In Vivo Investigation of Cardioprotective Effects of Melilotus officinalis and Melilotus albus Aerial Parts Extracts for Potential Therapeutic Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Preparation of Plant Extracts
2.4. Quantitative Analyses of Total Bioactive Compounds
2.5. LC-MS/MS Analysis of Polyphenols Apparatus and Chromatographic Conditions
2.6. The Evaluation of In Vitro Antioxidant Potential
2.7. The Evaluation of In Vivo Cardioprotective Effects
2.7.1. Experimental Protocol
2.7.2. Experimental Myocardial Ischemia
2.7.3. Electrocardiography Results
2.7.4. The Evaluation of In Vivo Antioxidant Properties
2.8. Multivariate Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Quantitative Analyses of Total Bioactive Compounds
3.2. LC-MS/MS Analysis of Polyphenols
3.3. In Vitro Antioxidant Properties
3.4. Evaluation of In Vivo Cardioprotective Activity
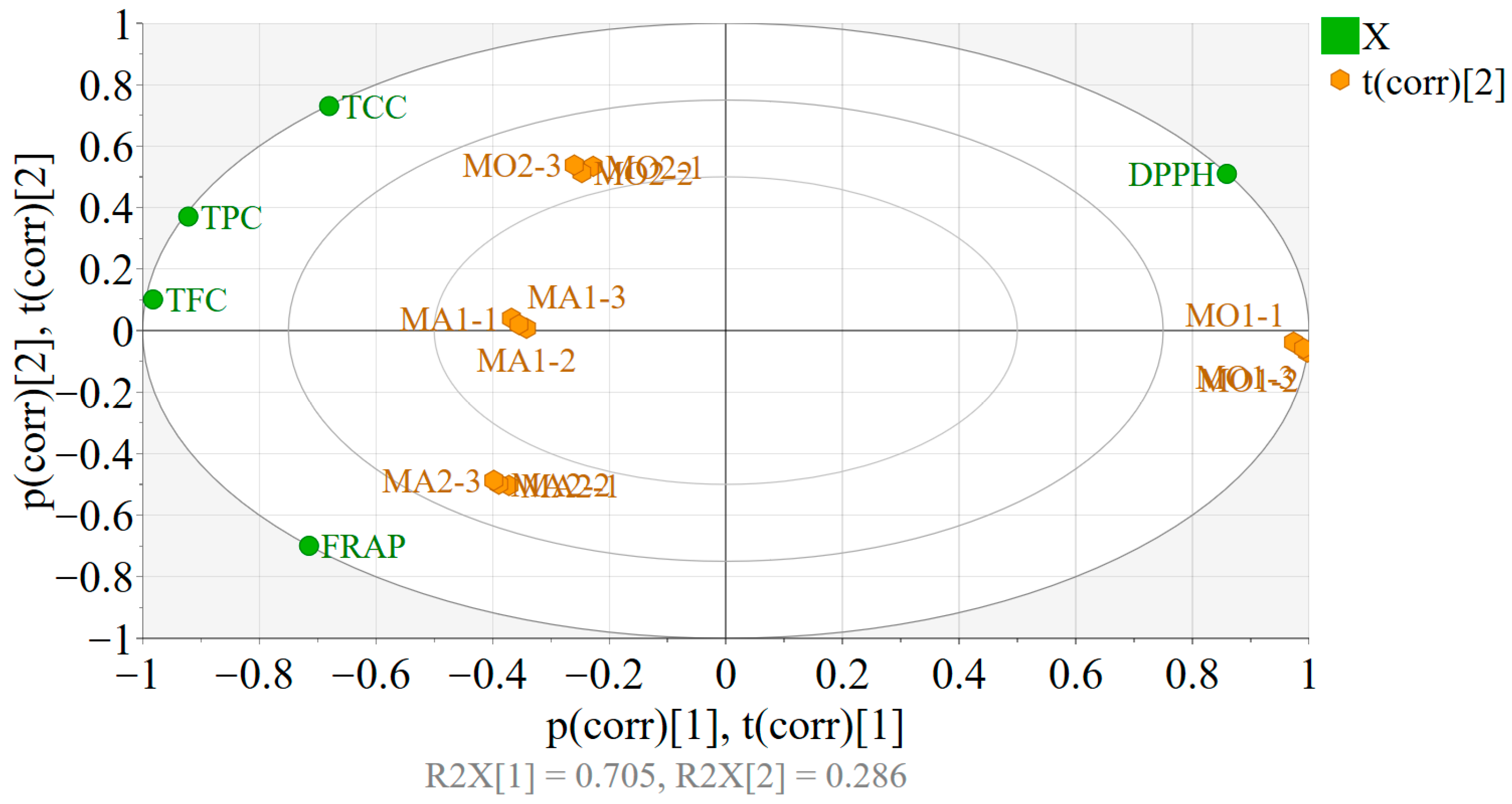
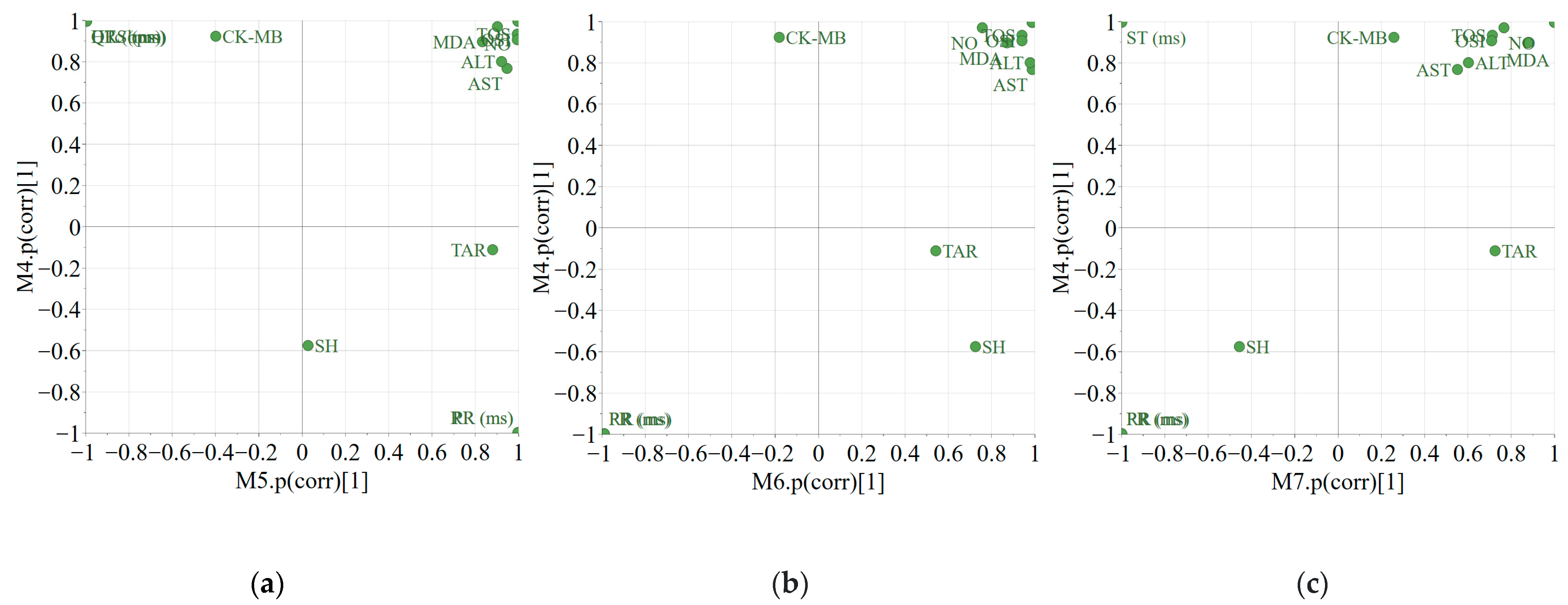
3.5. Multivariate Analysis
- PART 1
- PART 2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECG | Electrocardiogram |
| HPLC-MS | High-Performance Liquid Chromatography–Mass Spectrometry |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FRAP | Ferric Reducing Antioxidant Power |
| IC50 | Half Maximal Inhibitory Concentration |
| TOS | Total Oxidant Status |
| OSI | Oxidative Stress Index |
| TAC | Total Antioxidant Capacity |
| MDA | Malondialdehyde |
| NO | Nitric Oxide |
| SH | Total Thiols |
| GOT | Glutamate Oxaloacetate Transaminase |
| GPT | Glutamate Pyruvate Transaminase |
| CK-MB | Creatine Kinase–MB Isoenzyme |
| CVD | Cardiovascular Disease |
| IHD | Ischemic Heart Disease |
| LDL | Low-Density Lipoprotein |
| CRP | C-Reactive Protein |
| ROS | Reactive Oxygen Species |
| LDH | Lactate Dehydrogenase |
| ATP | Adenosine Triphosphate |
| NAD | Nicotinamide Adenine Dinucleotide |
| SOD | Superoxide Dismutase |
| MO | Melilotus officinalis |
| MAS | Melilotus albus |
| TPC | Total Phenolic Content |
| GAE | Gallic Acid Equivalents |
| TFC | Total Flavonoids Content |
| RE | Rutoside Equivalents |
| QE | Quercetin Equivalents |
| CUM | Coumarin |
| TCC | Total Coumarin Content |
| CE | Coumarin Equivalents |
| TPTZ | 2,4,6-Tripyridyl-s-triazine |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
| APCI | Atmospheric Pressure Chemical Ionization |
| BW | Body Weight |
| ISO | Isoprenaline |
| MAPK | Mitogen-Activated Protein Kinase |
| NF-KB | Nuclear Factor Kappa B |
| TE | Trolox Equivalents |
| INFL | Inflammation |
| CAT | Catalase |
| GSH | Glutathione |
| GPx | Glutathione Peroxidase |
| OPLS-DA | Orthogonal Projections to Latent Structures based Discriminant Analysis |
| PCA | Principal Component Analysis |
| TNF-alfa | Tumor Necrosis Factor-alpha |
| IL-6 | Interleukin-6 |
| MI | Myocardial Infarction |
| LOQ | Limit Of Quantification |
References
- Madhumitha, S.; Indhuleka, A. Cardioprotective Effect of Morus Alba L. Leaves in Isoprenaline Induced Rats. Int. J. Pharm. Sci. Res. 2012, 3, 1475–1480. [Google Scholar]
- Yang, Y.; Yang, M.; Ai, F.; Huang, C. Cardioprotective Effect of Aloe vera Biomacromolecules Conjugated with Selenium Trace Element on Myocardial Ischemia-Reperfusion Injury in Rats. Biol. Trace Elem. Res. 2016, 177, 345–352. [Google Scholar] [CrossRef]
- Epure, A.; Pârvu, A.E.; Vlase, L.; Benedec, D.; Hanganu, D.; Oniga, O.; Vlase, A.-M.; Ielciu, I.; Toiu, A.; Oniga, I. New Approaches on the Anti-Inflammatory and Cardioprotective Properties of Taraxacum officinale Tincture. Pharmaceuticals 2023, 16, 358. [Google Scholar] [CrossRef]
- Upaganlawar, A.; Gandhi, H.; Balaraman, R. Isoproterenol induced myocardial infarction: Protective role of natural products. J. Pharmacol. Toxicol. 2011, 6, 1–17. [Google Scholar] [CrossRef]
- Akter, H.; Rashid, M.M.; Islam, M.S.; Hossen, M.A.; Rahman, M.A.; Algheshairy, R.M.; Almujaydil, M.S.; Alharbi, H.F.; Alnajeebi, A.M. Biometabolites of Tamarindus indica play a remarkable cardioprotective role as a functional food in doxorubicin-induced cardiotoxicity models. J. Funct. Foods 2022, 1, 96. [Google Scholar] [CrossRef]
- Otręba, M.; Kośmider, L.; Stojko, J.; Rzepecka-Stojko, A. Cardioprotective activity of selected polyphenols based on epithelial and aortic cell lines. A review. Molecules 2020, 25, 5343. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.W.; Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea. Vol. 2. Rosaceae to Umbelliferae. J. Ecol. 1969, 57, 482. [Google Scholar] [CrossRef]
- Chorepsima, S.; Tentolouris, K.; Dimitroulis, D.; Tentolouris, N. Melilotus: Contribution to wound healing in the diabetic foot. J. Herb. Med. 2013, 3, 81–86. [Google Scholar] [CrossRef]
- Ciocârlan, V. Flora ilustrată a României Pteridophyta et Spermatophyta; Publishing House Ceres: Bucureşti, Romania, 2009; 1141p. [Google Scholar]
- Tang, C.N. Study on the extraction process of total flavonoids from Melilotus officinalis medicinal plant. J. Anhui Agric. Sci. 2012, 3, 23–25. [Google Scholar]
- Anwer, S.; Azhar, I.; Hasan, M.; Mohtasheem, M.; Ahmed, S.W.; Bano, H. Chemical constituents from Melilotus officinalis. J. Basic Appl. Sci. 2008, 4, 89–94. [Google Scholar]
- Stefanović, O.D.; Tešić, J.D.; Čomić, L.R. Melilotus albus and Dorycnium herbaceum extracts as source of phenolic compounds and their antimicrobial, antibiofilm, and antioxidant potentials. J. Food Drug Anal. 2015, 23, 417. [Google Scholar] [CrossRef]
- Sisay, M.A.; Mammo, W.; Yaya, E.E. Phytochemical studies of Melilotus officinalis. Bull. Chem. Soc. Ethiop. 2021, 35, 141–150. [Google Scholar] [CrossRef]
- Nogues, I.; Passatore, L.; Bustamante, M.Á.; Pallozzi, E.; Luz, J.; Traquete, F.; Ferreira, A.E.N.; Silva, M.S.; Cordeiro, C. Cultivation of Melilotus officinalis as a source of bioactive compounds in association with soil recovery practices. Front. Plant Sci. 2023, 14, 1218594. [Google Scholar] [CrossRef]
- Trouillas, P.; Calliste, C.-A.; Allais, D.-P.; Simon, A.; Marfak, A.; Delage, C.; Duroux, J.-L. Antioxidant, anti-inflammatory and antiproliferative properties of sixteen water plant extracts used in the Limousin countryside as herbal teas. Food Chem. 2003, 80, 399–407. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Pastorino, G.; Marchetti, C.; Borghesi, B.; Cornara, L.; Ribulla, S.; Burlando, B. Biological activities of the legume crops Melilotus officinalis and Lespedeza capitata for skin care and pharmaceutical applications. Ind. Crops Prod. 2017, 96, 158–164. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Melilotus officinalis (L.) Pall., Herba [Internet]; EMA: London, UK, 2017; Available online: https://www.fitoterapia.net/archivos/202505/addendum-assessment-report-melilotus-officinalis-l-lam-herba_en.pdf?1 (accessed on 1 May 2025).
- European Directorate for the Quality of Medicines & HealthCare (EDQM). Meliloti herba (Melilotus officinalis herba). In European Pharmacopoeia, 9th ed.; Council of Europe: Strasbourg, France, 2017. [Google Scholar]
- Stoker, J.R. The biosynthesis of coumarin in Melilotus alba. Biochem. Biophys. Res. Commun. 1963, 14, 17–20. [Google Scholar] [CrossRef]
- Nair, R.M.; Whittall, A.; Hughes, S.J.; Craig, A.D.; Revell, D.K.; Miller, S.M.; Powell, T.; Auricht, G.C. Variation in coumarin content of Melilotus species grown in South Australia. N. Z. J. Agric. Res. 2010, 53, 201–213. [Google Scholar] [CrossRef]
- Radmanesh, E.; Dianat, M.; Badavi, M.; Goudarzi, G.; Mard, S.A. The cardioprotective effect of vanillic acid on hemodynamic parameters, malondialdehyde, and infarct size in ischemia-reperfusion isolated rat heart exposed to PM10. Iran J. Basic Med. Sci. 2017, 20, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Safaeian, L.; Baniahmad, B.; Vaseghi, G.; Rabbani, M.; Mohammadi, B. Cardioprotective effect of vanillic acid against doxorubicin-induced cardiotoxicity in rat. Res. Pharm. Sci. 2020, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Ya, F.; Li, K.; Chen, H.; Tian, Z.; Fan, D.; Shi, Y.; Song, F.; Xu, X.; Ling, W.; Adili, R.; et al. Protocatechuic Acid Protects Platelets from Apoptosis via Inhibiting Oxidative Stress-Mediated PI3K/Akt/GSK3β Signaling. Thromb. Haemost. 2021, 121, 931–943. [Google Scholar] [CrossRef]
- Li, L.; Ma, H.; Zhang, Y.; Jiang, H.; Xia, B.; Al Sberi, H.; Elhefny, M.A.; Lokman, M.S.; Kassab, R.B. Protocatechuic acid reverses myocardial infarction mediated by β-adrenergic agonist via regulation of Nrf2/HO-1 pathway, inflammatory, apoptotic, and fibrotic events. J. Biochem. Mol. Toxicol. 2023, 37, e23270. [Google Scholar] [CrossRef] [PubMed]
- Toiu, A.; Vlase, L.; Vodnar, D.C.; Gheldiu, A.-M.; Oniga, I. Solidago graminifolia L. Salisb. (Asteraceae) as a Valuable Source of Bioactive Polyphenols: HPLC Profile, In Vitro Antioxidant and Antimicrobial Potential. Molecules 2019, 24, 2666. [Google Scholar] [CrossRef] [PubMed]
- Vlase, A.-M.; Toiu, A.; Tomuță, I.; Vlase, L.; Muntean, D.; Casian, T.; Fizeșan, I.; Nadăș, G.C.; Novac, C.Ș.; Tămaș, M.; et al. Epilobium Species: From Optimization of the Extraction Process to Evaluation of Biological Properties. Antioxidants 2023, 12, 91. [Google Scholar] [CrossRef]
- Hsieh, C.W.; Ko, W.C.; Ho, W.J.; Chang, C.K.; Chen, G.J.; Tsai, J.C. Antioxidant and hepatoprotective effects of Ajuga nipponensis extract by ultrasonic-assisted extraction. Asian Pac. J. Trop. Med. 2016, 9, 420–425. [Google Scholar] [CrossRef]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.E.; Vodnar, D.C.; Gheldiu, A.-M.; Moldovan, C.; Oniga, I. Phytochemical composition, antioxidant, antimicrobial and in vivo anti-inflammatory activity of traditionally used Romanian Ajuga laxmannii (Murray) Benth. (‘nobleman’s beard’ - barba împăratului). Front. Pharmacol. 2018, 9, 7. [Google Scholar] [CrossRef]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.E.; Vodnar, D.C.; Gheldiu, A.-M.; Moldovan, C.; Oniga, I. Comparative Phytochemical Profile, Antioxidant, Antimicrobial and In Vivo Anti-Inflammatory Activity of Different Extracts of Traditionally Used Romanian Ajuga genevensis L. and A. reptans L. (Lamiaceae). Molecules 2019, 24, 1597. [Google Scholar] [CrossRef] [PubMed]
- Osório Ade, C.; Martins, J.L.S. Determinação de cumarina em extrato fluido e tintura de guaco por espectrofotometria derivada de primeira ordem. Rev. Bras. Ciênc. Farm. 2004, 40, 481–486. [Google Scholar] [CrossRef]
- Balea, S.S.; Pârvu, A.E.; Pop, N.; Marín, F.Z.; Pârvu, M. Polyphenolic compounds, antioxidant, and cardioprotective effects of pomace extracts from Fetească neagră cultivar. Oxid. Med. Cell Longev. 2018, 2018, 8194721. [Google Scholar] [CrossRef]
- Sarac, F.; Yeniocak, S.; Erbin, A.; Yucetas, E.; Altundal, K.; Ucpinar, B.; Saygili, A.; Koldas, M. Ischemia Modified Albumin and D-dimer in the Diagnosis of Testicular Torsion: An Experimental Model. Urol. J. 2019, 16, 567–571. [Google Scholar] [PubMed]
- Ziaee, M.; Khorrami, A.; Ebrahimi, M.; Nourafcan, H.; Amiraslanzadeh, M.; Rameshrad, M.; Garjani, M.; Garjani, A. Cardioprotective Effects of Essential Oil of Lavandula angustifolia on Isoproterenol-induced Acute Myocardial Infarction in Rat. Iran J. Pharm. Res. 2015, 14, 279–289. [Google Scholar] [PubMed]
- Konopelski, P.; Ufnal, M. Electrocardiography in rats: A comparison to human. Physiol. Res. 2016, 65, 717–725. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Harma, M.; Erel, O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med. Wkly. 2003, 133, 563–566. [Google Scholar] [CrossRef]
- Muti, L.A.; Pârvu, A.E.; Crăciun, A.M.; Miron, N.; Acalovschi, M. Nitro-oxidative stress, VEGF and MMP-9 in patients with cirrhotic and non-cirrhotic portal hypertension. Clujul Med. 2015, 88, 140–145. [Google Scholar] [CrossRef]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar] [CrossRef]
- Hu, M.L. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994, 233, 380–385. [Google Scholar] [PubMed]
- Ghasemi, A.; Hedayati, M.; Biabani, H. Protein precipitation methods evaluated for determination of serum nitric oxide end products by the Griess Assay. JMSR 2007, 2, 29–32. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi- and Megavariate Data Analysis: Basic Principles and Applications, 3rd ed.; MKS Umetrics AB: Malmo, Sweden, 2013; Volume 1. [Google Scholar]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-Based Metabolomics Data for Identification of Biochemically Interesting Compounds Using OPLS Class Models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef]
- Ayadi, M.; Bennani, M.L.; Aarab, A.; Brigui, J.; Benicha, M. Content of polyphenolic compounds in Melilotus officinalis ecotypes from Morocco. Options Méditerranéennes 2021, 125, 559–563. [Google Scholar]
- Szymański, M.; Szajkowska, D.; Szymański, A. Analiza fitochemiczna gatunków Melilotus officinalis i Melilotus alba. Postępy Fitoter. 2020, 21, 207–213. [Google Scholar] [CrossRef]
- Merghem, M.; Hani, M.; Dahamna, S. Evaluation of Antioxidant Activity and Polyphenols Contents of Melilotus indicus Extracts. Indian J. Nat. Sci. 2021, 12, 125–130. [Google Scholar]
- Borhani, G.; Mazandarani, M.; Abbaspour, H. Antioxidant, Antibacterial Activity, Ethnopharmacology, Phytochemical in Different Extracts of Melilotus officinalis L. as an Anti-infection and Anti-diabetic in Traditional Uses of Two Northern Provinces From Iran. Crescent J. Med. Biol. Sci. 2024, 11, 83–91. [Google Scholar] [CrossRef]
- Nechita, M.-A.; Toiu, A.; Benedec, D.; Hanganu, D.; Ielciu, I.; Oniga, O.; Nechita, V.-I.; Oniga, I. Agastache species: A comprehensive review on phytochemical composition and therapeutic properties. Plants 2023, 12, 2937. [Google Scholar] [CrossRef]
- Toiu, A.; Vlase, L.; Gheldiu, A.-M.; Vodnar, D.; Oniga, I. Evaluation of the Antioxidant and Antibacterial Potential of Bioactive Compounds from Ajuga Reptans Extracts. Farmacia 2017, 65, 351–355. [Google Scholar]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K.; et al. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Magiera, A.; Kołodziejczyk-Czepas, J.; Olszewska, M.A. Antioxidant and Anti-Inflammatory Effects of Vanillic Acid in Human Plasma, Human Neutrophils, and Non-Cellular Models In Vitro. Molecules 2025, 30, 467. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef]
- Masella, R.; Santangelo, C.; D’Archivio, M.; LiVolti, G.; Giovannini, C.; Galvano, F. Protocatechuic Acid and Human Disease Prevention: Biological Activities and Molecular Mechanisms. Curr. Med. Chem. 2012, 19, 2901–2917. [Google Scholar] [CrossRef]
- Sowa-Borowiec, P.; Czernicka, M.; Jarecki, W.; Dżugan, M. Sweet Clover (Melilotus spp.) as a Source of Biologically Active Compounds. Molecules 2025, 30, 526. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Chemical Constituents and Pharmacological Effects of Melilotus Officinalis—A Review. IOSR J. Pharm. 2020, 10, 26–36. [Google Scholar]
- Hamad, I.; Arda, N.; Pekmez, M.; Karaer, S.; Temizkan, G. Intracellular scavenging activity of Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) in the fission yeast, Schizosaccharomyces pombe. J. Nat. Sci. Biol. Med. 2010, 1, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Bartel, I.; Mandryk, I.; Horbańczuk, J.O.; Wierzbicka, A.; Koszarska, M. Nutraceutical Properties of Syringic Acid in Civilization Diseases—Review. Nutrients 2023, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.; Mateen, S.; Naeem, S.S.; Akhtar, K.; Rizvi, W.; Moin, S. Syringic acid protects from isoproterenol induced cardiotoxicity in rats. Eur. J. Pharmacol. 2019, 849, 135–145. [Google Scholar] [CrossRef]
| Sample | Plant Species | Voucher Specimen | Harvesting Place (GPS Coordinates) |
|---|---|---|---|
| MO1 | Melilotus officinalis | MO-5 | Cluj County (Lat. 46°83′64.37″ N, Long. 23°63′19.88′′ E) |
| MO2 | Melilotus officinalis | MO-8 | Brasov County (Lat. 45°39′48.57″ N, Long. 25°30′21.13″ E) |
| MA1 | Melilotus albus | MA-4 | Cluj County (Lat. 46°48′22.88″ N, Long. 23°35′17.77″ E) |
| MA2 | Melilotus albus | MA-7 | Alba County (Lat. 46°18′22.51″ N, Long. 23°07′51.55″ E) |
| Extract | TPC (mg GAE/g de) | TFC (mg RE/g de) | TCC (mg CE/g de) |
|---|---|---|---|
| MO1 | 86.516 ± 0.98 | 34.752 ± 0.57 | 18.473 ± 0.63 |
| MO2 | 138.157 ± 3.91 | 78.201 ± 1.63 | 47.691 ± 0.95 |
| MA1 | 121.995 ± 3.05 | 77.278 ± 2.02 | 26.877 ± 0.55 |
| MA2 | 127.279 ± 3.48 | 86.105 ± 1.66 | 37.331 ± 0.58 |
| [M − H]− m/z | Polyphenolic Compound | LOQ μg/mL | Main Daughter Ions | RT a,b,c ± SD (min) | Polyphenol Content MO2 | Polyphenol Content MA2 |
|---|---|---|---|---|---|---|
| 311 | Caftaric acid b | 0.2 | 148.6, 178.6 | 3.34 b ± 0.05 | <LOQ | nd |
| 353 | Chlorogenic acid b | 0.2 | 178.7, 190.7 | 5.6 b ± 0.05 | 4.33 ± 0.25 | nd |
| 163 | p-Coumaric acid b | 0.2 | 118.7 | 9.18 b ± 0.08 | <LOQ | <LOQ |
| 193 | Ferulic acid b | 0.2 | 133.7, 148.7, 177.6 | 12.8 b ± 0.10 | 1.28 ± 0.04 | 0.69 ± 0.02 |
| 169 | Gallic acid c | 0.2 | 169 * | 1.5 c ± 0.09 | 12.06 ± 0.37 | nd |
| 153 | Protocatechuic acid c | 0.2 | 153 * | 2.8 c ± 0.15 | 40.64 ± 3.51 | 8.01 ± 0.85 |
| 167 | Vanillic acid c | 0.2 | 167 * | 6.7 c ± 0.17 | 57.37 ± 2.36 | 23.48 ± 1.91 |
| 197 | Syringic acid c | 0.2 | 197 * | 8.4 c ± 0.11 | 11.55 ± 1.74 | 2.6 ± 0.05 |
| 289 | Catechin c | 0.2 | 289 * | 6.00 c ± 0.03 | 2.02 ± 0.05 | 1.84 ± 0.06 |
| 359 | Rosmarinic acid a | 0.2 | 160.6, 178.6, 196.7 | 2.2 a ± 0.18 | 1.51 ± 0.09 | <LOQ |
| Extract | DPPH Method IC50 (μg TE /mL) | FRAP Method μM TE/100 mL |
|---|---|---|
| MO1 | 118.036 ± 2.84 | 193.55 ± 3.24 |
| MO2 | 92.192 ± 2.63 | 279.35 ± 4.96 |
| MA1 | 73.256 ± 2.04 | 489.89 ± 8.71 |
| MA2 | 52.193 ± 1.45 | 702.92 ± 9.65 |
| Group |
TAC
(mM TE/L) |
TOS
(μM H2O2 E/L) | OSI |
NOx (μM/L) |
MDA
(μM/L) |
SH
(μM/L) |
|---|---|---|---|---|---|---|
| CONTROL | 1.088 ± 0.001 | 18.008 ** ± 2.182 | 16.538 ** ± 1.996 | 27.564 *** ± 2.234 | 2.530 *** ± 0.334 | 444.400 *** ± 53.785 |
| ISO | 1.088 ± 0.001 | 32.456 ± 3.975 | 29.086 ± 4.559 | 41.820 ± 2.147 | 3.902 ± 0.417 | 377.200 ± 58.178 |
| MO2/ISO | 1.090 # ± 0.002 | 33.084 ± 6.415 | 31.247 ± 6.412 | 33.347 # ± 5.277 | 3.243 ### ± 0.042 | 561.000 ### ± 106.151 |
| MA2/ISO | 1.090 # ± 0.002 | 24.746 # ± 3.853 | 21.857 #± 4.158 | 31.407 ## ± 0.730 | 3.447 ### ± 0.163 | 404.750 # ± 16.029 |
| CUM/ISO | 1.091 ## ± 0.001 | 40.310 ± 1.210 | 36.944 ± 1.108 | 41.242 ± 4.833 | 3.528 ## ± 0.430 | 444.800 ### ± 83.125 |
| Group | GOT (U/L) | GPT (U/L) | CK-MB (U/L) |
|---|---|---|---|
| CONTROL | 34.400 *** ± 4.506 | 37.200 *** ± 4.266 | 8.000 *** ± 1.732 |
| ISO | 84.800 ± 32.568 | 87.600 ± 28.962 | 17.600 ± 3.209 |
| MO2/ISO | 76.667 ± 1.528 | 78.667 ± 11.060 | 7.333 ### ± 3.055 |
| MA2/ISO | 55.000 ## ± 29.597 | 60.667 ### ± 29.771 | 8.667 ### ± 0.577 |
| CUM/ISO | 77.800 ± 12.518 | 85.800 ± 17.584 | 6.800 ### ± 1.304 |
| Group | RR (ms) | HR (bpm) | PR (ms) | QRS (ms) | QT (ms) | QTc (ms) | ST (mV) |
|---|---|---|---|---|---|---|---|
| CONTROL | 0.231 *** ± 0.019 | 261.600 *** ± 21.338 | 0.049 ± 0.006 | 0.037 ± 0.006 | 0.066 *** ± 0.003 | 0.137 *** ± 0.007 | 0.030 ± 0.007 |
| ISO | 0.144 ± 0.016 | 420.200 ± 45.008 | 0.045 ± 0.006 | 0.039 ± 0.006 | 0.103 ± 0.006 | 0.273 ± 0.013 | 0.040 ± 0.008 |
| MO2/ISO | 0.192 ## ± 0.061 | 337.000 ### ± 96.374 | 0.043 ± 0.005 | 0.128 ± 0.197 | 0.093 # ± 0.014 | 0.221 ± 0.056 | 0.031 ± 0.008 |
| MA2/ISO | 0.157 # ± 0.028 | 393.250 # ± 71.672 | 0.041 ± 0.009 | 0.041 ± 0.005 | 0.101 ± 0.011 | 0.256 ± 0.015 | 0.027 ± 0.009 |
| CUM/ISO | 0.266 ### ± 0.017 | 226.600 ### ± 14.415 | 0.049 ± 0.004 | 0.035 ± 0.002 | 0.068 ### ± 0.005 | 0.133 ### ± 0.013 | 0.033 ± 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toiu, A.; Vlase, A.-M.; Vlase, L.; Casian, T.; Pârvu, A.E.; Oniga, I.
In Vivo Investigation of Cardioprotective Effects of
Toiu A, Vlase A-M, Vlase L, Casian T, Pârvu AE, Oniga I.
In Vivo Investigation of Cardioprotective Effects of
Toiu, Anca, Ana-Maria Vlase, Laurian Vlase, Tibor Casian, Alina Elena Pârvu, and Ilioara Oniga.
2025. "In Vivo Investigation of Cardioprotective Effects of
Toiu, A., Vlase, A.-M., Vlase, L., Casian, T., Pârvu, A. E., & Oniga, I.
(2025). In Vivo Investigation of Cardioprotective Effects of











