Climate-Driven Vegetation Distribution and Wetland Expansion at the Edge of Jiangjiadian Grassland, Northeastern China
Abstract
1. Introduction
- (1)
- Analyzing the plant community characteristics of grassland edge areas using indices related to plant frequency, density, height, and coverage.
- (2)
- Contributing to improving the understanding the differences in plant characteristics due to spatial differentiation of climatic conditions related to climate change.
- (3)
- Revealing the climate–vegetation relationship via structural equations and correlation analysis. This study aims to offer insights into the spatial correspondence between climate and vegetation distribution, focusing on plant community dynamics, and provide a reference for future research on climate–vegetation relationships under climate change.
2. Materials and Methods
2.1. Study Area
2.2. Grassland Sampling
2.3. Extraction and Analysis of Climate Spatial Data
2.4. Data Analysis
3. Results
3.1. Plant Characteristics
3.1.1. Common Plants
3.1.2. Species Relationship of Common Plants
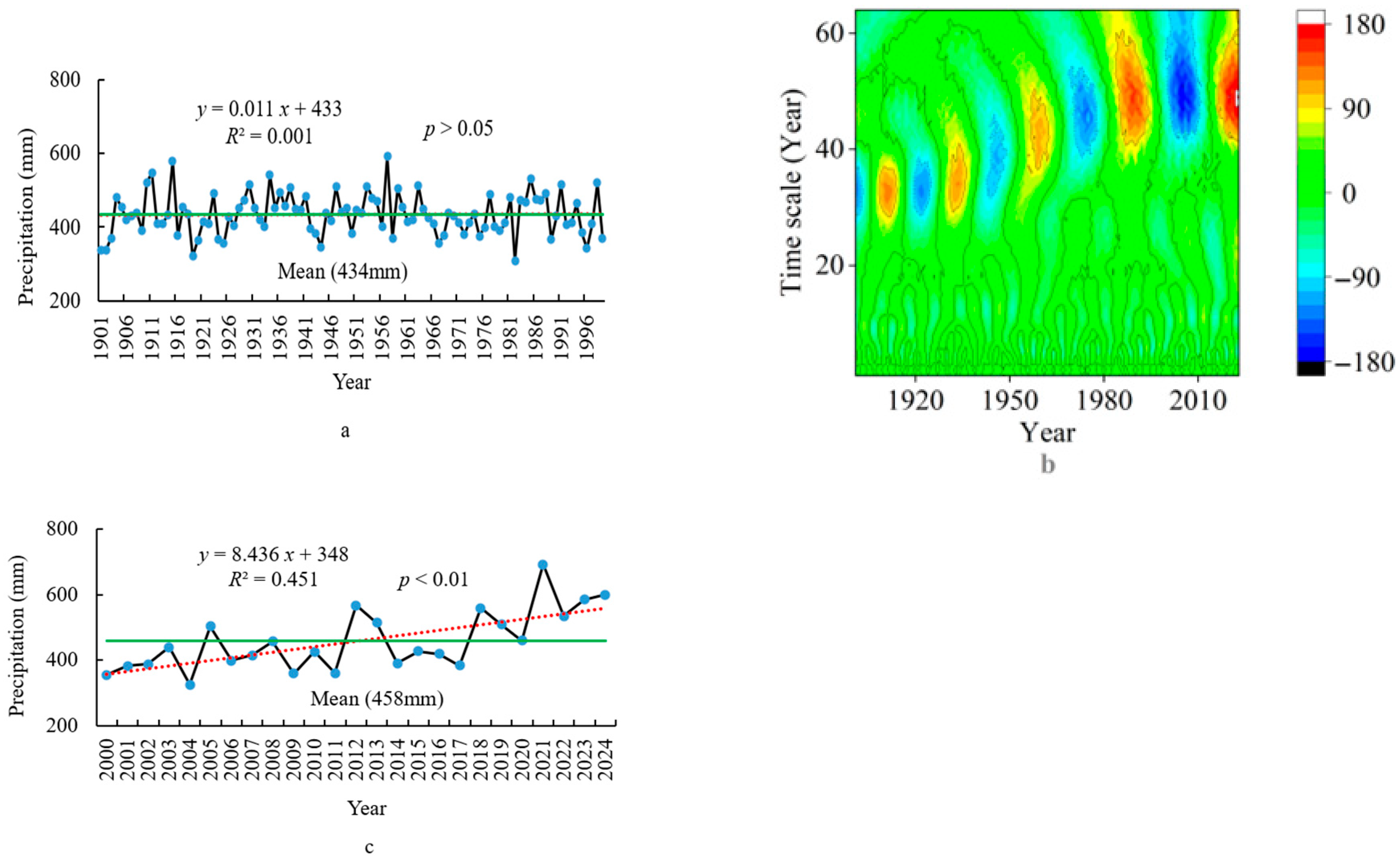
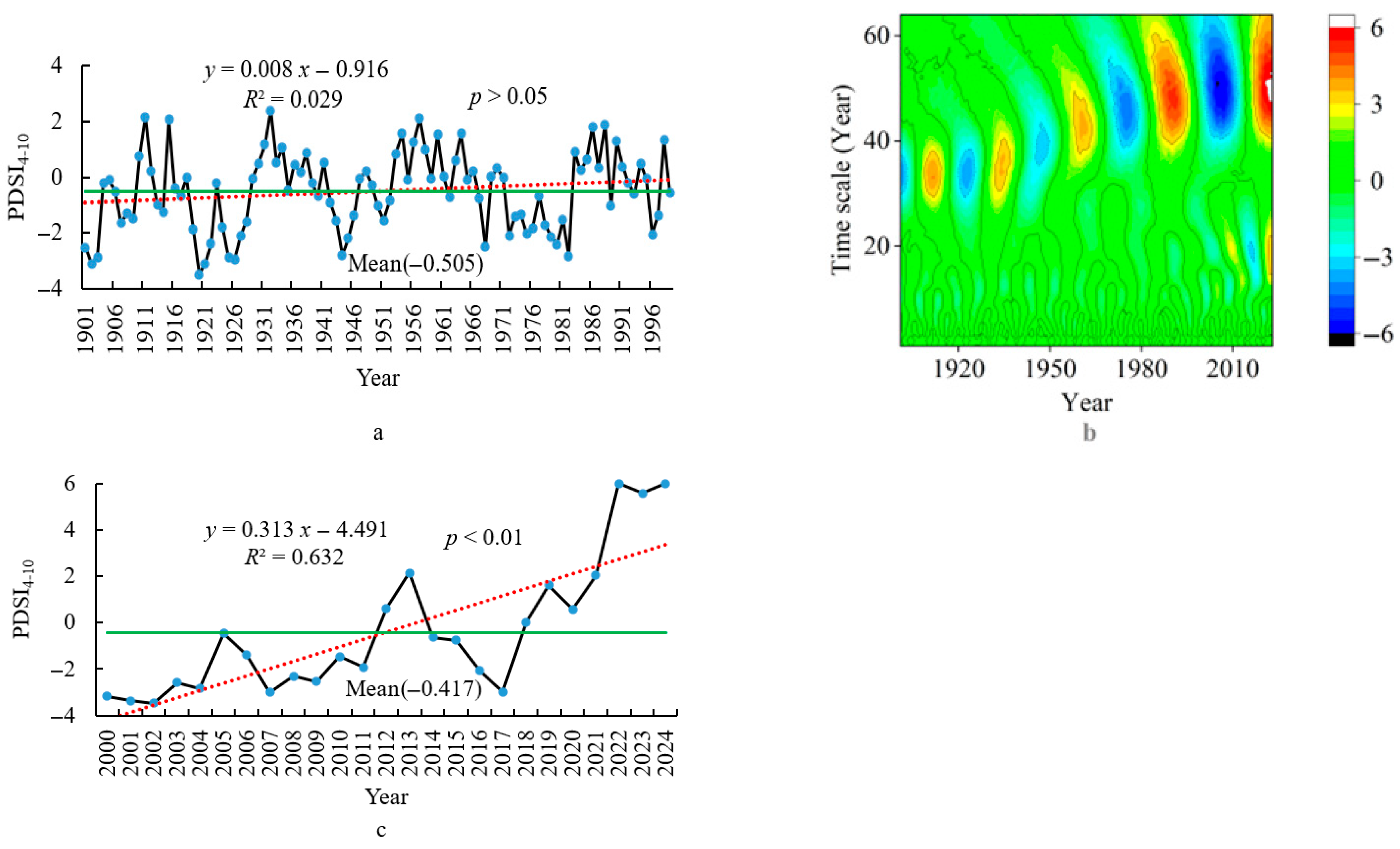
3.2. Climatic Characteristics
3.2.1. Temperature Characteristics
3.2.2. Precipitation Characteristics
3.2.3. Dry–Wet Characteristics
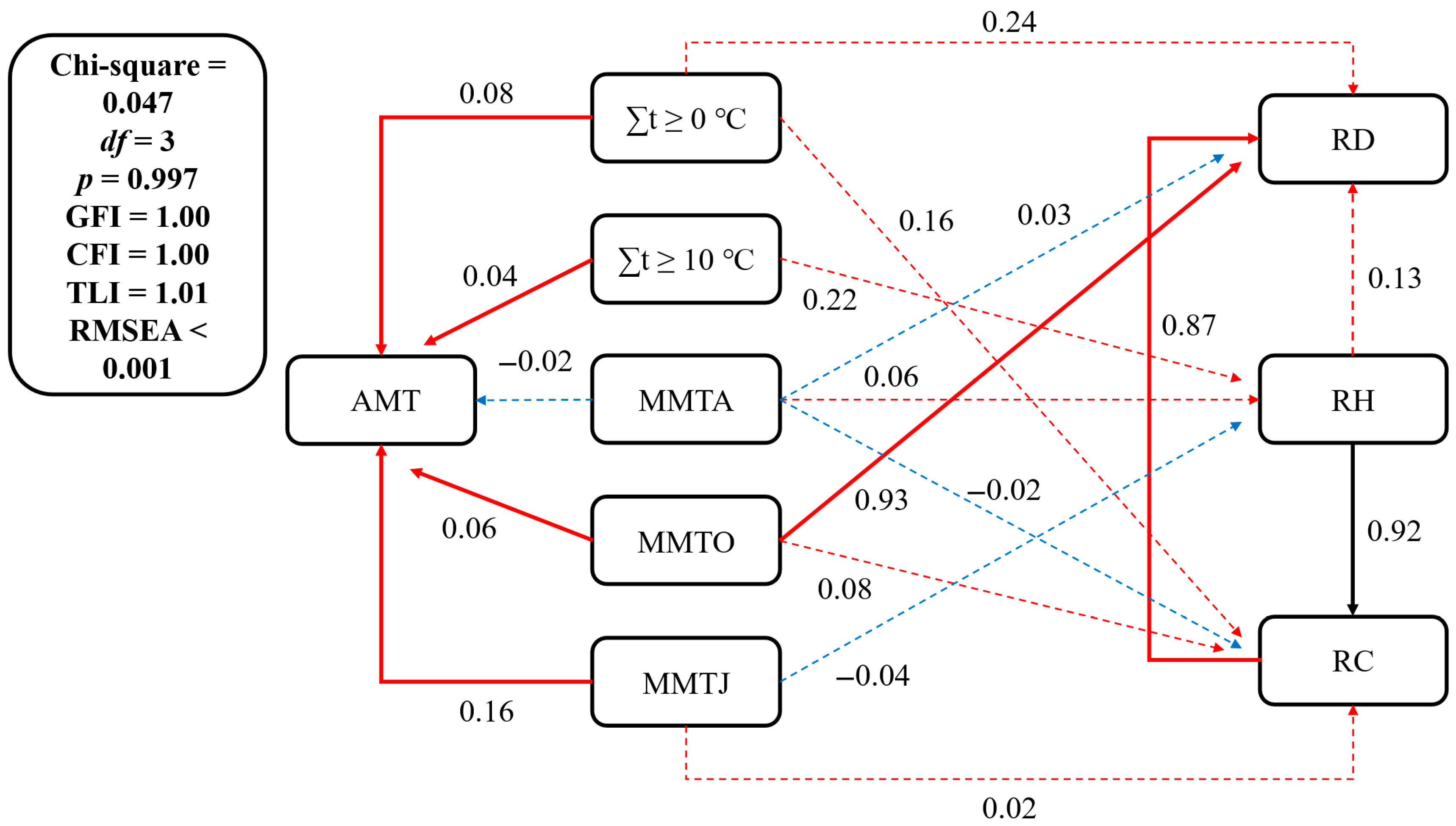
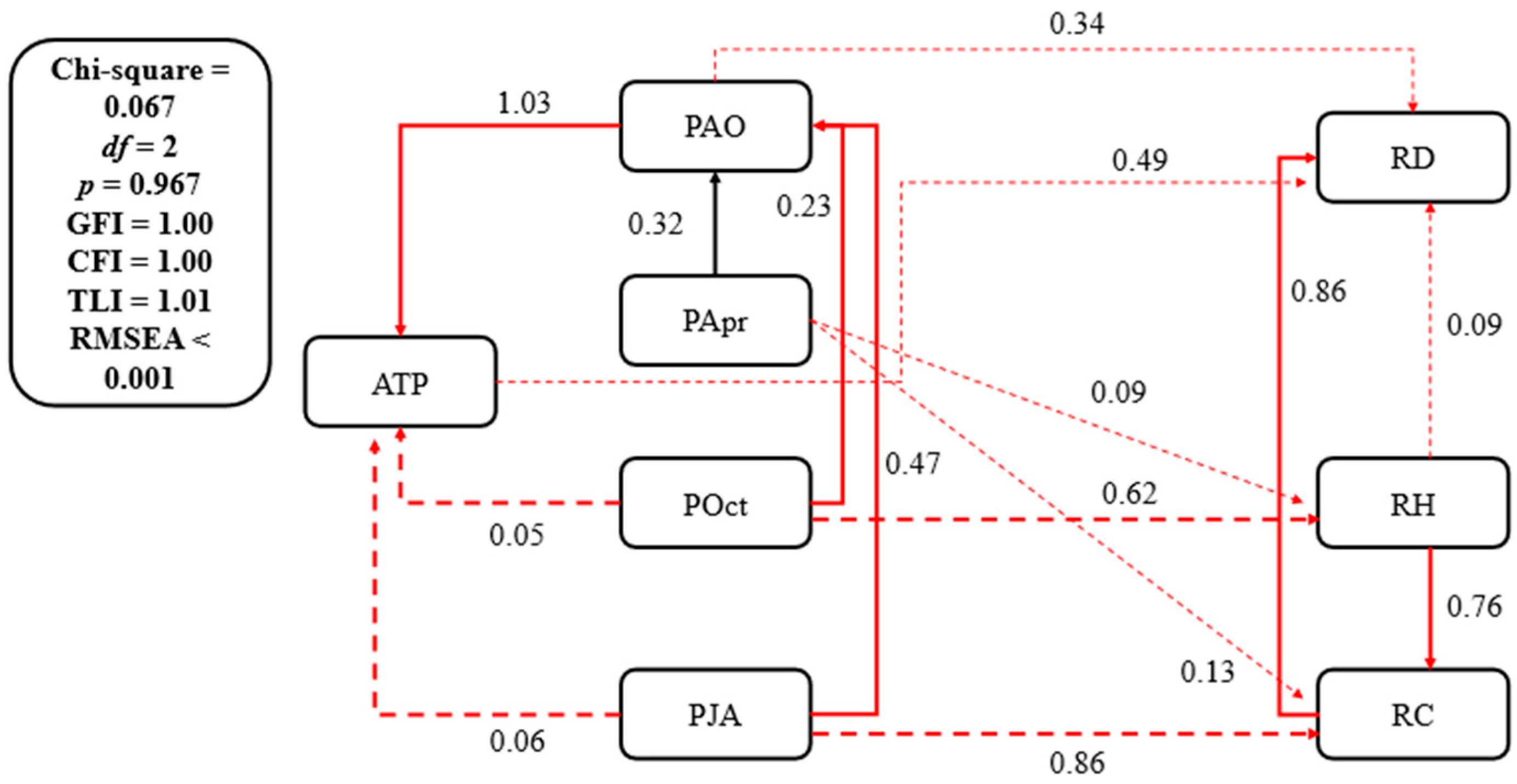
3.3. Climate–Vegetation Relationship
3.3.1. Temperature–Vegetation Relationship
3.3.2. Precipitation–Vegetation Relationship
3.3.3. Dry–Wet Condition–Vegetation Relationship
4. Discussion
4.1. The Resistance of Grassland Plants
4.2. The Salinization Phenomenon
4.3. The Complexity of Climate–Vegetation Relationship
4.4. The Study’s Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, Y.; Feng, J.C.; Sang, W.G.; Axmacher, J.C. Geographical divergence of species richness and local homogenization of plant assemblages due to climate change in grasslands. Biodivers. Conserv. 2022, 31, 797–810. [Google Scholar] [CrossRef]
- A, W.; Lu, W.W.; Sun, J.P.; Li, B.W.; Wang, S.P. Effects of climate warming and precipitation change on the relationship between plant diversity and community productivity and its stability in grassland. Acta Bot. Boreali-Occident. Sin. 2025, 45, 1–10, (In Chinese with English Abstract). [Google Scholar]
- Sasaki, T.; Nambu, M.; Iwachido, Y.; Yoshihara, Y.; Batdelger, G.; Kinugasa, T. Responses of plant productivity and carbon fluxes to short-term experimental manipulations of climate change and species loss in a Mongolian grassland. J. Arid. Environ. 2022, 198, 104690. [Google Scholar] [CrossRef]
- Lu, G.Y.; He, M.T.; Li, H.Y.; Ning, Y.N.; Li, Q.; Wang, C.J. Responses of desert steppe plant community characteristics and soil carbon and nitrogen contents to warming and increased precipitation. Chin. J. Grassl. 2024, 46, 60–69, (In Chinese with English Abstract). [Google Scholar]
- Wu, R.N.; Liu, B.Y. Spatial-temporal evolution of compound dry-hot events and their effects on vegetation vulnerability in Inner Mongolia Grassland. Glob. Ecol. Conserv. 2024, 54, e03105. [Google Scholar] [CrossRef]
- Chen, L.T.; Coomes, D.A.; Wang, J.L.; Jing, X.; He, J.S. Climate shapes the spatial pattern in local β-diversity of alpine grasslands on the Tibetan Plateau. Sci. Total Environ. 2025, 970, 178977. [Google Scholar] [CrossRef]
- Lian, J.L.; Chen, J.; Yang, X.Q.; Zhao, Y.; Luo, X.; Han, C.; Zhao, Y.X.; Li, J.P. Responses of desert steppe plant diversity and microbial to precipitation change. Biodivers. Sci. 2024, 32, 24044, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Abrha, H.; Dodiomon, S.; Ongoma, V.; Hagos, H.; Birhane, E.; Gebresamuel, G.; Manaye, A. Response of plant species to impact of climate change in Hugumbrda Grat-Kahsu forest, Tigray, Ethiopia: Implications for domestication and climate change mitigation. Trees Forests People 2024, 15, 100487. [Google Scholar] [CrossRef]
- Wang, Y.; Klaus, V.H.; Gilgen, K.; Buchmann, N. Temperate grasslands under climate extremes: Effects of plant di versity on ecosystem services. Agr. Ecosyst. Environ. 2025, 379, 109372. [Google Scholar] [CrossRef]
- Bookout, B.; Herzog, S.; Ratajczak, Z. Resilience and multi-faceted diversity of grazed and ungrazed great plains grassland plant communities to severe drought. Biol. Conserv. 2025, 305, 111088. [Google Scholar] [CrossRef]
- Deveautour, C.; Power, S.A.; Barnett, K.L.; Ochoa-Hueso, R.; Donn, S.; Bennett, A.E.; Powell, J.R. Temporal dynamics of mycorrhizal fungal communities and co-associations with grassland plant communities following experimental manipulation of rainfall. J. Ecol. 2020, 108, 515–527. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.P.; Austrheim, G.; Speed, J.D.M.; Zhao, W.W.; Cherubini, F. Disentangling the effects of climate variability and herbivory on spatial and temporal changes in northern vegetation greening. Ecol. Indic. 2024, 159, 111700. [Google Scholar] [CrossRef]
- Meng, Q.; Peng, X.B.; Zhang, S.H. Spatio-temporal response of vegetation coverage at multiple time scales to ex treme climate in the Qinling mountains in Northwest China. Res. Cold Arid. Reg. 2024, 16, 302–309. [Google Scholar]
- Liu, W.; Wang, C.T.; Zhao, J.Z.; Xu, Q.M.; Zhou, L. Responses of quantity characteristics of plant community to simulating warming in Alpine Kobresia humilis meadow ecosystem. Acta Bot. Boreali-Occident. Sin. 2010, 30, 995–1003. [Google Scholar]
- Yang, C.Y.; Ding, Y.; Ma, F.L.; Zhou, H.K.; Wang, X.L.; Zhang, Q.; Liu, X.W.; Mutalifu, W.; Guo, L. Climate change affects plant aboveground biomass by regulating the growth periods in alpine grasslands of the Tibetan Plateau, China. Chin. J. Appl. Ecol. 2024, 35, 1260–1268, (In Chinese with English Abstract). [Google Scholar]
- Liu, L.; Liu, L.P.; Sheng, L.X. Relationship between wetland change and local climate in the semi-arid zone of the Songnen Plain. J. Univ. Sci. Technol. China 2015, 45, 655–664, (In Chinese with English Abstract). [Google Scholar]
- Luo, S.S.; Tian, L.; Chang, C.L.; Wang, S.J.; Zhang, J.F.; Zhou, X.; Li, X.J.; Tran, L.P.; Tian, C.J. Grass and maize vegetation systems restore saline-sodic soils in the Songnen Plain of northeast China. Land Degrad. Dev. 2018, 29, 1107–1119. [Google Scholar] [CrossRef]
- Zhao, H.C.; Jia, G.S.; Wang, H.S.; Zhang, A.Z.; Xu, X.Y. Seasonal and interannual variations in carbon fluxes in East Asia semi-arid grasslands. Sci. Total Environ. 2019, 668, 1128–1138. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zang, P.; Guo, H.L.; Yang, G.D. Wetlands ecological security assessment in lower reaches of Taoerhe river connected with Nenjiang river using modified PSR model. HydroResearch 2023, 6, 156–165. [Google Scholar] [CrossRef]
- Li, J.P.; Zhang, B.; Zhang, S.Q. Spatio-Temporal pattern and driving forces of grassland change in west Jilin province. J. Nat. Resour. 2005, 20, 830–835, (In Chinese with English Abstract). [Google Scholar]
- Li, J.P.; Zhang, B.; Zhang, S.Q. Studies on dynamic changes of grassland in Daan city of Jilin province-remote sensing survey based on RS and GIS. Grassl. China 2004, 26, 23–29, (In Chinese with English Abstract). [Google Scholar]
- Wang, X.Y.; Zhong, J.F.; Li, Y.F. Ecological stoichiometry of multiple nutrients in Leymus chinensis and soils subjected to long-term saline-sodic stress in western Jilin province, China. Rangel. Ecol. Manag. 2025, 98, 125–133. [Google Scholar] [CrossRef]
- Gao, Y.; An, Y.; Qi, B.L.; Liu, J.; Yu, H.Z.; Wang, D.J. Grazing exclusion mediates the trade-off between plant diversity and productivity in Leymus chinensis meadows along a chronosequence on the Songnen Plain, China. Ecol. Indic. 2021, 126, 107655. [Google Scholar] [CrossRef]
- Wu, D.H.; Hu, K.; Yin, X.Q. Ecological characteristics of soil macro-animal community in mid-south Songnen degraded Leymus chinensis grasslands under restoration succession. Acta Prataculturae Sin. 2004, 13, 121–126, (In Chinese with English Abstract). [Google Scholar]
- Luo, S.Y.; Yuan, J.B.; Song, Y.Y.; Ren, J.S.; Qi, J.; Zhu, M.Y.; Feng, Y.S.; Li, M.N.; Wang, B.W.; Li, X.Y.; et al. Elevated salinity decreases microbial communities complexity and carbon, nitrogen and phosphorus metabolism in the Songnen Plain wetlands of China. Water Res. 2025, 276, 123285. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, M.; Wen, B.L.; Zhang, Q.L.; Chen, J.Z.; Li, X.J.; An, Y. Reed-mushroom-fertilizer ecological agriculture in wetlands: Harvesting reed to cultivate mushroom and returning waste substrates to restore saline-alkaline marshes. Sci. Total Environ. 2023, 878, 162987. [Google Scholar] [CrossRef]
- Zou, T.H.; Chang, Y.X.; Chen, P.; Liu, J.F. Spatial-temporal variations of ecological vulnerability in Jilin Province (China), 2000 to 2018. Ecol. Indic. 2021, 133, 108429. [Google Scholar] [CrossRef]
- Paramanik, S.; Behera, M.D.; Dash, J. Symbolic regression-based allometric model development of a mangrove forest LAI using structural variables and digital hemispherical photography. Appl. Geogr. 2022, 139, 102649. [Google Scholar] [CrossRef]
- Fogarty, D.T.; Beadle, M.; Allen, C.R.; Bielski, C.; Twidwell, D. Woody plant reinvasion shortens the lifespan of grassland restoration treatments. J. Environ. Manag. 2025, 374, 124020. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.L.; Liu, H.F.; Liang, L.Q.; Cai, Y.P.; Wang, X.; Li, C. Vegetation dynamics under water-level fluctuations: Implications for wetland restoration. J. Hydrol. 2020, 581, 124418. [Google Scholar] [CrossRef]
- Wen, B.L.; Li, X.Y.; Yang, F.; Lu, X.R.; Li, X.J.; Yang, F.Y. Growth and physiology responses of Phragmites australis to combined drought-flooding condition in inland saline-alkaline marsh, Northeast China. Ecol. Engineer. 2017, 108, 234–239. [Google Scholar] [CrossRef]
- Fischer, F.M.; Chytr, K.; Teitel, J.; Danihelka, J.; Chytr, M. Weather fluctuations drive short-term dynamics and long-term stability in plant communities: A 25-year study in a Central European dry grassland. J. Veg. Sci. 2020, 31, 711–721. [Google Scholar] [CrossRef]
- Dai, A.G. Drought under global warming: A review. Wiley Interdiscip. Rev. Clim. Change 2011, 2, 45–65. [Google Scholar] [CrossRef]
- Zhang, L.; He, J.; Zhou, Y.; Li, J.; Qiu, J.; Li, X. Assessing current status and exploring influencing factors and mechanisms of mothers’ discharge readiness for premature infants: A cross-sectional analysis using structural equation modeling. Int. J. Nurs. Stud. Adv. 2025, 27, 100372. [Google Scholar] [CrossRef]
- Zuo, X.Z.; Li, X.Y.; Yue, P.; Guo, A.X.; Yue, X.Y.; Xu, C.; Knapp, A.K.; Smith, M.D.; Luo, W.T.; Allington, G.R.H.; et al. Drought-driven shifts in relationships between plant biodiversity and productivity in temperate steppes. Funct. Ecol. 2022, 36, 2917–2928. [Google Scholar] [CrossRef]
- Sen, S.J.; Song, Z.Y.; Lin, C.C.; Liu, T.; Zhang, X.M.; Ji, B.M. Spatial pattern of plant communities along wetness gradient in Alpine grassland of Xizang and their driving factors. Acta Ecol. Sin. 2024, 44, 1996–2007, (In Chinese with English Abstract). [Google Scholar]
- Yan, Y.; Zhu, J.J.; Zhang, B.; Zhang, Y.J.; Lu, S.B.; Pan, Q.M. A review of belowground biomass allocation and its response to global climatic change in grassland ecosystems. Chin. J. Plant Ecol. 2017, 41, 585–596, (In Chinese with English Abstract). [Google Scholar]
- Huang, Y.B.; Lei, H.; Duan, L.M. Resistance of grassland productivity to drought and heatwave over a temperate semi-arid climate zone. Sci. Total Environ. 2024, 951, 175495. [Google Scholar] [CrossRef]
- Twumasi-Ankrah, M.J.; Zhan, J.Y.; Asamoah, E.F. Mapping ecoregional vulnerability to climate change for Africa. Sci. Total Environ. 2024, 953, 176219. [Google Scholar] [CrossRef]
- Li, X.G.; Han, G.X.; Eller, F.; Hui, D.F.; Zhu, L.Q.; Chen, L.; Chu, X.J.; Song, W.M.; Xu, J.W. Acclimation of coastal wetland vegetation to salinization results in the asymmetric response of soil respiration along an experimental precipitation gradient. Agric. For. Meteorol. 2021, 310, 108626. [Google Scholar] [CrossRef]
- Liang, C.; Yang, B.; Cao, Y.C.; Liu, K.Y.; Wu, J.; Hao, F.; Han, Y.; Han, W.L. Salinization mechanism of lakes and controls on organic matter enrichment: From present to deep-time records. Earth-Sci Rev. 2024, 251, 104720. [Google Scholar] [CrossRef]
- Perez-Vega, E.; Mulholland, M.R.; Crider, K.E.; Powell, K.E.; Chappell, P.D.; Bochdansky, A. The effect of temperature and salinity on Margalefidium polykrikoides group III VA, USA strain growth. Harmful Algae 2025, 146, 102837. [Google Scholar] [CrossRef]
- Swierszcz, S.; Kotowski, M.; Hebda, G.; Nowak, A. Climate-driven alterations reshape flower coloration and possible plant-pollinator interactions in wet grasslands. Agr. Ecosyst. Environ. 2025, 392, 109772. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Jin, B.C.; Zhang, X.L.; Wei, H.H.; Chang, Q.Q.; Huang, F.Q.; Liu, W.H.; Lv, Y.; Xu, Q.; Sun, G.J.; et al. Grazing alters the relationships between species diversity and biomass during community succession in a semiarid grassland. Sci. Total Environ. 2023, 887, 164155. [Google Scholar] [CrossRef]
- Silva, C.M.; Rowland, L.; Oliveira, S.R.; Pennington, R.T.; Moonlight, P. Elevation modulates the impacts of climate change on the Brazilian Cerrado flora. Divers. Distrib. 2024, 30, e13832. [Google Scholar] [CrossRef]
- Li, X.; Deng, Q.; Chen, L.L.; Liu, G.Y.; Shi, X.R.; Lock, T.R.; Kallenbach, R.L.; Yuan, Z.Y. Plant nutrient stoichiometry appears out of sync from soil: Increasing influences of changing climate on the grassland in inner Mongolia, China. Acta Oecol. 2024, 124, 104011. [Google Scholar] [CrossRef]
- Liu, Q.P.; Lin, Z.S.; Zhou, Q. Simulation on the responses of the first four dominant species′productivity to cli mate warming in Inner Mongolia Leymus chinensis grassland, China. Geogr. Res. 2007, 26, 866–976, (In Chinese with English Abstract). [Google Scholar]
- Lyu, J.X.; Lai, Y.; Geng, S.B.; Chen, S.Z. Characteristics and driving force of vegetation dynamics in Inner Mongolia Autonomous region from 1992 to 2018. Chin. J. Appl. Ecol. 2022, 33, 1240–1250, (In Chinese with English Abstract). [Google Scholar]
- Yao, Y.W.; Ren, H.R. Estimation of grassland aboveground biomass in northern China based on topography-climate-remote sensing data. Ecol. Indic. 2024, 165, 112230. [Google Scholar] [CrossRef]
- Reed, P.B.; Assour, H.R.; Okotie, O.A.; Bailes, G.T.; Johnson, B.R.; Nelson, A.A.; Pfeifer, M.L.; Roy, B.A.; Bridgham, S.D. Climate effects on prairie productivity partially ameliorated by soil nutrients and plant community responses. Ecosystems 2022, 26, 983–999. [Google Scholar] [CrossRef]
- Britton, T.G.; Hovenden, M.J.; Porter, M.; Flittner, A.; Brinkhoff, R.; Mayfield, M.M. Plant communities, populations and individuals have distinct responses to short-term warming and neighbour biomass removal in two montane grasslands. Appl. Veg. Sci. 2021, 24, e12557. [Google Scholar] [CrossRef]



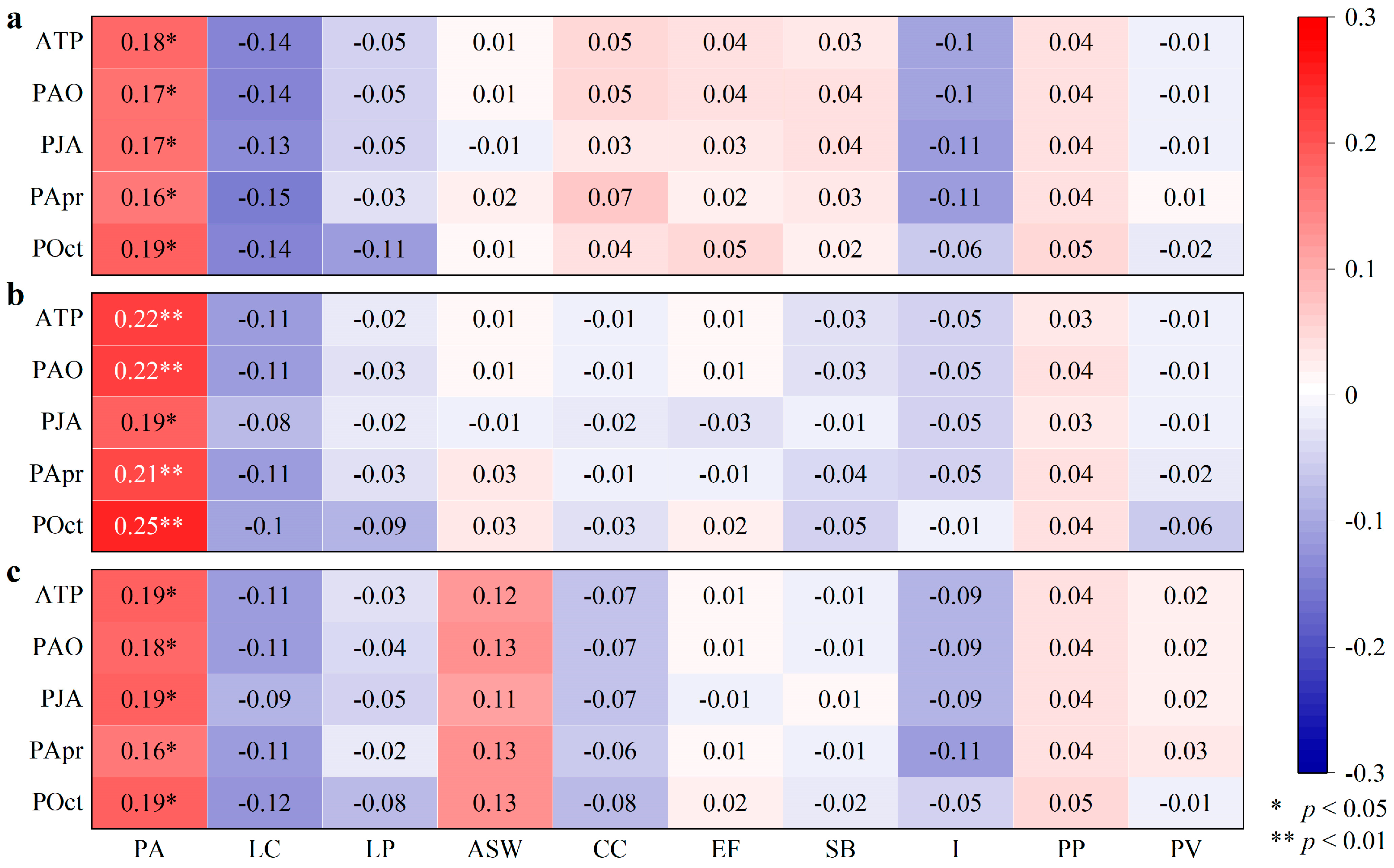
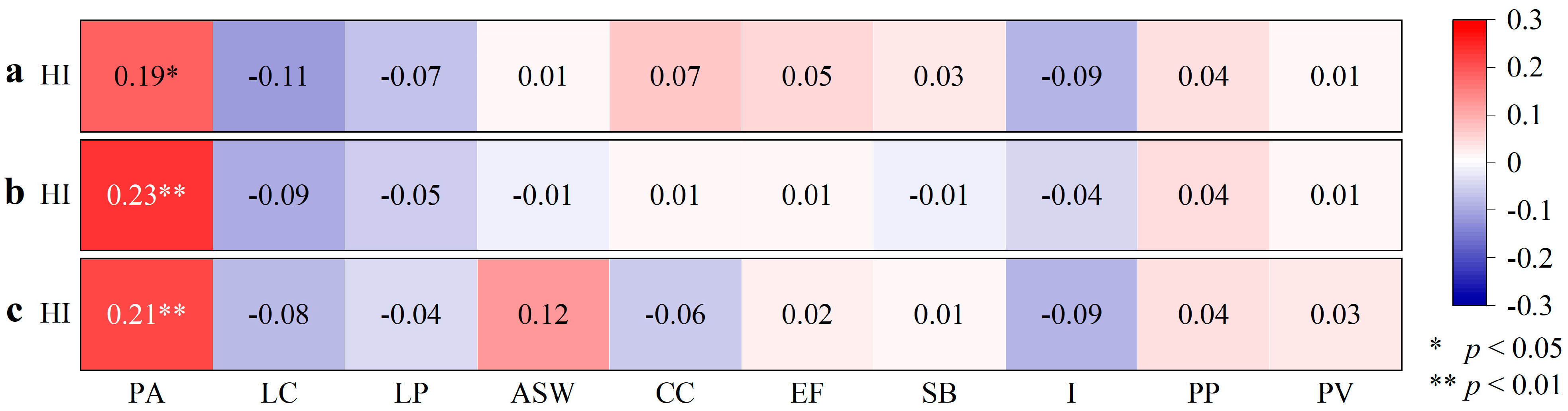
| Plants | PA | LC | LP | ASW | CC | EF | SB | I | PP | PV |
|---|---|---|---|---|---|---|---|---|---|---|
| RF | 0.69 | 0.19 | 0.43 | 0.31 | 0.14 | 0.11 | 0.13 | 0.11 | 0.11 | 0.11 |
| RD | 0.24 | 0.14 | 0.02 | 0.06 | 0.01 | 0.04 | 0.04 | 0.01 | 0.04 | 0.03 |
| RH | 0.19 | 0.1 | 0.04 | 0.07 | 0.01 | 0.03 | 0.04 | 0.02 | 0.04 | 0.04 |
| RC | 0.23 | 0.12 | 0.02 | 0.06 | 0.01 | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 |
| CT | Plants | PA | LC | LP | ASW | CC | EF | SB | IP | PP | PV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GC | RF | 0.73 | 1.00 | 0.13 | 0.47 | 0.27 | 0.00 | 0.00 | 0.07 | 0.00 | 0.07 |
| RD | 0.11 | 0.74 | 0.02 | 0.10 | 0.05 | 0.00 | 0.00 | 0.03 | 0.00 | 0.17 | |
| RH | 0.29 | 0.38 | 0.27 | 0.13 | 0.07 | 0.00 | 0.00 | 0.04 | 0.00 | 0.24 | |
| RC | 0.10 | 0.76 | 0.03 | 0.10 | 0.05 | 0.00 | 0.00 | 0.02 | 0.00 | 0.10 | |
| WC | RF | 1.00 | 0.00 | 0.24 | 0.29 | 0.06 | 0.12 | 0.18 | 0.00 | 0.29 | 0.12 |
| RD | 0.69 | 0.00 | 0.12 | 0.24 | 0.01 | 0.10 | 0.07 | 0.00 | 0.15 | 0.04 | |
| RH | 0.35 | 0.00 | 0.39 | 0.15 | 0.05 | 0.39 | 0.44 | 0.00 | 0.52 | 0.39 | |
| RC | 0.74 | 0.00 | 0.18 | 0.13 | 0.02 | 0.08 | 0.10 | 0.00 | 0.14 | 0.08 | |
| Area (km2) | PT () | Plot number (N) | PTP () | ||||||||
| GC | 15 | 9.43 | 15 | 9.43 | |||||||
| WC | 17 | 10.69 | 17 | 10.69 | |||||||
| Significant Correlation Among Plants | |
|---|---|
| RD | PA and LC (−0.31 **) |
| RH | PA and ASW (−0.20 *), PA and CC (−0.19 *), LC and LP (−0.19 *), ASW and CC (0.20 *), ASW and PV (−0.17 *), LC and PP (−0.21 **), LC and EF (−0.17 *) |
| RC | PA and LP (0.19 *), PA and LC (−0.30 **) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, X.; Fei, L.; Liu, X.; Zhang, M. Climate-Driven Vegetation Distribution and Wetland Expansion at the Edge of Jiangjiadian Grassland, Northeastern China. Plants 2025, 14, 2785. https://doi.org/10.3390/plants14172785
Wang X, Li X, Fei L, Liu X, Zhang M. Climate-Driven Vegetation Distribution and Wetland Expansion at the Edge of Jiangjiadian Grassland, Northeastern China. Plants. 2025; 14(17):2785. https://doi.org/10.3390/plants14172785
Chicago/Turabian StyleWang, Xiaodong, Xiaoqiang Li, Long Fei, Xiaohui Liu, and Mei Zhang. 2025. "Climate-Driven Vegetation Distribution and Wetland Expansion at the Edge of Jiangjiadian Grassland, Northeastern China" Plants 14, no. 17: 2785. https://doi.org/10.3390/plants14172785
APA StyleWang, X., Li, X., Fei, L., Liu, X., & Zhang, M. (2025). Climate-Driven Vegetation Distribution and Wetland Expansion at the Edge of Jiangjiadian Grassland, Northeastern China. Plants, 14(17), 2785. https://doi.org/10.3390/plants14172785






