Phytochemistry and Bioactivity of Essential Oil and Methanolic Extracts of Origanum vulgare L. from Central Italy
Abstract
1. Introduction
2. Results
2.1. Essential Oil Yield and Compositions
2.2. UHPLC-DAD Analysis
2.3. Antioxidant Activity and Polyphenolic Content
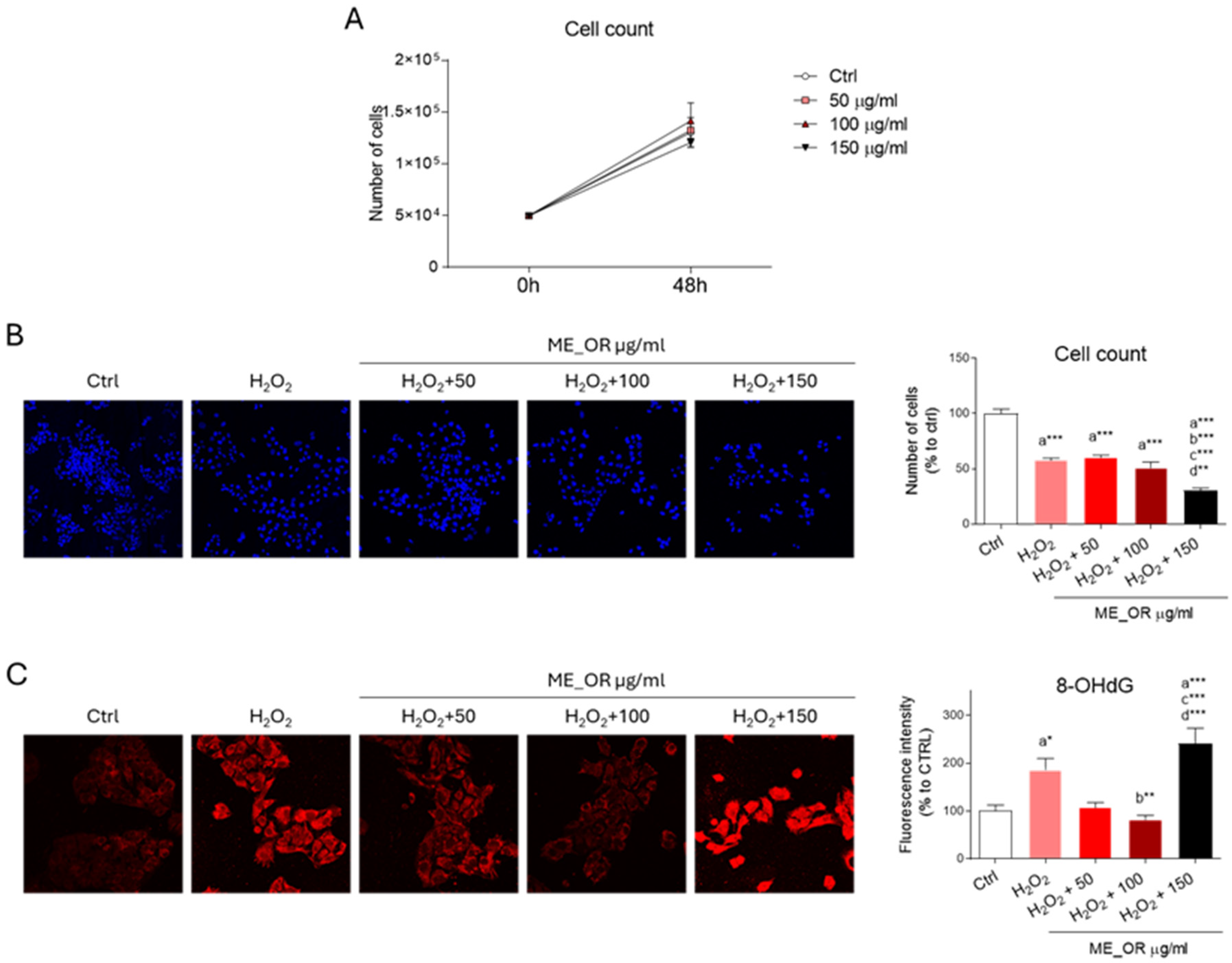
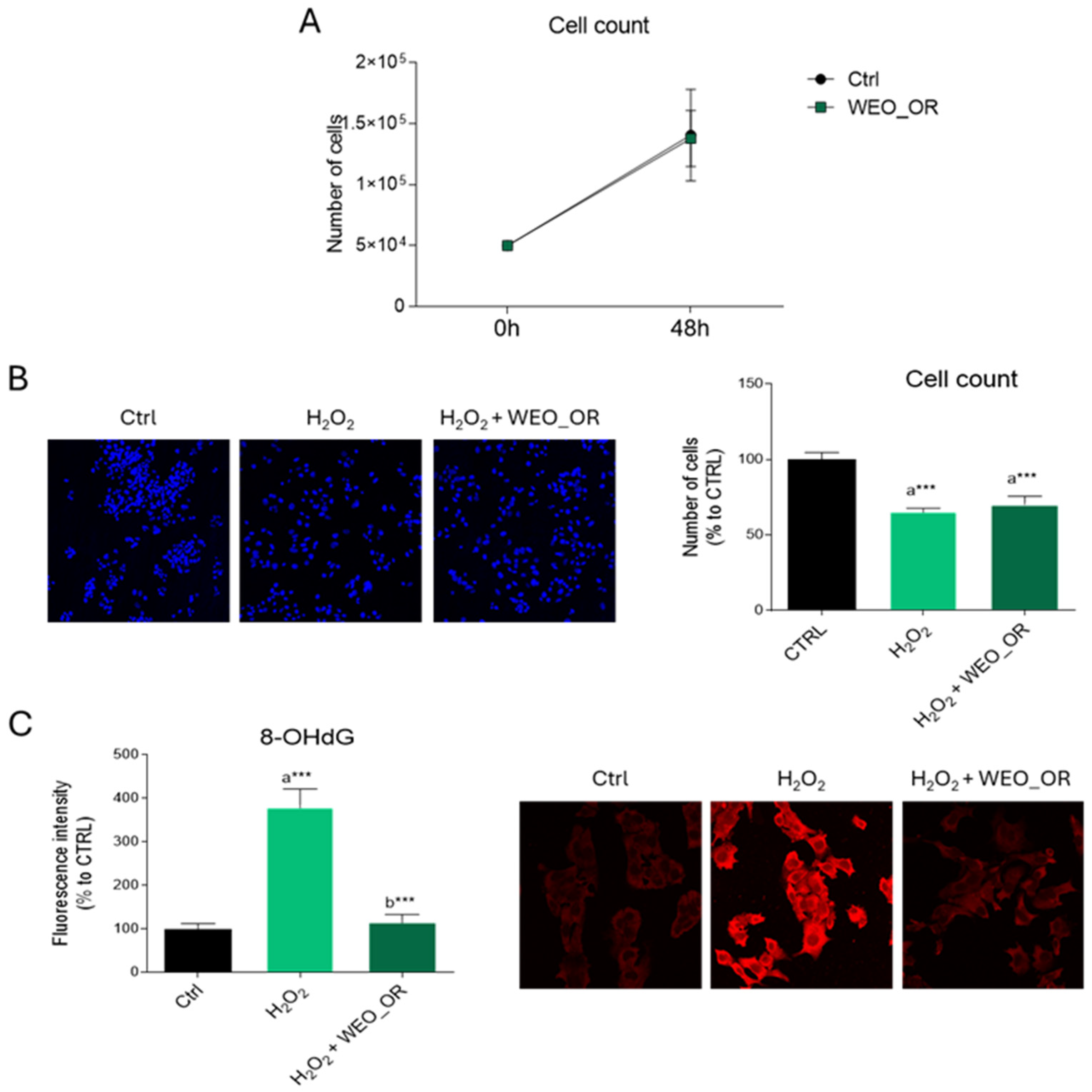
2.4. Biological Activity of the HepG2 Cell Line
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Plant Material
4.3. Essential Oil Isolation
4.4. GC-MS Analysis, Identification of EO Components
4.5. Hydroalcoholic Extraction
4.6. UHPLC-DAD Analysis
4.7. Antioxidant Activity
4.7.1. DPPH Radical Scavenging Activity
4.7.2. ABTS Radical Scavenging Activity
4.7.3. Ferric Reducing Power (FRAP)
4.8. Polyphenolic Content
4.9. Flavonoid Content
4.10. Cell Cultures
4.10.1. Cell Viability and Cytoprotection Assessment
4.10.2. Immunofluorescence and Confocal Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kintzios, S.E. Oregano: The Genera Origanum and Lippia (Medicinal and Aromatic Plants-Industrial Profiles); CRC Press: New York, NY, USA, 2002. [Google Scholar]
- Chishti, S.; Kaloo, Z.A.; Sultan, P. Medicinal importance of genus Origanum: A review. J. Pharmacogn. Phytother. 2013, 5, 170–177. [Google Scholar]
- García-Beltrán, J.M.; Esteban, M.A. Properties and applications of plants of Origanum sp. Genus. SM J. Biol. 2016, 2, 1006. [Google Scholar]
- Lukas, B.; Schmider, C.; Novak, J. Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Kang, J.; Yang, R.; Li, H.; Cui, H.; Bai, H.; Tsitsilin, A.; Li, J.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, L.; Di Stefano, A.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Baser, K.H.C. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef]
- Zhang, X.L.; Guo, Y.S.; Wang, C.H.; Li, G.Q.; Xu, J.J.; Chung, H.Y.; Ye, W.-C.; Li, Y.-L.; Wang, G.-C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef]
- Karakaya, S.; El, S.N.; Karagözlü, N.; Sahin, S. Antioxidant and antimicrobial activities of essential oils obtained from oregano (Origanum vulgare ssp. hirtum) by using different extraction methods. J. Med. Food 2011, 14, 645–652. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Perumalsamy, H.; Huq, M.A.; Balasubramanian, B. Anti-proliferative activity of Origanum vulgare inhibited lipogenesis and induced mitochondrial mediated apoptosis in human stomach cancer cell lines. Biomed. Pharmacother. 2018, 108, 1835–1844. [Google Scholar] [CrossRef]
- Coccimiglio, J.; Alipour, M.; Jiang, Z.H.; Gottardo, C.; Suntres, Z. Antioxidant, antibacterial, and cytotoxic activities of the ethanolic Origanum vulgare extract and its major constituents. Oxid. Med. Cell Longev. 2016, 2016, 1404505. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Decoction, infusion and hydroalcoholic extract of Origanum vulgare L.: Different performances regarding bioactivity and phenolic compounds. Food Chem. 2014, 158, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shetty, K. Stimulation of rosmarinic acid in shoot cultures of oregano (Origanum vulgare) clonal line in response to proline, proline analogue, and proline precursors. J. Agric. Food Chem. 1998, 46, 2888–2893. [Google Scholar] [CrossRef]
- De Mastro, G.; Tarraf, W.; Verdini, L.; Brunetti, G.; Ruta, C. Essential oil diversity of Origanum vulgare L. populations from Southern Italy. Food Chem. 2017, 235, 1–6. [Google Scholar] [CrossRef]
- Napoli, E.; Giovino, A.; Carrubba, A.; How Yuen Siong, V.; Rinoldo, C.; Nina, O.; Ruberto, G. Variations of essential oil constituents in oregano (Origanum vulgare subsp. viridulum (= O. heracleoticum) over Cultivation Cycles. Plants 2020, 9, 1174. [Google Scholar] [CrossRef]
- De Falco, E.; Roscigno, G.; Landolfi, S.; Scandolera, E.; Senatore, F. Growth, essential oil characterization, and antimicrobial activity of three wild biotypes of oregano under cultivation condition in Southern Italy. Ind. Crops Prod. 2014, 62, 242–249. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Sheng, Z.; Li, X.; Chang, Y.; Dai, W.; Chang, S.K.; Liu, J.; Yang, Y. Effect of pinolenic acid on oxidative stress injury in HepG2 cells induced by H2O2. Food Sci Nutr. 2021, 9, 5689–5697. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, M.; Huang, L.; Wang, J.; Gong, L.; Liu, J.; Sun, B. Evaluation of the Cellular and Animal Model for the Study of Antioxidant Activity: A Review. J. Food Sci. 2017, 82, 278–288. [Google Scholar] [CrossRef]
- Sharath Babu, G.R.; Anand, T.; Ilaiyaraja, N.; Khanum, F.; Gopalan, N. Pelargonidin Modulates Keap1/Nrf2 Pathway Gene Expression and Ameliorates Citrinin-Induced Oxidative Stress in HepG2 Cells. Front. Pharmacol. 2017, 8, 868. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Co.: Carol Stream, IL, USA, 2007; ISBN 978-1932633214. [Google Scholar]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Dragićević, M.; Stupar, A.; Uysal, A.; Şenkardes, I.; Sinan, K.I.; Picot-Allain, M.C.N.; Ak, G.; et al. UHPLC-LTQ OrbiTrap MS Analysis and Biological Properties of Origanum vulgare subsp. viridulum Obtained by Different Extraction Methods. Ind. Crops Prod. 2020, 154, 112747. [Google Scholar] [CrossRef]
- de Torre, M.P.; Vizmanos, J.L.; Cavero, R.Y.; Calvo, M.I. Improvement of antioxidant activity of oregano (Origanum vulgare L.) with an oral pharmaceutical form. Biomed. Pharmacother. 2020, 129, 110424. [Google Scholar] [CrossRef] [PubMed]
- Parra, C.; Munoz, P.; Bustos, L.; Parra, F.; Simirgiotis, M.J.; Escobar, H. UHPLC-DAD Characterization of Origanum vulgare L. from Atacama Desert Andean Region and Antioxidant, Antibacterial and Enzyme Inhibition Activities. Molecules 2021, 26, 2100. [Google Scholar] [CrossRef]
- Yan, F.; Azizi, A.; Janke, S.; Schwarz, M.; Zeller, S.; Honermeier, B. Antioxidant capacity variation in the oregano (Origanum vulgare L.) collection of the German national Genebank. Ind. Crops Prod. 2016, 92, 19–25. [Google Scholar] [CrossRef]
- Di Liberto, D.; Iacuzzi, N.; Pratelli, G.; Porrello, A.; Maggio, A.; La Bella, S.; De Blasio, A.; Notaro, A.; D’Anneo, A.; Ema-nuele, S.; et al. Cytotoxic Effect Induced by Sicilian Oregano Essential Oil in Human Breast Cancer Cells. Cells 2023, 12, 2733. [Google Scholar] [CrossRef]
- Novak, J.; Lukas, B.; Franz, C. Temperature influences thymol and carvacrol differentially in Origanum spp.(Lamiaceae). J. Essent. Oil Res. 2010, 22, 412–415. [Google Scholar] [CrossRef]
- Mugao, L. Factors influencing yield, chemical composition and efficacy of essential oils. Int. J. Multidiscip. Res. Growth Eval. 2024, 5, 169–178. [Google Scholar] [CrossRef]
- Sahin, F.; Gulluce, M.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M.; Agar, G.; Ozer, H. Biological activities of the essential oils and methanol extract of Origanum vulgare in the Eastern Anatolia region of Turkey. Food Control. 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Boroski, M.; Giroux, H.J.; Sabik, H.; Petit, H.V.; Visentainer, J.V.; Matumoto-Pintro, P.T.; Britten, M. Use of oregano extract and oregano essential oil as antioxidants in functional dairy beverage formulations. Food Sci. Technol. 2012, 47, 167–174. [Google Scholar] [CrossRef]
- Spiridon, I.; Bodirlau, R.; Teaca, C.A. Total phenolic content and antioxidant activity of plants used in traditional Romanian herbal medicine. Open Life Sci. 2011, 6, 388–396. [Google Scholar] [CrossRef]
- Stanojević, L.P.; Stanojević, J.S.; Cvetković, D.J.; Ilić, D.P. Antioxidant activity of oregano essential oil (Origanum vulgare L.). Biol. Nyssana 2016, 7, 131–139. [Google Scholar]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655, 9–16. [Google Scholar] [CrossRef]
- Spiridon, I.; Colceru, S.; Anghel, N.; Teaca, C.A.; Bodirlau, R.; Armatu, A. Antioxidant capacity and total phenolic contents of oregano (Origanum vulgare), lavender (Lavandula angustifolia) and lemon balm (Melissa officinalis) from Romania. Nat. Prod. Res. 2011, 25, 1657–1661. [Google Scholar] [CrossRef]
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Moghrovyan, A.; Sahakyan, N.; Babayan, A.; Chichoyan, N.; Petrosyan, M.; Trchounian, A. Essential oil and ethanol extract of oregano (Origanum vulgare L.) from Armenian flora as a natural source of terpenes, flavonoids and other phytochemicals with antiradical, antioxidant, metal chelating, tyrosinase inhibitory and antibacterial activity. Curr. Pharm. Des. 2019, 25, 1809–1816. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and phenolic acids from oregano: Occurrence, biological activity and health benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef]
- Kosakowska, O.; Węglarz, Z.; Pióro-Jabrucka, E.; Przybył, J.L.; Kraśniewska, K.; Gniewosz, M.; Bączek, K. Antioxidant and Antibacterial Activity of Essential Oils and Hydroethanolic Extracts of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare). Molecules 2021, 26, 988. [Google Scholar] [CrossRef] [PubMed]
- Ravaria, P.; Saxena, P.; Laksmi Bs, S.; Ranjan, V.; Abidi, S.W.F.; Saha, P.; Ramamoorthy, S.; Ahmad, F.; Rana, S.S. Molecular mechanisms of neuroprotective offerings by rosmarinic acid against neurodegenerative and other CNS pathologies. Phytother. Res. 2023, 37, 2119–2143. [Google Scholar] [CrossRef] [PubMed]
- Varone, M.; Scavo, G.; Colardo, M.; Martella, N.; Pensabene, D.; Bisesto, E.; Del Busso, A.; Segatto, M. p75NTR Modulation Reduces Oxidative Stress and the Expression of Pro-Inflammatory Mediators in a Cell Model of Rett Syn-drome. Biomedicines 2024, 12, 2624. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Y.; Wang, Z.; Chen, T.; Qian, H.; He, J.; Li, J.; Han, B.; Wang, T. Rosmarinic acid protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity in zebrafish embryos. Toxicol. Vitr. 2020, 65, 104823. [Google Scholar] [CrossRef]
- Chen, C.; Ma, J.; Ren, L.; Sun, B.; Shi, Y.; Chen, L.; Wang, D.; Wei, J.; Sun, Y.; Cao, X. Rosmarinic Acid Activates the Nrf2/ARE Signaling Pathway via the miR-25-3p/SIRT6 Axis to Inhibit Vascular Remodeling. J. Agric. Food Chem. 2024, 72, 4008–4022. [Google Scholar] [CrossRef]
- Charoensin, S.; Dansakda, S. Modulatory Effect of Rosmarinic Acid on H2O2-Induced Adaptive Glycolytic Response in Dermal Fibroblasts. Molecules 2023, 28, 5599. [Google Scholar] [CrossRef]
- Savini, I.; Arnone, R.; Catani, M.V.; Avigliano, L. Origanum vulgare induces apoptosis in human colon cancer caco2 cells. Nutr. Cancer 2009, 61, 381–389. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A.; De Feo, V.; Camele, I. Cytotoxic activity of Origanum vulgare L. on hepatocellular carcinoma cell line HepG2 and evaluation of its biological activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef]
- Zeytinoglu, H.S.; Tomsuk, O. Antiproliferative and apoptotic effects of the essential oil of Origanum onites and carvacrol on Hep-G2 cells. Life Sci. Biotechnol. 2011, 1, 171–180. [Google Scholar]
- Caldez, M.J.; Van Hul, N.; Koh, H.W.L.; Teo, X.Q.; Fan, J.J.; Tan, P.Y.; Dewhurst, M.R.; Too, P.G.; Talib, S.Z.A.; Chiang, B.E.; et al. Metabolic remodeling during liver regeneration. Dev. Cell 2018, 47, 425–438.e5. [Google Scholar] [CrossRef]
- Lu, Q.; Tian, X.; Wu, H.; Huang, J.; Li, M.; Mei, Z.; Zhou, L.; Xie, H.; Zheng, S. Metabolic changes of hepatocytes in NAFLD. Front. Physiol. 2021, 12, 710420. [Google Scholar] [CrossRef]
- Raskov, H.; Gaggar, S.; Tajik, A.; Orhan, A.; Gogenur, I. Metabolic switch in cancer-survival of the fittest. Eur. J. Cancer 2022, 180, 30–51. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Peng, J.; Wei, H. Oregano essential oil induces SOD1 and GSH expression through Nrf2 activation and alleviates hydrogen peroxide-induced oxidative damage in IPEC-J2 cells. Oxidative Med. Cell Longev. 2016, 2016, 5987183. [Google Scholar] [CrossRef]
- Özkan, A.; Erdogan, A. Membrane and DNA damaging/protective effects of eugenol, eucalyptol, terpinen-4-ol, and camphor at various concentrations on parental and drug resistant H1299 cells. Turk. J. Biol. 2013, 37, 405–413. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol Vitr. 2015, 29, 647–656. [Google Scholar] [CrossRef]
- Ozkan, A.; Erdogan, A. A comparative study of the antioxidant/prooxidant effects of carvacrol and thymol at various concentrations on membrane and DNA of parental and drug resistant H1299 cells. Nat. Prod. Commun. 2012, 7, 1934578X1200701201. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2004; Volume 1, p. 217. [Google Scholar]
- Kováts, E. Gas Chromatographic Characterization of Organic Substances in the Retention Index System. In Advances in Chromatography; Marcel Dekker: New York, NY, USA, 1965; pp. 229–247. [Google Scholar]
- van den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas–Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- NIST; EPA; NIH. Mass Spectral Library; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020.
- McLafferty, F.W. Wiley Registry of Mass Spectral Data, with NIST Spectral Data CD-ROM, 7th ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Grob, R.L.; Kaiser, M.A. Qualitative and Quantitative Analysis by Gas Chromatography. In Modern Practice of Gas Chromatography; Grob, B., Ed.; John Wiley & Sons: New York, NY, USA, 2004; ISBN 0471229830. [Google Scholar]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J. Agric. Food Chem. 2010, 58, 7218–7225. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Kim, D.-O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]



| N | Compound | Exp RI | Ref RI | Area % ± SD WEO_OR | Area % ± SD CEO_OR | Abbr. |
|---|---|---|---|---|---|---|
| 1 | α-Thujene | 929 | 930 | 0.23 ± 0.05 | 0.32 ± 0.03 | MM |
| 2 | α-Pinene | 935 | 939 | 0.16 ± 0.02 | 0.51 ± 0.01 | BM |
| 3 | Camphene | 948 | 954 | - | 0.06 ± 0.01 | BM |
| 4 | β-Pinene | 975 | 979 | 1.94 ± 0.14 | 0.09 ± 0.01 | BM |
| 5 | Decene | 984 | 989 | - | 0.09 ± 0.01 | OT |
| 6 | Myrcene | 993 | 990 | 0.99 ± 0.02 | 0.58 ± 0.03 | AM |
| 7 | α-Phellandrene | 1002 | 1002 | - | 0.11 ± 0.02 | MM |
| 8 | 3-Carene | 1008 | 1011 | - | 0.05 ± 0.01 | BM |
| 9 | α-Terpinene | 1016 | 1017 | 3.72 ± 0.13 | 1.18 ± 0.03 | MM |
| 10 | p-Cymene | 1024 | 1024 | 3.47 ± 0.13 | 6.81 ± 0.17 | MM |
| 11 | o-Cymene | 1030 | 1026 | - | 0.35 ± 0.03 | MM |
| 12 | Limonene | 1030 | 1029 | 1.49 ± 0.18 | - | MM |
| 13 | (Z)-b-Ocimene | 1043 | 1037 | 0.19 ± 0.02 | - | AM |
| 14 | (E)-b-Ocimene | 1053 | 1050 | 0.32 ± 0.11 | - | AM |
| 15 | γ-Terpinene | 1061 | 1059 | 17.25 ± 0.60 | 3.70 ± 0.16 | MM |
| 16 | p-Mentha-3.8-diene | 1070 | 1072 | 1.26 ± 0.10 | 0.12 ± 0.01 | MM |
| 17 | Terpinolene | 1089 | 1088 | 1.91 ± 0.04 | 0.14 ± 0.01 | MM |
| 18 | Linalool | 1100 | 1096 | 9.7 ± 0.36 | 0.78 ± 0.04 | AMO |
| 19 | Octen-3-yl-acetate | 1116 | 1112 | 0.89 ± 0.08 | - | OT |
| 20 | Borneol | 1168 | 1169 | - | 0.18 ± 0.00 | BMO |
| 21 | Terpinen-4-ol | 1179 | 1177 | 3.97 ± 0.05 | 0.58 ± 0.02 | MMO |
| 22 | α-Terpineol | 1192 | 1188 | 0.65 ± 0.03 | 0.02 ± 0.02 | MMO |
| 23 | Methyl cavicol (Estragole) | 1198 | 1196 | 0.47 ± 0.28 | - | OT |
| 24 | γ-Terpineol | 1197 | 1199 | - | 0.09 ± 0.00 | MMO |
| 25 | Dihydrocarvone | 1205 | 1200 | 0.10 ± 0.03 | 0.04 ± 0.01 | MMO |
| 26 | Carvacrol-methyl ether | 1247 | 1244 | 0.19 ± 0.04 | 0.09 ± 0.01 | MMO |
| 27 | Linalyl acetate | 1260 | 1257 | 4.18 ± 0.15 | - | AMO |
| 28 | Thymol | 1298 | 1290 | - | 4.39 ± 0.06 | MMO |
| 29 | Carvacrol | 1305 | 1299 | 19.17 ± 0.32 | 75.95 ± 0.40 | MMO |
| 30 | δ-Elemene | 1339 | 1338 | 1.19 ± 0.14 | - | MS |
| 31 | Neryl acetate | 1369 | 1361 | 0.23 ± 0.02 | - | AMO |
| 32 | Geranil acetate | 1387 | 1381 | 0.50 ± 0.08 | - | AMO |
| 33 | β-Caryophyllene | 1420 | 1419 | 4.79 ± 0.11 | 2.28 ± 0.03 | BS |
| 34 | β-Copaene | 1431 | 1432 | 0.21 ± 0.02 | - | BS |
| 35 | trans α-Bergamotene | 1438 | 1434 | 0.08 ± 0.01 | - | BS |
| 36 | Aromadendrene | 1446 | 1441 | 0.11 ± 0.01 | - | BS |
| 37 | allo Aromadendrene | 1455 | 1460 | 0.48 ± 0.02 | 0.14 ± 0.02 | BS |
| 38 | trans β-Farnesene | 1460 | 1456 | 0.21 ± 0.05 | - | AS |
| 39 | Germacrene D | 1483 | 1485 | 2.88 ± 0.15 | - | MS |
| 40 | γ-Amorphene | 1498 | 1495 | 2.02 ± 0.20 | - | BS |
| 41 | Germacrene A | 1510 | 1509 | 7.11 ± 0.19 | 0.29 ± 0.02 | MS |
| 42 | δ-Amorphene | 1516 | 1512 | 0.32 ± 0.02 | - | BS |
| 43 | δ-Cadinene | 1526 | 1523 | 0.77 ± 0.07 | 0.14 ± 0.01 | BS |
| 44 | Spatulenol | 1581 | 1578 | 0.51 ± 0.04 | - | BSO |
| 45 | Caryophyllene-oxide | 1586 | 1583 | 0.30 ± 0.03 | 0.19 ± 0.02 | BSO |
| 46 | Diethyl phthalate | 1599 | 1590 | 0.29 ± 0.14 | - | OT |
| 47 | α-Muurolol | 1645 | 1646 | 0.45 ± 0.01 | - | BSO |
| 48 | Eudesmol 7-epi-α | 1659 | 1663 | 0.60 ± 0.06 | - | BSO |
| 49 | Cedren-13-ol 8 | 1687 | 1689 | 0.85 ± 0.08 | - | BSO |
| Abbreviation | Area % WEO_OR | Area % CEO_OR | |
|---|---|---|---|
| Aliphatic monoterpenes | AM | 1.5 | 0.58 |
| Monocyclic monoterpenes | MM | 29.33 | 12.73 |
| Bi- and tricyclic monoterpenes | BM | 2.1 | 0.71 |
| Monoterpenes | M | 32.93 | 14.02 |
| Aliphatic oxygenated monoterpenes | AMO | 14.61 | 0.78 |
| Monocyclic oxygenated monoterpenes | MMO | 24.08 | 81.16 |
| Bi- and tricyclic oxygenated monoterpenes | BMO | - | - |
| Oxygenate monoterpenes | MO | 38.69 | 81.94 |
| Aliphatic sesquiterpenes | AS | 0.21 | - |
| Monocyclic sesquiterpenes | MS | 11.18 | 0.29 |
| Bi- and tricyclic sesquiterpenes | BS | 8.78 | 2.56 |
| Sesquiterpenes | S | 20.17 | 2.85 |
| Aliphatic oxygenated sesquiterpenes | ASO | - | - |
| Monocyclic oxygenated sesquiterpenes | MSO | - | - |
| Bi- and tricyclic oxygenated sesquiterpenes | BSO | 2.71 | 0.19 |
| Oxygenate sesquiterpenes | SO | 2.71 | 0.19 |
| Others | OT | 1.65 | 0.00 |
| Peak | Phenolic Compounds | Abbr. | RT | λ (nm) | ME_OR ± SD (mg g−1 DW) | R2 |
|---|---|---|---|---|---|---|
| 1 | Gallic acid | GA | 1.1 | 280 | 13.1 ± 1.3 | 0.9998 |
| 2 | Protocatechuic acid | PRCA | 1.5 | 260 | 1.2 ± 0.4 | 0.9989 |
| 3 | 4-Hydroxibenzoic acid | 4-HBA | 3.0 | 260 | 0.4 ± 0.1 | 0.9997 |
| 4 | Chlorogenic acid | CLA | 4.0 | 320 | 0.2 ± 0.1 | 0.9996 |
| 5 | Catechin | CAT | 4.1 | 280 | 2.2 ± 0.2 | 0.9990 |
| 6 | Vanillic acid | VA | 4.4 | 260 | 0.9 ± 0.2 | 0.9991 |
| 7 | Caffeic acid | CA | 4.6 | 320 | 1.1 ± 0.1 | 0.9988 |
| 8 | Vanillin | VAN | 6.0 | 280 | 8.1 ± 0.6 | 0.9989 |
| 9 | p-Coumaric acid | PCA | 6.5 | 320 | 0.4 ± 0.1 | 0.9998 |
| 10 | Rutin | RUT | 7.7 | 260 | 5.2 ± 0.5 | 0.9993 |
| 11 | Naringin | NAR | 8.8 | 280 | 4.4 ± 0.3 | 0.9996 |
| 12 | Rosmarinic acid | RA | 9.2 | 320 | 38.8 ± 2.8 | 0.9983 |
| 13 | Quercetin | QUE | 10.4 | 260 | 1.7 ± 0.4 | 0.9986 |
| 14 | Naringenin | NAN | 11.2 | 280 | 1.2 ± 0.3 | 0.9987 |
| 15 | Carvacrol | CAR | 13.2 | 280 | 0.3 ± 0.1 | 0.9997 |
| Sample | DPPH | ABTS | FRAP | |
|---|---|---|---|---|
| IC50 (mg mL−1) | IC50 (mg mL−1) | (mg TE mL−1) | (mg TE g−1) | |
| ME_OR | 0.052 ± 0.01 | 0.044 ± 0.006 | 3.94 ± 0.07 | 30.58 ± 3.7 |
| CEO_OR | 0.45 ± 0,11 | 0.033 ± 0.015 | 9.57 ± 0.52 | 7.33 ± 0.31 |
| WEO_OR | 1.54 ± 0.22 | 0.56 ± 0.07 | 0.10 ± 0.02 | - |
| Positive control | 0.00375 * | 0.00347 ** | 0.00506 ** | |
| Sample | Total Polyphenols | Flavonoids | |
|---|---|---|---|
| (mg g−1 GAE) | (mg QUE g−1 DM) | (mg CAE g−1 DM) | |
| ME_OR | 75.49 ± 0.9 | 147.2 ± 2.1 | 34.7 ± 0.5 |
| Time | Flow (mL/min) | % B |
|---|---|---|
| 0 | 0.4 | 5 |
| 5 | 0.4 | 16 |
| 8 | 0.4 | 30 |
| 14 | 0.4 | 85 |
| 16 | 0.4 | 5 |
| 20 | 0.4 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantasma, F.; Segatto, M.; Colardo, M.; Di Matteo, F.; Chini, M.G.; Iorizzi, M.; Saviano, G. Phytochemistry and Bioactivity of Essential Oil and Methanolic Extracts of Origanum vulgare L. from Central Italy. Plants 2025, 14, 2468. https://doi.org/10.3390/plants14162468
Fantasma F, Segatto M, Colardo M, Di Matteo F, Chini MG, Iorizzi M, Saviano G. Phytochemistry and Bioactivity of Essential Oil and Methanolic Extracts of Origanum vulgare L. from Central Italy. Plants. 2025; 14(16):2468. https://doi.org/10.3390/plants14162468
Chicago/Turabian StyleFantasma, Francesca, Marco Segatto, Mayra Colardo, Francesca Di Matteo, Maria Giovanna Chini, Maria Iorizzi, and Gabriella Saviano. 2025. "Phytochemistry and Bioactivity of Essential Oil and Methanolic Extracts of Origanum vulgare L. from Central Italy" Plants 14, no. 16: 2468. https://doi.org/10.3390/plants14162468
APA StyleFantasma, F., Segatto, M., Colardo, M., Di Matteo, F., Chini, M. G., Iorizzi, M., & Saviano, G. (2025). Phytochemistry and Bioactivity of Essential Oil and Methanolic Extracts of Origanum vulgare L. from Central Italy. Plants, 14(16), 2468. https://doi.org/10.3390/plants14162468








