Climate Change Drives Northwestward Migration of Betula alnoides: A Multi-Scenario MaxEnt Modeling Approach
Abstract
1. Introduction
2. Materials and Methods
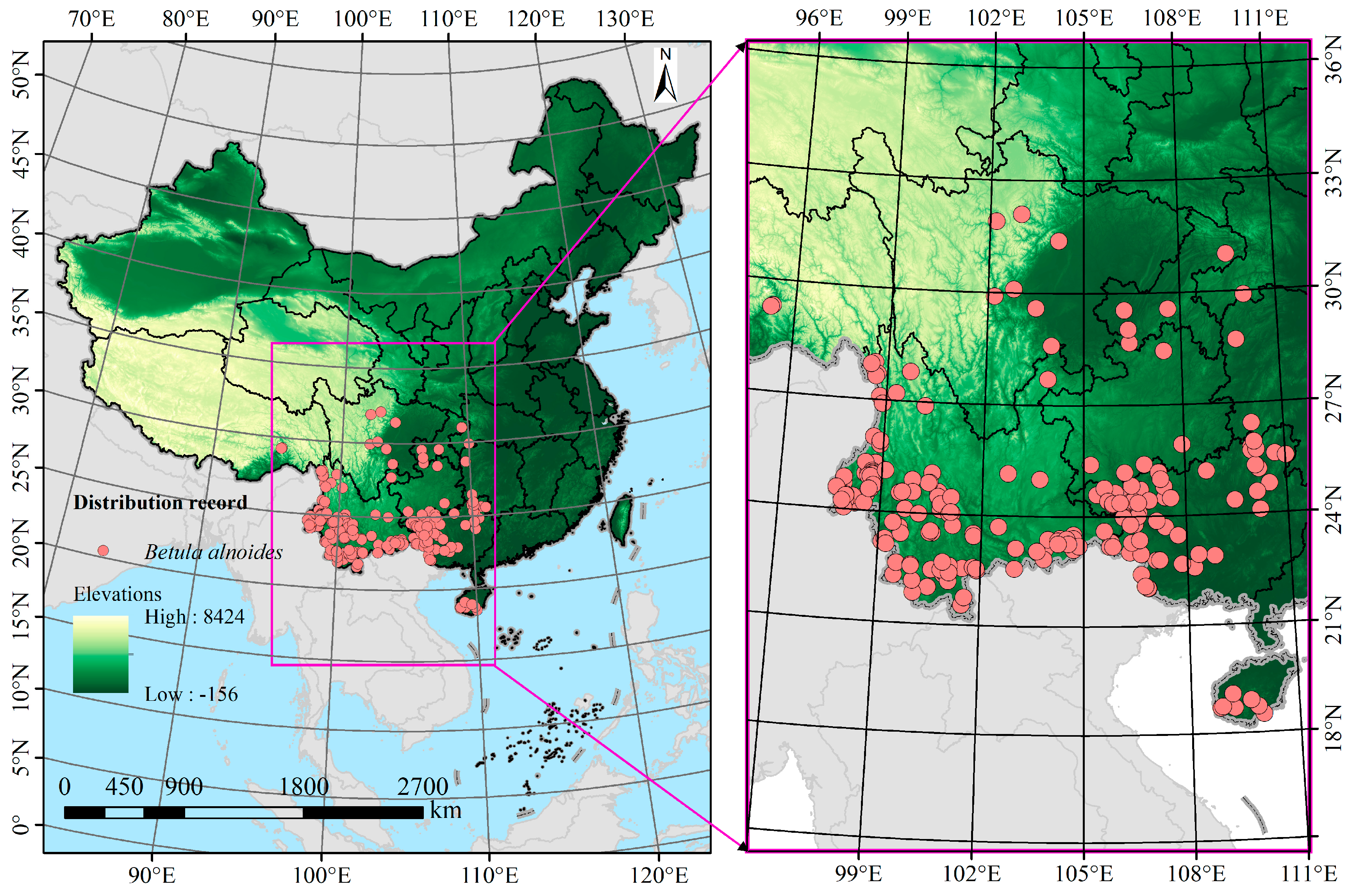
2.1. Species Occurrence Data Collection and Preprocessing
2.2. Environmental Variable Selection and Spatial Data Preparation
2.3. MaxEnt Model Construction and Optimization
2.3.1. Parameter Tuning Using ENMeval
2.3.2. Model Configuration and Cross-Validation
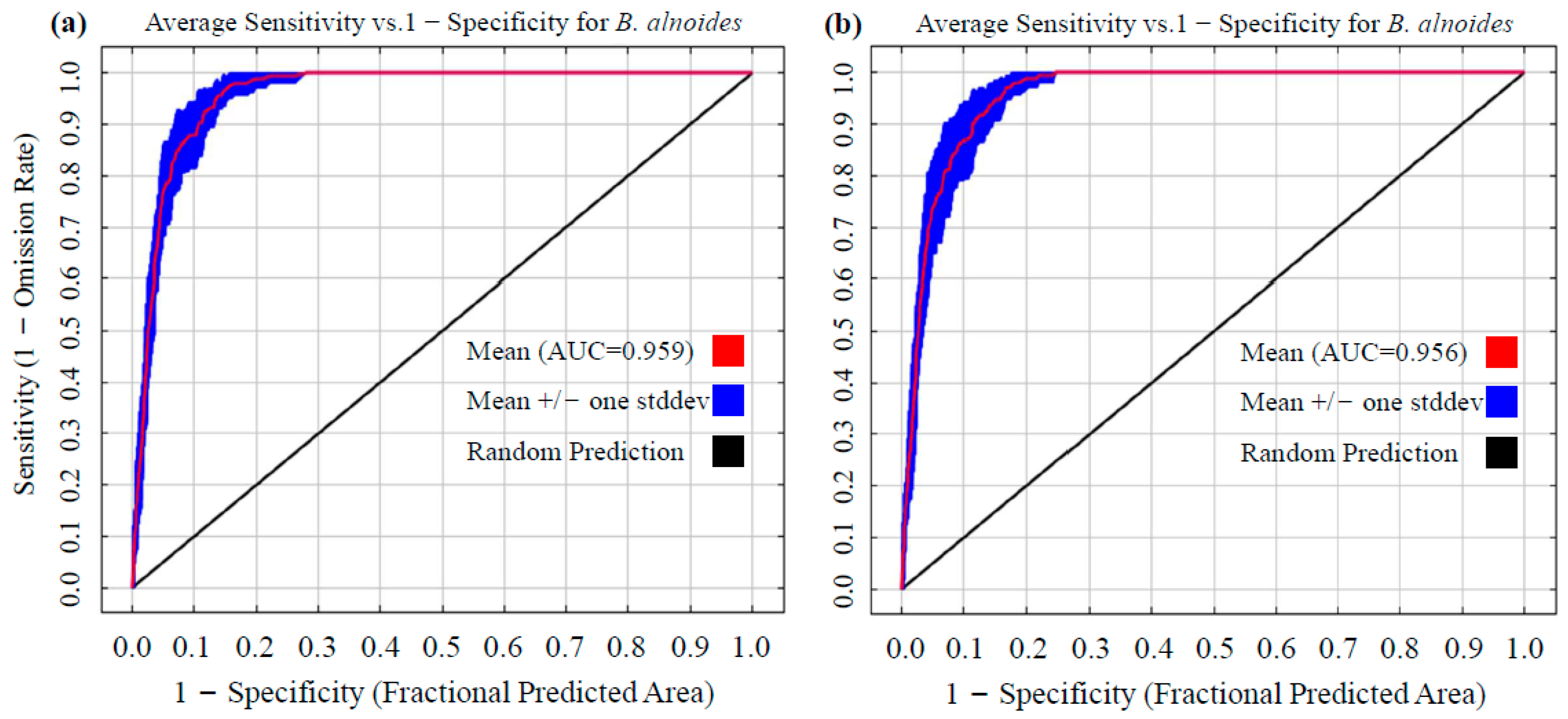
2.3.3. Model Performance Evaluation via AUC
2.4. Habitat Suitability Classification and Mapping
2.5. Spatiotemporal Dynamics of Suitable Habitats Under Climate Scenarios
2.6. Centroid Migration Analysis of Suitable Habitat Range
3. Results
3.1. Model Optimization Outcomes and Predictive Accuracy
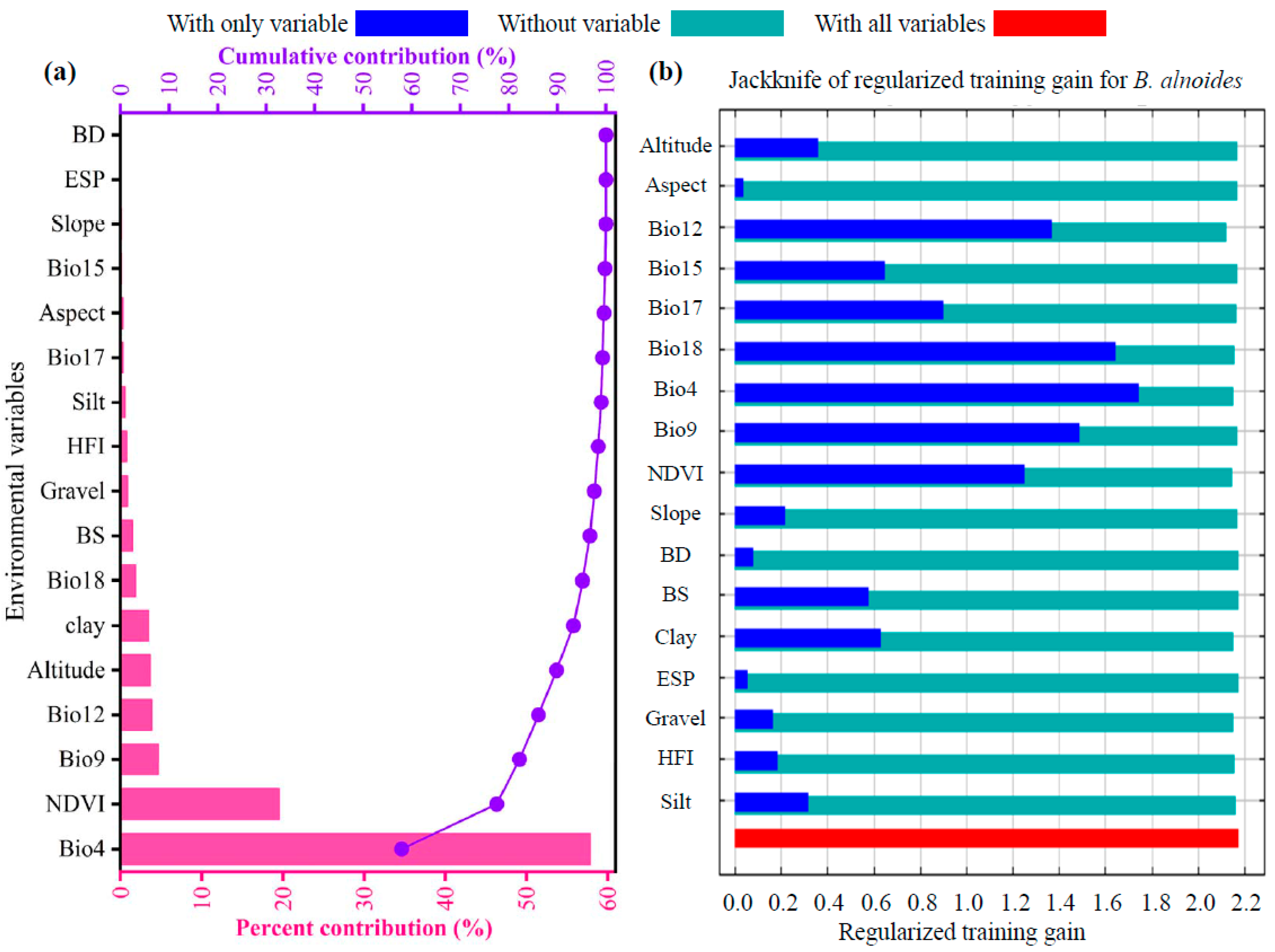
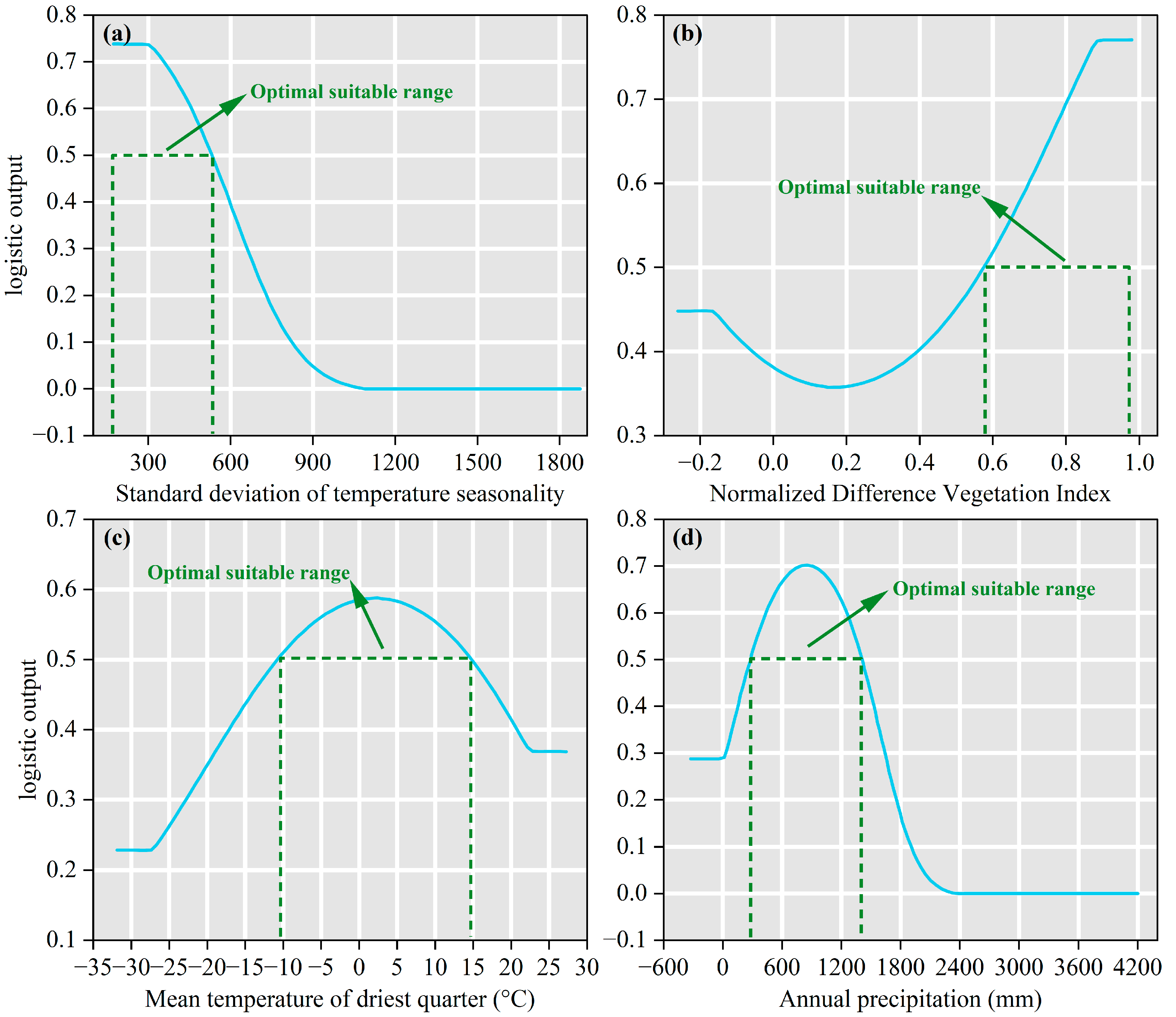
3.2. Identification of Key Environmental Drivers of Species Distribution
3.3. Current Distribution Pattern of Suitable Habitats for B. alnoides
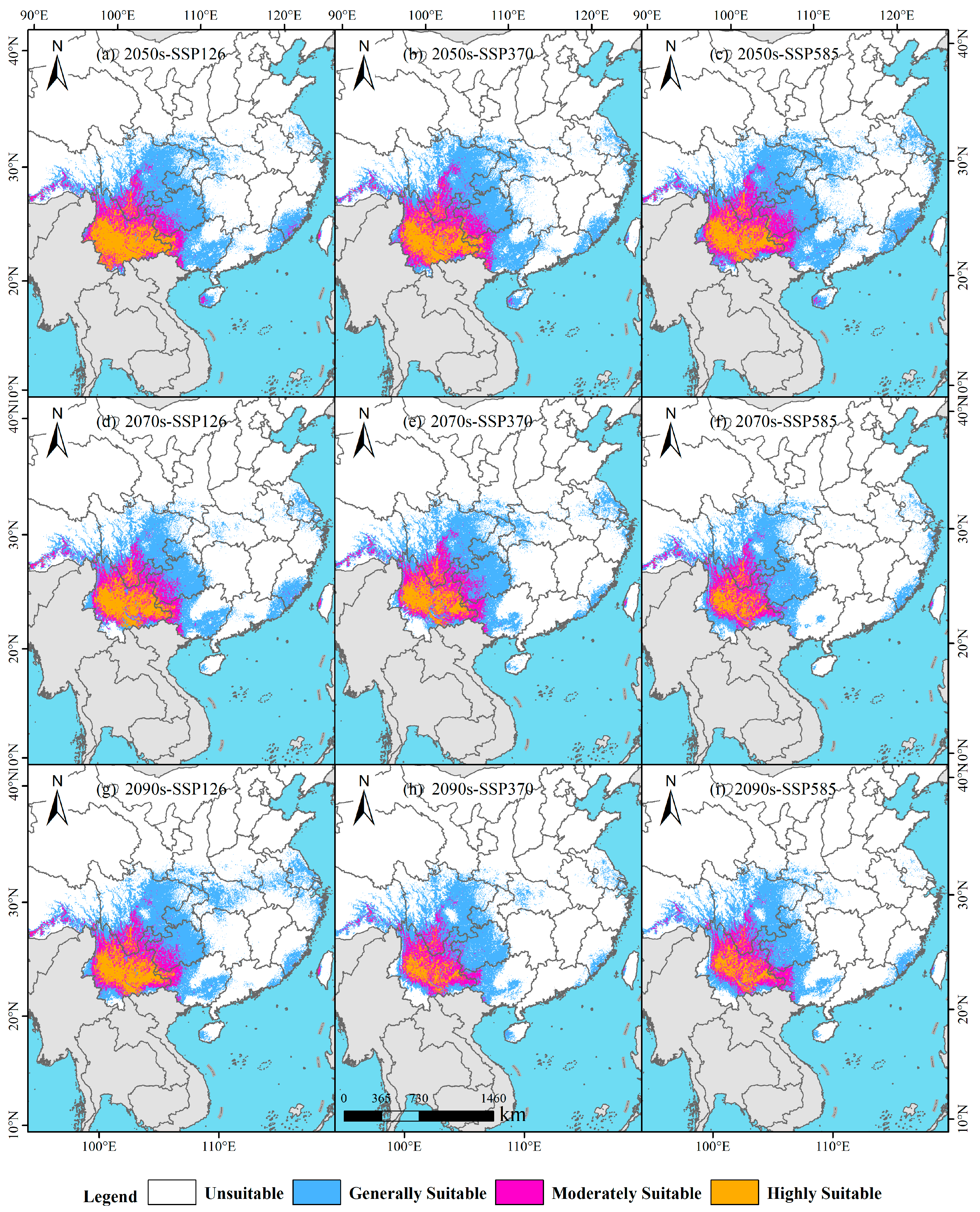
3.4. Projected Future Distribution Under Multiple Climate Scenarios
3.5. Temporal Trends in Habitat Stability, Loss, and Expansion
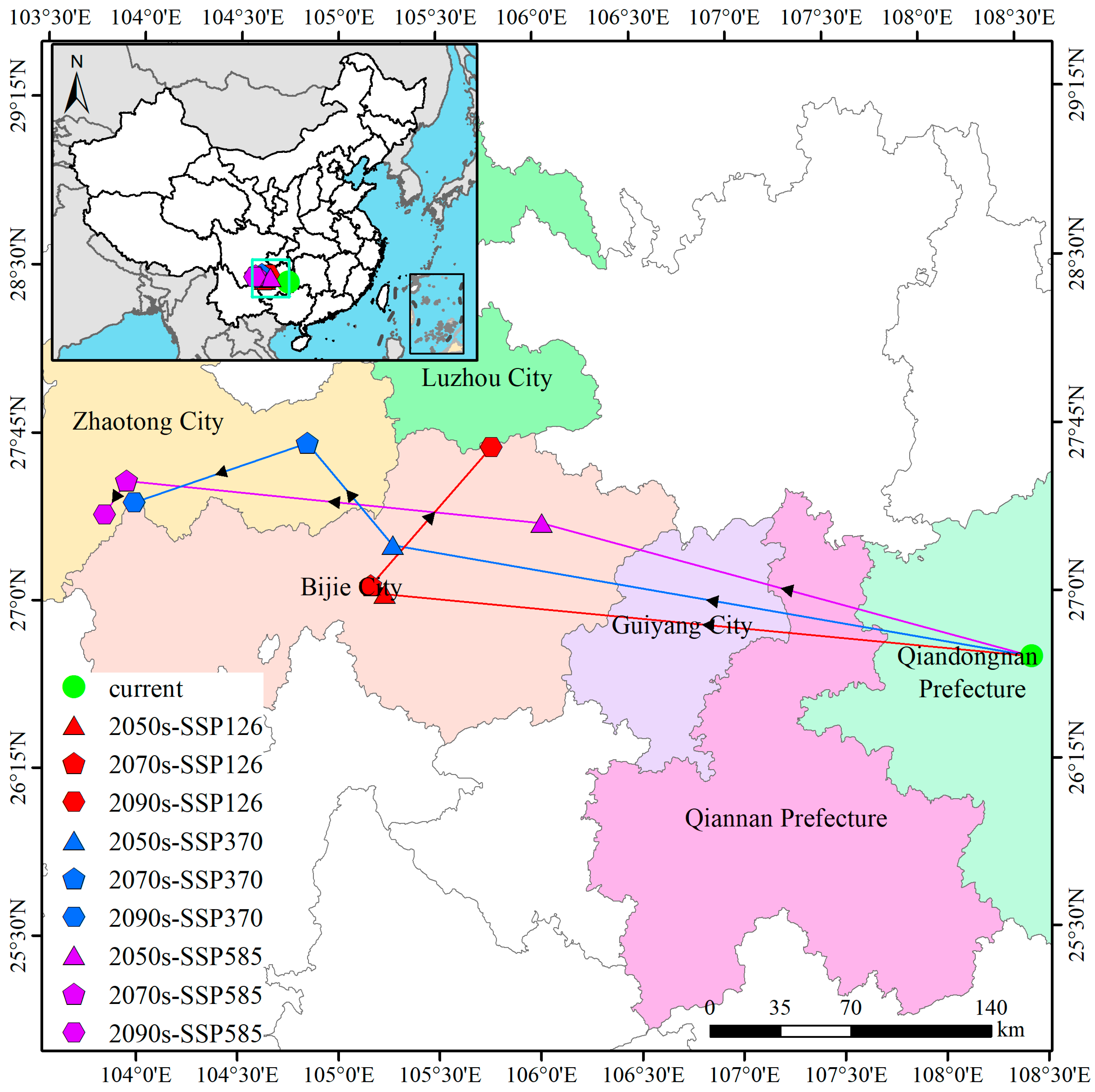
3.6. Directional Shifts in the Geographic Centroid of Suitable Habitat
4. Discussion
4.1. Advances in MaxEnt Model Optimization and Its Ecological Implications
4.2. Dominant Environmental Factors Shaping the Distribution of B. alnoides
4.3. Climate Change Impacts on Range Dynamics and Habitat Suitability
4.4. Quantifying Non-Climatic Drivers Through Model Integration
4.5. Study Limitations and Directions for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, X.; Zheng, X.; Liang, E.; Piao, S.; Babst, F.; Elliott, G.P.; Sigdel, S.R.; Wang, T.; Wang, Y.; Li, X.; et al. Patterns, dynamics and drivers of alpine treelines and shrublines. Nat. Rev. Earth Environ. 2025, 6, 489–502. [Google Scholar] [CrossRef]
- Franklin, J.; MacDonald, G.M. Climate change and California sustainability—Challenges and solutions. Proc. Natl. Acad. Sci. USA 2024, 121, e2405458121. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Z.; Lin, Y.; Kaewmano, A.; Wei, X.; Fu, P.; Grießinger, J.; Bräuning, A. Impact of extreme pre-monsoon drought on xylogenesis and intra-annual radial increments of two tree species in a tropical montane evergreen broad-leaved forest, southwest China. Tree Physiol. 2024, 44, tpae086. [Google Scholar] [CrossRef]
- Chen, B.; Liu, K.; Wang, C.; Guo, J.; Lu, J.; Chen, L.; Zhao, Z.; Zeng, J. Tree allometry responses to competition and complementarity in mixed-species plantations of Betula alnoides. For. Ecosyst. 2024, 11, 100207. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Haung, G.; Wang, T.; Wang, Q.; Su, X.; Ren, Z.; Chotamonsak, C.; Limsakul, A.; Torsri, K. Characteristics of super drought in Southwest China and the associated compounding effect of multiscalar anomalies. Sci. China Earth Sci. 2024, 67, 2084–2102. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, X.; Zhao, S.; Zhou, J. Memory effects of vegetation after extreme weather events under various geological conditions in a typical karst watershed in southwestern China. Agric. For. Meteorol. 2024, 345, 109840. [Google Scholar] [CrossRef]
- Bedair, H.; Badawy, N.K.; Morsy, A.; Rashad, H.; Dakhil, M.A. Impact of climate change on the spatial distribution of the endemic shrub Rubus asirensis in the Arabian Peninsula. Plant Ecol. 2024, 225, 441–450. [Google Scholar] [CrossRef]
- Lawlor, J.A.; Comte, L.; Grenouillet, G.; Lenoir, J.; Baecher, J.A.; Bandara, R.M.W.J.; Bertrand, R.; Chen, I.C.; Diamond, S.E.; Lancaster, L.T.; et al. Mechanisms, detection and impacts of species redistributions under climate change. Nat. Rev. Earth Environ. 2024, 5, 351–368. [Google Scholar] [CrossRef]
- Fiorentino, D.; Núñez-Riboni, I.; Pierce, M.E.; Oesterwind, D.; Akimova, A. Improving species distribution models for climate change studies: Ecological plausibility and performance metrics. Ecol. Model. 2025, 508, 111207. [Google Scholar] [CrossRef]
- Soley-Guardia, M.; Alvarado-Serrano, D.F.; Anderson, R.P. Top ten hazards to avoid when modeling species distributions: A didactic guide of assumptions, problems, and recommendations. Ecography 2024, 2024, e06852. [Google Scholar] [CrossRef]
- Wen, X.; Fang, G.; Chai, S.; He, C.; Sun, S.; Zhao, G.; Lin, X. Can ecological niche models be used to accurately predict the distribution of invasive insects? A case study of Hyphantria cunea in China. Ecol. Evol. 2024, 14, e11159. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Pirtle, J.L.; Laman, E.A.; Siple, M.C.; Thorson, J.T. An ensemble approach to species distribution modelling reconciles systematic differences in estimates of habitat utilization and range area. J. Appl. Ecol. 2024, 61, 351–364. [Google Scholar] [CrossRef]
- Tang, J.; Sun, B.; Cheng, R.; Shi, Z.; Luo, D.; Liu, S.; Centritto, M. Variation in leaf N allocation and mesophyll conductance to CO2 in four tree species under low soil P stress in subtropical China. Acta Physiol. Plant. 2024, 46, 86. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Qiu, Z.; Wang, C.; He, Y.; Chen, Q.; Ma, H. Meteorological Drivers and Forest Structural Prevention of the Canker Disease in Betula alnoides—A Case Study in South China. Forests 2025, 16, 440. [Google Scholar] [CrossRef]
- Wang, S.; Han, W.; Yi, S. Afforestation species and slope as key drivers of soil carbon sequestration in plantations of the tropical-subtropical transition zone: A case study from Xishuangbanna, Southwest China. J. For. Res. 2025, 36, 96. [Google Scholar] [CrossRef]
- Li, X.; Zhang, E.; Zhao, M.; He, M.; Li, M.; Dong, S.; Cai, W.; Li, F.; Feng, Y. Selection and evaluation of 17 superior clonal seedlings of Betula alnoides in young forests. For. Sci. Technol. 2023, 101–104. [Google Scholar] [CrossRef]
- Mu, H.; Li, X.; Wen, Y.; Huang, J.; Du, P.; Su, W.; Miao, S.; Geng, M. A global record of annual terrestrial Human Footprint dataset from 2000 to 2018. Sci. Data 2022, 9, 176. [Google Scholar] [CrossRef]
- Didan, K. MODIS/Terra Vegetation Indices Monthly L3 Global 1km SIN Grid V006; NASA Land Processes Distributed Active Archive Center: Sioux Falls, SD, USA, 2021.
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L.; et al. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Mendes, P.; Velazco, S.J.E.; Andrade, A.F.A.d.; De Marco, P. Dealing with overprediction in species distribution models: How adding distance constraints can improve model accuracy. Ecol. Model. 2020, 431, 109180. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, X.; Jiang, X.; Fan, T.; Liang, X.; Yan, W. MaxEnt modeling for predicting suitable habitat for endangered tree Keteleeria davidiana (Pinaceae) in China. Forests 2023, 14, 394. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Zhang, L.; Chen, S.; Zhao, A.; Ning, X.; Fan, G.; Wu, N.; Zhang, L.; Wang, Z. Prediction of the potentially suitable areas of Litsea cubeba in China based on future climate change using the optimized MaxEnt model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z.; Zhu, B.; Cheng, X.; Yang, L.; Gao, M.; Kong, R. MaxEnt Modeling Based on CMIP6 Models to Project Potential Suitable Zones for Cunninghamia lanceolata in China. Forests 2021, 12, 752. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, T.; Luo, Y.; Shi, J. Prediction of the global potential geographical distribution of Hylurgus ligniperda using a maximum entropy model. For. Ecosyst. 2022, 9, 100042. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Zhu, Z.; Tao, Y.; Xiang, J. Predicting the potential suitable distribution area of Emeia pseudosauteri in Zhejiang Province based on the MaxEnt model. Sci. Rep. 2023, 13, 1806. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Ngawang, N.; Chen, Y.; Bonjor, N.; Jia, X.; Zeng, Z.; Qiong, L. The potential habitat of Phlomoides rotata in Tibet was based on an optimized MaxEnt model. Front. Plant Sci. 2025, 16, 1560603. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; He, Q.; Ye, J.; Tian, B. Differential distribution shifts in two subregions of East Asian subtropical evergreen broadleaved forests—A case of Magnoliaceae. Front. Plant Sci. 2024, 14, 1326207. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- He, Y.; Ma, J.; Chen, G. Potential geographical distribution and its multi-factor analysis of Pinus massoniana in China based on the maxent model. Ecol. Indic. 2023, 154, 110790. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.P.; Gonzalez, I. Species-specific tuning increases robustness to sampling bias in models of species distributions: An implementation with Maxent. Ecol. Model. 2011, 222, 2796–2811. [Google Scholar] [CrossRef]
- Coelho, M.T.P.; Diniz-Filho, J.A.; Rangel, T.F. A parsimonious view of the parsimony principle in ecology and evolution. Ecography 2019, 42, 968–976. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, H.; Zhang, W.; Wu, J.; Yang, Y.; Yang, T.; Zhao, J.; Luo, C.; Yang, X.; Li, G. Potential distribution prediction of Terminalia chebula Retz. in China under current and future climate scenarios. Ecol. Evol. 2025, 15, e70908. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, Y.; Liu, Y.; Yuan, Y.; Li, S.; Yang, Q.; Zhang, J. Assessing climate change impacts on distribution dynamics of Lysimachia Christinae in China through MaxEnt modeling. Ecol. Evol. 2025, 15, e71664. [Google Scholar] [CrossRef]
- Zong, M.; Han, G.; Li, Y.; Wang, G.; Wang, A.; Yang, X. Predicting the potential distribution of dominant species of the coastal wetland in the Yellow River Delta, China using MaxEnt model. Chin. J. Appl. Ecol. 2017, 28, 1833–1842. [Google Scholar]
- Yang, S.; Wang, H.; Tong, J.; Bai, Y.; Alatalo, J.M.; Liu, G.; Fang, Z.; Zhang, F. Impacts of environment and human activity on grid-scale land cropping suitability and optimization of planting structure, measured based on the MaxEnt model. Sci. Total Environ. 2022, 836, 155356. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, L.; Xue, J.; Shi, P.; Liang, S. Habitat Suitability Shifts of Eucommia ulmoides in Southwest China Under Climate Change Projections. Biology 2025, 14, 451. [Google Scholar] [CrossRef]
- Shen, L.; Deng, H.; Zhang, G.; Ma, A.; Mo, X. Effect of climate change on the potentially suitable distribution pattern of Castanopsis hystrix Miq. in China. Plants 2023, 12, 717. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, G.G.; Zhu, M.; Jin, P.; Hu, Y.; Shu, P.; Wang, Z.; Fan, A.; Qian, P.; Han, Y.; et al. Potentially suitable habitat prediction of Pinus massoniana Lamb. in China under climate change using Maxent model. Front. For. Glob. Change 2023, 6, 1144401. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, K.; Zhao, R.; Xie, L.; Li, Y.; Zhao, G.; Zhang, F. Prediction of potential suitable habitats in the 21st century and GAP analysis of priority conservation areas of Chionanthus retusus based on the MaxEnt and Marxan models. Front. Plant Sci. 2024, 15, 1304121. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, Z.; Song, J.; Xie, T.; Xue, Y.; Yang, Q. Prediction of potential habitat of Verbena officinalis in China under climate change based on optimized MaxEnt model. Front. Plant Sci. 2025, 16, 1563070. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, H.; Chen, L.; Xiao, J.; Weng, F.; Liu, J.; He, T.; Chen, L.; Rong, J.; Chen, L.; et al. Forecasting appropriate habitats for rare and endangered indocalamus Species in China in response to climate change. Forests 2024, 15, 1693. [Google Scholar] [CrossRef]
- Bradie, J.; Leung, B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2017, 44, 1344–1361. [Google Scholar] [CrossRef]
- de Souza, R.A.; De Marco, P. The use of species distribution models to predict the spatial distribution of deforestation in the western Brazilian Amazon. Ecol. Model. 2014, 291, 250–259. [Google Scholar] [CrossRef]
- Bradley, B.A.; Olsson, A.D.; Wang, O.; Dickson, B.G.; Pelech, L.; Sesnie, S.E.; Zachmann, L.J. Species detection vs. habitat suitability: Are we biasing habitat suitability models with remotely sensed data? Ecol. Model. 2012, 244, 57–64. [Google Scholar] [CrossRef]
- Wang, M.; Qi, J.; Wei, L.; Qi, F.; Zhou, P.; Liang, W.; Hao, J. Effect of human disturbance on species diversity and soil physiochemical properties of Castanopsis fargesii secondary forest. J. Trop. Subtrop. Bot. 2018, 26, 355–362. [Google Scholar]
- Tang, M.; Li, S.; Xu, C.; Liu, J. The analysis on tree population niche of Betula alnoides natural secondary forest in Taiyanghe Nature Reserve. J. West China For. Sci. 2017, 46, 139–143. [Google Scholar]
- Dong, C.; Qi, S.; Dai, Z.; Qiu, X.; Luo, T. Research on accurate and effective identification of ecosystem surface based on human footprint index. Ecol. Indic. 2024, 162, 112013. [Google Scholar] [CrossRef]
- Li, F.; Ll, R.; Ma, C.; Yang, J.; Wang, L.; Chai, Y.; Sun, Y. Prediction of potential suitable areas of Podocarpus neriifolius in China under climate change scenarios. J. West China For. Sci. 2024, 53, 38–44. [Google Scholar]
- Xu, C.; Zhang, L.; Zhang, K.; Tao, J. MaxEnt Modeling and the Impact of Climate Change on Pistacia chinensis Bunge Habitat Suitability Variations in China. Forests 2023, 14, 1579. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, J.; Liu, L.; Zhang, K. MaxEnt Modeling and Effects of Climate Change on Shifts in Habitat Suitability for Sorbus alnifolia in China. Plants 2025, 14, 677. [Google Scholar] [CrossRef]
- Meng, J.; Li, M.; Guo, J.; Zhao, D.; Tao, J. Predicting Suitable Environments and Potential Occurrences for Cinnamomum camphora (Linn.) Presl. Forests 2021, 12, 1126. [Google Scholar] [CrossRef]
- Li, Y.; Shao, W.; Huang, S.; Zhang, Y.; Fang, H.; Jiang, J. Prediction of Suitable Habitats for Sapindus delavayi Based on the MaxEnt Model. Forests 2022, 13, 1611. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Xie, C.; Qiu, J.; Liu, X. Prediction of the potential distribution area of Jacaranda mimosifolia in China under climate change using the MaxEnt model. Front. For. Glob. Change 2024, 7, 1377689. [Google Scholar] [CrossRef]
- Yang, Q.; Xiang, Y.; Li, S.; Zhao, L.; Liu, Y.; Luo, Y.; Long, Y.; Yang, S.; Luo, X. Modeling the Impacts of Climate Change on Potential Distribution of Betula luminifera H. Winkler in China Using MaxEnt. Forests 2024, 15, 1624. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.; Zhang, L.; Wang, C.; Xu, Y. Predicting possible distribution of Tea (Camellia sinensis L.) under climate change scenarios using MaxEnt model in China. Agriculture 2021, 11, 1122. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, L.; Zhang, J.; He, L.; Sun, R. Maxent Modeling for Predicting the Potential Geographical Distribution of Castanopsis carlesii under Various Climate Change Scenarios in China. Forests 2023, 14, 1397. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Zhou, B.; Feng, X.; Meng, M.; Liu, Y.; Wang, D. Comparison of community characteristics between artificial Betula alnoides forest and mountainous rain forest in Xishuangbanna of Yunnan Province. Chin. Bull. Bot. 2006, 23, 169–176. [Google Scholar]
- Huang, Z.; Bai, Y.; Alatalo, J.M.; Yang, Z. Mapping biodiversity conservation priorities for protected areas: A case study in Xishuangbanna Tropical Area, China. Biol. Conserv. 2020, 249, 108741. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, Z.; Wang, C.; Guo, J.; Wang, H.; Zeng, J. Suitable cultivation regionalization and supporting cultivation techniques of Betula alnoides in warm areas of southern Guizhou. Guizhou For. Sci. Technol. 2017, 45, 33–38. [Google Scholar]
- Wang, C.; Guo, J.; Zhao, Z.; Wang, H.; Zeng, J. Spatial patterns and seasonal dynamics of foliar nutrients in 5-year-old Betula alnoides plantations. For. Ecol. Manag. 2021, 480, 118683. [Google Scholar] [CrossRef]
- Liu, H.; Xu, C.; Allen, C.D.; Hartmann, H.; Wei, X.; Yakir, D.; Wu, X.; Yu, P. Nature-based framework for sustainable afforestation in global drylands under changing climate. Glob. Change Biol. 2022, 28, 2202–2220. [Google Scholar] [CrossRef]
- Raiho, A.M.; Scharf, H.R.; Roland, C.A.; Swanson, D.K.; Stehn, S.E.; Hooten, M.B. Searching for refuge: A framework for identifying site factors conferring resistance to climate-driven vegetation change. Divers. Distrib. 2022, 28, 793–809. [Google Scholar] [CrossRef]
- Sigdel, S.R.; Zheng, X.; Babst, F.; Camarero, J.J.; Gao, S.; Li, X.; Lu, X.; Pandey, J.; Dawadi, B.; Sun, J.; et al. Accelerated succession in Himalayan alpine treelines under climatic warming. Nat. Plants 2024, 10, 1909–1918. [Google Scholar] [CrossRef]
- Li, G.; Fang, C.; Watson, J.E.M.; Sun, S.; Qi, W.; Wang, Z.; Liu, J. Mixed effectiveness of global protected areas in resisting habitat loss. Nat. Commun. 2024, 15, 8389. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.; VanDerWal, J.; Price, J.; Welbergen, J.A.; Atkinson, I.; Ramirez-Villegas, J.; Osborn, T.J.; Jarvis, A.; Shoo, L.P.; Williams, S.E.; et al. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Change 2013, 3, 678–682. [Google Scholar] [CrossRef]
- Liu, L.; Wang, R.; Zhang, Y.; Mou, Q.; Gou, Y.; Liu, K.; Huang, N.; Ouyang, C.; Hu, J.; Du, B. Simulation of potential suitable distribution of Alnus cremastogyne Burk. in China under climate change scenarios. Ecol. Indic. 2021, 133, 108396. [Google Scholar] [CrossRef]
- Song, H.; Zhang, X.; Wang, X.; Wang, Y.; Li, S.; Xu, Y. Not the expected poleward migration: Impact of climate change scenarios on the distribution of two endemic evergreen broad-leaved Quercus species in China. Sci. Total Environ. 2023, 889, 164273. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, Z.; Ou, J.; Sha, E.; Lin, K.; Zeng, J.; Xu, D. Spatiotemporal dynamics of seed rain in natural forest of Betula alnoides in Jingxi County, Guangxi, China. Chin. J. Plant Ecol. 2012, 36, 729–738. [Google Scholar] [CrossRef]
- Toledo-Aceves, T.; Sáenz-Romero, C.; Laura Cruzado-Vargas, A.; Vásquez-Reyes, V. Quercus insignis seedling response to climatic transfer distance in the face of climate change. For. Ecol. Manag. 2023, 533, 120855. [Google Scholar] [CrossRef]
- Li, Y.; Huang, S.; Wang, H.; Huang, Q.; Li, P.; Zheng, X.; Wang, Z.; Jiang, S.; Leng, G.; Li, J.; et al. Warming and greening exacerbate the propagation risk from meteorological to soil moisture drought. J. Hydrol. 2023, 622, 129716. [Google Scholar] [CrossRef]
- Zeng, Z.; Wu, W.; Peñuelas, J.; Li, Y.; Jiao, W.; Li, Z.; Ren, X.; Wang, K.; Ge, Q. Increased risk of flash droughts with raised concurrent hot and dry extremes under global warming. npj Clim. Atmos. Sci. 2023, 6, 134. [Google Scholar] [CrossRef]
- Kijowska-Oberc, J.; Staszak, A.M.; Ratajczak, E. Climate change affects seed aging? Initiation mechanism and consequences of loss of forest tree seed viability. Trees 2021, 35, 1099–1108. [Google Scholar] [CrossRef]
- Teskey, R.; Wertin, T.; Bauweraerts, I.; Ameye, M.; McGuire, M.A.; Steppe, K. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 2015, 38, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Yang, J.; Zhang, Z. Analysis on growth performance of betula alnoides at different altitude in Dehong Prefecture. J. Green Sci. Technol. 2022, 24, 173–176. [Google Scholar]
- Zeng, J.; Zheng, H.; Weng, Q. Geographic distributions and ecological conditions of Betula alnoides in China. For. Res. 1999, 12, 479–484. [Google Scholar]
- Liu, K.; Wang, C.; Chen, B.; Wang, R.; Zeng, J. Branch development in monoculture and mixed-species plantations of Betula alnoides, Erythrophleum fordii and Pinus kesiya var. langbianensis in southwestern China. For. Ecol. Manag. 2023, 528, 120643. [Google Scholar] [CrossRef]
- Lu, S.; Zhou, S.; Yin, X.; Zhang, C.; Li, R.; Chen, J.; Ma, D.; Wang, Y.; Yu, Z.; Chen, Y. Patterns of tree species richness in Southwest China. Environ. Monit. Assess. 2021, 193, 97. [Google Scholar] [CrossRef]


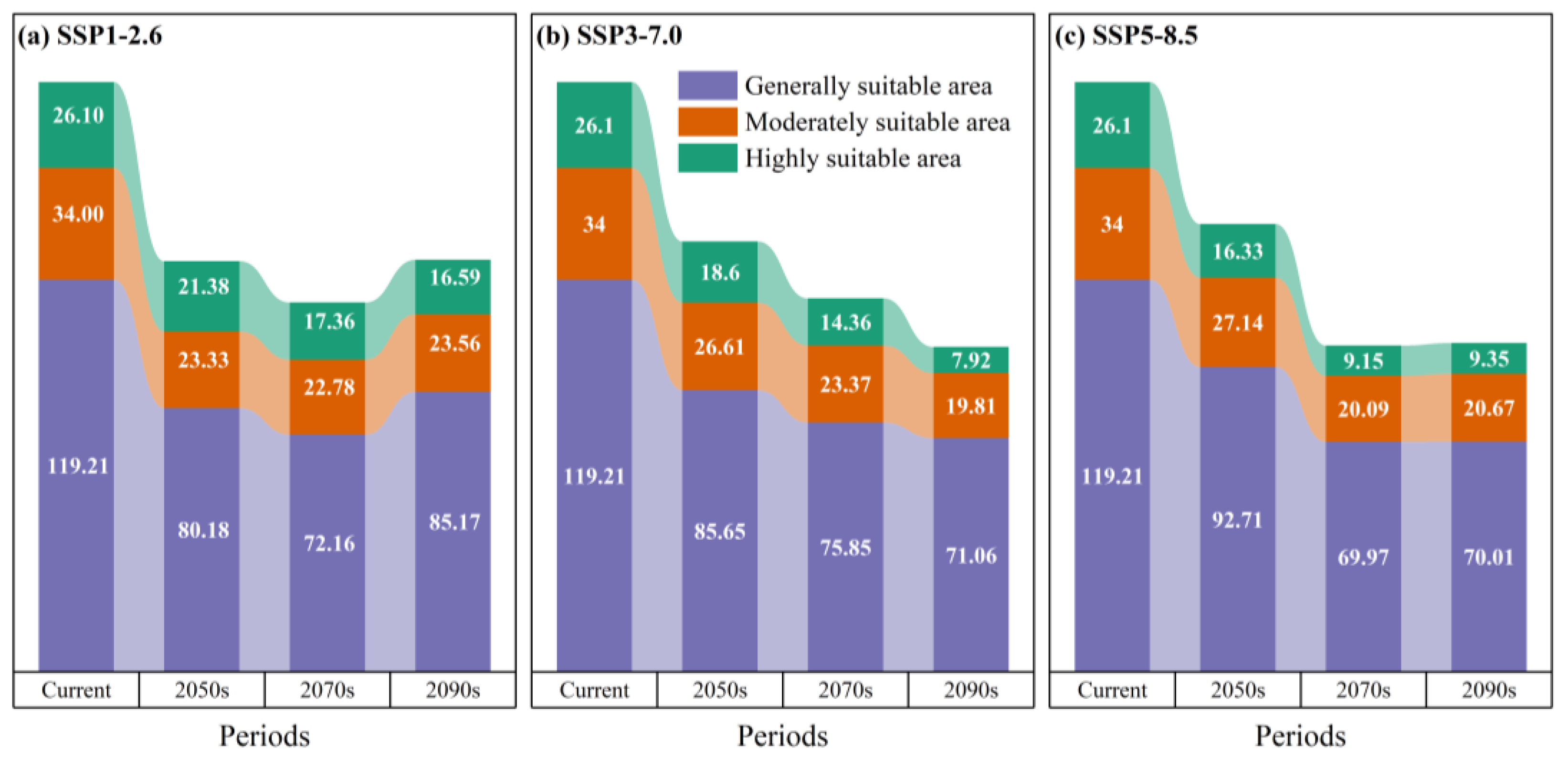
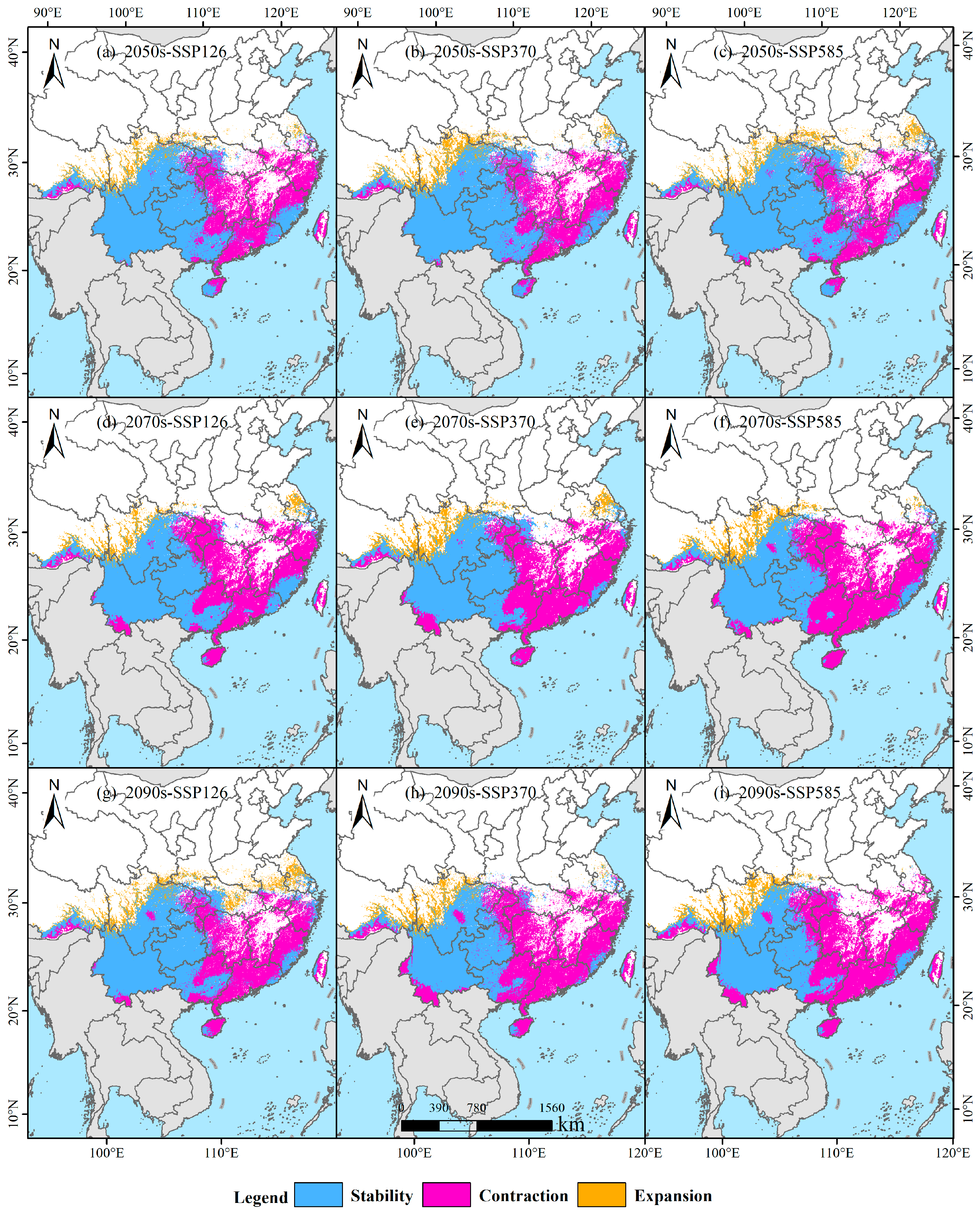
| Period | Area (104 km2) | Rate of Change (%) | ||||
|---|---|---|---|---|---|---|
| Stability | Expansion | Contraction | Stability | Expansion | Contraction | |
| 2050s-SSP126 | 136.22 | 15.71 | 82.60 | 58.08 | 6.70 | 35.22 |
| 2070s-SSP126 | 119.92 | 15.26 | 98.98 | 51.21 | 6.52 | 42.27 |
| 2090s-SSP126 | 128.04 | 24.21 | 90.69 | 52.7 | 9.97 | 37.33 |
| 2050s-SSP370 | 138.80 | 20.55 | 79.95 | 58.00 | 8.59 | 33.41 |
| 2070s-SSP370 | 115.76 | 20.86 | 103.25 | 48.26 | 8.70 | 43.04 |
| 2090s-SSP370 | 102.59 | 17.18 | 116.25 | 43.47 | 7.28 | 49.25 |
| 2050s-SSP585 | 143.69 | 21.85 | 75.09 | 59.72 | 9.08 | 31.21 |
| 2070s-SSP585 | 100.54 | 19.63 | 118.32 | 42.16 | 8.23 | 49.61 |
| 2090s-SSP585 | 102.02 | 19.08 | 116.88 | 42.87 | 8.02 | 49.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Y.; Yang, Q.; Li, S.; Liu, Y.; Li, Y.; Ren, J.; Yao, J.; Luo, X.; Luo, Y.; Yao, B. Climate Change Drives Northwestward Migration of Betula alnoides: A Multi-Scenario MaxEnt Modeling Approach. Plants 2025, 14, 2539. https://doi.org/10.3390/plants14162539
Xiang Y, Yang Q, Li S, Liu Y, Li Y, Ren J, Yao J, Luo X, Luo Y, Yao B. Climate Change Drives Northwestward Migration of Betula alnoides: A Multi-Scenario MaxEnt Modeling Approach. Plants. 2025; 14(16):2539. https://doi.org/10.3390/plants14162539
Chicago/Turabian StyleXiang, Yangzhou, Qiong Yang, Suhang Li, Ying Liu, Yuan Li, Jun Ren, Jiaxin Yao, Xuqiang Luo, Yang Luo, and Bin Yao. 2025. "Climate Change Drives Northwestward Migration of Betula alnoides: A Multi-Scenario MaxEnt Modeling Approach" Plants 14, no. 16: 2539. https://doi.org/10.3390/plants14162539
APA StyleXiang, Y., Yang, Q., Li, S., Liu, Y., Li, Y., Ren, J., Yao, J., Luo, X., Luo, Y., & Yao, B. (2025). Climate Change Drives Northwestward Migration of Betula alnoides: A Multi-Scenario MaxEnt Modeling Approach. Plants, 14(16), 2539. https://doi.org/10.3390/plants14162539






