with an in silico approach. Biol. Futur. 2022, 73, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Carnat, A.; Fraisse, D.; Carnat, A.P.; Felgines, C.; Chaud, D.; Lamaison, J.L. Influence of drying mode on iridoid bitter constituent levels in gentian root. J. Sci. Food Agric. 2005, 85, 598–602. [Google Scholar] [CrossRef]
- Aberham, A.; Pieri, V.; Croom, E.M., Jr.; Ellmerer, E.; Stuppner, H. Analysis of iridoids, secoiridoids and xanthones in Centaurium erythraea, Frasera caroliniensis and Gentiana lutea using LC–MS and RP-HPLC. J. Pharm. Biomed. Anal. 2011, 54, 517–525. [Google Scholar] [CrossRef]
- Jovanović, M.; Ćujić-Nikolić, N.; Drinić, Z. Spray drying of Gentiana asclepiadea L. root extract: Successful encapsulation into powders with preserved stability of bioactive compounds. Ind. Crops Prod. 2021, 172, 114044. [Google Scholar] [CrossRef]
- Ponticelli, M.; Lela, L.; Moles, M.; Mangieri, C.; Bisaccia, D.; Faraone, I.; Falabella, R.; Milella, L. The healing bitterness of Gentiana lutea L., phytochemistry and biological activities: A systematic review. Phytochemistry 2023, 206, 113518. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Gentiana lutea L., radix. Ref.: EMA/HMPC/607863/2017. Available online: https://www.ema.europa.eu/en/documents/herbal-report/assessment-report-gentiana-lutea-l-radix-revision-1_en.pdf (accessed on 15 June 2025).
- Fiorito, S.; Epifano, F.; Palumbo, L.; Collevecchio, C.; Mascioli, F.; Spogli, R.; Genovese, S. Leaves of Yellow Gentian (Gentiana lutea) as an Alternative Source of Bitter Secoiridoid Glycosides. J. Nat. Prod. 2022, 85, 2232–2235. [Google Scholar] [CrossRef]
- Janković, T.; Mudrić, J.; Radojičić, V.; Pejić, L.; Nikolić, N.Ć.; Marković, S.; Menković, N. Smoking of Gentiana lutea leaves: Validation of its traditional use. J. Pharm. Biomed. Anal. 2025, 264, 116968. [Google Scholar] [CrossRef]
- Balijagić, J.; Janković, T.; Zdunić, G.; Bošković, J.; Šavikin, K.; Gođevac, D.; Stanojković, T.; Jovančević, M.; Menković, N. Chemical Profile, Radical Scavenging and Cytotoxic Activity of Yellow Gentian Leaves (Genitaneae luteae folium) Grown in Northern Regions of Montenegro. Nat. Prod. Commun. 2012, 7, 1934578X1200701119. [Google Scholar] [CrossRef]
- Menković, N.; Šavikin-Fodulović, K.; Savin, K. Chemical Composition and Seasonal Variations in the Amount of Secondary Compounds in Gentiana lutea Leaves and Flowers. Planta Med. 2000, 66, 178–180. [Google Scholar] [CrossRef]
- Oubannin, S.; Bijla, L.; Ahmed, M.N.; Ibourki, M.; El Kharrassi, Y.; Devkota, K.; Bouyahya, A.; Maggi, F.; Caprioli, G.; Sakar, E.H.; et al. Recent advances in the extraction of bioactive compounds from plant matrices and their use as potential antioxidants for vegetable oils enrichment. J. Food Compos. Anal. 2024, 128, 105995. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- Yusoff, I.M.; Taher, Z.M.; Rahmat, Z.; Chua, L.S. A Review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Živković, J.; Janković, T.; Menković, N.; Šavikin, K. Optimization of ultrasound-assisted extraction of isogentisin, gentiopicroside and total polyphenols from gentian root using response-surface methodology. Ind. Crops Prod. 2019, 139, 111567. [Google Scholar] [CrossRef]
- Mudrić, J.; Janković, T.; Šavikin, K.; Bigović, D.; Đukić-Ćosić, D.; Ibrić, S.; Đuriš, J. Optimization and modelling of gentiopicroside, isogentisin and total phenolics extraction from Gentiana lutea L. Roots. Ind. Crops Prod. 2020, 155, 112767. [Google Scholar] [CrossRef]
- Shi, M.; Tang, J.; Zhang, T.; Han, H. Swertiamarin, an active iridoid glycoside from Swertia pseudochinensis H. Hara, protects against alpha-naphthylisothiocyanate-induced cholestasis by activating the farnesoid X receptor and bile acid excretion pathway. J. Ethnopharmacol. 2022, 291, 115164. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Pandey, D.K.; Gupta, R.C.; Dey, A. Simultaneous microwave assisted extraction and HPTLC quantification of mangiferin, amarogentin, and swertiamarin in Swertia species from Western Himalayas. Ind. Crops Prod. 2019, 132, 449–459. [Google Scholar] [CrossRef]
- Dao, T.A.T.; Webb, H.K.; Malherbe, F. Optimization of pectin extraction from fruit peels by response surface method: Conventional versus microwave-assisted heating. Food Hydrocoll. 2021, 113, 106475. [Google Scholar] [CrossRef]
- Kshirsagar, P.R.; Gaikwad, N.B.; Pai, S.R.; Bapat, V.A. Optimization of extraction techniques and quantification of swertiamarin and mangiferin by using RP-UFLC method from eleven Swertia species. S. Afr. J. Bot. 2017, 108, 81–89. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, R.; Gao, H.; Wang, Z.; Yang, X.; Ruan, M.; Gu, H.; Yang, L.; Tian, H.; Fan, C.; et al. β-Cyclodextrin as a booster for ultrasound-assisted extraction of secoiridoids from Gentiana rigescens using a biobased deep eutectic solvent. Ind. Crops Prod. 2023, 204, 117410. [Google Scholar] [CrossRef]
- Schmieder, A.; Schwaiger, S.; Csordas, A.; Backovic, A.; Messner, B.; Wick, G.; Stuppner, H.; Bernhard, D. Isogentisin—A novel compound for the prevention of smoking-caused endothelial injury. Atherosclerosis 2007, 194, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Şamlı, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Zhang, Y.; Pan, C.; Jia, Q.; Guo, F.; Li, Y.; Zhu, W.; Chen, K. Advances in studying of the pharmacological activities and structure-activity relationships of natural C-glycosylflavonoids. Acta Pharm. Sin. B 2013, 3, 154–162. [Google Scholar] [CrossRef][Green Version]
- Beelders, T.; de Beer, D.; Kidd, M.; Joubert, E. Modeling of thermal degradation kinetics of the C-glucosyl xanthone mangiferin in an aqueous model solution as a function of pH and temperature and protective effect of honeybush extract matrix. Food Res. Int. 2018, 103, 103–109. [Google Scholar] [CrossRef]
- Yugoslavian Pharmacopeia (Pharmacopoea Jugoslavica), 5th ed.; National Institute for Health Protection, Contemporaneous Administration: Belgrade, Serbia, 2000; Section 2.1.4; p. 18.

| Std | Run | Independent Variables | Dependent Variables | ||||
|---|---|---|---|---|---|---|---|
| A Extraction Time | B Ethanol Concentration | C Liquid-to-Solid Ratio | D Extraction Temperature | Swertiamarin | Isogentisin | ||
| min | % v/v | mL/g | °C | mg/g DW * | mg/g DW | ||
| 1 | 23 | 20 (−1) | 30 (−1) | 20 (−1) | 35 (−1) | 0.66 | 2.54 |
| 2 | 26 | 50 (+1) | 30 (−1) | 20 (−1) | 35 (−1) | 0.80 | 2.93 |
| 3 | 17 | 20 (−1) | 70 (+1) | 20 (−1) | 35 (−1) | 0.69 | 0.83 |
| 4 | 12 | 50 (+1) | 70 (+1) | 20 (−1) | 35 (−1) | 0.71 | 1.02 |
| 5 | 10 | 20 (−1) | 30 (−1) | 40 (+1) | 35 (−1) | 0.00 | 3.32 |
| 6 | 20 | 50 (+1) | 30 (−1) | 40 (+1) | 35 (−1) | 0.57 | 3.20 |
| 7 | 9 | 20 (−1) | 70 (+1) | 40 (+1) | 35 (−1) | 2.91 | 1.26 |
| 8 | 13 | 50 (+1) | 70 (+1) | 40 (+1) | 35 (−1) | 0.83 | 1.11 |
| 9 | 16 | 20 (−1) | 30 (−1) | 20 (−1) | 65 (+1) | 1.32 | 1.28 |
| 10 | 18 | 50 (+1) | 30 (−1) | 20 (−1) | 65 (+1) | 4.63 | 1.74 |
| 11 | 15 | 20 (−1) | 70 (+1) | 20 (−1) | 65 (+1) | 1.19 | 1.14 |
| 12 | 4 | 50 (+1) | 70 (+1) | 20 (−1) | 65 (+1) | 0.90 | 1.04 |
| 13 | 19 | 20 (−1) | 30 (−1) | 40 (+1) | 65 (+1) | 0.68 | 1.44 |
| 14 | 14 | 50 (+1) | 30 (−1) | 40 (+1) | 65 (+1) | 5.17 | 2.32 |
| 15 | 22 | 20 (−1) | 70 (+1) | 40 (+1) | 65 (+1) | 0.60 | 0.99 |
| 16 | 5 | 50 (+1) | 70 (+1) | 40 (+1) | 65 (+1) | 0.56 | 0.85 |
| 17 | 27 | 5 (−2) | 50 (0) | 30 (0) | 50 (0) | 1.20 | 1.42 |
| 18 | 11 | 65 (+2) | 50 (0) | 30 (0) | 50 (0) | 0.84 | 1.37 |
| 19 | 3 | 35 (0) | 10 (−2) | 30 (0) | 50 (0) | 0.92 | 2.65 |
| 20 | 2 | 35 (0) | 90 (+2) | 30 (0) | 50 (0) | 1.13 | 1.22 |
| 21 | 24 | 35 (0) | 50 (0) | 10 (−2) | 50 (0) | 0.93 | 1.16 |
| 22 | 28 | 35 (0) | 50 (0) | 50 (+2) | 50 (0) | 0.59 | 1.23 |
| 23 | 7 | 35 (0) | 50 (0) | 30 (0) | 20 (−2) | 0.84 | 1.91 |
| 24 | 25 | 35 (0) | 50 (0) | 30 (0) | 80 (+2) | 0.86 | 1.10 |
| 25 | 1 | 35 (0) | 50 (0) | 30 (0) | 50 (0) | 1.29 | 1.39 |
| 26 | 21 | 35 (0) | 50 (0) | 30 (0) | 50 (0) | 1.31 | 1.47 |
| 27 | 29 | 35 (0) | 50 (0) | 30 (0) | 50 (0) | 1.34 | 1.42 |
| 28 | 6 | 35 (0) | 50 (0) | 30 (0) | 50 (0) | 0.74 | 1.32 |
| 29 | 8 | 35 (0) | 50 (0) | 30 (0) | 50 (0) | 0.67 | 1.20 |
| Terms | p-Value | |
|---|---|---|
| Swertiamarin | Isogentisin | |
| Linear | ||
| A—Extraction time | 0.1213 | / |
| B—Ethanol concentration | 0.1498 | <0.0001 *** |
| C—Liquid-to-solid ratio | / | / |
| D—Extraction temperature | 0.0275 * | 0.0002 *** |
| Interaction | ||
| AB | 0.0006 *** | / |
| AC | / | / |
| AD | 0.0038 ** | / |
| BC | / | / |
| BD | 0.0003 *** | 0.0028 ** |
| CD | / | / |
| Quadratic | ||
| A2 | / | / |
| B2 | / | 0.0237 * |
| C2 | / | / |
| D2 | / | / |
| Model fitting assessment | ||
| Model | <0.0001 *** | <0.0001 *** |
| R2 | 0.7143 | 0.8429 |
| Lack-of-fit | 0.0671 | 0.0619 |
| Response Values | Predicted Content (mg/g DW) | Measured Content (mg/g DW *) |
|---|---|---|
| Swertiamarin | 3.68 | 3.75 ± 0.19 |
| Isogentisin | 1.62 | 1.57 ± 0.08 |
| Sample | Swertiamarin | Gentiopicrin | Mangiferin | Isoorientin | Isovitexin | Isogentisin |
|---|---|---|---|---|---|---|
| −18 °C dark | 100.2 ± 0.2% | 100.1 ± 0.2% | 99.9 ± 0.2% | 100.1 ± 0.2% | 100.1 ± 0.1% | 100.3 ± 0.2% |
| 4 °C dark | 100.2 ± 0.2% | 100.2 ± 0.2% | 100.0 ± 0.3% | 100.1 ± 0.1% | 100.1 ± 0.2% | 100.2 ± 0.2% |
| 25 °C dark | 100.1 ± 0.2% | 100.2 ± 0.1% | 94.5 ± 0.2% | 100.2 ± 0.2% | 100.2 ± 0.3% | 100.1 ± 0.2% |
| 25 °C light | 100.4 ± 0.2% | 100.3 ± 0.2% | 57.7 ± 0.9% | 100.2 ± 0.3% | 100.2 ± 0.2% | 99.9 ± 0.3% |
| 40 °C dark | 98.2 ± 0.5% | 96.3 ± 0.4% | 86.1 ± 0.3% | 99.9 ± 0.2% | 99.8 ± 0.4% | 100.1 ± 0.2% |
| Class of Phytocompounds | G. lutea Roots | G. lutea Leaves |
|---|---|---|
| Iridoids and secoiridoids | Loganic Acid Sweroside Swertiamarin Gentiopicrin Amarogentin | Swertiamarin Gentiopicrin Eustomoside Eustomorusside Septemfidoside |
| Flavonoids | / | Isovitexin Isosaponarin Isoorientin Isoorientin 2″-O-glucoside Isoorientin 4′-O-glucoside |
| Xanthones | Gentioside Gentisin Isogentisin | Mangiferin Isogentisin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šavikin, K.; Jovanović, M.S.; Zdunić, G.; Živković, J.; Kitić, D.; Bigović, D.; Janković, T. Optimization of Swertiamarin and Isogentisin Extraction from Gentiana lutea L. Leaves by Response Surface Methodology. Plants 2025, 14, 2538. https://doi.org/10.3390/plants14162538
Šavikin K, Jovanović MS, Zdunić G, Živković J, Kitić D, Bigović D, Janković T. Optimization of Swertiamarin and Isogentisin Extraction from Gentiana lutea L. Leaves by Response Surface Methodology. Plants. 2025; 14(16):2538. https://doi.org/10.3390/plants14162538
Chicago/Turabian StyleŠavikin, Katarina, Miloš S. Jovanović, Gordana Zdunić, Jelena Živković, Dušanka Kitić, Dubravka Bigović, and Teodora Janković. 2025. "Optimization of Swertiamarin and Isogentisin Extraction from Gentiana lutea L. Leaves by Response Surface Methodology" Plants 14, no. 16: 2538. https://doi.org/10.3390/plants14162538
APA StyleŠavikin, K., Jovanović, M. S., Zdunić, G., Živković, J., Kitić, D., Bigović, D., & Janković, T. (2025). Optimization of Swertiamarin and Isogentisin Extraction from Gentiana lutea L. Leaves by Response Surface Methodology. Plants, 14(16), 2538. https://doi.org/10.3390/plants14162538









