Early Sowing Approach for Developing Climate Resilient Maize: Cold Stress Impact on Germination of Adapted Inbred Lines with High Nutritive Value
Abstract
1. Introduction
2. Results
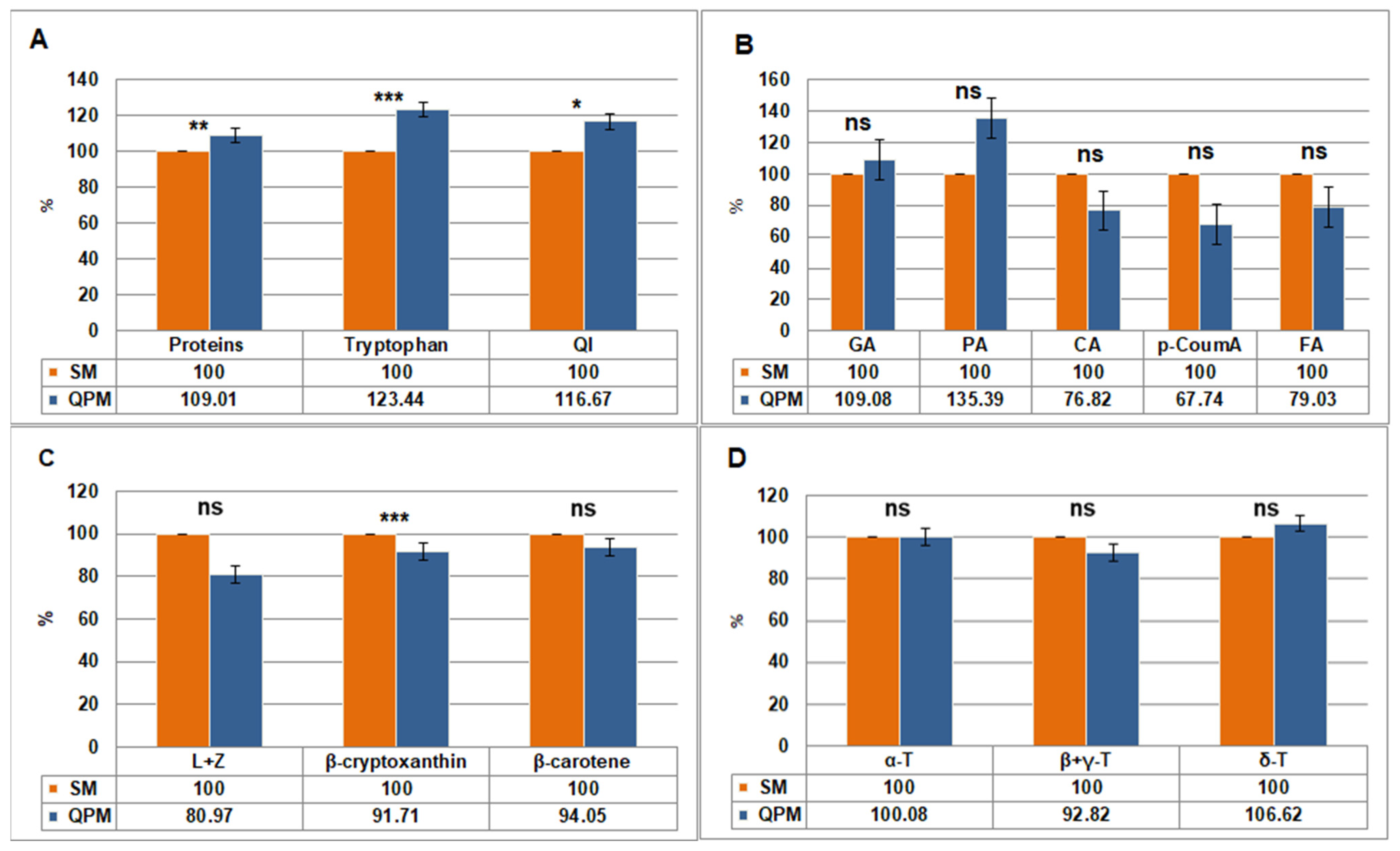
2.1. Seed Biochemical Analyses
2.1.1. Protein and Tryptophan Content
2.1.2. Free Phenolic Acid Content
2.1.3. Carotenoid Content
2.1.4. Tocopherol Content
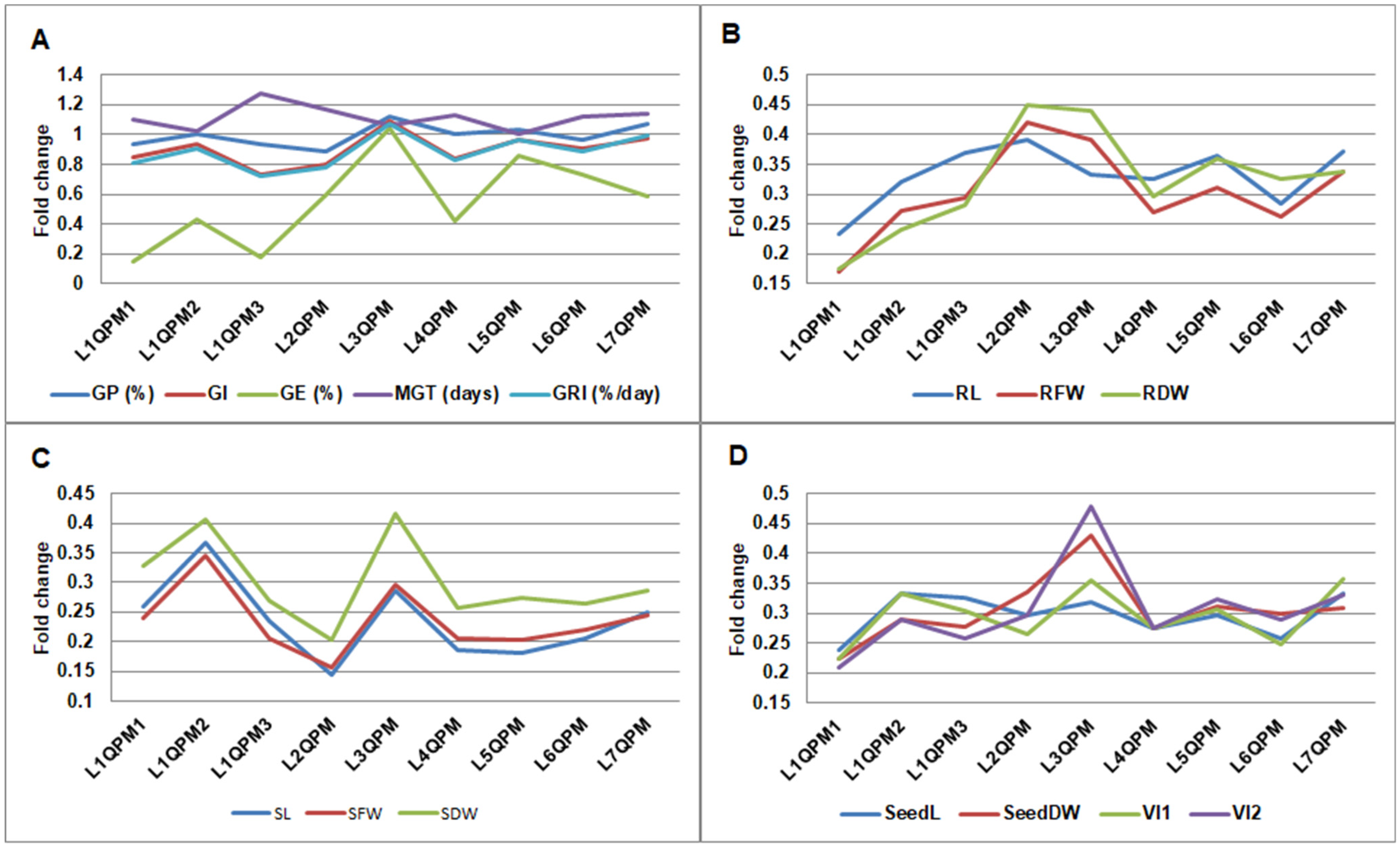
2.2. Germination Parameters
2.2.1. Seed Germination
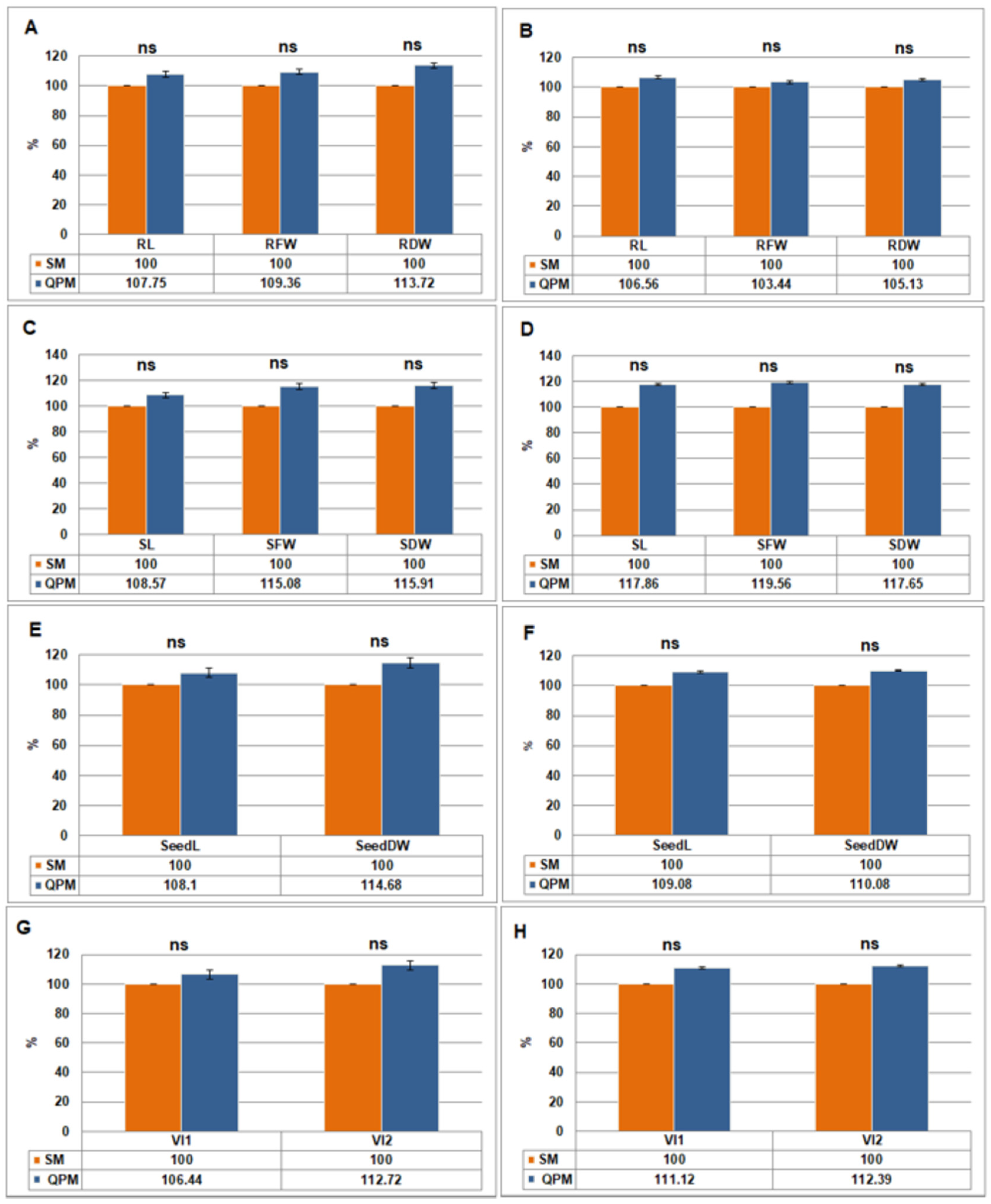
2.2.2. Seedling Morphological and Physiological Traits
2.3. Pearson’s Correlations Analysis
2.3.1. Germination Parameters and Biochemical Traits
2.3.2. Morphological/Physiological Traits and Biochemical Traits
2.3.3. Morphological/Physiological Traits and Germination Parameters
3. Discussion
3.1. Seed Nutritive Composition
3.2. Cold Stress Tolerance
4. Materials and Methods
4.1. Plant Material
4.2. Seed Biochemical Analyses
4.2.1. Protein and Tryptophan Content Determination
4.2.2. Tocopherols, Carotenoids, and Free Phenolic Acids Determination
4.3. Cold Stress Experiment
4.3.1. Experimental Design
4.3.2. Germination and Vigor Determination
4.3.3. Seedling Morphological and Physiological Traits
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Goredema-Matongera, N.; Ndhlela, T.; Magorokosho, C.; Kamutando, C.N.; Van Biljon, A.; Labuschagne, M. Multinutrient Biofortification of Maize (Zea Mays L.) in Africa: Current Status, Opportunities and Limitations. Nutrients 2021, 13, 1039. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Beshir Issa, A.; Khokhar, E.S. Quality Protein Maize (QPM): Importance, Genetics, Timeline of Different Events, Breeding Strategies and Varietal Adoption. Plant Breed. 2021, 140, 375–399. [Google Scholar] [CrossRef]
- Joshi, S.; Sharma, R.; Sharma, S.; Gupta, A.; Singh, B. Quality Protein Maize: Nutritional and Bioactive Composition, Technological Attributes and Potential Food Applications. Int. J. Food Sci. Technol. 2022, 57, 5600–5610. [Google Scholar] [CrossRef]
- Worral, H.M.; Scott, M.P.; Hallauer, A.R. Registration of Temperate Quality Protein Maize (QPM) Lines BQPM9, BQPM10, BQPM11, BQPM12, BQPM13, BQPM14, BQPM15, BQPM16, and BQPM17. J. Plant Regist. 2015, 9, 371–375. [Google Scholar] [CrossRef]
- Carena, M.J.; Dong, N. The ND EarlyQPM Program: Developing the next Generation of Healthier Maize (Zea Mays L.) Products. Euphytica 2017, 213, 150. [Google Scholar] [CrossRef]
- Ignjatovic-Micic, D.; Kostadinovic, M.; Bozinovic, S.; Djordjevic-Melnik, O.; Stankovic, G.; Delic, N.; Vancetovic, J. Evaluation of Temperate Quality Protein Maize (QPM) Hybrids for Field Performance and Grain Quality. Chil. J. Agric. Res. 2020, 80, 598–607. [Google Scholar] [CrossRef]
- Kostadinovic, M.; Ignjatovic-Micic, D.; Vancetovic, J.; Ristic, D.; Bozinovic, S.; Stankovic, G.; Mladenovic Drinic, S. Development of High Tryptophan Maize Near Isogenic Lines Adapted to Temperate Regions through Marker Assisted Selection—Impediments and Benefits. PLoS ONE 2016, 11, e0167635. [Google Scholar] [CrossRef]
- Ignjatović-Micić, D.; Vančetović, J.; Marković, K.; Ristić, D.; Pavlov, J.; Čamdžija, Z.; Kostadinović, M. Advantages of Quality Protein Maize Use in Broiler Diets. Sci. Agric. 2025, 82, e20240028. [Google Scholar] [CrossRef]
- Jägermeyr, J.; Müller, C.; Ruane, A.C.; Elliott, J.; Balkovic, J.; Castillo, O.; Faye, B.; Foster, I.; Folberth, C.; Franke, J.A.; et al. Climate Impacts on Global Agriculture Emerge Earlier in New Generation of Climate and Crop Models. Nat. Food 2021, 2, 873–885. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations, Strategic Framework 2022-31, © OECD/FAO 2021. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/29404c26-c71d-4982-a899-77bdb2937eef/content (accessed on 1 April 2025).
- Rising, J.; Devineni, N. Crop Switching Reduces Agricultural Losses from Climate Change in the United States by Half under RCP 8.5. Nat. Commun. 2020, 11, 4991. [Google Scholar] [CrossRef]
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Babar, M.A.; et al. Climate Change Impact and Adaptation for Wheat Protein. Glob. Change Biol. 2019, 25, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Bassu, S.; Asseng, S.; Motzo, R.; Giunta, F. Optimising Sowing Date of Durum Wheat in a Variable Mediterranean Environment. Field Crop. Res. 2009, 111, 109–118. [Google Scholar] [CrossRef]
- Landa, B.B.; Navas-Cortes, J.A.; Jimenez-Díaz, R.M. Integrated Management of Fusarium Wilt of Chickpea with Sowing Date, Host Resistance, and Biological Control. Phytopatholog 2004, 94, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C. Agronomic options for improving rainfall-use efficiency of crops in dryland farming systems. J. Exp. Bot. 2004, 55, 2413–2425. [Google Scholar] [CrossRef]
- Wu, W.; Yue, W.; Bi, J.; Zhang, L.; Xu, D.; Peng, C.; Chen, X.; Wang, S. Influence of Climatic Variables on Maize Grain Yield and Its Components by Adjusting the Sowing Date. Front. Plant Sci. 2024, 15, 1411009. [Google Scholar] [CrossRef]
- Abendroth, L.J.; Woli, K.P.; Myers, A.J.W.; Elmore, R.W. Yield-Based Corn Planting Date Recommendation Windows for Iowa. Crop Forage Turf. Man. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Dadrassi, A.; Soltani, E.; Makowski, D.; Lamichhane, J.R. Does Shifting from Normal to Early or Late Sowing Dates Provide Yield Benefits? A Global Meta-Analysis. Field Crops Res. 2024, 318, 109600. [Google Scholar] [CrossRef]
- Beck, E.H.; Fettig, S.; Knake, C.; Hartig, K.; Bhattarai, T. Specific and Unspecific Responses of Plants to Cold and Drought Stress. J. Biosci. 2007, 32, 501–510. [Google Scholar] [CrossRef]
- Serbia Climate Change Adaptation Programme, 2023–2030. Available online: https://www.preventionweb.net/publication/serbia-climate-change-adaptation-programme-period-2023-2030 (accessed on 29 July 2025).
- Petrović, G.; Ivanović, T.; Knežević, D.; Radosavac, A.; Obhođaš, I.; Brzaković, T.; Golić, Z.; Dragičević Radičević, T. Assessment of Climate Change Impact on Maize Production in Serbia. Atmosphere 2023, 14, 110. [Google Scholar] [CrossRef]
- Twumasi-Afriyie, S.; Palacios-Rojas, N.; Friesen, D.; Teklewold, A.; Wegary, D.; De Groote, H.; Prasanna, B.M. Guidelines for the Quality Control of Quality Protein Maize (QPM) Seed and Grain; CIMMYT: Addis Ababa, Ethiopia, 2016; pp. 1–48. ISBN 978-607-8263-60-8. [Google Scholar]
- Zaidi, P.H.; Vasal, S.K.; Maniselvan, P.; Jha, G.C.; Mehrajjudin, M.; Singh, R.P. Stability in Performance of Quality Protein Maize under Abiotic Stress. Maydica 2008, 53, 249–260. [Google Scholar]
- Ignjatovic-Micic, D.; Vancetovic, J.; Trbovic, D.; Dumanovic, Z.; Kostadinovic, M.; Bozinovic, S. Grain Nutrient Composition of Maize (Zea Mays L.) Drought-Tolerant Populations. J. Agric. Food Chem. 2015, 63, 1251–1260. [Google Scholar] [CrossRef]
- Amegbor, I.; Van Biljon, A.; Shargie, N.; Tarekegne, A.; Labuschagne, M. Identifying Quality Protein Maize Inbred Lines for Improved Nutritional Value of Maize in Southern Africa. Foods 2022, 11, 898. [Google Scholar] [CrossRef]
- Panda, A.K.; Zaidi, P.H.; Rama Rao, S.V.; Raju, M.V.L.N. Efficacy of Quality Protein Maize in Meeting Energy and Essential Amino Acid Requirements in Broiler Chicken Production. J. Appl. Anim. Res. 2014, 42, 133–139. [Google Scholar] [CrossRef]
- Khan, N.A.; Alam, M.; Khan, R.; Khan, K.; Rahman, S.U. Evaluating the Nutritional Value of the Newly Developed Quality Protein Maize in Pakistan: Impact on Broiler Performance and Profitability. Pak. J. Zool. 2020, 52, 425–824. [Google Scholar] [CrossRef]
- Rajasekhar, K.V.; Prakash, B.; Vijaya Lakshmi, K.; Rama Rao, S.V.; Raju, M.V.L.N. Effect of Feeding Diet with Alternate Protein Sources and Quality Protein Maize on Performance and Nutrient Utilization in Broiler Chickens. Trop. Anim. Health Prod. 2020, 52, 2297–2302. [Google Scholar] [CrossRef]
- Elangovan, A.V.; Anandan, S.; Giridhar, K.; Rao, S.B.N.; Awachat, V.B.; Parthipan, S.; Nalina, M.; Bagath, M.; Bhatta, R. Comparative Feeding Value of Quality Protein Maize and Normal Maize in Broiler Chicken. Indian J. Anim. Nutr. 2022, 39, 325–331. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A Comprehensive Review on Carotenoids in Foods and Feeds: Status Quo, Applications, Patents, and Research Needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Pinaffi-Langley, A.C.C.; Fuentes, J.; Speisky, H.; De Camargo, A.C. Vitamin E as an Essential Micronutrient for Human Health: Common, Novel, and Unexplored Dietary Sources. Free Radic. Biol. Med. 2021, 176, 312–321. [Google Scholar] [CrossRef]
- Calugar, R.E.; Muntean, E.; Varga, A.; Vana, C.D.; Has, V.V.; Tritean, N.; Ceclan, L.A. Improving the Carotenoid Content in Maize by Using Isonuclear Lines. Plants 2022, 11, 1632. [Google Scholar] [CrossRef]
- Šarčević, H.; Bukan, M.; Galić, V.; Jambrović, A.; Kljak, K.; Buhiniček, I.; Pejić, I.; Kiš, G.; Šimić, D. Genetic Variability of Tocol Content in a Genebank Collection of Temperate Maize Inbred Lines from Southeastern Europe. Agronomy 2024, 14, 269. [Google Scholar] [CrossRef]
- Hadinezhad, M.; Harris, L.J.; Miller, S.S.; Schneiderman, D. Genetic Variability of Kernel Phenolics in Maize (Zea Mays L.) Inbreds with Differing Levels of Resistance to Gibberella Ear Rot Mehri. Crop Sci. 2023, 63, 2162–2183. [Google Scholar] [CrossRef]
- Shumskaya, M.; Wurtzel, E.T. The Carotenoid Biosynthetic Pathway: Thinking in All Dimensions. Plant Sci. 2013, 208, 58–63. [Google Scholar] [CrossRef]
- Xiang, N.; Qi, X.; Hu, J.; Wang, S.; Guo, X. L-Tryptophan Synergistically Increased Carotenoid Accumulation with Blue Light in Maize (Zea Mays L.) Sprouts. Food Chem. Mol. Sci. 2023, 6, 100161. [Google Scholar] [CrossRef]
- Tarasevičienė, Ž.; Velička, A.; Paulauskienė, A. Impact of Foliar Application of Amino Acids on Total Phenols, Phenolic Acids Content of Different Mints Varieties under the Field Condition. Plants 2021, 10, 599. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Gątarek, P.; Chirumbolo, S.; Chartrand, M.S.; Bjørklund, G. How Important Is Tryptophan in Human Health? Crit. Rev. Food Sci. Nutr. 2019, 59, 72–88. [Google Scholar] [CrossRef]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. γ-Tocopherol, the Major Form of Vitamin E in the US Diet, Deserves More Attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar] [CrossRef]
- Pavlíková, N. Caffeic Acid and Diseases—Mechanisms of Action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological Effects of Gallic Acid in Health and Disease: A Mechanistic Review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Kader, M.A. A Comparison of Seed Germination Calculation Formulae and the Associated Interpretation of Resulting Data. J. Proceeding R. Soc. N. S. W. 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Meng, A.; Wen, D.; Zhang, C. Dynamic Changes in Seed Germination under Low-Temperature Stress in Maize. Int. J. Mol. Sci. 2022, 23, 5495. [Google Scholar] [CrossRef]
- Dueñas, M.; Hernández, T.; Estrella, I.; Fernández, D. Germination as a Process to Increase the Polyphenol Content and Antioxidant Activity of Lupin Seeds (Lupinus angustifolius L.). Food Chem. 2009, 117, 599–607. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Han, Z.; He, X.; Herrera-Balandrano, D.D.; Xiang, J. Comprehensive Evaluation on Phenolic Derivatives and Antioxidant Activities of Diverse Yellow Maize Varieties. Food Chem. 2025, 464, 141602. [Google Scholar] [CrossRef]
- Ashraf, M.; Mao, Q.; Hong, J.; Shi, L.; Ran, X.; Liaquat, F.; Uzair, M.; Liang, W.; Fernie, A.R.; Shi, J. HSP70 -16 and VDAC3 Jointly Inhibit Seed Germination under Cold Stress in Arabidopsis. Plant Cell Environ. 2021, 44, 3616–3627. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, S.; Yuan, X.; Chen, J.; Tian, M.; Zhao, Z.; Guo, T.; Xiao, W. OsNAL11 and OsBURP12 Affect Rice Seed Germination at Low Temperature. Plant Cell Environ. 2025, 48, 6118–6139. [Google Scholar] [CrossRef]
- Ma, Y.; Tan, R.; Zhao, J. Chilling Tolerance in Maize: Insights into Advances—Toward Physio-Biochemical Responses’ and QTL/Genes’ Identification. Plants 2022, 11, 2082. [Google Scholar] [CrossRef]
- Aroca, R.; Amodeo, G.; Fernández-Illescas, S.; Herman, E.M.; Chaumont, F.; Chrispeels, M.J. The Role of Aquaporins and Membrane Damage in Chilling and Hydrogen Peroxide Induced Changes in the Hydraulic Conductance of Maize Roots. Plant Physiol. 2005, 137, 341–353. [Google Scholar] [CrossRef]
- Guan, Y.; Li, Z.; He, F.; Huang, Y.; Song, W.; Hu, J. “On-Off” Thermoresponsive Coating Agent Containing Salicylic Acid Applied to Maize Seeds for Chilling Tolerance. PLoS ONE 2015, 10, e0120695. [Google Scholar] [CrossRef] [PubMed]
- Di Fenza, M.; Hogg, B.; Grant, J.; Barth, S. Transcriptomic Response of Maize Primary Roots to Low Temperatures at Seedling Emergence. PeerJ 2017, 5, e2839. [Google Scholar] [CrossRef]
- Ren, C.; Cheng, T.; Jia, J.; Cao, L.; Zhang, W.; Zhang, S.; Li, W.; Zhang, Y.; Yu, G. Exogenous Tryptophan Enhances Cold Resistance of Soybean Seedlings by Promoting Melatonin Biosynthesis. Physiol. Plant. 2025, 177, e70189. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, E.; Cheng, X.; Wang, L.; Jiang, S.; Yang, X.; Ma, H.; Zhang, T.; Li, T.; Yang, X. Effects of Chilling at Different Growth Stages on Rice Photosynthesis, Plant Growth, and Yield. Environ. Exp. Bot. 2022, 203, 105045. [Google Scholar] [CrossRef]
- Hmmam, I.; Abdelaal, R.A.; Gomaa, A.H. Insight into Chilling Stress Response of Key Citrus Grafting Combinations Grown in Egypt. Plant Stress 2023, 8, 100155. [Google Scholar] [CrossRef]
- Sowiński, P.; Rudzińska-Langwald, A.; Adamczyk, J.; Kubica, I.; Fronk, J. Recovery of Maize Seedling Growth, Development and Photosynthetic Efficiency after Initial Growth at Low Temperature. J. Plant Physiol. 2005, 162, 67–80. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of Photosynthetic Processes and the Accumulation of Secondary Metabolites in Plants in Response to Monochromatic Light Environments: A Review. Biochim. Biophys. Acta BBA Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Ke, D.; Guo, J.; Li, K.; Wang, Y.; Han, X.; Fu, W.; Miao, Y.; Jia, K.-P. Carotenoid-Derived Bioactive Metabolites Shape Plant Root Architecture to Adapt to the Rhizospheric Environments. Front. Plant Sci. 2022, 13, 986414. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Lyu, J.; Chen, Y.; Zhang, J.; Ye, N. Effects of Stress-Induced ABA on Root Architecture Development: Positive and Negative Actions. Crop J. 2023, 11, 1072–1079. [Google Scholar] [CrossRef]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed Germination and Vigor: Ensuring Crop Sustainability in a Changing Climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Manjeet, M.A.; Singh, V.K.; Verma, P.K. Character Association and Path Analysis for Seed Vigor Traits in Sesame (Sesamum indicum L.). J. Exp. Biol. Agric. Sci. 2020, 8, 111–114. [Google Scholar] [CrossRef]
- Barik, S.R.; Pandit, E.; Sanghamitra, P.; Mohanty, S.P.; Behera, A.; Mishra, J.; Nayak, D.K.; Bastia, R.; Moharana, A.; Sahoo, A.; et al. Unraveling the Genomic Regions Controlling the Seed Vigour Index, Root Growth Parameters and Germination per Cent in Rice. PLoS ONE 2022, 17, e0267303. [Google Scholar] [CrossRef]
- Zhang, T.; Fan, S.; Xiang, Y.; Zhang, S.; Wang, J.; Sun, Q. Non-Destructive Analysis of Germination Percentage, Germination Energy and Simple Vigour Index on Wheat Seeds during Storage by Vis/NIR and SWIR Hyperspectral Imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118488. [Google Scholar] [CrossRef]
- Vivek, B.S.; Krivanek, A.F.; Palacios-Rojas, N.; Twumasi-Afriyie, S.; Diallo, A.O. Breeding Quality Protein Maize (QPM): Protocols for Developing QPM Cultivars; AgEcon Search: Buford Ave, CA, USA, 2008. [Google Scholar] [CrossRef]
- Vukadinović, J.; Srdić, J.; Tosti, T.; Dragičević, V.; Kravić, N.; Drinić, S.M.; Milojković-Opsenica, D. Alteration in Phytochemicals from Sweet Maize in Response to Domestic Cooking and Frozen Storage. J. Food Compos. Anal. 2022, 114, 104637. [Google Scholar] [CrossRef]
- Mesarović, J.; Srdić, J.; Mladenović-Drinić, S.; Dragičević, V.; Simić, M.; Brankov, M.; Milojković-Opsenica, D. Evaluation of the Nutritional Profile of Sweet Maize after Herbicide and Foliar Fertilizer Application. J. Cereal Sci. 2019, 87, 132–137. [Google Scholar] [CrossRef]
- Mesarovic, J.; Dragicevic, V.; Mladenovic-Drinic, S.; Ristic, D.; Kravic, N. Determination of Free Phenolic Acids from Leaves within Different Colored Maize. J. Serbian Chem. Soc. 2017, 82, 63–72. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor Determination in Soybean Seed by Multiple Criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- MSTAT Development Team. MSTAT-C: A Microcomputer Program for the Design, Management and Analysis of Agronomic Research Experiments; Michigan State University, East Lansing, MI, USA, 1989; MSTAT Development Team: Princeton, NJ, USA, 1989. [Google Scholar]
- Table of Critical Values: Pearson Correlation—Statistics Solutions. Available online: https://www.statisticssolutions.com/free-resources/directory-of-statistical-analyses/pearsons-correlation-coefficient/table-of-critical-values-pearson-correlation/ (accessed on 15 January 2025).

| Group of Biochemical Compounds | Biochemical Compounds | SM | QPM | Difference (QPM-SM) | Level of Difference Significance | ||
|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | ||||
| Proteins, amino acids (%) | Total proteins | 11.76 | 0.06 | 12.82 | 0.06 | 1.06 | 0.01 |
| Tryptophan | 0.064 | 0.001 | 0.079 | 0.003 | 0.015 | 0.001 | |
| QI | 0.54 | 0.07 | 0.63 | 0.08 | 0.09 | 0.05 | |
| Phenolic acids (μg/g dm) | GA | 22.36 | 0.58 | 24.39 | 0.85 | 2.03 | ns |
| PA | 8.28 | 0.18 | 11.21 | 0.14 | 2.93 | ns | |
| CA | 8.80 | 0.13 | 6.76 | 0.09 | −2.04 | ns | |
| p-CoumA | 3.10 | 0.05 | 2.10 | 0.03 | −1.00 | ns | |
| FA | 4.72 | 0.06 | 3.73 | 0.04 | −0.99 | ns | |
| Carotenoids (μg/g dm) | L + Z | 24.65 | 1.28 | 19.96 | 1.39 | −4.69 | ns |
| β-cryptoxanthin | 5.31 | 0.23 | 4.87 | 0.26 | −0.44 | 0.001 | |
| β-carotene | 4.87 | 0.09 | 4.58 | 0.14 | −0.29 | ns | |
| Tocopherols (μg/g dm) | A − T | 12.59 | 0.27 | 12.60 | 0.27 | 0.01 | ns |
| β + γ − T | 43.86 | 1.16 | 40.71 | 1.08 | −3.15 | ns | |
| Δ − T | 1.51 | 0.04 | 1.61 | 0.05 | 0.10 | ns | |
| Group of Traits | Traits | Average | t-Test (C/T) | ||||
|---|---|---|---|---|---|---|---|
| SM | QPM | ||||||
| Control | Treatment | Control | Treatment | SM | QPM | ||
| Germination parameters | GP (%) | 97.46 | 93.97 | 96.30 | 95.56 | ns | ns |
| GI | 415.86 | 360.00 | 426.67 | 383.67 | ns | ns | |
| GE (%) | 76.98 | 46.67 | 82.96 | 48.15 | * | *** | |
| MGT (days) | 3.26 | 3.20 | 3.06 | 3.41 | ns | ns | |
| GRI (%/day) | 31.01 | 27.60 | 33.01 | 29.36 | ns | ns | |
| Root traits | RL (cm) | 14.58 | 4.88 | 15.71 | 5.20 | *** | *** |
| RFW (g) | 0.2649 | 0.0843 | 0.2897 | 0.0872 | *** | *** | |
| RDW (g) | 0.0226 | 0.0078 | 0.0257 | 0.0082 | *** | *** | |
| Shoot traits | SL (cm) | 6.77 | 1.40 | 7.35 | 1.65 | *** | *** |
| SFW (g) | 0.2222 | 0.0496 | 0.2557 | 0.0593 | *** | *** | |
| SDW (g) | 0.0176 | 0.0051 | 0.0204 | 0.0060 | *** | *** | |
| Seedling traits | SeedL (cm) | 21.34 | 6.28 | 23.07 | 6.85 | *** | *** |
| SeedDW (g) | 0.0402 | 0.0129 | 0.0461 | 0.0142 | *** | *** | |
| Vigor indices | VI1 | 2075.91 | 589.92 | 2238.47 | 669.48 | *** | *** |
| VI2 | 3.91 | 1.21 | 4.34 | 1.43 | *** | *** | |
| # | Inbred Line | Type | * Cold Tolerance | FAO Group | Heterotic Group |
|---|---|---|---|---|---|
| 1 | L1 | SM | moderately tolerant | FAO 550 | Lancaster |
| 2 | L1QPM1 | QPM | - | ||
| 3 | L1QPM2 | QPM | - | ||
| 4 | L1QPM3 | QPM | - | ||
| 5 | L2 | SM | susceptible | FAO 350–400 | Lancaster |
| 6 | L2QPM | QPM | - | ||
| 7 | L3 | SM | tolerant | FAO 300 | BSSS/ID |
| 8 | L3QPM | QPM | - | ||
| 9 | L4 | SM | tolerant | FAO 300 | BSSS/ID |
| 10 | L4QPM | QPM | - | ||
| 11 | L5 | SM | tolerant | FAO 450–500 | BSSS/ID |
| 12 | L5QPM | QPM | - | ||
| 13 | L6 | original | moderately tolerant | FAO 300 | BSSS/ID |
| 14 | L6QPM | QPM | - | ||
| 15 | L7 | original | moderately tolerant | FAO 600 | BSSS |
| 16 | L7QPM | QPM | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostadinović, M.; Milovanović, M.; Nikolić, A.; Marković, K.; Vukadinović, J.; Vančetović, J.; Ignjatović Micić, D. Early Sowing Approach for Developing Climate Resilient Maize: Cold Stress Impact on Germination of Adapted Inbred Lines with High Nutritive Value. Plants 2025, 14, 2540. https://doi.org/10.3390/plants14162540
Kostadinović M, Milovanović M, Nikolić A, Marković K, Vukadinović J, Vančetović J, Ignjatović Micić D. Early Sowing Approach for Developing Climate Resilient Maize: Cold Stress Impact on Germination of Adapted Inbred Lines with High Nutritive Value. Plants. 2025; 14(16):2540. https://doi.org/10.3390/plants14162540
Chicago/Turabian StyleKostadinović, Marija, Mirjana Milovanović, Ana Nikolić, Ksenija Marković, Jelena Vukadinović, Jelena Vančetović, and Dragana Ignjatović Micić. 2025. "Early Sowing Approach for Developing Climate Resilient Maize: Cold Stress Impact on Germination of Adapted Inbred Lines with High Nutritive Value" Plants 14, no. 16: 2540. https://doi.org/10.3390/plants14162540
APA StyleKostadinović, M., Milovanović, M., Nikolić, A., Marković, K., Vukadinović, J., Vančetović, J., & Ignjatović Micić, D. (2025). Early Sowing Approach for Developing Climate Resilient Maize: Cold Stress Impact on Germination of Adapted Inbred Lines with High Nutritive Value. Plants, 14(16), 2540. https://doi.org/10.3390/plants14162540






