Characterization of Biological Components of Leaves and Flowers in Moringa peregrina and Their Effect on Proliferation of Staurogyne repens in Tissue Culture Conditions
Abstract
1. Introduction
2. Results
Amount of Quercetin, Gallic Acid, Caffeic Acid, and Myricetin in the Aqueous Extract of M. peregrina
3. Discussion
4. Materials and Methods
4.1. Collection and Preparation of Aqueous Extract of the Moringa Plant
4.1.1. Preparation of Ethanolic Extracts
4.1.2. Preparation of Aqueous Extracts
4.2. Measurement of the Amount of Secondary Metabolites in the Aqueous Extract of Moringa via HPLC
4.3. Disinfection and Establishment of Staurogyne Plant Buds
4.4. Seedling Rooting
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassanein, A.M.A.; Al-Soqeer, A.A. Morphological and genetic diversity of Moringa oleifera and Moringa peregrina genotypes. Hortic. Environ. Biotechnol. 2018, 59, 251–261. [Google Scholar] [CrossRef]
- Senthilkumar, A.; Karuvantevida, N.; Rastrelli, L.; Kurup, S.S.; Cheruth, A.J. Traditional Uses, Pharmacological Efficacy, and Phytochemistry of Moringa peregrina (Forssk.) Fiori. A Review. Front. Pharmacol. 2018, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.; Mansour, S. Moringa peregrina a natural medicine for increasing immunity defense against the COVID-19. Med. Aromat. Plants 2020, 9, 358. [Google Scholar] [CrossRef]
- Mansour, M.; Mohamed, M.F.; Elhalwagi, A.; El-Itriby, H.A.; Shawki, H.H.; Abdelhamid, I.A. Moringa peregrina Leaves Extracts Induce Apoptosis and Cell Cycle Arrest of Hepatocellular Carcinoma. BioMed Res. Int. 2019, 2019, 2698570. [Google Scholar] [CrossRef]
- Khajeh, H.; Fazeli-Nasab, B.; Pourshahdad, A.; Mirzaei, A.R.; Ghorbanpour, M. Green-synthesized silver nanoparticles induced apoptotic cell death in CACO2 cancer cells by activating MLH1 gene expression. Sci. Rep. 2024, 14, 29601. [Google Scholar] [CrossRef]
- Saleem, F.; Sarkar, D.; Ankolekar, C.; Shetty, K. Phenolic bioactives and associated antioxidant and anti-hyperglycemic functions of select species of Apiaceae family targeting for type 2 diabetes relevant nutraceuticals. Ind. Crops Prod. 2017, 107, 518–525. [Google Scholar] [CrossRef]
- Pietruś, W.; Kafel, R.; Bojarski, A.J.; Kurczab, R. Hydrogen Bonds with Fluorine in Ligand–Protein Complexes-the PDB Analysis and Energy Calculations. Molecules 2022, 27, 1005. [Google Scholar] [CrossRef] [PubMed]
- Garda, T.; Riba, M.; Vasas, G.; Beyer, D.; M-Hamvas, M.; Hajdu, G.; Tándor, I.; Máthé, C. Cytotoxic effects of cylindrospermopsin in mitotic and non-mitotic Vicia faba cells. Chemosphere 2015, 120, 145–153. [Google Scholar] [CrossRef]
- Roughani, A.; Miri, S.M.; Kashi, A.K.; Khiabani, B.N. Increasing the ploidy level in spinach (Spinacia oleracea L.) using mitotic inhibitors. Plant Cell Biotechnol. Mol. Biol. 2017, 18, 124–130. [Google Scholar]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Fazeli-Nasab, B.; Rahmani, A.F. Microbial Genes, Enzymes, and Metabolites: To Improve Rhizosphere and Plant Health Management. In Microbiological Activity for Soil and Plant Health Management; Soni, R., Suyal, D.C., Bhargava, P., Goel, R., Eds.; Springer: Singapore, 2021; pp. 459–506. [Google Scholar] [CrossRef]
- Salehi-Sardoei, A.; Mousavinasab, F.; Sayyed, R.Z.; Bameri, F.; Beheshtizadeh, H.; Fazeli-Nasab, B.; Mirzaei, A.R. Chapter 11—Endophytic fungi: Perspectives for microbial engineering. In Microbial Biostimulants for Plant Growth and Abiotic Stress Amelioration; Chauhan, P.S., Bisht, N., Agarwal, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 167–220. [Google Scholar] [CrossRef]
- Gulzar, A.; Siddiqui, M.B.; Bi, S. Phenolic acid allelochemicals induced morphological, ultrastructural, and cytological modification on Cassia sophera L. and Allium cepa L. Protoplasma 2016, 253, 1211–1221. [Google Scholar] [CrossRef]
- Ain, Q.; Mushtaq, W.; Shadab, M.; Siddiqui, M.B. Allelopathy: An alternative tool for sustainable agriculture. Physiol. Mol. Biol. Plants 2023, 29, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Alhakmani, F.; Khan, S.A.; Ahmad, A. Determination of total phenol, in-vitro antioxidant and anti-inflammatory activity of seeds and fruits of Zizyphus spina-christi grown in Oman. Asian Pac. J. Trop. Biomed. 2014, 4, S656–S660. [Google Scholar] [CrossRef]
- Salih, E.Y.A.; Julkunen-Tiitto, R.; Luukkanen, O.; Fahmi, M.K.M.; Fyhrquist, P. Hydrolyzable tannins (ellagitannins), flavonoids, pentacyclic triterpenes and their glycosides in antimycobacterial extracts of the ethnopharmacologically selected Sudanese medicinal plant Combretum hartmannianum Schweinf. Biomed. Pharmacother. 2021, 144, 112264. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Boland, R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Zare, N.; Farjaminezhad, R.; Asghari-Zakaria, R.; Farjaminezhad, M. Enhanced thebaine production in Papaver bracteatum cell suspension culture by combination of elicitation and precursor feeding. Nat. Prod. Res. 2014, 28, 711–717. [Google Scholar] [CrossRef]
- Bahmani, M.; Zargaran, A.; Rafieian-Kopaei, M. Identification of medicinal plants of Urmia for treatment of gastrointestinal disorders. Rev. Bras. Farmacogn. 2014, 24, 468–480. [Google Scholar] [CrossRef]
- Mahajan, S.G.; Mehta, A.A. Immunosuppressive activity of ethanolic extract of seeds of Moringa oleifera Lam. in experimental immune inflammation. J. Ethnopharmacol. 2010, 130, 183–186. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, G.; Guo, M. Correlations between phytochemical fingerprints of Moringa oleifera leaf extracts and their antioxidant activities revealed by chemometric analysis. Phytochem. Anal. 2021, 32, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, G.; Martorell, M.; Ramírez-Alarcón, K.; Salehi, B.; Sharifi-Rad, J. Phytochemical screening of Moringa oleifera leaf extracts and their antimicrobial activities. Cell. Mol. Biol. 2020, 66, 20–26. [Google Scholar] [CrossRef]
- Rayorath, P.; Jithesh, M.N.; Farid, A.; Khan, W.; Palanisamy, R.; Hankins, S.D.; Critchley, A.T.; Prithiviraj, B. Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L.) Le Jol. using a model plant, Arabidopsis thaliana (L.) Heynh. J. Appl. Phycol. 2008, 20, 423–429. [Google Scholar] [CrossRef]
- Tabin, S.; Gupta, R.C.; Kamili, A.N.; Parray, J.A. Medical and medicinal importance of Rheum. spp. collected from different altitudes of the Kashmir Himalayan range. Cell. Mol. Biomed. Rep. 2022, 2, 187–201. [Google Scholar] [CrossRef]
- Alavi, M.; Hamblin, M.R.; Aghaie, E.; Mousavi, S.A.R.; Hajimolaali, M. Antibacterial and antioxidant activity of catechin, gallic acid, and epigallocatechin-3-gallate: Focus on nanoformulations. Cell. Mol. Biomed. Rep. 2023, 3, 62–72. [Google Scholar] [CrossRef]
- Kakaei, M.; Rehman, F.U.; Fazeli, F. The effect of chickpeas metabolites on human diseases and the application of their valuable nutritional compounds suitable for human consumption. Cell. Mol. Biomed. Rep. 2024, 4, 30–42. [Google Scholar] [CrossRef]
- Natarajan, A.; Elavazhagan, P.; Prabhakaran, J. Allelopathic potential of billy goat weed Ageratum conyzoides L. and Cleome viscosa L. on germination and growth of Sesamum indicum L. Int. J. Curr. Biotechnol. 2014, 2, 21–24. [Google Scholar]
- Sarkar, E.; Chatterjee, S.N.; Chakraborty, P. Allelopathic effect of Cassia tora on seed germination and growth of mustard. Turk. J. Bot. 2012, 36, 488–494. [Google Scholar] [CrossRef]
- Abd-Elkareem, M.; Abd El-Rahman, M.A.M.; Khalil, N.S.A.; Amer, A.S. Antioxidant and cytoprotective effects of Nigella sativa L. seeds on the testis of monosodium glutamate challenged rats. Sci. Rep. 2021, 11, 13519. [Google Scholar] [CrossRef]
- Ptak, A.; Morańska, E.; Saliba, S.; Zieliński, A.; Simlat, M.; Laurain-Mattar, D. Elicitation of galanthamine and lycorine biosynthesis by Leucojum aestivum L. and L. aestivum ‘Gravety Giant’ plants cultured in bioreactor RITA®. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 128, 335–345. [Google Scholar] [CrossRef]
- Naz, R.; Bano, A. Effects of Calotropis procera and Citrullus colosynthis on germination and seedling growth of maize. Allelopath. J. 2013, 31, 105–116. [Google Scholar]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Nikbakht, M.; Pour Ali, P. Biological Production and antibacterial effect of synthesized Ag with aqua extract and methanol anab. Med. Sci. J. Islam. Azad Univ. 2015, 12, 112–118. [Google Scholar]
- Hurst, W.; Martin Jr, R.; Zoumas, B. Application of HPLC to characterization of individual carbohydrates in foods. J. Food Sci. 1979, 44, 892–895. [Google Scholar] [CrossRef]
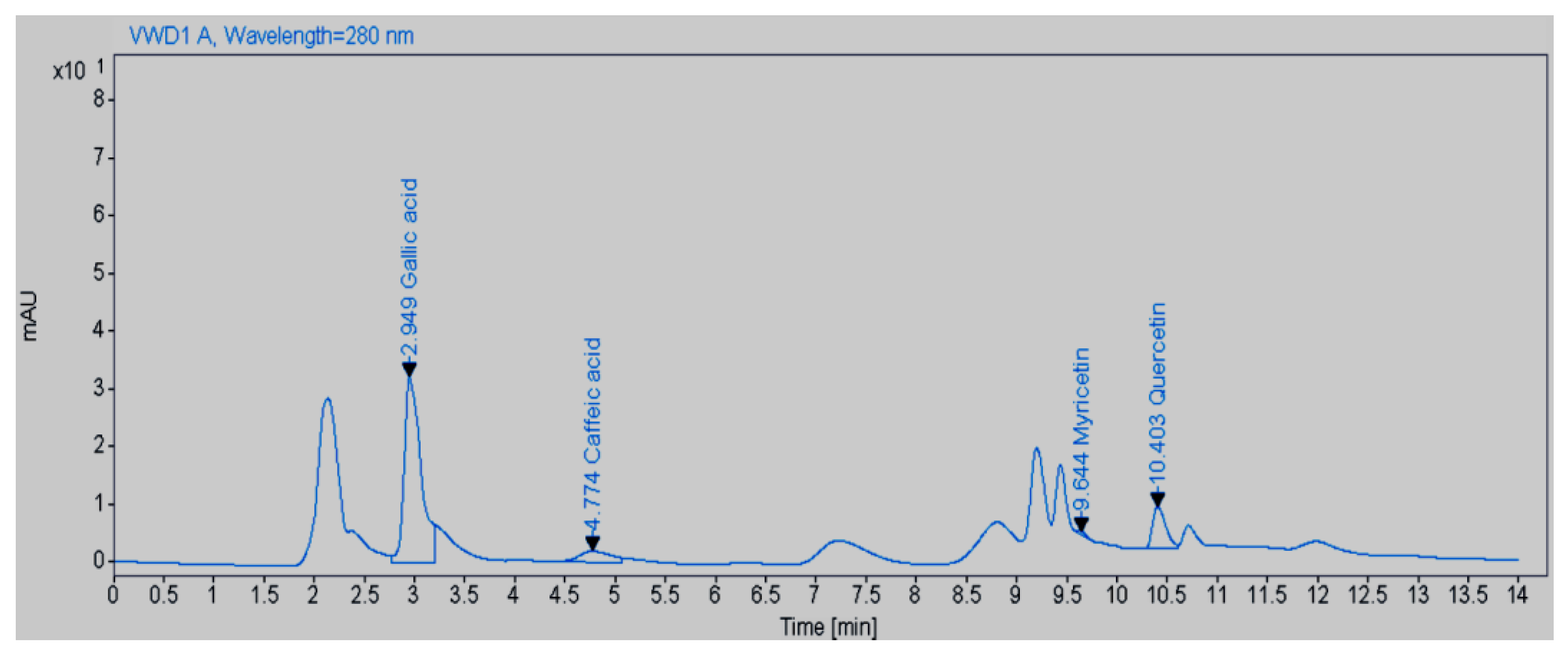
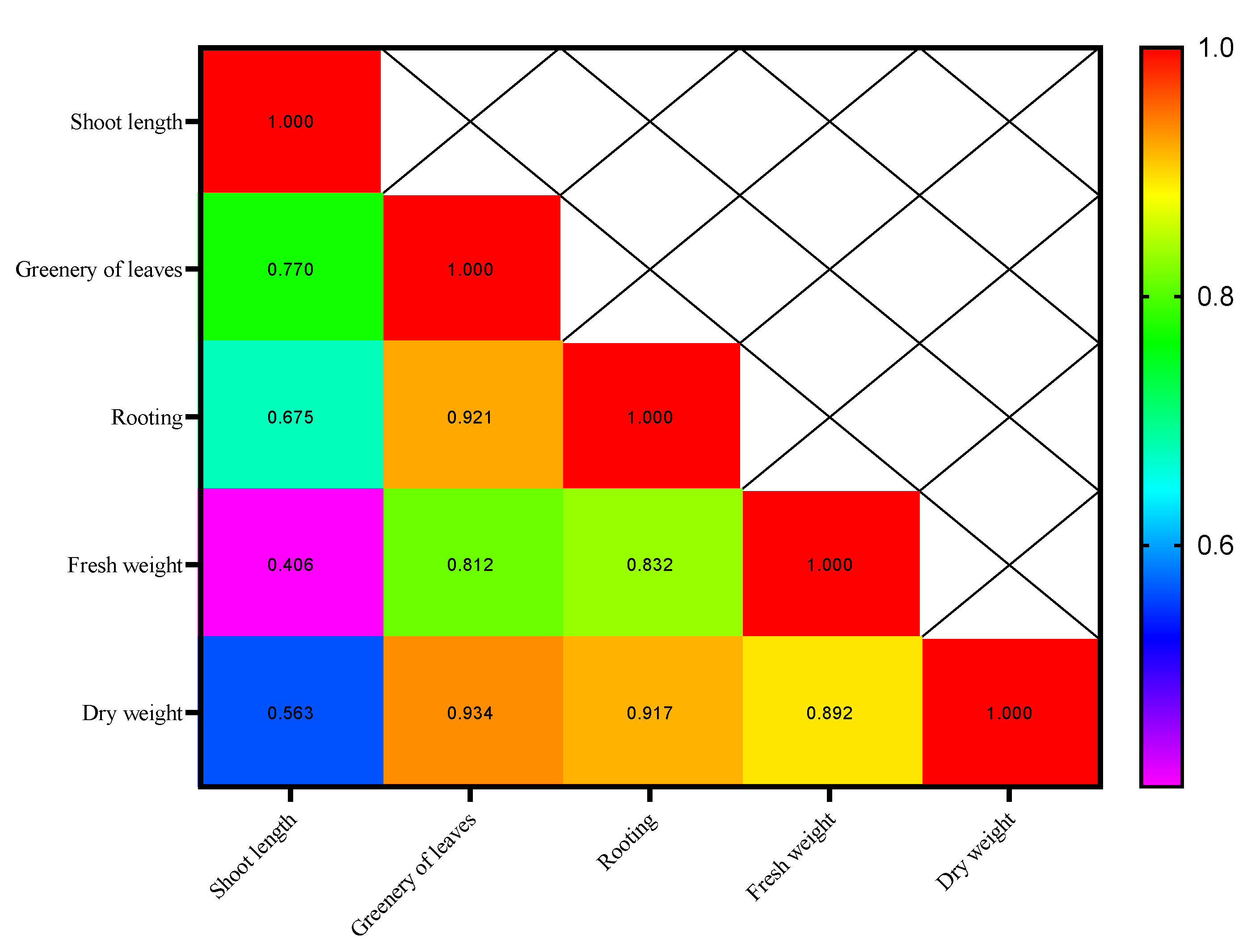
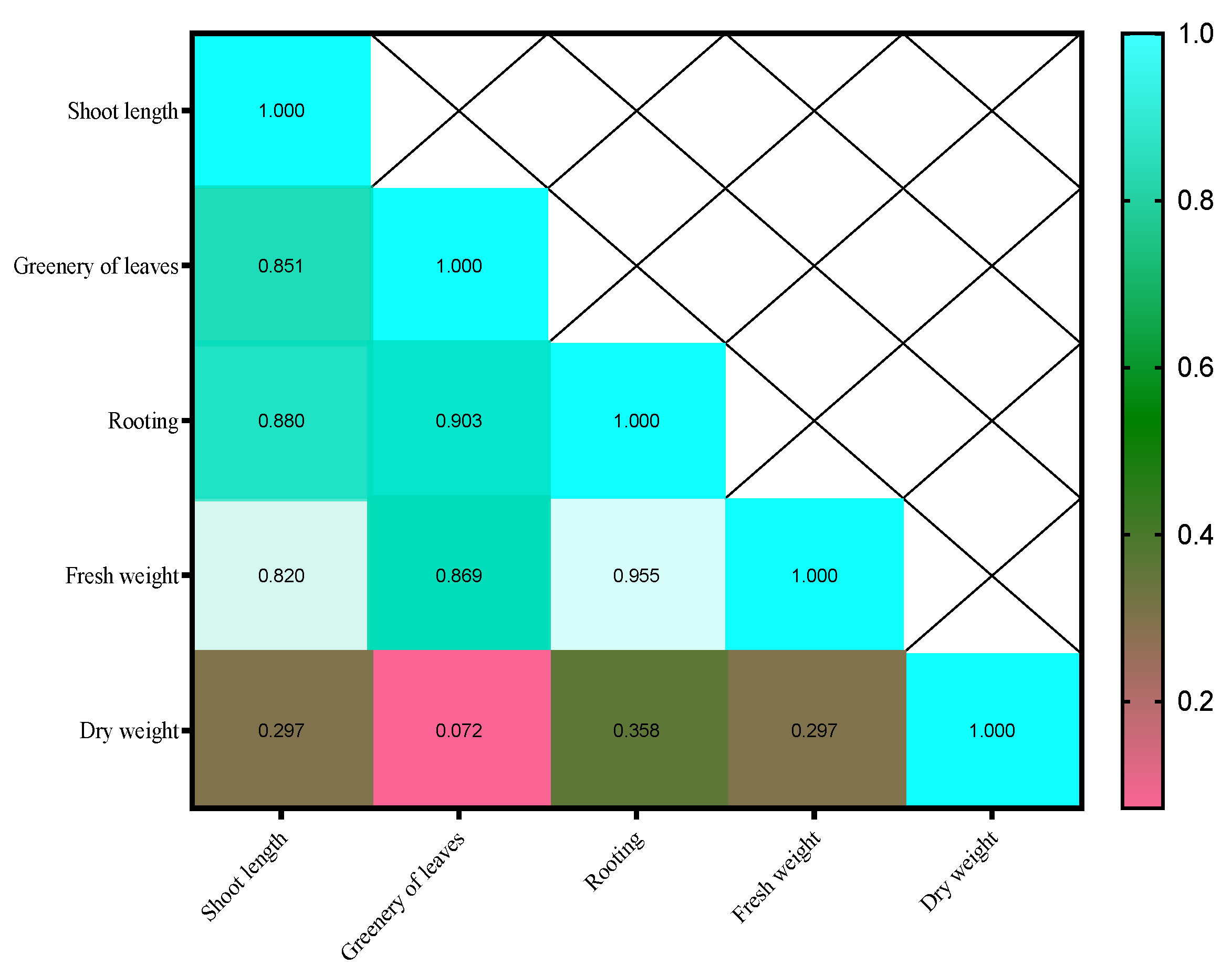


| Sample | Myricetin (mg.g−1) | Caffeic Acid (mg.g−1) | Gallic Acid (mg.g−1) | Quercetin (mg.g−1) |
|---|---|---|---|---|
| Amount in plant water sample | 4.6 | 42 | 374.8 | 64.9 |
| % | 0.14 | 8.62 | 77.07 | 13.34 |
| Culture Media | Different Concentrations of M. peregrina Leaf Aqueous Extract | Shoot Length (cm) | ||
|---|---|---|---|---|
| First Week | Second Week | Third Week | ||
| MS | 0 mg/L | 0.033 ± 0.033 d | 0.133 ± 0.033 de | 0.733 ± 0.037 de |
| 1 mg/L | 1.067 ± 0.067 ab | 1.333 ± 0.076 c | 2.100 ± 0.100 c | |
| 1.5 mg/L | 1.300 ± 0.058 a | 1.800 ± 0.015 b | 3.400 ± 0.035 b | |
| 3 mg/L | 1.300 ± 0.416 a | 2.600 ± 0.650 a | 5.333 ± 0.0.033 a | |
| ½ MS | 0 mg/L | 0.000 ± 0.000 d | 0.000 ± 0.000 e | 0.233 ± 0.088 e |
| 1 mg/L | 0.333 ± 0.145 cd | 0.200 ± 0.058 de | 0.893 ± 0.052 de | |
| 1.5 mg/L | 0.733 ± 0.033 bc | 0.517 ± 0.073 d | 1.167 ± 0.088 d | |
| 3 mg/L | 0.800 ± 0.115 abc | 1.367 ± 0.120 c | 1.833 ± 0.088 c | |
| C.V. | - | 11.61 | 12.28 | 19.22 |
| Culture Media | Different Concentrations of M. peregrina Leaf Aqueous Extract | Greenery of Leaves (%) | ||
|---|---|---|---|---|
| First Week | Second Week | Third Week | ||
| MS | 0 mg/L | 0.000 ± 0.000 d | 1.000 ± 0.001 e | 4.333 ± 0.088 cd |
| 1 mg/L | 1.667 ± 0.033 bc | 2.667 ± 0.033 c | 5.333 ± 0.667 bc | |
| 1.5 mg/L | 2.467 ± 0.027 b | 5.000 ± 0.577 b | 7.667 ± 0.333 b | |
| 3 mg/L | 6.000 ± 0.577 a | 8.000 ± 0.577 a | 14.000 ± 1.082 a | |
| ½ MS | 0 mg/L | 0.000 ± 0.000 d | 0.667 ± 0.033 e | 0.333 ± 0.033 f |
| 1 mg/L | 0.667 ± 0.033 cd | 1.333 ± 0.033 de | 1.333 ± 0.033 ef | |
| 1.5 mg/L | 1.333 ± 0.033 c | 2.333 ± 0.033 cd | 1.667 ± 0.033 def | |
| 3 mg/L | 1.667 ± 0.033 bc | 1.333 ± 0.033 de | 4.000 ± 0.577 cde | |
| C.V. | - | 3.28 | 14.25 | 19.06 |
| Culture Media | Different Concentrations of M. peregrina Leaf Aqueous Extract | Rooting (%) | ||
|---|---|---|---|---|
| First Week | Second Week | Third Week | ||
| MS | 0 mg/L | 0.000 ± 0.000 d | 0.000 ± 0.000 d | 0.667 ± 0.003 c |
| 1 mg/L | 0.733 ± 0.0.37 bcd | 1.300 ± 0.030 bc | 1.833 ± 0.166 bc | |
| 1.5 mg/L | 1.333 ± 0.333 b | 2.333 ± 0.333 b | 3.000 ± 0.557 b | |
| 3 mg/L | 3.333 ± 0.333 a | 6.000 ± 0.577 a | 9.667 ± 0.145 a | |
| ½ MS | 0 mg/L | 0.000 ± 0.000 d | 0.000 ± 0.000 d | 1.000 ± 0.000 c |
| 1 mg/L | 0.233 ± 0.014 cd | 0.867 ± 0.133 cd | 1.833 ± 0.166 bc | |
| 1.5 mg/L | 0.333 ± 0.033 cd | 1.333 ± 0.033 bc | 2.333 ± 0.333 bc | |
| 3 mg/L | 0.933 ± 0.067 bc | 2.000 ± 0.057 bc | 3.667 ± 0.333 b | |
| C.V. | - | 15.80 | 15.55 | 14.36 |
| Culture Media | Different Concentrations of M. peregrina Leaf Aqueous Extract | Fresh Weight (g) | ||
|---|---|---|---|---|
| First Week | Second Week | Third Week | ||
| MS | 0 mg/L | 0.000 ± 0.000 d | 0.100 ± 0.005 ef | 0.233 ± 0.014 de |
| 1 mg/L | 0.133 ± 0.033 bc | 0.400 ± 0.005 cd | 0.800 ± 0.015 cde | |
| 1.5 mg/L | 0.333 ± 0.008 bc | 0.633 ± 0.003 bc | 1.733 ± 0.267 b | |
| 3 mg/L | 1.333 ± 0.033 a | 2.967 ± 0.057 a | 6.000 ± 0.577 a | |
| ½ MS | 0 mg/L | 0.000 ± 0.000 d | 0.000 ± 0.000 f | 0.000 ± 0.000 e |
| 1 mg/L | 0.067 ± 0.033 d | 0.100 ± 0.001 ef | 0.200 ± 0.005 de | |
| 1.5 mg/L | 0.133 ± 0.033 bc | 0.400 ± 0.005 cd | 0.900 ± 0.058 cd | |
| 3 mg/L | 0.500 ± 0.115 b | 0.867 ± 0.133 b | 1.400 ± 0.030 bc | |
| C.V. | - | 13.21 | 8.06 | 12.41 |
| Culture Media | Different Concentrations of M. peregrina Leaf Aqueous Extract | Dry Weight (g) | ||
|---|---|---|---|---|
| First Week | Second Week | Third Week | ||
| MS | 0 mg/L | 0.000 ± 0.000 d | 0.000 ± 0.000 b | 0.001 ± 0.000 c |
| 1 mg/L | 0.009 ± 0.000 c | 0.005 ± 0.001 b | 0.047 ± 0.002 bc | |
| 1.5 mg/L | 0.020 ± 0.006 b | 0.017 ± 0.001 b | 0.113 ± 0.011 b | |
| 3 mg/L | 0.087 ± 0.003 a | 0.039 ± 0.011 b | 1.100 ± 0.057 a | |
| ½ MS | 0 mg/L | 0.000 ± 0.000 d | 0.000 ± 0.000 b | 0.000 ± 0.000 c |
| 1 mg/L | 0.002 ± 0.001 cd | 0.003 ± 0.001 b | 0.000 ± 0.000 c | |
| 1.5 mg/L | 0.005 ± 0.001 cd | 0.059 ± 0.002 b | 0.007 ± 0.001 c | |
| 3 mg/L | 0.007 ± 0.001 cd | 0.127 ± 0.003 a | 0.036 ± 0.001 c | |
| C.V. | - | 1.23 | 1.05 | 2.79 |
| SOV | Area | Area in Mix | MeOH 70 | Area Average | SD | RSD% | |
|---|---|---|---|---|---|---|---|
| Quercetin 10.38 | 2 | 21,264 | 7983 | 64.9 | 21,546 | 398.8082 | 1.850962 |
| 1 | 21,828 | ||||||
| 21,546 | |||||||
| Gallic acid 2.93 | 3 | 29,416 | 10,116 | 374.8 | 29,439 | 32.52691 | 0.110489 |
| 1 | 29,462 | ||||||
| 29,439 | |||||||
| Caffeic acid 4.99 | 60,295 | 21,341 | 42 | ||||
| Myricetin 9.73 | 26,018 | 4.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khajeh, H.; Fazeli-Nasab, B.; Salehi Sardoei, A.; Fotoohiyan, Z.; Hatami, M.; Mirzaei, A.; Ghorbanpour, M.; Maggi, F. Characterization of Biological Components of Leaves and Flowers in Moringa peregrina and Their Effect on Proliferation of Staurogyne repens in Tissue Culture Conditions. Plants 2025, 14, 2340. https://doi.org/10.3390/plants14152340
Khajeh H, Fazeli-Nasab B, Salehi Sardoei A, Fotoohiyan Z, Hatami M, Mirzaei A, Ghorbanpour M, Maggi F. Characterization of Biological Components of Leaves and Flowers in Moringa peregrina and Their Effect on Proliferation of Staurogyne repens in Tissue Culture Conditions. Plants. 2025; 14(15):2340. https://doi.org/10.3390/plants14152340
Chicago/Turabian StyleKhajeh, Hamideh, Bahman Fazeli-Nasab, Ali Salehi Sardoei, Zeinab Fotoohiyan, Mehrnaz Hatami, Alireza Mirzaei, Mansour Ghorbanpour, and Filippo Maggi. 2025. "Characterization of Biological Components of Leaves and Flowers in Moringa peregrina and Their Effect on Proliferation of Staurogyne repens in Tissue Culture Conditions" Plants 14, no. 15: 2340. https://doi.org/10.3390/plants14152340
APA StyleKhajeh, H., Fazeli-Nasab, B., Salehi Sardoei, A., Fotoohiyan, Z., Hatami, M., Mirzaei, A., Ghorbanpour, M., & Maggi, F. (2025). Characterization of Biological Components of Leaves and Flowers in Moringa peregrina and Their Effect on Proliferation of Staurogyne repens in Tissue Culture Conditions. Plants, 14(15), 2340. https://doi.org/10.3390/plants14152340







