Abstract
Wild blackberry (Rubus ulmifolius Schott) is a culinary and medicinal plant traditionally used to treat various ailments, including hypertension. This study evaluated the vasorelaxant effects of five crude leaf extracts of R. ulmifolius (hexane, dichloromethane, ethyl acetate, methanol, and aqueous), as well as the hypotensive and antioxidant activities of its methanolic extract (MERu), and analyzed its phytochemical profile. Crude extracts, obtained using a Soxhlet apparatus, were tested in vitro on isolated rat aortic rings precontracted with phenylephrine. The hypotensive effect of MERu was examined in vivo in normotensive rats, and its antioxidant activity was assessed using the DPPH assay. Total phenolic and tannin contents were quantified by the Folin–Ciocalteu and hide powder methods, respectively, while UHPLC-MS was used to identify its phytochemicals. All crude extracts induced concentration-dependent vasorelaxation, with MERu showing the strongest effect (59.31% relaxation at 10−1 g/L). Intravenous MERu induced significant blood pressure reductions in rats, starting at 1 mg/kg. At 20 mg/kg, systolic, diastolic, and mean arterial pressures dropped by 38.61%, 51.58%, and 45.19%, respectively. MERu also demonstrated potent antioxidant activity and was rich in polyphenols, particularly tannins. Sixteen compounds were identified, notably rubanthrone A, a galloyl-bis-HHDP glucose derivative, ellagic acid, and quercetin-3-O-β-D-glucuronide. These results suggest that R. ulmifolius may have therapeutic potential for hypertension and exhibits promising characteristics as a functional food.
1. Introduction
Hypertension is a major risk factor for cardiovascular diseases, accounting for 47% of ischemic heart disease cases and 54% of strokes, making it one of the leading causes of mortality worldwide [1]. This condition is defined by a persistent elevation of systolic blood pressure (SBP) at or above 140 mmHg and/or diastolic blood pressure (DBP) at or above 90 mmHg [2]. More than 90% of hypertensive individuals suffer from primary or essential hypertension, the causes of which remain unknown. In comparison, the remaining 10% are affected by secondary hypertension linked to other treatable conditions such as kidney or thyroid diseases [3]. Several factors contribute to the development of hypertension. Non-modifiable risk factors include age, male sex, premature menopause, and heredity. In contrast, modifiable risk factors are largely associated with lifestyle. These include unfavorable socioeconomic conditions, stress, physical inactivity, smoking, obesity, excessive sodium and alcohol intake, and an unbalanced diet [1,4]. In 2000, approximately 25% of the global population was affected by this condition. Projections indicated that this prevalence would increase by 60% by 2025, reaching 1.56 billion people [5]. The burden of hypertension is particularly significant in Africa, where nearly 50% of individuals aged 25 and older (over 150 million people) are affected. In 2019, hypertension was the leading risk factor for mortality in North Africa, accounting for 26% of deaths, while in sub-Saharan Africa, it ranked fourth among risk factors, contributing to 9% of deaths [6]. The prevention and management of hypertension primarily rely on lifestyle modifications, which also enhance the efficacy of pharmacological treatments. These interventions include adopting a healthy and balanced diet, reducing sodium and alcohol intake, increasing the consumption of plant-based foods, engaging in regular physical activity, achieving weight reduction, effectively managing stress, and quitting smoking [7]. Pharmacological treatment involves the use of various drug classes, including calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, and beta-blockers [8].
Alongside pharmacological approaches, there is growing global interest in using medicinal plants to manage hypertension. Many individuals rely on these plants to treat various ailments, including hypertension, driving increased scientific interest in their potential therapeutic benefits [9].
Rubus species, belonging to the Rosaceae family and including blackberries and raspberries, have been utilized as a source of food and in traditional medicine across various cultures for centuries. Previous research has established that leaves, roots, and fruits have astringent, anti-inflammatory, and antimicrobial effects, and have been utilized to treat digestive issues, skin wounds, respiratory problems, and menstrual disorders. Additionally, Rubus species are rich in antioxidants, contributing to their use in promoting overall health and combating oxidative stress. Rubus ulmifolius, commonly known as wild blackberry, is a perennial shrub widely distributed across Europe, Asia, and North Africa [10]. It is valued not only for its medicinal properties but also for its culinary uses. Its nutrient-rich fruits are consumed fresh or processed into jams and juices [11]. The leaves are used as natural food additives, particularly as colorants [12]. In Moroccan traditional medicine, leaf powder is applied topically to treat burns [13]. Leaf macerations are used to relieve gastrointestinal disorders and diarrhea [14], while infusions are employed to alleviate menstrual pain [15] and cardiac arrhythmia [16]. Leaf decoctions are used to treat oral infections and as facial rinses to firm the skin [17]. Additionally, decoctions combined with Cynara cardunculus L., Marrubium vulgare L., Lavandula dentata L., and eucalyptus are traditionally used to treat headaches [17]. The leaves are also used in the management of diabetes [12]. In Algerian traditional medicine, leaf infusions are administered to treat hypertension [18] and diarrhea [19] and are also valued for their anti-inflammatory properties [20]. Furthermore, they are applied as poultices to treat skin disorders [21].
From a phytochemical perspective, R. ulmifolius leaves are a significant source of secondary metabolites, including flavonoids such as catechin, hyperoside, and kaempferol 3-O-rutinoside; phenolic acids such as 3-O-caffeoylquinic acid and 3-p-coumaroylquinic acid; anthrones such as rubanthrone A; and tannins such as ellagic acid, methyl gallate, and the glucose derivative galloyl-bis-HHDP [22,23,24,25]. Previous pharmacological studies have highlighted various biological activities of R. ulmifolius leaves, notably their antibacterial [23], antiviral [26], antifungal [27], antiproliferative [28], and antioxidant properties [24]. More recently, we evaluated the hypotensive and vasodilatory activities of the aqueous extract, both in vivo and ex vivo, in rats. The results were promising, particularly in terms of blood pressure reduction [25]. Although the aqueous extract exhibited a notable cardiovascular effect, it likely represents only a fraction of the plant’s phytochemical content. To broaden the range of extracted compounds and further investigate the cardiovascular effects of R. ulmifolius, we employed the Soxhlet extraction technique using solvents of increasing polarity, resulting in five successive crude extracts (hexane, dichloromethane, ethyl acetate, methanol, and aqueous). Regarding the toxicity of the leaf extracts, an acute toxicity study was previously conducted using the methanolic extract obtained by maceration of the aerial parts of Rubus ulmifolius, administered to mice at various doses (0.01, 0.1, 1, 2, 4, and 6 g/kg). The results indicated that the extract was non-toxic up to a dose of 6 g/kg body weight [29]. Similarly, administration of the methanolic extract of R. ulmifolius’s aerial parts at a dose of 2.5 g/kg body weight did not cause any mortality or behavioral changes in mice [30].
This study aims to evaluate the vasorelaxant effects of crude extracts of varying polarity, obtained from R. ulmifolius using a Soxhlet apparatus, on isolated rat aorta. Additionally, it seeks to investigate the hypotensive effect of the most active extract, the methanolic extract, assess its antioxidant activity, and determine its phytochemical composition.
2. Materials and Methods
2.1. Plant Material
The leaves of Rubus ulmifolius Schott (wild blackberry) were collected in September 2023 in Berkane (34°53′25.0″ N, 2°20′44.8″ W), located in the Oriental region of Morocco. The plant was identified by Professor Mostafa Elachouri, and a voucher specimen was deposited in the herbarium of the Faculty of Sciences at Mohamed First University (Oujda, Morocco) under the accession number HUMPOM741.
2.2. Crude Extracts Preparation
The leaves of R. ulmifolius were dried in the shade and ground into a fine powder. A total of 170 g of the powder was sequentially extracted using a Soxhlet apparatus with solvents of increasing polarity: hexane, dichloromethane, ethyl acetate, methanol, and distilled water. For each extraction, 600 mL of solvent was used. The extraction process was carried out over several cycles and continued until the solvent became colorless. The extraction time for each solvent was approximately 8 h. The resulting extracts were evaporated under reduced pressure at 40 °C using a rotary evaporator and subsequently stored at −20 °C until further use. These extracts are referred to as crude extracts. The extraction yields were calculated using the following formula:
Extraction yield (%) = (Weight of obtained extract [g]/Weight of dried plant material [g]) × 100
2.3. Experimental Animals
The experiments were conducted on male Wistar rats raised under standard breeding conditions in the animal house of the Department of Biology at the Faculty of Sciences, Mohammed First University, Oujda, Morocco. The rats were maintained on a 12 h light–dark cycle with a temperature of 22 °C ± 2 and provided with ad libitum access to standardized food and water. All animal procedures followed the guidelines outlined in the Guide for the Care and Use of Laboratory Animals, published by the United States National Research Council [31]. Additionally, the experiments were conducted in compliance with ethical principles and were approved by the Faculty of Sciences, Mohammed First University, Oujda, Morocco (Approval No. 7/24-LBBES).
2.4. Ex Vivo Assessment of the Vasorelaxant Effect of Crude Extracts
The vasorelaxant effect of crude extracts of R. ulmifolius was evaluated ex vivo using isolated aorta rings from normotensive male Wistar rats (n = 8) weighing between 200 and 300 g. The rats were anesthetized via intraperitoneal injection of sodium thiopental (0.1 mL/100 g body weight), and their thoracic aortas were excised, placed in a physiological solution, cleaned of adipose tissue, and cut into small rings measuring 2–3 mm in length. From each rat, three to four rings were obtained, and five rings were used per experimental condition. Each ring was suspended between two hooks and placed in an isolated organ chamber containing 11 mL of physiological solution composed of glucose (11 mM), magnesium sulfate (MgSO4, 1.2 mM), sodium chloride (NaCl, 119 mM), potassium dihydrogen phosphate (KH2PO4, 1.2 mM), potassium chloride (KCl, 4.7 mM), sodium bicarbonate (NaHCO3, 25 mM), and calcium chloride (CaCl2, 2.6 mM). The solution was continuously oxygenated (95% O2 and 5% CO2) and maintained at a pH of 7.4 and a temperature of 37 °C. The upper hook was connected to a force transducer (Emka technologies, France), and a tension of 1 g was applied to the mounted ring. The rings were allowed to stabilize for 30 min. After stabilization, the endothelial integrity of the aorta was assessed by inducing contraction with phenylephrine (PHE, 10−6 M) followed by relaxation with carbachol (CCh, 10−4 M). The experiment proceeded only if the relaxation exceeded 50%. Once endothelial integrity was confirmed, the rings were exposed to cumulative concentrations of each extract (10−3, 10−2, 10−1 g/L). No washing was performed between concentrations. Each subsequent concentration was added only after the vasorelaxant response to the previous concentration had reached a stable plateau, typically within 1 to 5 min. Methanol was used as the vehicle, and its effect was negligible. The percentage of relaxation was calculated as follows:
Vasodilation (%) = (Amplitude of relaxation/Amplitude of PHE contraction) × 100
2.5. In Vivo Assessment of the Hypotensive Effect of Methanolic Extract
The hypotensive effect was studied solely in the R. ulmifolius methanolic extract, identified as the most active in the vasorelaxant effect study. This investigation was conducted on normotensive male Wistar rats (n = 3) weighing between 220 and 360 g. The rats were anesthetized via intraperitoneal injection of sodium thiopental (0.1 mL/100 g body weight) and placed on a heating plate to maintain their body temperature at 37 °C. Cannulation was performed on the femoral vein and artery using pre-heparinized catheters to prevent blood coagulation. The catheter inserted into the femoral vein was used for administering the drug. In contrast, the one placed in the femoral artery enabled the recording and visualization of arterial signals using a National Instruments data acquisition system and LabVIEW 6.1 software. After a 30 min stabilization period, the baroreflex response was assessed by analyzing changes in blood pressure and heart rate following intravenous injection of phenylephrine (PHE, 3 µg/kg, an α-adrenergic agonist) and sodium nitroprusside (SNP, 10 µg/kg, an exogenous NO donor). Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR) were subsequently recorded before (control) and after intravenous administration of the methanolic extract at doses of 0.5, 1, 5, 10, and 20 mg/kg body weight, with a 10 min interval between each injection. The extract was administered by intravenous bolus injection at a volume of 25 µL/100 g of body weight. Each dose was individually adjusted according to the animal’s weight. Blood pressure variations were evaluated intra-individually by comparing the values recorded before and after each injection in the same rat. The vehicle used was 0.9% NaCl, and it had no effect.
2.6. Antioxidant Activity
The antioxidant activity of the methanolic extract of R. ulmifolius was evaluated in vitro using the DPPH assay with some modifications. A volume of 180 µL of a DPPH solution (150 µM in MeOH/H2O, 80:20) was added to 20 µL of each extract at varying concentrations (12.5, 25, 50, 100, 125, 150, and 250 µg/mL in methanol). The mixture was vigorously shaken and incubated in the dark at room temperature for 40 min. Trolox (12.5–250 µg/mL in methanol) was used as a standard. Absorbance was measured at 515 nm using a microplate reader. All experiments were performed in triplicate. The DPPH radical scavenging activity was calculated using the following equation:
where Ab (blank absorbance) corresponds to the absorbance of MeOH/H2O (80:20) (180 µL) mixed with distilled water (20 µL); Ac (control absorbance) corresponds to the absorbance of the DPPH solution (180 µL) mixed with distilled water (20 µL); As (sample absorbance) corresponds to the absorbance of the DPPH solution (180 µL) mixed with the extract or Trolox (20 µL).
% Radical scavenging activity = [1 − (As − Ab)/(Ac − Ab)] × 100
2.7. Quantitative Analysis of Total Phenolic and Tannin Content of Methanolic Extract
2.7.1. Determination of Total Phenolic Content
The total phenolic content of the R. ulmifolius methanolic extract was determined using the Folin–Ciocalteu method, with some modifications. Briefly, 20 µL of the extract (0.42 mg/mL) was mixed with 100 µL of Folin–Ciocalteu reagent (diluted 1:10). The mixture was thoroughly shaken for 4 min. Subsequently, 80 µL of sodium carbonate (Na2CO3, 106 g/L) was added. The absorbance was measured at 760 nm. A standard curve was prepared using gallic acid at concentrations ranging from 0 to 100 mg/L. All measurements were performed in triplicate. The total phenolic content was expressed as milligrams of gallic acid equivalents per gram of extract (mg GAE/g).
2.7.2. Determination of Total Tannin Content
The total tannin content of the R. ulmifolius methanolic extract was determined using the hide powder method, with some modifications. Initially, 1.5 mL of the extract (0.42 mg/mL) was heated in a water bath at 95 °C for 30 min. After heating, the extract was centrifuged for 5 min. Then, 500 µL of the supernatant was used to determine the total polyphenol content. An additional 500 µL of the supernatant was mixed with 10 mg of non-chromated hide powder (SIGMA®). The mixture was thoroughly stirred for one hour in the dark and then centrifuged for 5 min. The resulting supernatant was used to determine the total non-tannic polyphenol content (phenolic compounds not adsorbed by the non-chromated hide powder). The total tannin content was calculated as the difference between total polyphenols and non-tannic phenolic compounds. The results were expressed as milligrams of gallic acid equivalents per gram of extract (mg GAE/g).
2.8. Ultra-High-Performance Liquid Chromatography Coupled with Mass Spectrometry (UHPLC-MS) Analysis of Methanolic Extract
The separation of compounds from R. ulmifolius methanolic extract was performed using an Acquity UPLC H-Class Waters® system (Guyancourt, France). The system comprised two independent pumps, a diode array detector (DAD), an automatic injector, a controller, and a QDa electrospray quadrupole mass spectrometer. Ionization was conducted in negative mode, with a mass range of 50–1250 Da. The cone voltage was set to 15 V, and the capillary voltage was maintained at 0.8 kV. The wavelength range for detection spanned 190–400 nm, with a resolution of 1.2 nm. The stationary phase consisted of an Uptisphere C18-AQ column (2.6 µm, 2.1 × 100 mm). The mobile phase was composed of two solvents: solvent (A) was ultrapure water with 0.1% formic acid (Carlo Erba Reagents®, Val de Reuil, France), and solvent (B) was acetonitrile (Carlo Erba Reagents®, Val de Reuil, France) with 0.1% formic acid. The elution gradient was programmed as follows: 5% to 80% (B) from 0 to 9 min, followed by 100% (B) from 9.5 to 10.5 min, and then a return to 5% (B) from 11 to 14 min. For the analysis, 1 mg of the extract was dissolved in 1 mL of methanol. The solution was filtered through a 20 µm Millipore filter, and 2 µL of the filtrate was injected. The analysis was carried out at 30 °C with a flow rate of 0.3 mL/min. The correspondence of each mass (m/z) to a molecule was essentially based on literature data. In addition, standards were used for some identified compounds [32,33,34]. Neochlorogenic acid, quercetin-3-O-β-D-glucuronide, kaempferol-3-O-β-D-glucuronide, and tiliroside were isolated from R. ulmifolius and characterized by NMR by our team. Chlorogenic acid, 3-p-coumaroylquinic acid, and ellagic acid were purchased from MedChemExpress (Nanterre, France) and Sigma-Aldrich (Saint-Quentin-Fallavier, France), respectively.
To quantify the different molecules identified in MERu, we used the same method as before, with some modifications. The wavelength ranges were 313 nm for 3-p-coumaroylquinic acid, 326 nm for chlorogenic acid, and 367 nm for ellagic acid. The elution gradient was programmed as follows: 5% (B) from 0 to 1 min, followed by 5% to 60% (B) from 1.01 to 9 min, 100% (B) from 9.01 to 11 min, and then a return to 5% (B) from 11.01 to 14 min.
2.9. Statistical Analysis
Results are expressed as mean ± SEM. Data were analyzed using a two-way ANOVA (Analysis of Variance), followed by Bonferroni’s post hoc test. All statistical analyses were performed using GraphPad Prism version 5 software. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Extraction Yield
The extraction yield was determined by calculating the ratio of the mass of the extract obtained after evaporation to the mass of the dried plant material. Among the extracts, the methanolic extract exhibited the highest yield. The yields of the hexane, dichloromethane, ethyl acetate, methanol, and aqueous extracts were 2.34%, 1.58%, 1.75%, 14.66%, and 11.62%, respectively.
3.2. Vasorelaxant Effect of the Crude Extracts
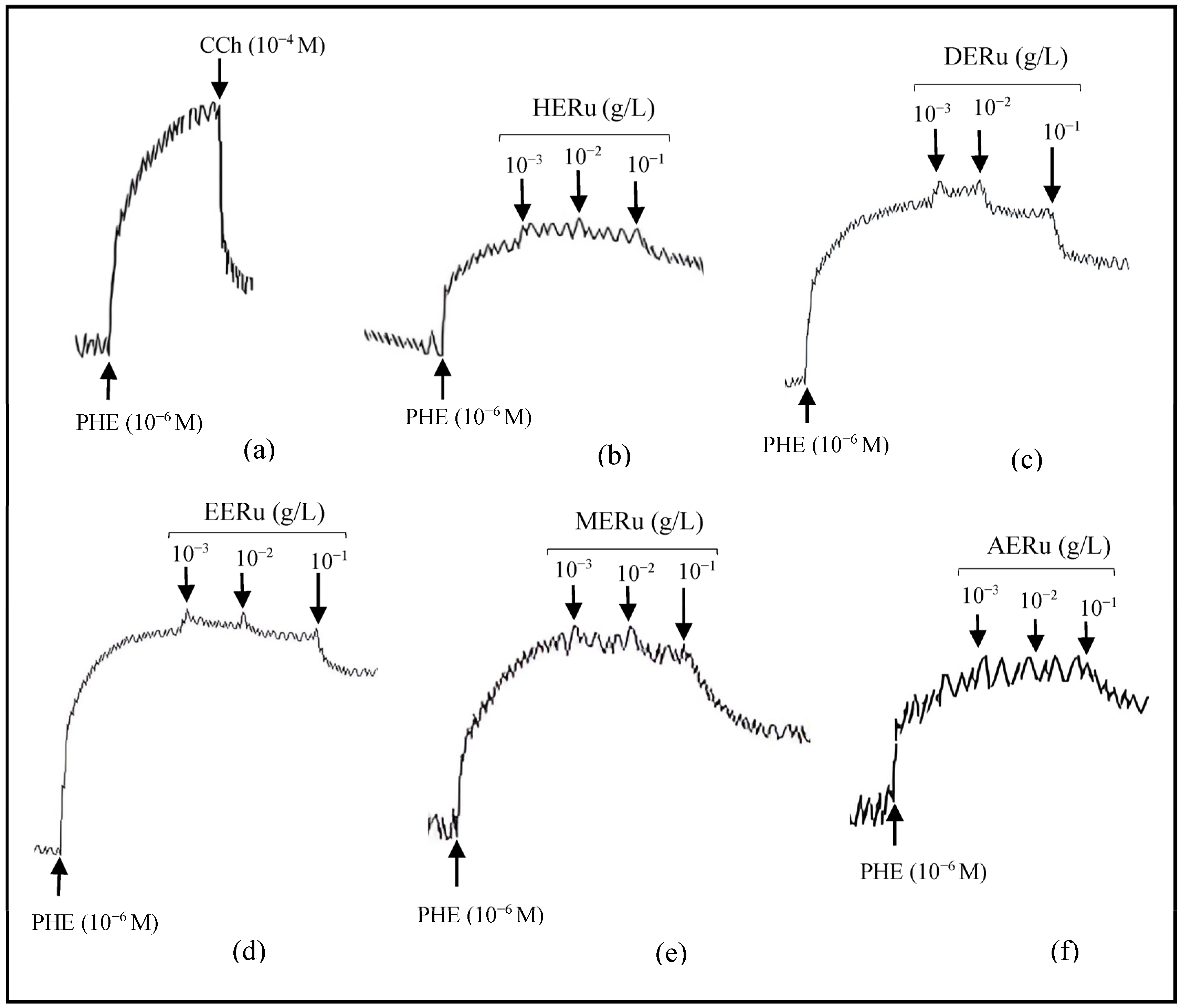
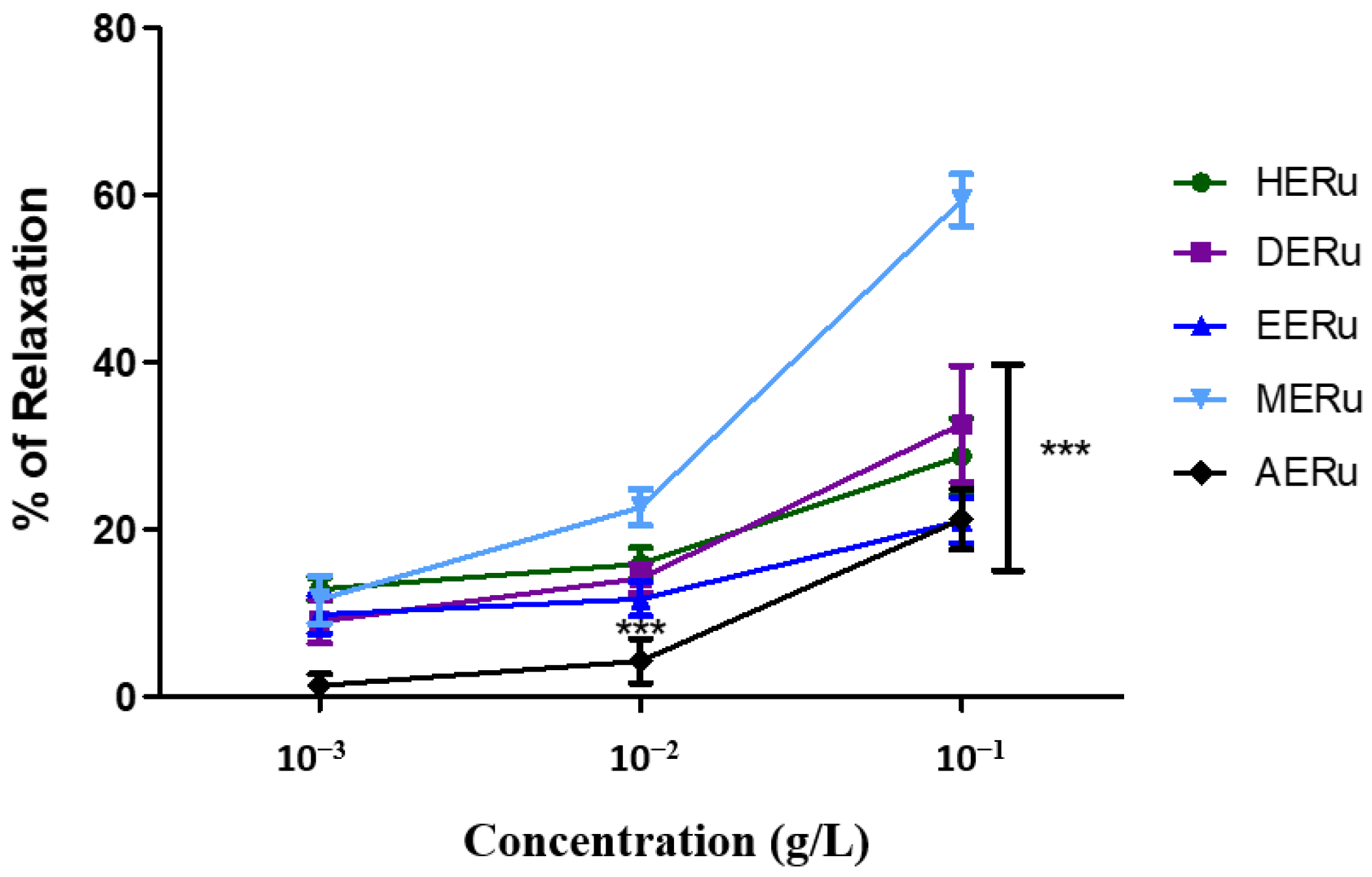
Crude extracts of R. ulmifolius leaves—prepared using hexane (HERu), dichloromethane (DERu), ethyl acetate (EERu), methanol (MERu), and water (AERu)—induced concentration-dependent relaxation of aortic rings precontracted with phenylephrine. As shown in Figure 1 and Figure 2, the maximum vasorelaxation was observed at a concentration of 10−1 g/L, with the most pronounced effect exhibited by MERu, followed by DERu, HERu, AERu, and EERu. The percentages of maximum relaxation were 59.31 ± 3.09%, 32.57 ± 6.97%, 28.72 ± 4.49%, 21.22 ± 3.59%, and 21.03 ± 2.74%, respectively.
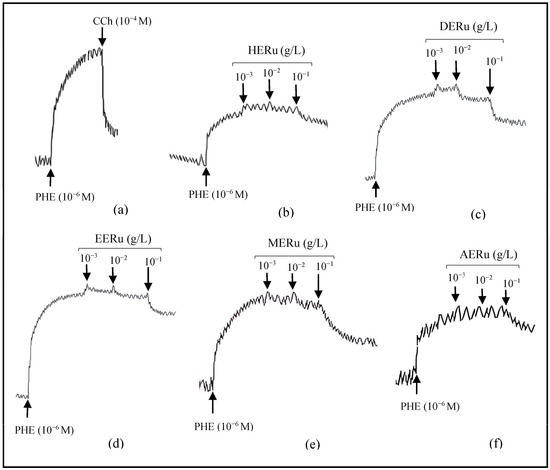
Figure 1.
Original tracings demonstrating the vascular effects of carbachol (CCh, 10−4 M) (a) and cumulative doses (10−3, 10−2, 10−1 g/L) of crude extracts of R. ulmifolius: hexane (b), dichloromethane (c), ethyl acetate (d), methanol (e), and aqueous (f), on aorta precontracted with phenylephrine (PHE, 10−6 M).
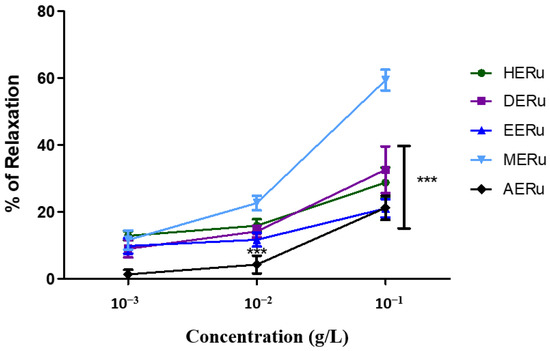
Figure 2.
The vasorelaxant effect of cumulative doses (10−3, 10−2, 10−1 g/L) of the hexane (HERu), dichloromethane (DERu), ethyl acetate (EERu), methanol (MERu), and aqueous (AERu) extracts of R. ulmifolius on aorta precontracted with phenylephrine (PHE; 10−6 M). Results are expressed as mean ± standard error of the mean (SEM) (n = 5 rings, obtained from 5 rats per condition). *** p < 0.001 (MERu vs HERu, DERu, EERu, AERu), determined using two-way ANOVA followed by Bonferroni post hoc test.
Since the methanolic extract (MERu) showed the best vasorelaxant effect, we decided to further investigate the biological effects of this extract.
3.3. Hypotensive Effect of the Methanolic Extract (MERu)
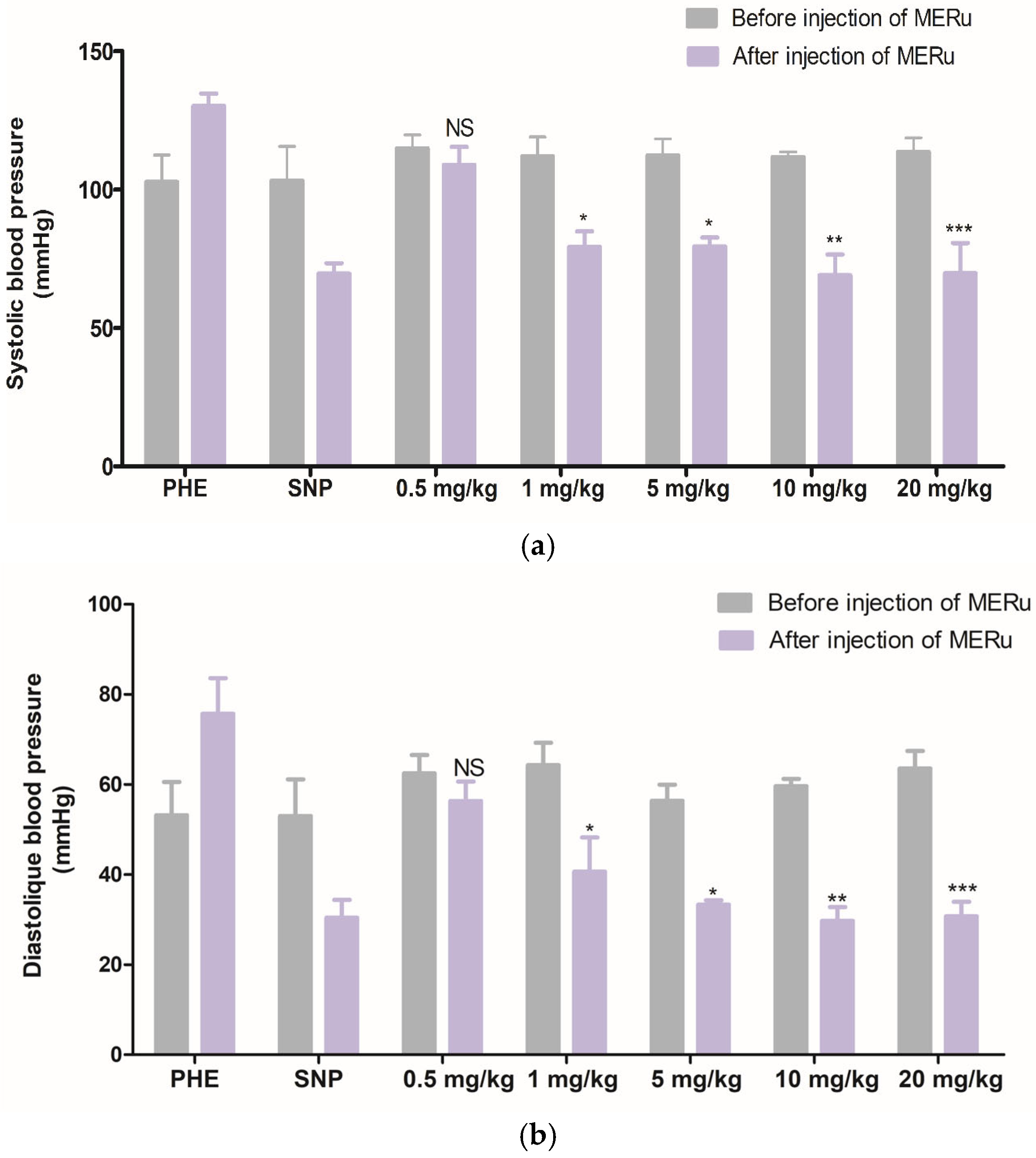
The intravenous injection of the methanolic extract at doses of 0.5, 1, 5, 10, and 20 mg/kg body weight induced a decrease in systolic blood pressure (SBP) by 5.13 ± 1.68%, 29.24 ± 1.32%, 29.16 ± 3.18%, 38.24 ± 7.74%, and 38.61 ± 7.26%, respectively, and in diastolic blood pressure (DBP) 9.85 ± 4.46%, 36.74 ± 7.61%, 40.92 ± 4.25%, 50.11 ± 5.18%, and 51.58 ± 8.15%, respectively (Figure 3).
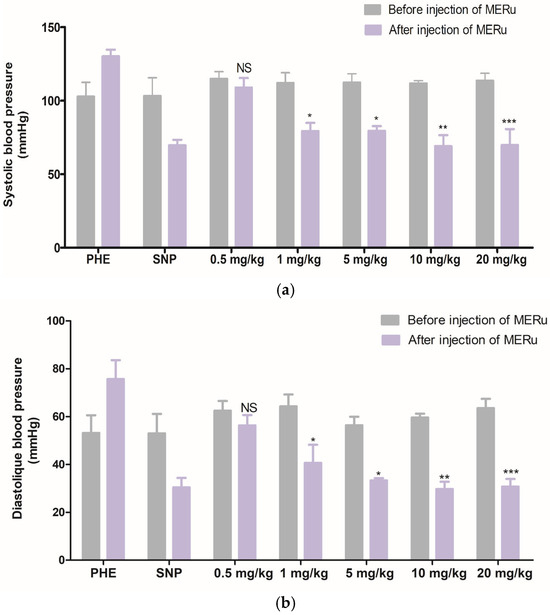
Figure 3.
Effect of intravenous injection of the methanolic extract of R. ulmifolius (MERu) on systolic blood pressure (a) and diastolic blood pressure (b) in normotensive anesthetized rats. Results are expressed as mean ± SEM (n = 3 rats) and were analyzed using two-way ANOVA followed by Bonferroni post hoc test. NS (not significant), * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control (before injection of the methanolic extract). PHE: phenylephrine; SNP: sodium nitroprusside.
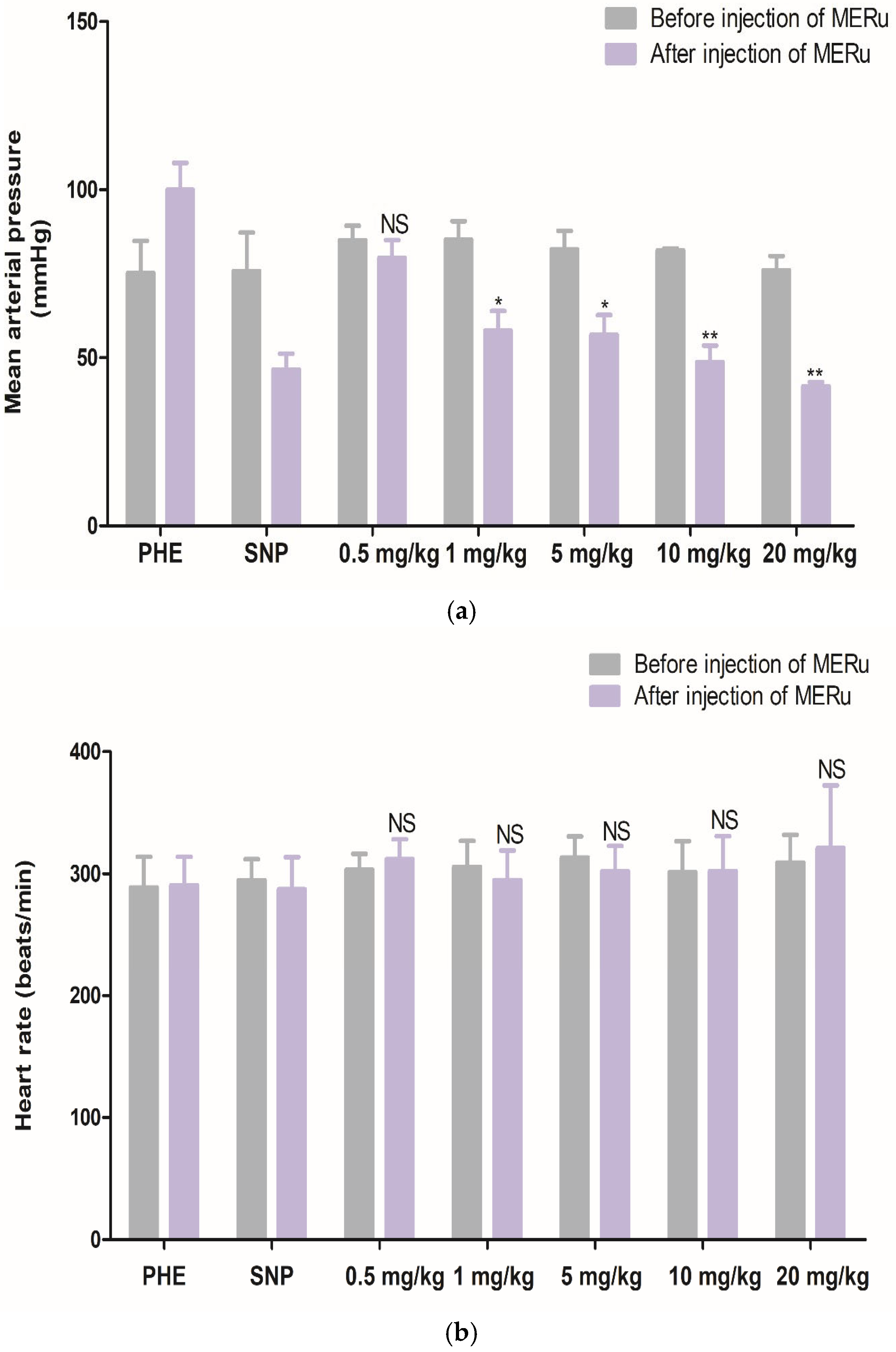
For mean arterial pressure (MAP), reductions of 6.15 ± 2.83%, 31.75 ± 3.44%, 30.89 ± 5.28%, 39.96 ± 5.94%, and 45.19 ± 3.72% were observed at doses of 0.5, 1, 5, 10, and 20 mg/kg body weight, respectively. However, heart rate (HR) was not significantly affected by the methanolic extract (Figure 4).
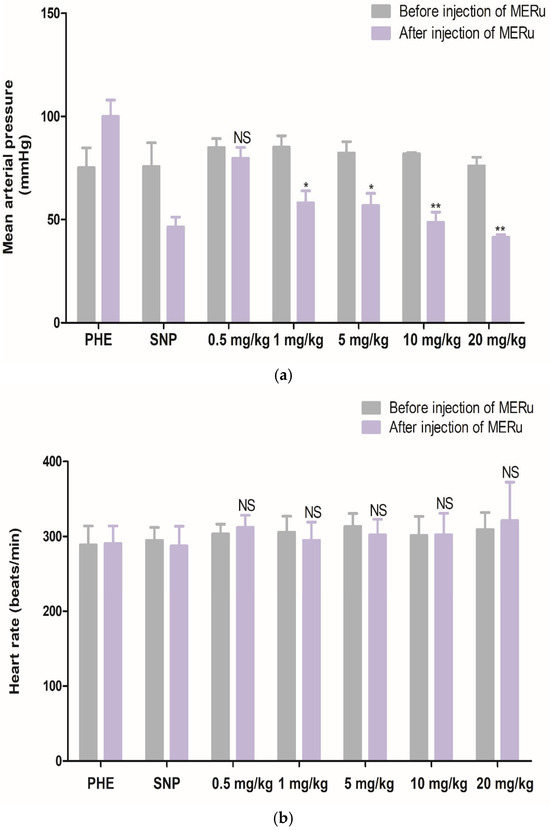
Figure 4.
Effect of intravenous injection of the methanolic extract of R. ulmifolius (MERu) on mean arterial pressure (a) and heart rate (b) in normotensive anesthetized rats. Results are expressed as mean ± SEM (n = 3 rats) and were analyzed using two-way ANOVA followed by Bonferroni post hoc test. NS (not significant), * p < 0.05, ** p < 0.01 vs. control (before injection of the methanolic extract). PHE: phenylephrine; SNP: sodium nitroprusside.
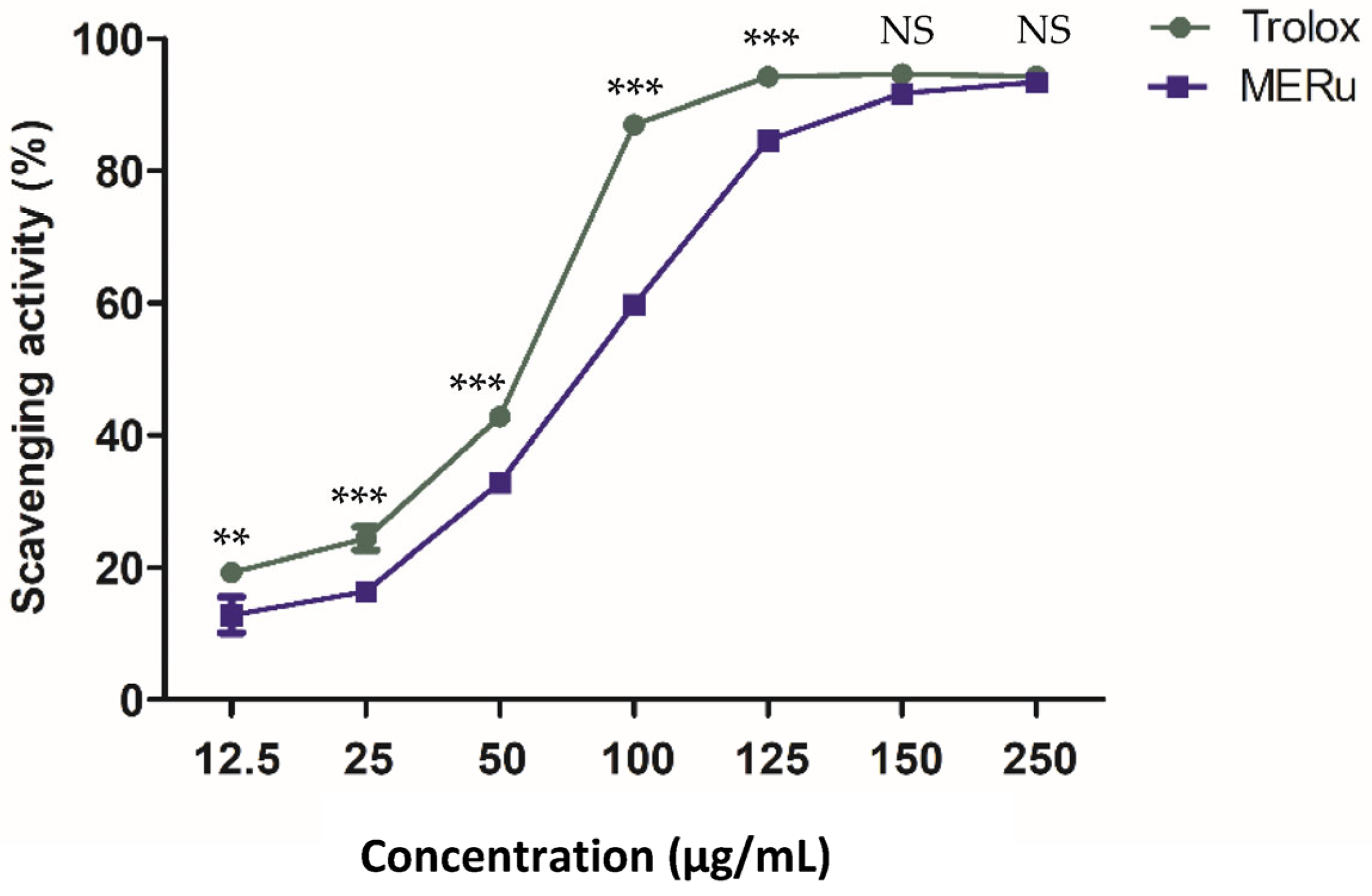
3.4. Antioxidant Activity (DPPH Assay)
The antioxidant effect of the methanolic extract of R. ulmifolius (MERu) was evaluated using the DPPH assay. The extract demonstrated a marked capacity to neutralize DPPH free radicals, reaching a maximum inhibition of 93.35 ± 0.39% at a concentration of 250 µg/mL, with an inhibitory concentration 50% (IC50) value of 86.49 µg/mL. As a reference, Trolox used as a positive control exhibited a maximum activity of 94.30 ± 0.23% at the same concentration, with an IC50 value of 59.18 µg/mL (Figure 5).
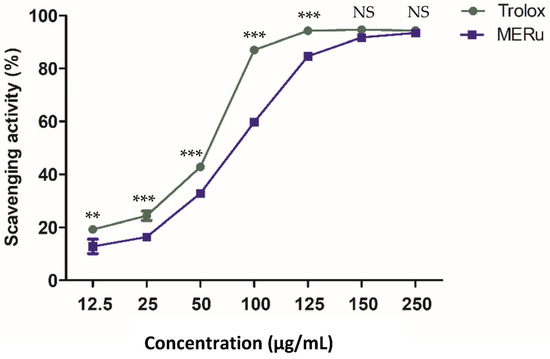
Figure 5.
Antioxidant effect of the methanolic extract of R. ulmifolius (MERu) on DPPH radical scavenging (0.1 mM). Results are expressed as mean ± SEM (n = 3) and were analyzed using two-way ANOVA followed by Bonferroni post hoc test. NS (not significant), ** p < 0.01, *** p < 0.001 vs. Trolox.
3.5. Total Phenolic, Tannin, and Pure Compound Contents of the Methanolic Extract
The phenolic and tannin contents of the methanolic extract of R. ulmifolius were determined using the Folin–Ciocalteu and the hide powder methods, respectively. The results are presented in Table 1. The extract exhibited phenolic and tannin contents of 218.36 ± 10.65 mg GAE/g and 139.58 ± 5.86 mg GAE/g, respectively.

Table 1.
Total phenolic, tannin, and pure compound contents of R. ulmifolius methanolic extract.
Additionally, chlorogenic acid, 3-p-coumaroylquinic acid, and ellagic acid were quantified in MERu using standard ranges of their standard. However, neochlorogenic acid, quercetin-3-O-β-D-glucuronide, kaempferol-3-O-β-D-glucuronide, and tiliroside, isolated by our team, had a purity lower than 95%, so their quantification could not be performed. The different concentrations for the standard ranges of each standard, along with the evaluated parameters and the amount of each molecule in MERu, are presented in Table 1.
3.6. Ultra-High-Performance Liquid Chromatography Coupled with Mass Spectrometry (UHPLC-MS) Analysis of the Methanolic Extract
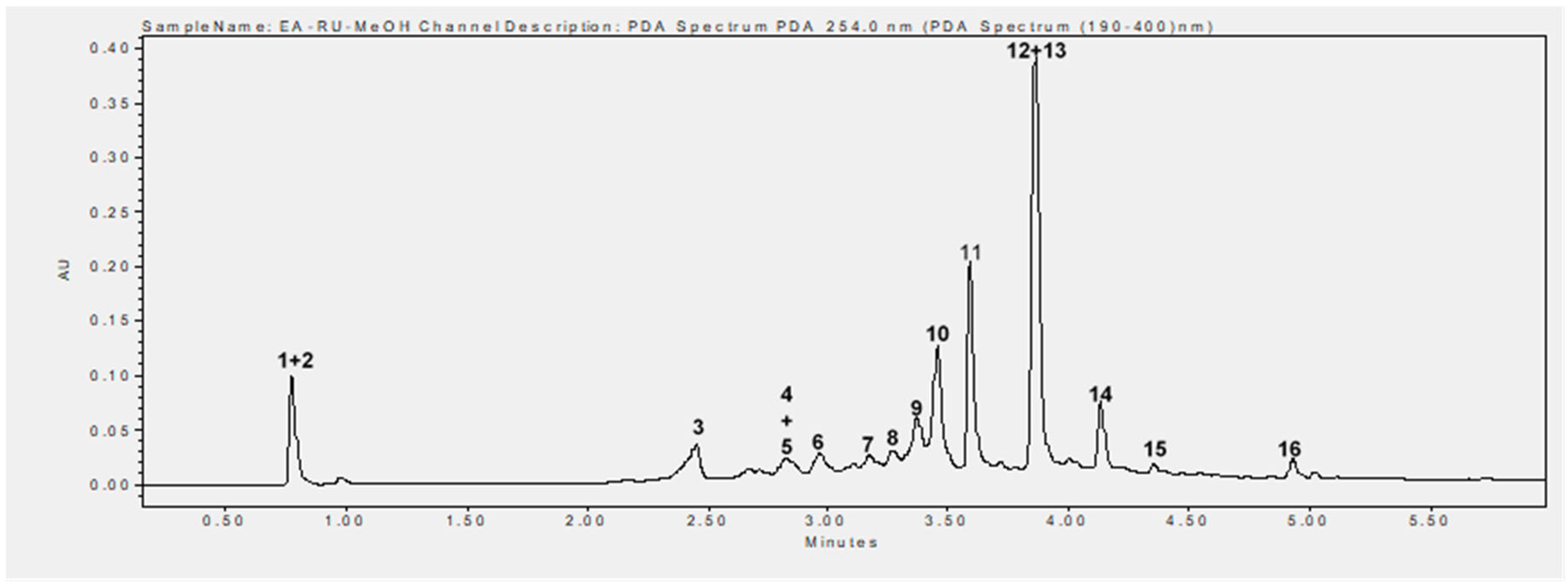
Phytochemical analysis of the methanolic extract using UHPLC-MS identified 16 phenolic compounds, including flavonoids, tannins, and phenolic acids. The main compounds identified were rubanthrone A (peak 2), a galloyl-bis-HHDP glucose derivative (peak 10), ellagic acid (peak 12), and quercetin-3-O-β-D-glucuronide (peak 13) (Table 2, Figure 6). These compounds were identified by comparing their mass spectral data and retention times with those reported in the literature [32,34].

Table 2.
UHPLC-MS analysis of MERu.
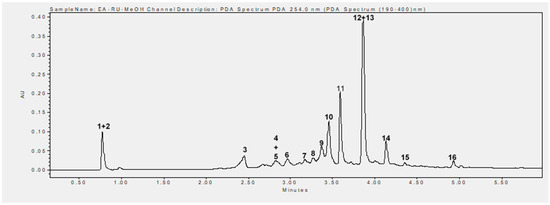
Figure 6.
UHPLC chromatogram recorded at 254 nm. 1. Not identified, 2. rubanthrone A, 3. neochlorogenic acid, 4. p-coumaroylquinic acid derivative, 5. kaempferol-3-O-arabinoside, 6. chlorogenic acid, 7. kaempferin, 8. epicatechin, 9. 3-p-coumaroylquinic acid, 10. galloyl-bis-HHDP glucose derivative, 11. not identified, 12. ellagic acid, 13. quercetin-3-O-β-D-glucuronide, 14. kaempferol-3-O-β-D-glucuronide, 15. not identified, 16. tiliroside.
Additionally, the retention times of neochlorogenic acid, chlorogenic acid, 3-p-coumaroylquinic acid, ellagic acid, quercetin-3-O-β-D-glucuronide, kaempferol-3-O-β-D-glucuronide, and tiliroside were compared with those of their respective standards.
4. Discussion
In our previous study, a nutrigenomic experiment was conducted using mouse models with R. ulmifolius Schott (wild blackberry) and Artemisia campestris L. (mugwort) leaves. We only focused on the aqueous extracts prepared by infusion, with the aim of associating biomarker genes with the vasorelaxant and hypotensive effects of these extracts [25]. Despite the promising cardiovascular effects observed from the aqueous extracts, this approach did not allow for the precise identification of the active compounds, nor did it reveal whether more potent molecules, not extracted by water, might be present. This encouraged us to continue investigating Rubus ulmifolius. Thus, the current study aimed to investigate the vasorelaxant effect of different crude extracts of R. ulmifolius (HERu, DERu, EERu, MERu, AERu), using solvents of increasing polarity, on the isolated rat aorta precontracted with phenylephrine (10−6 M). Based on the results obtained, the addition of cumulative concentrations (10−3, 10−2, 10−1 g/L) of the different extracts induced vasorelaxation, with the maximal effect observed at a concentration of 10−1 g/L. The methanolic extract (MERu) exhibited the most potent effect, with a maximum relaxation of 59.31%.
To understand through which pathway this effect occurs, further mechanistic investigations, particularly using nitric oxide synthase inhibitors such as L-NAME, would be useful to determine whether the observed vasorelaxant effect is endothelium-dependent.
Vasodilation (dilation of blood vessels) contributes to maintaining normal blood pressure by reducing vascular resistance (resistance to blood flow). It is mediated by the endothelium, the innermost layer of blood vessels, which plays a central role in regulating vascular homeostasis [35]. The endothelium is responsible for secreting vasodilatory substances such as nitric oxide (NO), endothelium-derived hyperpolarizing factors (EDHFs), and prostacyclin (PGI2). Under pathological conditions, blood vessels become rigid, and the endothelium loses its functions, leading to a reduction in the secretion of these substances. This promotes vasoconstriction and, consequently, the development of hypertension [36].
The vasorelaxant effect of MERu, reaching 59.31% at a concentration of 10−1 g/L, is comparable to that of the methanolic extract of Tetraclinis articulata, obtained by Soxhlet extraction, which induced a relaxation of 56% at the same concentration in isolated rat aortic rings [37]. Similarly, a vasorelaxant effect of the methanolic extract of Chenopodium ambrosioides, also obtained by Soxhlet extraction and tested on isolated rat aortic rings, was observed at various concentrations (10−3, 10−2, 10−1, and 1 mg/mL), with a maximal relaxation of 35.5% at 10−1 mg/mL [38]. Thus, at an equivalent concentration, the vasorelaxant effect of MERu appears more pronounced than that of the methanolic extract of C. ambrosioides.
Due to its potent vasorelaxant effect, MERu was selected to investigate its hypotensive effect in anesthetized normotensive rats. Our results showed that intravenous injection of MERu at increasing doses (0.5, 1, 5, 10, 20 mg/kg) induced a significant reduction in blood pressure starting at the dose of 1 mg/kg of body weight. At the dose of 20 mg/kg of body weight, the extract reduced systolic blood pressure, diastolic blood pressure, and mean arterial pressure by 38.61%, 50.33%, and 45.33%, respectively. However, the heart rate remained unchanged. This suggests that the hypotensive effect of MERu is mediated through the vascular pathway and is independent of the cardiac pathway.
Previous studies on other berry species have highlighted beneficial effects on vascular relaxation and blood pressure reduction. Anthocyanin-rich extracts of Aronia melanocarpa (chokeberry) and Vaccinium myrtillus (bilberry) induced concentration-dependent relaxation in isolated porcine coronary artery rings, achieving maximal effects of 68% and 59%, respectively, at a concentration of 5 mg/L [39]. In another study, an Aronia melanocarpa extract reduced blood pressure by 21% in L-NAME-treated hypertensive rats [40]. It has also been suggested that blueberry supplementation in rats fed a high-fat, high-cholesterol diet improved relaxation in isolated aortic rings in response to acetylcholine and significantly reduced systolic blood pressure [41]. Additionally, the ethanol extract of Rubus coreanus Miq. (black raspberry) fruits induced concentration- and endothelium-dependent relaxation in isolated rat aorta precontracted with phenylephrine, with a maximal effect of 93.84% at a concentration of 10 μg/mL [42]. Furthermore, the aqueous and butanol fractions of Crataegus songarica (hawthorn berry) demonstrated endothelium-dependent vasorelaxant effects, with the aqueous fraction being the most active, inducing 94% relaxation in isolated porcine coronary artery rings at a concentration of 3 mg/mL. In contrast, the butanol fraction caused 73.5% vasorelaxation at a concentration of 10 mg/mL [43].
Another part of this work focused on studying the antioxidant activity of MERu using the DPPH assay. Oxidative stress plays a key role in the development of hypertension. It results from an imbalance between the formation of reactive oxygen species (ROS) and their neutralization by antioxidant systems. Among the main sources of ROS production in blood vessels is the NADPH oxidase enzyme, which contributes to the inactivation of NO. It has been demonstrated that isolated arteries from hypertensive animals or humans exhibited a significant increase in ROS production [44]. The results of the DPPH assay showed that MERu possessed a potent antioxidant effect (Figure 5).
Previous studies on R. ulmifolius have also revealed antioxidant activity through this test. Chloroform, methanolic, and aqueous extracts demonstrated antioxidant activity with respective IC50 values of 45.54 μg/mL, 2.13 μg/mL, and 4.13 μg/mL [45]. Additionally, it was observed that the methanolic extract exhibited a potent antioxidant effect, with an IC50 value of 5.10 μg/mL [46]. Antioxidants are molecules capable of neutralizing ROS and act through various mechanisms. Among them, polyphenols represent an important class of antioxidants. They have shown beneficial effects on endothelial function and blood pressure regulation, particularly by inhibiting NADPH oxidase and increasing the expression of the endothelial nitric oxide synthase (eNOS), the enzyme responsible for NO synthesis [44].
Our study revealed that MERu contained high levels of polyphenols such as tannins (Table 1). To identify the specific compounds, present in this extract, a phytochemical analysis was conducted using ultra-high-performance liquid chromatography coupled with mass spectrometry (UHPLC-MS). This analysis showed that MERu is primarily composed of phenolic acids (neochlorogenic acid, p-coumaroylquinic acid derivative, chlorogenic acid, 3-p-coumaroylquinic acid), tannins (epicatechin, ellagic acid, galloyl-bis-HHDP glucose derivative), and flavonoids (kaempferol-3-O-arabinoside, kaempferin, quercetin-3-O-β-D-glucuronide, kaempferol-3-O-β-D-glucuronide, tiliroside).
Flavonoids are diverse polyphenolic compounds known for their beneficial effects on the cardiovascular system, particularly due to their ability to reduce the risk of hypertension and other cardiovascular diseases. Quercetin and kaempferol are flavonols that can exist in glycosylated forms. It has been demonstrated that quercetin lowers blood pressure through several mechanisms, including improving endothelial function, exerting antioxidant activity, and inhibiting angiotensin-converting enzyme (ACE) [47]. Additionally, a study revealed that kaempferol reduced blood pressure and induced relaxation of isolated aortic rings from rats precontracted with phenylephrine by stimulating endothelial NO production [48]. Moreover, it was reported that tiliroside reduced blood pressure in DOCA-salt hypertensive rats and dilated mesenteric arteries precontracted with phenylephrine. This vasorelaxant effect was endothelium-independent and occurred through the blockade of L-type calcium channels in smooth muscle cells [49]. Furthermore, a molecular docking study revealed that pentose ellagic acid, tiliroside, and galloyl-HHDP glucose had binding affinity for ACE, which is responsible for converting angiotensin I to angiotensin II, a potent vasoconstrictor. This affinity for ACE suggests an inhibition of its activity [50]. Additionally, research has shown that epicatechin reduced blood pressure and preserved cardiac function in DOCA-salt hypertensive rats with cardiac hypertrophy [51].
Regarding phenolic acids, chlorogenic acid is recognized for its health benefits, particularly its antihypertensive properties. A study demonstrated that it induced concentration and endothelium-dependent relaxation in phenylephrine-precontracted rat aortic rings, with a maximal effect of 67% at a concentration of 10−4 M. This effect was mediated through the activation of signaling pathways involving EDHF, eNOS, and cyclooxygenase [52]. Furthermore, a randomized study conducted on healthy individuals demonstrated that chlorogenic acid significantly reduced systolic and diastolic blood pressure [53].
Based on these results, chlorogenic acid, epicatechin, quercetin-3-O-β-D-glucuronide, kaempferol-3-O-β-D-glucuronide, and tiliroside, identified in the methanolic extract of R. ulmifolius, may play a key role in the observed vasorelaxant and hypotensive effects.
However, it is well established that the in vivo efficacy of phytochemicals largely depends on their bioavailability and pharmacokinetic profile. Taking these parameters into account is therefore essential to accurately assess the extent of their systemic effects. Among the compounds identified in the methanolic extract of R. ulmifolius, ellagic acid, galloyl-bis-HHDP glucose derivatives, and quercetin-3-O-β-D-glucuronide are particularly noteworthy, as they are major constituents of the extract and have been previously investigated for their pharmacokinetic behavior.
Ellagic acid is known for its low oral bioavailability, primarily due to poor absorption and limited solubility. A study conducted in rats by Lei et al. (2003) showed that following oral administration of a pomegranate leaf extract (0.8 g/kg) rich in ellagic acid, the compound reached a maximum plasma concentration of 213 ng/mL at 33 min post-ingestion, but was rapidly eliminated [54]. In humans, Seeram et al. (2004) demonstrated that after ingestion of pomegranate juice containing 25 mg of ellagic acid, the compound was detected in plasma one hour after administration at a peak concentration of only 31.9 ng/mL and became undetectable after four hours [55]. González-Sarrías et al. (2015) reported similar findings even after higher doses, highlighting extensive microbial metabolism into urolithins [56].
Regarding galloyl-bis-HHDP glucose derivatives, these compounds belong to the ellagitannin class. A study conducted in Iberian pigs, a physiological model closely related to humans, showed that after consumption of an acorn-rich diet containing ellagitannins, these compounds were no longer detectable in plasma. They were first hydrolyzed into ellagic acid, which was then metabolized by the gut microbiota into urolithins. These more lipophilic metabolites are better absorbed and represent the predominant forms detected in systemic circulation [57]. Similarly, a study in human volunteers demonstrated that after consumption of red raspberries rich in ellagitannins, the parent compounds were not detected in plasma. However, their metabolites, mainly conjugated urolithins, were present. This suggests that this metabolic transformation is essential to account for the systemic effects of ellagitannins despite their poor absorption in native form [58]. Additionally, a recent pharmacokinetic study in rabbits on geraniin, another ellagitannin, revealed that it is not absorbed as such but is hydrolyzed in the digestive tract into ellagic acid, which itself is poorly absorbed. However, the authors developed an optimized formulation, a geraniin–phosphatidylcholine complex, to improve the solubility and lipophilicity of ellagic acid and thereby enhance its absorption. With this formulation, ellagic acid was detected in plasma as early as 0.5 h post-administration, with two concentration peaks (Cmax) of 588.82 ng/mL at 2 h (Tmax1) and 711.13 ng/mL at 24 h (Tmax2), reflecting a marked improvement in bioavailability [59].
As for quercetin-3-O-β-D-glucuronide, a comparative pharmacokinetic study in rats evaluated the profiles of quercetin, isoquercitrin, and quercetin-3-O-β-D-glucuronide following oral administration of each compound at a dose of 50 mg/kg. All three compounds were detected in plasma, confirming systemic absorption. Moreover, following administration of quercetin-3-O-β-D-glucuronide, free quercetin was also detected in plasma as early as 1.5 h, indicating in vivo metabolic conversion. The bioavailability of quercetin, estimated from the area under the plasma concentration–time curve (AUC0–t), was 3505.7 ± 1565.0 mg × min/L, while that of quercetin-3-O-β-D-glucuronide was 962.7 ± 602.3 mg × min/L, suggesting that part of the latter is converted into quercetin, the more bioavailable form [60].
These pharmacokinetic limitations considerably reduce the systemic efficacy of these compounds when administered orally. In our study, intravenous administration of the methanolic extract of R. ulmifolius allowed the active constituents to reach the systemic circulation directly, thereby bypassing first-pass metabolism and intestinal absorption barriers, which may explain the cardiovascular effects observed.
These findings provide important insights into the pharmacological potential of R. ulmifolius and support its relevance in the management of cardiovascular disorders. Further investigations into the pharmacokinetics of isolated compounds from R. ulmifolius, as well as into their long-term cardiovascular effects, would be valuable to confirm and extend these findings.
5. Conclusions
The findings of this study demonstrated the concentration-dependent vasorelaxant effect of crude extracts of R. ulmifolius (hexane, dichloromethane, ethyl acetate, methanol, and aqueous) on isolated rat aortic rings precontracted with phenylephrine, with the most pronounced effect induced by the methanolic extract (MERu). Furthermore, intravenous administration of MERu in anesthetized normotensive rats resulted in a significant reduction in blood pressure starting at a dose of 1 mg/kg body weight. The extract also exhibited notable antioxidant activity. Phytochemical analysis of MERu, conducted using UHPLC-MS, confirmed the presence of flavonoids, phenolic acids, and tannins, suggesting that these compounds may contribute to the observed effects. These findings highlight the significant vasodilatory and hypotensive effects of the MERu of R. ulmifolius.
Rubus ulmifolius shows promising characteristics as a potential functional food, owing to its rich phytochemical content and demonstrated antioxidant, vasorelaxant, and hypotensive activities. These results align with the fact that no toxicity has been reported in the leaves, supporting their use in food.
Further studies are needed to isolate the major bioactive compounds, evaluate their effects in pure form, elucidate the underlying mechanisms of action, and validate the extract’s potential role in cardiovascular health through human trials.
Author Contributions
Conceptualization, A.M., C.A., A.Z. and S.S.; methodology, Z.L.E.A., A.Z. and S.S.; software, A.M., C.A. and Z.L.E.A.; validation, Z.L.E.A., A.Z. and S.S.; formal analysis, A.M., C.A. and Z.L.E.A.; investigation, A.M. and C.A.; resources, I.D., S.A., A.B., H.M. and A.L.; data curation, A.M. and C.A.; writing—original draft preparation, A.M. and C.A.; writing—review and editing, Z.L.E.A., I.D., S.A., A.B., H.M., A.L., A.Z. and S.S.; visualization, A.M., C.A. and Z.L.E.A.; supervision, Z.L.E.A., A.Z. and S.S.; project administration, A.Z. and S.S.; funding acquisition, A.Z. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the CNRST within the framework of the Research Excellence Grants Program (CNRST grants for A.M. and C.A.), and by the aromatic and medicinal plants project 2nd PMA 2020/5 (period 2020–2025) jointly funded by the Ministry of Higher Education, the National Agency for Aromatic and Medicinal Plants of Taounate, and the Presidency of Mohammed First University. A.M. also received a Moblilex Scholarship from the University of Lille. CPER BiHauts Eco de France also supported this project.
Institutional Review Board Statement
All animal procedures followed the guidelines outlined in the Guide for the Care and Use of Laboratory Animals, published by the United States National Research Council [19]. The experiments were conducted in compliance with ethical principles and were approved by the Faculty of Sciences, Mohammed First University, Oujda, Morocco (Approval No. 7/24-LBBES).
Data Availability Statement
All data supporting the results of this study are presented in the manuscript.
Acknowledgments
We express our utmost gratitude to the CNRST, the Ministry of Higher Education, the National Agency for Aromatic and Medicinal Plants of Taounate, and the Presidency of Mohammed First University for their financial support. The authors would also like to thank the Faculty of Sciences of Oujda, the University of Lille, and CPER BiHauts Eco de France for their logistical and financial support.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MERu | Methanolic extract of Rubus ulmifolius |
| HERu | Hexanic extract of Rubus ulmifolius |
| DERu | Dichloromethane extract of Rubus ulmifolius |
| EERu | Ethyl acetate extract of Rubus ulmifolius |
| AERu | Aqueous extract of Rubus ulmifolius |
| PHE | Phenylephrine |
| CCh | Carbachol |
| SNP | Sodium nitroprusside |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| MAP | Mean arterial pressure |
| HR | Heart rate |
References
- Jordan, J.; Kurschat, C.; Reuter, H. Arterial hypertension: Diagnosis and treatment. Dtsch. Arztebl. Int. 2018, 115, 557. [Google Scholar]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and control of hypertension: JACC health promotion series. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Lackland, D.T.; Campbell, N.R.; Ojo Owolabi, M.; Bavuma, C.; Mamoun Beheiry, H.; Dzudie, A.; Ibrahim, M.M.; El Aroussy, W.; Singh, S. How to improve awareness, treatment, and control of hypertension in Africa, and how to reduce its consequences: A call to action from the World Hypertension League. Hypertension 2022, 79, 1949–1961. [Google Scholar] [CrossRef]
- Charchar, F.J.; Prestes, P.R.; Mills, C.; Ching, S.M.; Neupane, D.; Marques, F.Z.; Sharman, J.E.; Vogt, L.; Burrell, L.M.; Korostovtseva, L. Lifestyle management of hypertension: International Society of Hypertension position paper endorsed by the World Hypertension League and European Society of Hypertension. J. Hypertens. 2024, 42, 23–49. [Google Scholar] [CrossRef]
- Ram, C.V.S. Antihypertensive drugs: An overview. Am. J. Cardiovasc. Drugs 2002, 2, 77–89. [Google Scholar] [CrossRef]
- Romano, B.; Lucariello, G.; Capasso, R. Topical collection pharmacology of medicinal plants. Biomolecules 2021, 11, 101. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Pereira, E.; Prieto, M.A.; Simal-Gandara, J.; Pires, T.C.; Alves, M.J.; Calhelha, R.; Barros, L.; Ferreira, I.C. Rubus ulmifolius Schott as a novel source of food colorant: Extraction optimization of coloring pigments and incorporation in a bakery product. Molecules 2019, 24, 2181. [Google Scholar] [CrossRef]
- Chauhan, E.S.; Chauhan, U. Nutritional and bioactive properties of Rubus ulmifolius Schott (blackberry): A review. Asian J. Dairy Food Res. 2022, 41, 249–255. [Google Scholar] [CrossRef]
- Drioiche, A.; Benhlima, N.; Kchibale, A.; Boutahiri, S.; Ailli, A.; El Hilali, F.; Moukaid, B.; Zair, T. Ethnobotanical investigation of herbal food additives of Morocco used as natural dyes. Ethnobot. Res. Appl. 2021, 21, 1–43. [Google Scholar] [CrossRef]
- Salhi, S.; Fadli, M.; Zidane, L.; Douira, A. Études floristique et ethnobotanique des plantes médicinales de la ville de Kénitra (Maroc). Mediterr. Bot. 2010, 31, 133. [Google Scholar] [CrossRef]
- El-Hilaly, J.; Hmammouchi, M.; Lyoussi, B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (northern Morocco). J. Ethnopharmacol. 2003, 86, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, S.; Dahmani, J.; Belahbib, N.; Zidane, L. Ethnopharmacological and ethnobotanical study of medicinal plants in the Central High Atlas, Morocco الدراسة الإيثنوفرمكولوجية والإ ثنوبولوجیة للنباتات الطبية في الأطلس المتوسط الكبير للمغرب. Ethnobot. Res. Appl. 2020, 20, 1–40. [Google Scholar]
- Chaachouay, N.; Azeroual, A.; Bencharki, B.; Zidane, L. Herbal medicine used in the treatment of cardiovascular diseases in the Rif, north of Morocco. Front. Pharmacol. 2022, 13, 921918. [Google Scholar] [CrossRef]
- Bouayyadi, L.; El Hafian, M.; Zidane, L. Étude floristique et ethnobotanique de la flore médicinale dans la région du Gharb, Maroc. J. Appl. Biosci. 2015, 93, 8770–8788. [Google Scholar] [CrossRef]
- Meddour, R.; Sahar, O.; Ouyessad, M. Ethnobotanical survey on medicinal plants in the Djurdjura National Park and its influence area, Algeria. Ethnobot. Res. Appl. 2020, 20, 1–25. [Google Scholar] [CrossRef]
- Hamel, T.; Zaafour, M.; Boumendjel, M. Ethnomedical knowledge and traditional uses of aromatic and medicinal plants of the Wetlands Complex of the Guerbes-Sanhadja Plain (wilaya of Skikda in northeastern Algeria). Herbal Med. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Kaci, Z.; Tirchi, N.; Dahmane, T.; Berrai, H.; Holgado, R.; Boubekeur, S.; Chebli, A.; Biche, M. First ethnobotanical study relating to usage of medicinal plants in province of Ain Defla region, south-west of Algeria. Indian J. Ecol. 2022, 49, 655–664. [Google Scholar]
- Chohra, D.; Ferchichi, L. Ethnobotanical study of Belezma National Park (BNP) plants in Batna: East of Algeria. Acta Sci. Nat. 2019, 6, 40–54. [Google Scholar] [CrossRef]
- Martini, S.; d’Addario, C.; Colacevich, A.; Focardi, S.; Borghini, F.; Santucci, A.; Figura, N.; Rossi, C. Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int. J. Antimicrob. Agents 2009, 34, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Tabarki, S.; Aouadhi, C.; Mechergui, K.; Hammi, K.M.; Ksouri, R.; Raies, A.; Toumi, L. Comparison of phytochemical composition and biological activities of Rubus ulmifolius extracts originating from four regions of Tunisia. Chem. Biodivers. 2017, 14, e1600168. [Google Scholar] [CrossRef] [PubMed]
- Bramki, A.; Benouchenne, D.; Salvatore, M.M.; Benslama, O.; Andolfi, A.; Rahim, N.; Moussaoui, M.; Ramoul, S.; Nessah, S.; Barboucha, G. In vitro and in silico biological activities investigation of ethyl acetate extract of Rubus ulmifolius Schott leaves collected in Algeria. Plants 2024, 13, 3425. [Google Scholar] [CrossRef]
- Mehiou, A.; Lucau-Danila, A.; Akissi, Z.L.; Alla, C.; Bouanani, N.; Legssyer, A.; Hilbert, J.-L.; Sahpaz, S.; Ziyyat, A. Nutrigenomic insights and cardiovascular benefits of blackberry (Rubus ulmifolius Schott.) and mugwort (Artemisia campestris L.). Exp. Physiol. 2025, 1–16. [Google Scholar] [CrossRef]
- Sanna, G.; Farci, P.; Busonera, B.; Murgia, G.; La Colla, P.; Giliberti, G. Antiviral properties from plants of the Mediterranean flora. Nat. Prod. Res. 2015, 29, 2065–2070. [Google Scholar] [CrossRef]
- Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.; Msanda, F.; Saadi, B.; Ait Ben Aoumar, A. Antifungal activity of Moroccan medicinal plants against citrus sour rot agent Geotrichum candidum. Lett. Appl. Microbiol. 2012, 55, 155–161. [Google Scholar] [CrossRef]
- Triggiani, D.; Ceccarelli, D.; Cusi, M.G.; Paffetti, A.; Braconi, D.; Millucci, L.; Bernardini, G.; Santucci, A. Rubus ulmifolius leaf extract inhibits proliferation of murine myeloma cells. Med. Aromat. Plant Sci. Biotechol. 2012, 6, 37–41. [Google Scholar]
- Ali, N.; Shaoib, M.; Shah, S.W.A.; Shah, I.; Shuaib, M. Pharmacological profile of the aerial parts of Rubus ulmifolius Schott. BMC Complement. Altern. Med. 2017, 17, 59. [Google Scholar] [CrossRef]
- Akhtar, K.; Shah, S.W.A.; Shah, A.A.; Shoaib, M.; Haleem, S.K.; Sultana, N. Pharmacological effect of Rubus ulmifolius Schott as antihyperglycemic and antihyperlipidemic on streptozotocin (STZ)-induced albino mice. Appl. Biol. Chem. 2017, 60, 411–418. [Google Scholar] [CrossRef]
- National Research Council (NRC). Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011.
- Flamini, G.; Catalano, S.; Caponi, C.; Panizzi, L.; Morelli, I. Three anthrones from Rubus ulmifolius. Phytochemistry 2002, 59, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, L.; Caponi, C.; Catalano, S.; Cioni, P.; Morelli, I. In vitro antimicrobial activity of extracts and isolated constituents of Rubus ulmifolius. J. Ethnopharmacol. 2002, 79, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Mehiou, A.; Alla, C.; Akissi, Z.L.; Lucau-Danila, A.; Bouanani, N.; Legssyer, A.; Hilbert, J.-L.; Sahpaz, S.; Ziyyat, A. Rubus ulmifolius schott: A comprehensive review of its botany, traditional uses, phytochemistry and pharmacological effects. J. Berry Res. 2024, 14, 301–328. [Google Scholar] [CrossRef]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef]
- Giles, T.D.; Sander, G.E.; Nossaman, B.D.; Kadowitz, P.J. Impaired vasodilation in the pathogenesis of hypertension: Focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J. Clin. Hypertens. 2012, 14, 198–205. [Google Scholar] [CrossRef]
- Zidane, A.; Tits, M.; Angenot, L.; Wauters, J.-N.; Frederich, M.; Dib, I.; Mekhfi, H.; Aziz, M.; Bnouham, M.; Abdelkhaleq, L.; et al. Phytochemical analysis of Tetraclinis articula in relation to its vasorelaxant property. J. Mater. Environ. Sci. 2014, 5, 1368–1375. [Google Scholar]
- Assaidi, A.; Dib, I.; Tits, M.; Angenot, L.; Bellahcen, S.; Bouanani, N.; Legssyer, A.; Aziz, M.; Mekhfi, H.; Bnouham, M. Chenopodium ambrosioides induces an endothelium-dependent relaxation of rat isolated aorta. J. Integr. Med. 2019, 17, 115–124. [Google Scholar] [CrossRef]
- Bell, D.R.; Gochenaur, K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J. Appl. Physiol. 2006, 100, 1164–1170. [Google Scholar] [CrossRef]
- Cebová, M.; Klimentová, J.; Janega, P.; Pecháňová, O. Effect of bioactive compound of Aronia melanocarpa on cardiovascular system in experimental hypertension. Oxid. Med. Cell. Longev. 2017, 2017, 8156594. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Ishisaka, A.; Mawatari, K.; Vidal-Diez, A.; Spencer, J.P.; Terao, J. Blueberry intervention improves vascular reactivity and lowers blood pressure in high-fat-, high-cholesterol-fed rats. Br. J. Nutr. 2013, 109, 1746–1754. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.H.; Kang, W.S.; Kim, J.S.; Kim, S. Endothelium-dependent and endothelium-independent vasorelaxant effects of unripe Rubus coreanus Miq. and Dendropanax morbiferus H. Lév. extracts on rat aortic rings. BMC Complement. Med. Ther. 2020, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Younis, W.; Schini-Kerth, V.B.; Farooq, M.A.; Althobaiti, M.; Roberts, R.E. Hawthorn berry (Crataegus songarica) causes endothelium-dependent relaxation of the porcine coronary artery: Role of estrogen receptors. J. Berry Res. 2021, 11, 249–265. [Google Scholar] [CrossRef]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J. Res. Med. Sci. 2014, 19, 358. [Google Scholar]
- Akkari, H.; Hajaji, S.; B’chir, F.; Rekik, M.; Gharbi, M. Correlation of polyphenolic content with radical-scavenging capacity and anthelmintic effects of Rubus ulmifolius (Rosaceae) against Haemonchus contortus. Vet. Parasitol. 2016, 221, 46–53. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Cervellati, R.; Loi, M.C.; Innocenti, G. Evaluation of in vitro antioxidant properties of some traditional Sardinian medicinal plants: Investigation of the high antioxidant capacity of Rubus ulmifolius. Food Chem. 2008, 106, 745–749. [Google Scholar] [CrossRef]
- Maaliki, D.; Shaito, A.A.; Pintus, G.; El-Yazbi, A.; Eid, A.H. Flavonoids in hypertension: A brief review of the underlying mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65. [Google Scholar] [CrossRef]
- Leeya, Y.; Mulvany, M.J.; Queiroz, E.F.; Marston, A.; Hostettmann, K.; Jansakul, C. Hypotensive activity of an n-butanol extract and their purified compounds from leaves of Phyllanthus acidus (L.) Skeels in rats. Eur. J. Pharmacol. 2010, 649, 301–313. [Google Scholar] [CrossRef]
- Silva, G.C.; Pereira, A.C.; Rezende, B.A.; da Silva, J.P.F.; Cruz, J.S.; Maria de Fátima, V.; Gomes, R.A.; Teles, Y.C.; Cortes, S.F.; Lemos, V.S. Mechanism of the antihypertensive and vasorelaxant effects of the flavonoid tiliroside in resistance arteries. Planta Med. 2013, 79, 1003–1008. [Google Scholar] [CrossRef]
- Radović, J.; Suručić, R.; Niketić, M.; Kundaković-Vasović, T. Alchemilla viridiflora Rothm.: The potent natural inhibitor of angiotensin I-converting enzyme. Mol. Cell. Biochem. 2022, 477, 1893–1903. [Google Scholar] [CrossRef]
- Jackson, D.; Connolly, K.; Batacan, R.; Ryan, K.; Vella, R.; Fenning, A. (−)-Epicatechin reduces blood pressure and improves left ventricular function and compliance in deoxycorticosterone acetate-salt hypertensive rats. Molecules 2018, 23, 1511. [Google Scholar] [CrossRef]
- Tom, E.N.L.; Girard-Thernier, C.; Demougeot, C. The Janus face of chlorogenic acid on vascular reactivity: A study on rat isolated vessels. Phytomedicine 2016, 23, 1037–1042. [Google Scholar] [CrossRef]
- Mubarak, A.; Bondonno, C.P.; Liu, A.H.; Considine, M.J.; Rich, L.; Mas, E.; Croft, K.D.; Hodgson, J.M. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: A randomized trial. J. Agric. Food Chem. 2012, 60, 9130–9136. [Google Scholar] [CrossRef]
- Lei, F.; Xing, D.-M.; Xiang, L.; Zhao, Y.-N.; Wang, W.; Zhang, L.-J.; Du, L.-J. Pharmacokinetic study of ellagic acid in rat after oral administration of pomegranate leaf extract. J. Chromatogr. B 2003, 796, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Zafrilla, P.; Mulero, J.; Tomás-Barberán, F.A.; Espín, J.C. Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J. Funct. Foods 2015, 19, 225–235. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Barrio, R.; Cerdá, B.; López-Bote, C.; Rey, A.I.; Tomás-Barberán, F.A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agric. Food Chem. 2007, 55, 10476–10485. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769. [Google Scholar] [CrossRef]
- Elendran, S.; Kumar, V.S.; Sundralingam, U.; Tow, W.-K.; Palanisamy, U.D. Enhancing the bioavailability of the ellagitannin, geraniin: Formulation, characterization, and in vivo evaluation. Int. J. Pharm. 2024, 660, 124333. [Google Scholar] [CrossRef]
- Yin, H.; Ma, J.; Han, J.; Li, M.; Shang, J. Pharmacokinetic comparison of quercetin, isoquercitrin, and quercetin-3-O-β-D-glucuronide in rats by HPLC-MS. PeerJ 2019, 7, e6665. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).