Patterns and Determinants of Ecological Uniqueness in Plant Communities on the Qinghai-Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas and Plant Community Survey
2.2. Environmental Variables
2.3. Calculation of Beta Diversity and the Ecological Uniqueness of Plant Communities
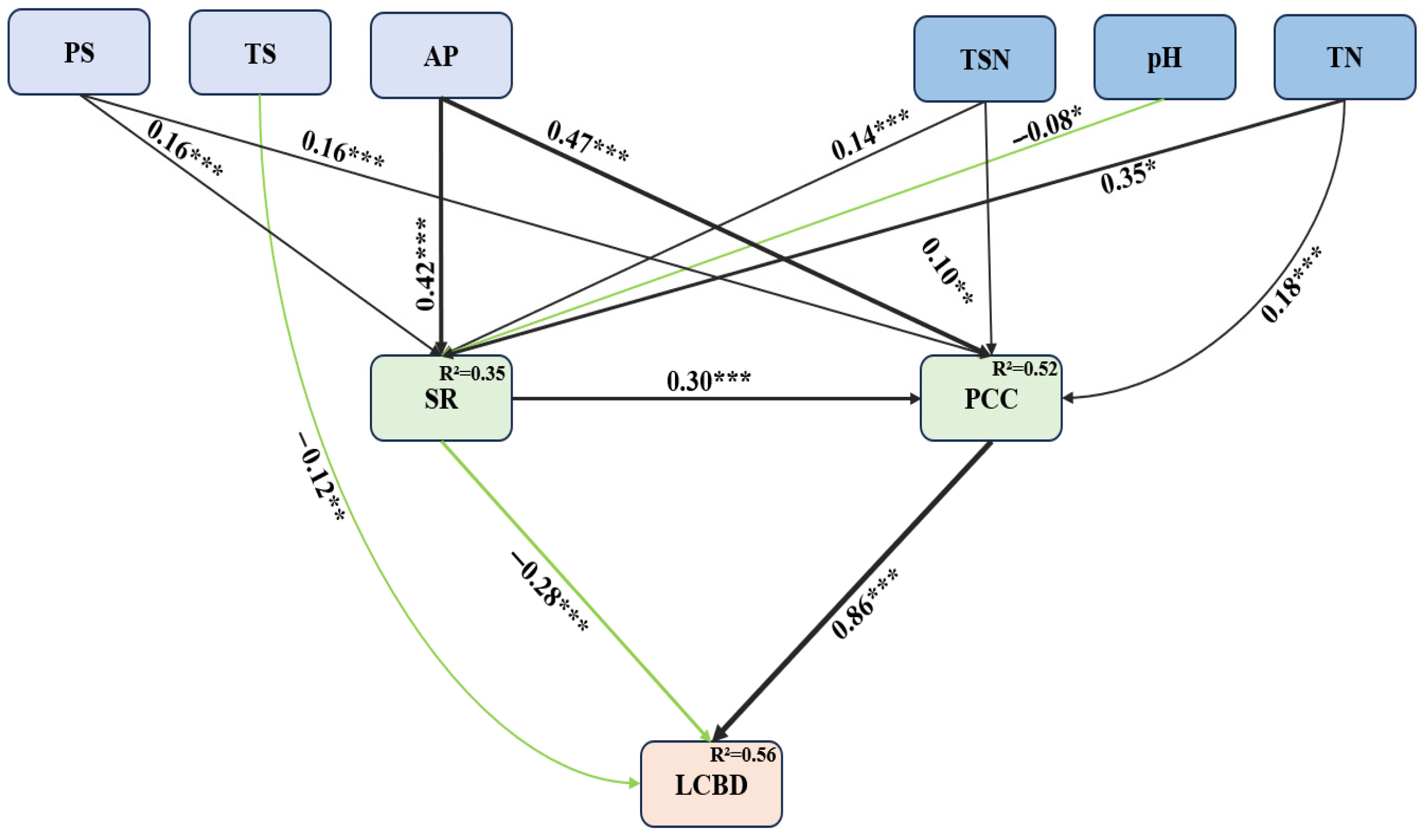
2.4. Statistical Analysis
3. Results
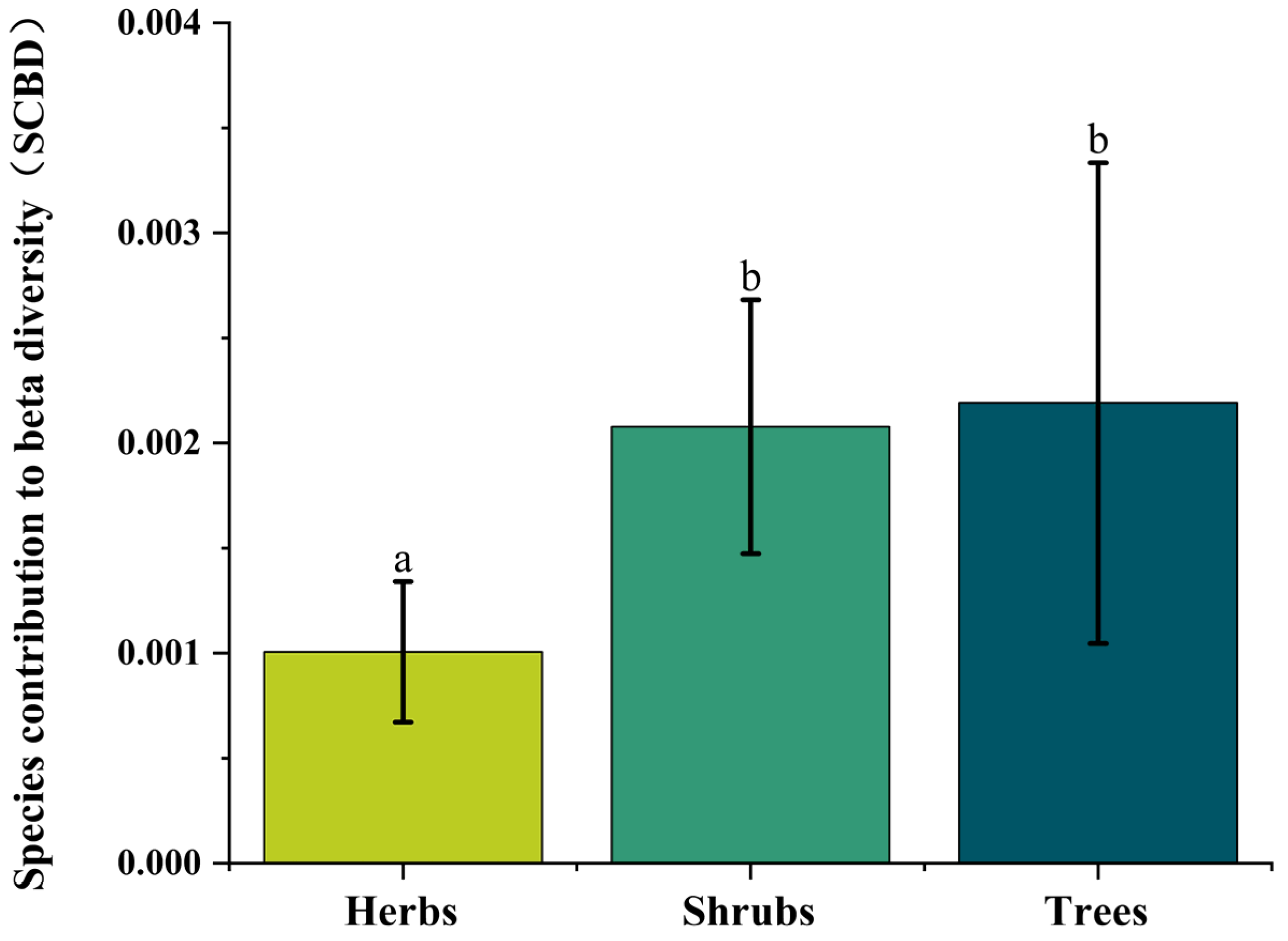
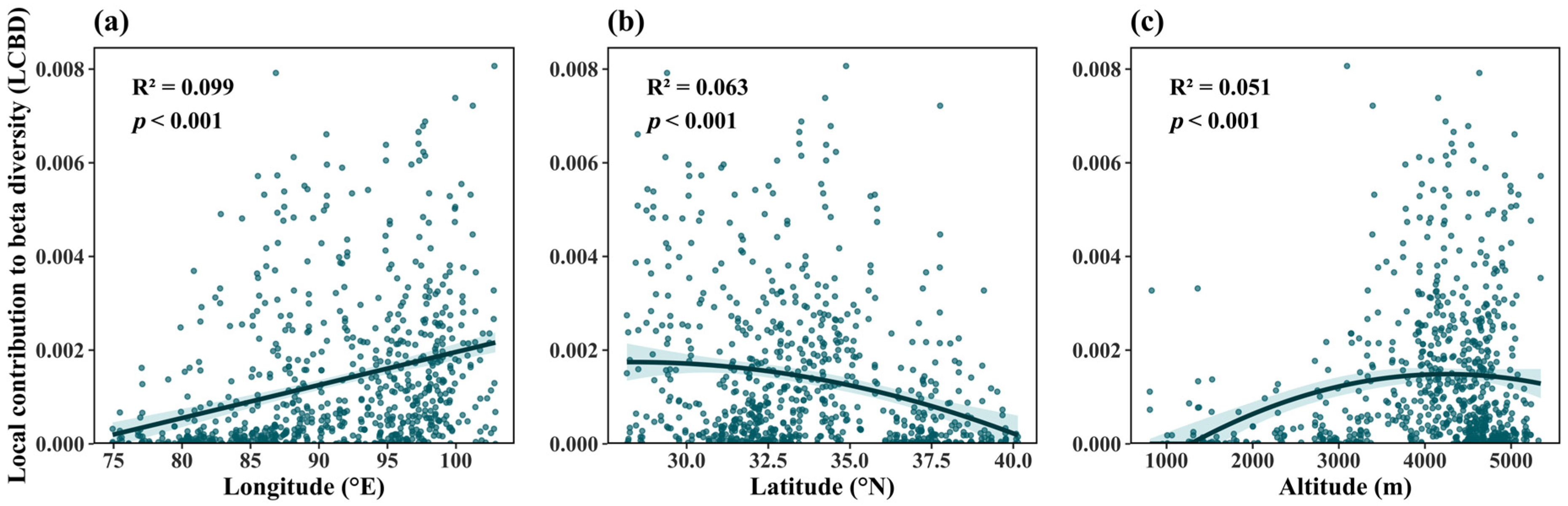
3.1. Geographic Patterns of Plant Community Ecological Uniqueness
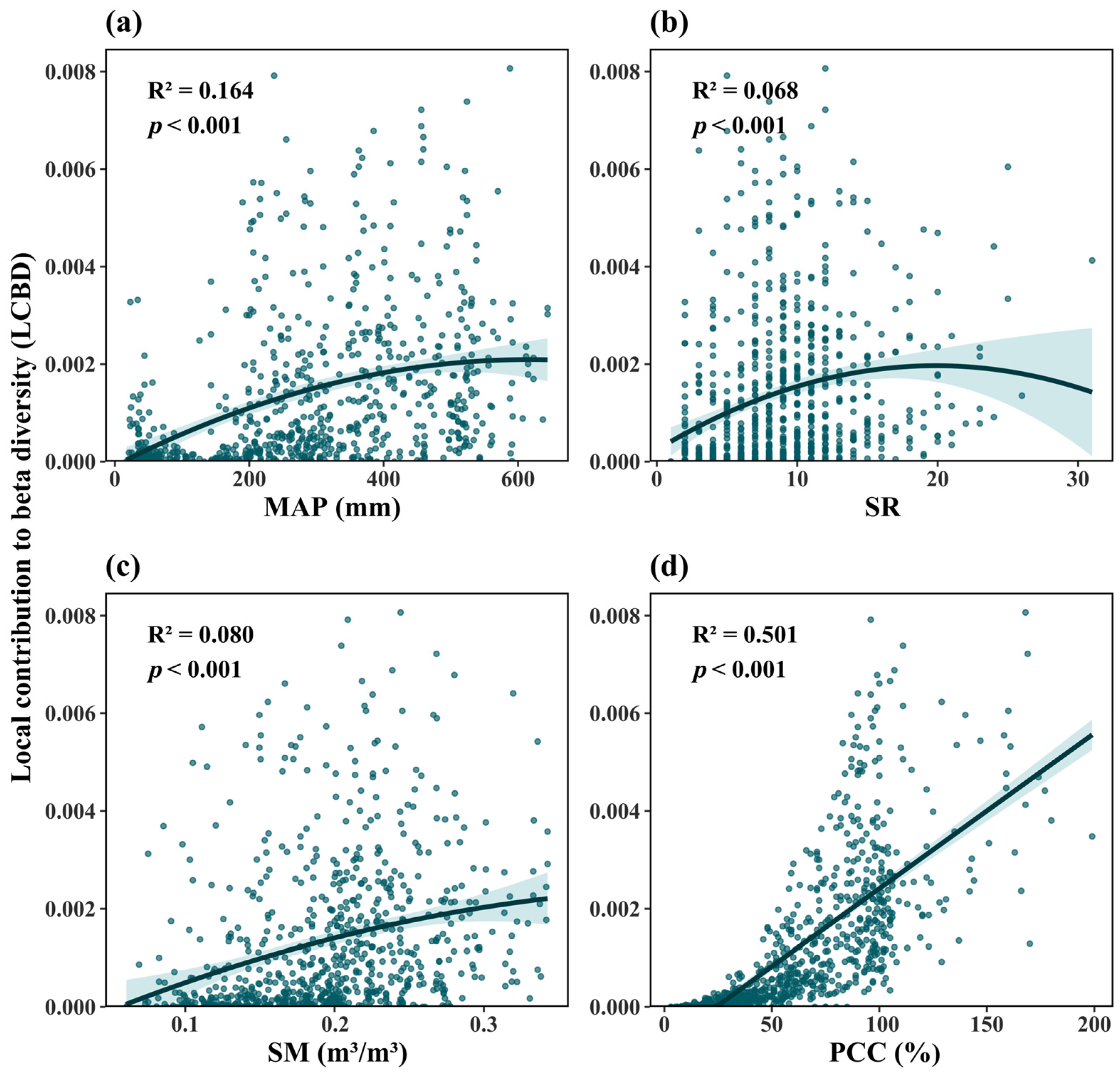
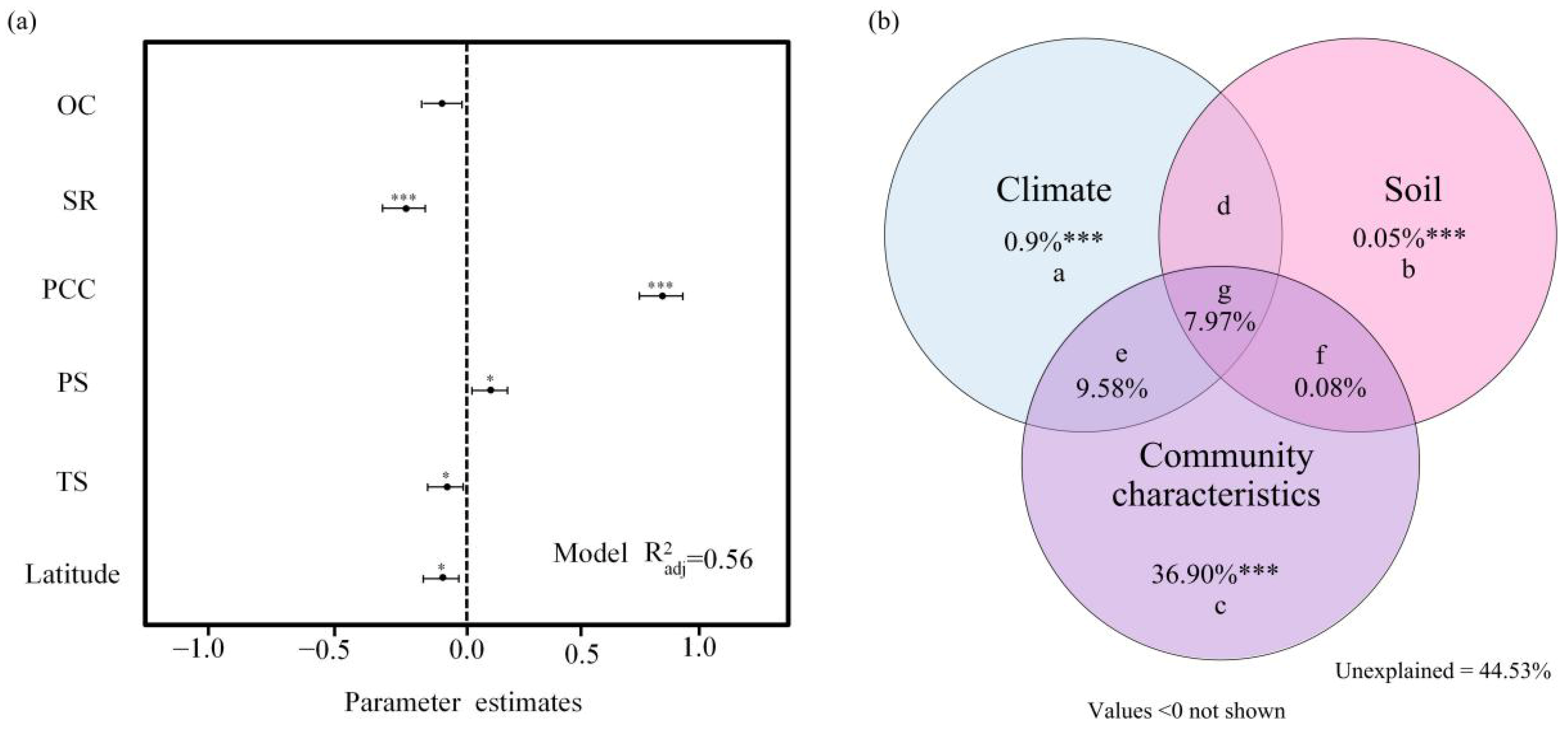
3.2. Relationships Between Ecological Uniqueness of Plant Communities and Influencing Factors
4. Discussion
4.1. Spatial Patterns of Ecological Uniqueness in Plant Communities
4.2. Drivers of Ecological Uniqueness Patterns in Plant Communities
4.3. Non-Linear Relationship Between Ecological Uniqueness and Species Richness
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hutchinson, G. The paradox of plankton. Am. Nat. 1961, 95, 882. [Google Scholar] [CrossRef]
- Ricklefs, R.E. Environmental heterogeneity and plant species diversity: A hypothesis. Am. Nat. 1977, 111, 376–381. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Chave, J. Neutral theory and community ecology. Ecol. Lett. 2004, 7, 241–253. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 407. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Gaston, K.J. The distribution of bird species in the New World: Patterns in species turnover. Oikos 1996, 77, 146–152. [Google Scholar] [CrossRef]
- Moritz, C.; Richardson, K.S.; Ferrier, S.; Monteith, G.B.; Stanisic, J.; Williams, S.E.; Whiffin, T. Biogeographical concordance and efficiency of taxon indicators for establishing conservation priority in a tropical rainforest biota. Proc. R. Soc. Lond. B. 2001, 268, 1875–1881. [Google Scholar] [CrossRef]
- Lennon, J.J.; Koleff, P.; Gaston, G.K.J. The geographical structure of British bird distributions: Diversity, spatial turnover and scale. J. Anim. Ecol. 2001, 70, 966–979. [Google Scholar] [CrossRef]
- Graham, C.H.; Moritz, C.; Williams, S.E. Habitat history improves prediction of biodiversity in rainforest fauna. Proc. Natl. Acad. Sci. USA 2006, 103, 632–636. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, Y.; Zhang, H.; Xu, C.; Zhang, K.; Cadotte, M.W.; Cheng, L. The proportion of low abundance species is a key predictor of plant β-diversity across the latitudinal gradient. J. Ecol. 2025, 113, 795–805. [Google Scholar] [CrossRef]
- He, S.; Qin, C.; Janne Soininen, A. A flexible framework to assess patterns and drivers of beta diversity across spatial scales. Ecography 2024, 2024, e06901. [Google Scholar] [CrossRef]
- Van Rensburg, B.J.; Koleff, P.; Gaston, K.J.; Chown, S.L. Spatial congruence of ecological transition at the regional scale in South Africa. J. Biogeogr. 2004, 31, 843–854. [Google Scholar] [CrossRef]
- Guo, Y.; Xiang, W.; Wang, B.; Li, D.; Mallik, A.U.; Chen, H.Y.; Huang, F.; Ding, T.; Wen, S.; Li, X.; et al. Partitioning beta diversity in a tropical karst seasonal rainforest in Southern China. Sci. Rep. 2018, 8, 17408. [Google Scholar] [CrossRef]
- Hu, G.; Pang, Q.; Hu, C.; Xu, C.; Zhang, Z.; Zhong, C. Beta Diversity Patterns and Determinants among Vertical Layers of Tropical Seasonal Rainforest in Karst Peak-Cluster Depressions. Forests 2024, 15, 365. [Google Scholar] [CrossRef]
- Li, W. A Study of the Diversity Patterns of Desert Vegetation Communities in an Arid Zone of China. Plants 2024, 13, 2783. [Google Scholar] [CrossRef]
- Al-Mutairi, K.A. Effect of environmental conditions on the taxonomic diversity of plant species in the arid region of Tabuk. Arid. Ecosyst. 2017, 7, 271–276. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M.; Morlon, H. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Sor, R.; Legendre, P.; Lek, S. Uniqueness of sampling site contributions to the total variance of macroinvertebrate communities in the Lower Mekong Basin. Ecol. Indic. 2018, 84, 425–432. [Google Scholar] [CrossRef]
- Vilmi, A.; Karjalainen, S.M.; Heino, J. Ecological uniqueness of stream and lake diatom communities shows different macroecological patterns. Divers. Distrib. 2017, 23, 1042–1053. [Google Scholar] [CrossRef]
- Lopes, P.M.; Bini, L.M.; Declerck, S.A.J.; Farjalla, V.F.; Bozelli, R.L. Correlates of zooplankton beta diversity in tropical lake systems. PLoS ONE 2014, 9, e109581. [Google Scholar] [CrossRef] [PubMed]
- Tonkin, J.D.; Heino, J.; Sundermann, A.; Haase, P.; Jähnig, S.C. Context dependency in biodiversity patterns of central German stream metacommunities. Freshw. Biol. 2016, 61, 607–620. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Siqueira, T.; Bini, L.M.; Cianciaruso, M.V.; Oliveira, F. The role of niche measures in explaining the abundance–distribution relationship in tropical lotic chironomids. Hydrobiologia 2009, 636, 163–172. [Google Scholar] [CrossRef]
- Yao, J.; Huang, J.; Ding, Y.; Xu, Y.; Xu, H.; Zang, R. Ecological uniqueness of species assemblages and their determinants in forest communities. Divers. Distrib. 2021, 27, 454–462. [Google Scholar] [CrossRef]
- da Silva, P.G.; Hernández, M.I.M.; Heino, J. Disentangling the correlates of species and site contributions to beta diversity in dung beetle assemblages. Divers. Distrib. 2018, 24, 1674–1686. [Google Scholar] [CrossRef]
- Heino, J.; Grönroos, M. Exploring species and site contributions to beta diversity in stream insect assemblages. Oecologia 2017, 183, 151–160. [Google Scholar] [CrossRef]
- Heino, J.; Bini, L.M.; Andersson, J.; Bergsten, J.; Bjelke, U.; Johansson, F. Unravelling the correlates of species richness and ecological uniqueness in a metacommunity of urban pond insects. Ecol. Indic. 2017, 73, 422–431. [Google Scholar] [CrossRef]
- Kong, H.; Chevalier, M.; Laffaille, P.; Lek, S. Spatio-temporal variation of fish taxonomic composition in a South-East Asian flood-pulse system. PLoS ONE 2017, 12, e0174582. [Google Scholar] [CrossRef]
- Vad, C.F.; Péntek, A.L.; Cozma, N.J.; Földi, A.; Tóth, A.; Tóth, B.; Böde, N.A.; Móra, A.; Ptacnik, R.; Ács, É.; et al. Wartime scars or reservoirs of biodiversity? The value of bomb crater ponds in aquatic conservation. Biol. Conserv. 2017, 209, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Gauthier, O. Statistical methods for temporal and space–time analysis of community composition data. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132728. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Li, Q.; Jiang, Q.; Lu, J.; Franklin, S.; Tang, Z.; Wang, Q.; Zhang, J.; Lu, Z.; Bao, D. Beta diversity determinants in Badagongshan, a subtropical forest in central China. Sci. Rep. 2015, 5, 17043. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, C.; De Cáceres, M.; Legendre, P.; Zhao, X.; Roxburgh, S. Variation in compositional and structural components of community assemblage and its determinants. J. Veg. Sci. 2019, 30, 257–268. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Fonseca, G.A.B.D.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Deng, T.; Wu, F.; Zhou, Z.; Su, T. Tibetan Plateau: An evolutionary junction for the history of modern biodiversity. Sci. China Earth Sci. 2020, 63, 172–187. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Change Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Yue, J.P.; Sun, W.G.; Xu, B.; Li, Z.M.; Comes, H.P.; Sun, H. Evolutionary history of the subnival flora of the Himalaya-Hengduan Mountains: First insights from comparative phylogeography of four perennial herbs. J. Biogeogr. 2016, 43, 31–43. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, H.; Wei, L.; Hou, Y.; Hu, J.; Wu, K.; Xia, H.; Xia, J.; Zhou, B.; Li, K.; et al. A plot-based dataset of plant community on the Qingzang Plateau. Chin. J. Plant Ecol. 2022, 46, 846–854. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Kreft, H.; Jetz, W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 5925–5930. [Google Scholar] [CrossRef]
- Willig, M.R.; Kaufman, D.M.; Stevens, R.D. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Pohe, S.R.; Winterbourn, M.J.; Harding, J.S. Species Richness and Similarity of New Zealand Mayfly Communities (Ephemeroptera) Decline with Increasing Latitude and Altitude. Insects 2024, 15, 757. [Google Scholar] [CrossRef]
- Dubois, R.; Proulx, R.; Pellerin, S. Ecological uniqueness of plant communities as a conservation criterion in lake-edge wetlands. Biol. Conserv. 2020, 243, 108491. [Google Scholar] [CrossRef]
- Dansereau, G.; Legendre, P.; Poisot, T. Evaluating ecological uniqueness over broad spatial extents using species distribution modelling. Oikos 2022, 2022, e09063. [Google Scholar] [CrossRef]
- Fuller, B.; Wood, K.; Rapoport, T.; Dornbusch, S.M. The organizational context of individual efficacy. Rev. Educ. Res. 1982, 52, 7–30. [Google Scholar] [CrossRef]
- Koleff, P.; Lennon, J.J.; Gaston, K.J. Are there latitudinal gradients in species turnover? Glob. Ecol. Biogeogr. 2003, 12, 483–498. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, R.; Dong, W. Interannual and Interdecadal Variations of Air Temperature and Precipitation over the Tibetan Plateau. Chin. J. Atmos. Sci. 2003, 27, 157–170. [Google Scholar]
- Zhang, W.; Li, S.; Pang, Q. Changes of precipitation spatial-temporal over the Qinghai-Tibet Plateau during last 40 years. Adv. Water Sci. 2009, 20, 168–176. [Google Scholar]
- Wang, H.; Zhang, R.; Cai, Y.; Yang, Q.; Lv, G. Ecological uniqueness and the determinants in arid desert ecosystems of Northwest China. Glob. Ecol. Conserv. 2022, 34, e02005. [Google Scholar] [CrossRef]
- Wang, J.; Lei, X.; Feng, Y.; Wu, B.; Lu, Q.; He, N.; Li, J. The ecological uniqueness of plant communities and their determinants across the temperate deserts of China. Biodivers. Sci. 2023, 31, 23144. [Google Scholar] [CrossRef]
- Wang, J.M.; Dong, F.Y.; Nasina, B.H.; Li, J.W.; Li, J.Q.; Feng, Y.M.; Lu, Q. Plant distribution patterns and the factors influencing plant diversity in the Black Gobi Desert of China. Acta Ecol. Sin. 2016, 36, 3488–3498. [Google Scholar]
- Akram, M.A.; Wang, X.; Shrestha, N.; Zhang, Y.; Sun, Y.; Yao, S.; Li, J.; Hou, Q.; Hu, W.; Ran, J. Variations and driving factors of leaf functional traits in the dominant desert plant species along an environmental gradient in the drylands of China. Sci. Total Environ. 2023, 897, 165394. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Sun, Y.; Ran, J.; Hu, W.; Ji, M.; Du, Q.; Xiong, J.; Gong, H.; Yao, S.; Akram, M.A.; et al. Ecosystem organic carbon storage and their drivers across the drylands of China. CATENA 2022, 214, 106280. [Google Scholar] [CrossRef]
- Gough, L.; Shaver, G.R.; Carroll, J.; Royer, D.L.; Laundre, J.A. Vascular plant species richness in Alaskan arctic tundra: The importance of soil pH. J. Ecol. 2000, 88, 54–66. [Google Scholar] [CrossRef]
- Ceulemans, T.; Stevens, C.J.; Duchateau, L.; Jacquemyn, H.; Gowing, D.J.G.; Merckx, R.; Wallace, H.; Rooijen, N.V.; Goethem, T.; Bobbink, R. Soil phosphorus constrains biodiversity across European grasslands. Glob. Change Biol. 2014, 20, 3814–3822. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Y.; Li, L.L.; Hu, H.J.; Yan, Y. Factors influencing the diversity of terrestrial plant communities in China. Chin. J. Appl. Ecol. 2025, 36, 113–120. [Google Scholar]
- Palpurina, S.; Wagner, V.; von Wehrden, H.; Hájek, M.; Horsák, M.; Brinkert, A.; Hölzel, N.; Wesche, K.; Kamp, J.; Hájková, P. The relationship between plant species richness and soil pH vanishes with increasing aridity across Eurasian dry grasslands. Glob. Ecol. Biogeogr. 2017, 26, 425–434. [Google Scholar] [CrossRef]
- Sternberg, M.; Brown, V.K.; Masters, G.J.; Clarke, I.P. Plant community dynamics in a calcareous grassland under climate change manipulations. Plant Ecol. 1999, 143, 29–37. [Google Scholar] [CrossRef]
- Wu, J.S.; Li, S.J.; Shen, Z.X.; Zhang, X.Z.; Shi, P.L.; Yu, C.Q.; Wang, J.S.; Zhou, Y.T. Species diversity distribution pattern of alpine grasslands communities along a precipitation gradient across Northern Tibetan Plateau. Acta Prataculturae Sin. 2012, 21, 17–25. [Google Scholar]
- Hernandez-Garcia, E.; Lopez, C.; Pigolotti, S.; Andersen, K.H. Species competition: Coexistence, exclusion and clustering. Philos. Trans. R. Soc. A 2008, 367, 3183–3195. [Google Scholar] [CrossRef]
- Genxu, W.; Yuanshou, L.I.; Qingbai, W.U.; Wang, Y. Impacts of permafrost changes on alpine ecosystem in Qinghai-Tibet Plateau. Sci. China Ser. D Earth Sci. 2006, 49, 1156–1169. [Google Scholar] [CrossRef]
- Yi, S.; Zhou, Z.; Ren, S.; Xu, M.; Qin, Y.; Chen, S.; Ye, B. Effects of permafrost degradation on alpine grassland in a semi-arid basin on the Qinghai–Tibetan Plateau. Environ. Res. Lett. 2011, 6, 45403. [Google Scholar] [CrossRef]
- Kang, S.; Xu, Y.; You, Q.; Flügel, W.A.; Pepin, N.; Yao, T. Review of climate and cryospheric change in the Tibetan Plateau. Environ. Res. Lett. 2010, 5, 15101. [Google Scholar] [CrossRef]
- Jin, X.; Deng, A.; Fan, Y.; Ma, K.; Zhao, Y.; Wang, Y.; Zheng, K.; Zhou, X.; Lu, G. Diversity, functionality, and stability: Shaping ecosystem multifunctionality in the successional sequences of alpine meadows and alpine steppes on the Qinghai-Tibet Plateau. Front. Plant Sci. 2025, 16, 1436439. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, X.; Wang, Q.; Wang, C.; Zhan, Z.; Chen, L.; Yan, J.; Qu, R. Vegetation net primary productivity and its response to climate change during 2001–2008 in the Tibetan Plateau. Sci. Total Environ. 2013, 444, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Lavorel, S.; de Bello, F.; Robson, T.M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef]
- Wang, S.; Guo, L.; He, B.; Lyu, Y.; Li, T. The stability of Qinghai-Tibet Plateau ecosystem to climate change. Phys. Chem. Earth Parts A/B/C 2020, 115, 102827. [Google Scholar] [CrossRef]
- Windmaißer, T.; Reisch, C. Long-term study of an alpine grassland: Local constancy in times of global change. Alp. Bot. 2013, 123, 1–6. [Google Scholar] [CrossRef]
- Latif, A.; Ilyas, S.; Zhang, Y.; Xin, Y.; Zhou, L.; Zhou, Q. Review on global change status and its impacts on the Tibetan Plateau environment. J. Plant Ecol. 2019, 12, 917–930. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Li, J.; Chen, Y. Current status and future directions of the Tibetan Plateau ecosystem research. Sci. Bull. 2019, 64, 428–430. [Google Scholar] [CrossRef]
- Gupta, A.; Srivastava, P.K.; Satish, K.V.; Chauhan, A.; Pandey, P.C. Challenges and Future Possibilities Toward Himalayan Forest Monitoring. Adv. Remote Sens. For. Monit. 2022, 329–350. [Google Scholar] [CrossRef]

| Variable | R2 | Slope | p |
|---|---|---|---|
| Species richness (SR) | 0.068 | UM | <0.001 |
| Soil moisture (SM) | 0.080 | DU | <0.001 |
| Plant community coverage (PCC) | 0.501 | 0.0006/L+ | <0.001 |
| Soil total nitrogen content (TSN) | 0.014 | UD | <0.001 |
| Soil pH (pH) | 0.015 | DU | <0.001 |
| Mean annual precipitation (MAP) | 0.164 | DU | <0.001 |
| Precipitation seasonality (PS) | 0.046 | DU | <0.001 |
| Solar radiation (SRAD) | 0.0215 | DD | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Gheyret, G. Patterns and Determinants of Ecological Uniqueness in Plant Communities on the Qinghai-Tibetan Plateau. Plants 2025, 14, 2379. https://doi.org/10.3390/plants14152379
Li L, Gheyret G. Patterns and Determinants of Ecological Uniqueness in Plant Communities on the Qinghai-Tibetan Plateau. Plants. 2025; 14(15):2379. https://doi.org/10.3390/plants14152379
Chicago/Turabian StyleLi, Liangtao, and Gheyur Gheyret. 2025. "Patterns and Determinants of Ecological Uniqueness in Plant Communities on the Qinghai-Tibetan Plateau" Plants 14, no. 15: 2379. https://doi.org/10.3390/plants14152379
APA StyleLi, L., & Gheyret, G. (2025). Patterns and Determinants of Ecological Uniqueness in Plant Communities on the Qinghai-Tibetan Plateau. Plants, 14(15), 2379. https://doi.org/10.3390/plants14152379







