Uptake, Partitioning, and Accumulation of High and Low Rates of Carbamazepine in Hydroponically Grown Lettuce (Lactuca sativa var. capitata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Plant Measurements
2.3. Carbamazepine Extraction and Analysis
2.4. Statistical Analysis
3. Results
3.1. Environmental Conditions
3.2. Trial 1 Results
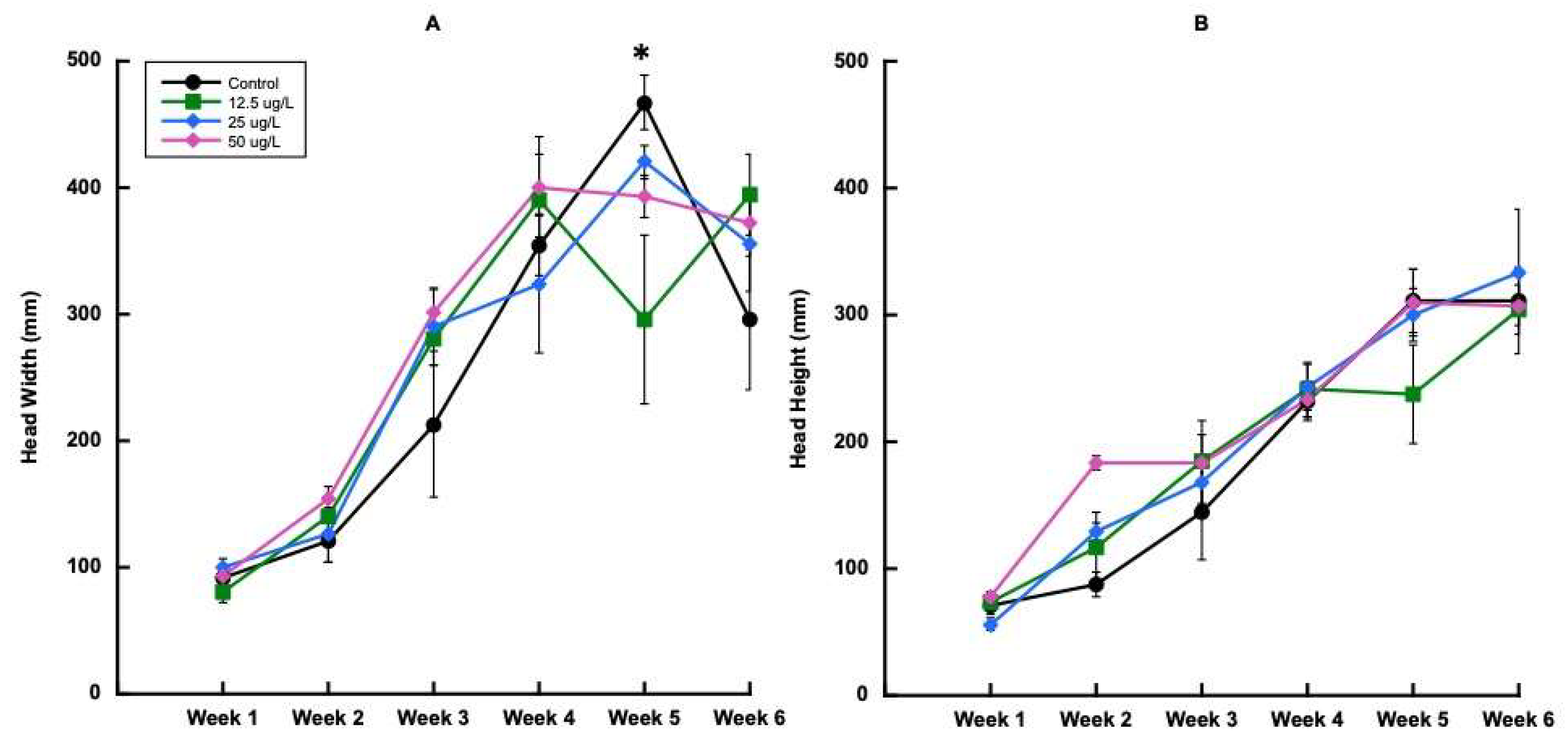
3.2.1. Plant Growth and Physiology for Trial 1
3.2.2. Carbamazepine Concentrations in Plant Tissues
3.2.3. Correlations Between Carbamazepine and Environmental Factors
3.3. Trial 2
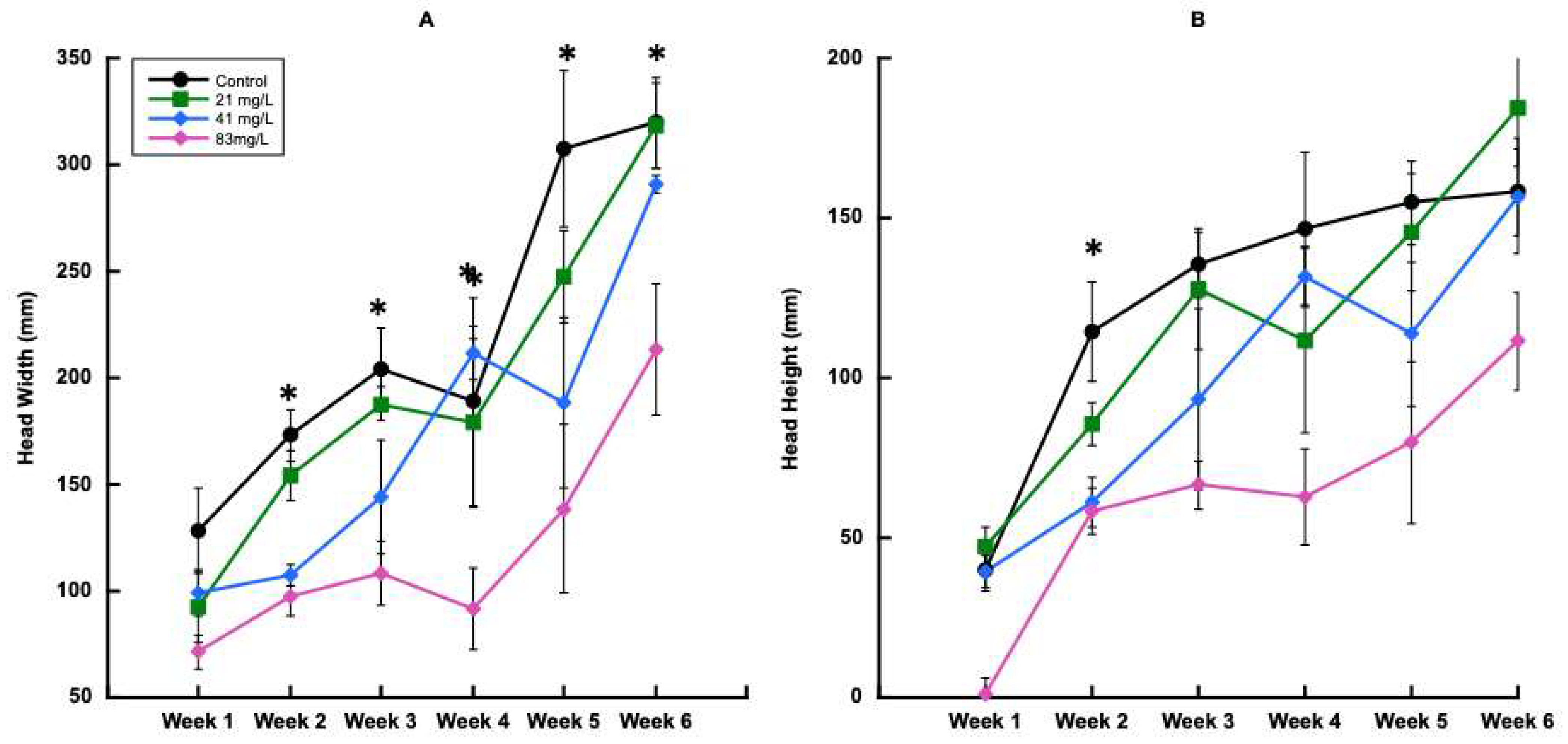
3.3.1. Plant Growth and Physiology for Trial 2
3.3.2. Carbamazepine
3.3.3. Correlations
4. Discussion
4.1. Environmental Influence of Carbamazepine Uptake and Plant Growth
4.2. Plant Growth Effects
4.3. Carbamazepine Accumulation and Partitioning in Plant Tissues
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shenker, M.; Harush, D.; Ben-Ari, J.; Chefetz, B. Uptake of Carbamazepine by Cucumber Plants—A Case Study Related to Irrigation with Reclaimed Wastewater. Chemosphere 2011, 82, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Conkle, J.L.; Ernst, F.; Gan, J. Treated Wastewater Irrigation: Uptake of Pharmaceutical and Personal Care Products by Common Vegetables under Field Conditions. Environ. Sci. Technol. 2014, 48, 11286–11293. [Google Scholar] [CrossRef]
- Malchi, T.; Maor, Y.; Tadmor, G.; Shenker, M.; Chefetz, B. Irrigation of Root Vegetables with Treated Wastewater: Evaluating Uptake of Pharmaceuticals and the Associated Human Health Risks. Environ. Sci. Technol. 2014, 48, 9325–9333. [Google Scholar] [CrossRef]
- Batchelder, A.R. Chlortetracycline and Oxytetracycline Effects on Plant Growth and Development in Soil Systems. J. Environ. Qual. 1982, 11, 675–678. [Google Scholar] [CrossRef]
- González García, M.; Fernández-López, C.; Pedrero-Salcedo, F.; Alarcón, J.J. Absorption of Carbamazepine and Diclofenac in Hydroponically Cultivated Lettuces and Human Health Risk Assessment. Agric. Water Manag. 2018, 206, 42–47. [Google Scholar] [CrossRef]
- Dodgen, L.K.; Ueda, A.; Wu, X.; Parker, D.R.; Gan, J. Effect of Transpiration on Plant Accumulation and Translocation of PPCP/EDCs. Environ. Pollut. 2015, 198, 144–153. [Google Scholar] [CrossRef]
- Leitão, I.; Mourato, M.P.; Carvalho, L.; Oliveira, M.C.; Marques, M.M.; Martins, L.L. Antioxidative Response of Lettuce (Lactuca sativa) to Carbamazepine-Induced Stress. Environ. Sci. Pollut. Res. 2021, 28, 45920–45932. [Google Scholar] [CrossRef] [PubMed]
- Benmore, C.J.; Edwards, A.; Alderman, O.L.G.; Cherry, B.R.; Smith, P.; Smith, D.; Byrn, S.; Weber, R.; Yarger, J.L. The Structure of Liquid and Glassy Carbamazepine. Quantum Beam Sci. 2022, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information PubChem Compound Summary for CID 2554, Carbamazepine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Carbamazepine (accessed on 26 June 2024).
- Tolou-Ghamari, Z.; Zare, M.; Habibabadi, J.M.; Najafi, M.R. A Quick Review of Carbamazepine Pharmacokinetics in Epilepsy from 1953 to 2012. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, S81. [Google Scholar]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Williams, C.F.; Watson, J.E.; Nelson, S.D. Comparison of Equilibrium and Non-Equilibrium Distribution Coefficients for the Human Drug Carbamazepine in Soil. Chemosphere 2014, 95, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Ricart, M.; Köck-Schulmeyer, M.; Guasch, H.; Bonnineau, C.; Proia, L.; De Alda, M.L.; Sabater, S.; Barceló, D. Pharmaceuticals and Pesticides in Reclaimed Water: Efficiency Assessment of a Microfiltration–Reverse Osmosis (MF–RO) Pilot Plant. J. Hazard. Mater. 2015, 282, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.R.; Carter, L.J.; McLaughlin, M.J. Bioaccumulation, Uptake, and Toxicity of Carbamazepine in Soil–Plant Systems. Enviro Toxic Chem. 2018, 37, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.J.; Harris, E.; Williams, M.; Ryan, J.J.; Kookana, R.S.; Boxall, A.B.A. Fate and Uptake of Pharmaceuticals in Soil–Plant Systems. J. Agric. Food Chem. 2014, 62, 816–825. [Google Scholar] [CrossRef]
- Carter, L.J.; Williams, M.; Böttcher, C.; Kookana, R.S. Uptake of Pharmaceuticals Influences Plant Development and Affects Nutrient and Hormone Homeostases. Environ. Sci. Technol. 2015, 49, 12509–12518. [Google Scholar] [CrossRef]
- Kinney, C.A.; Furlong, E.T.; Werner, S.L.; Cahill, J.D. Presence and Distribution of Wastewater-derived Pharmaceuticals in Soil Irrigated with Reclaimed Water. Environ. Toxic Chem. 2006, 25, 317–326. [Google Scholar] [CrossRef]
- Żur, J.; Piński, A.; Marchlewicz, A.; Hupert-Kocurek, K.; Wojcieszyńska, D.; Guzik, U. Organic Micropollutants Paracetamol and Ibuprofen—Toxicity, Biodegradation, and Genetic Background of Their Utilization by Bacteria. Environ. Sci. Pollut. Res. 2018, 25, 21498–21524. [Google Scholar] [CrossRef]
- Zhou, J.L.; Zhang, Z.L.; Banks, E.; Grover, D.; Jiang, J.Q. Pharmaceutical Residues in Wastewater Treatment Works Effluents and Their Impact on Receiving River Water. J. Hazard. Mater. 2009, 166, 655–661. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Smyth, S.A.; Barclay, K.; Sauvé, S.; Gagnon, C. Distribution of Antidepressant Residues in Wastewater and Biosolids Following Different Treatment Processes by Municipal Wastewater Treatment Plants in Canada. Water Res. 2012, 46, 5600–5612. [Google Scholar] [CrossRef]
- Feijoo, S.; Kamali, M.; Dewil, R. A Review of Wastewater Treatment Technologies for the Degradation of Pharmaceutically Active Compounds: Carbamazepine as a Case Study. Chem. Eng. J. 2023, 455, 140589. [Google Scholar] [CrossRef]
- Williams, C.F.; Williams, C.F.; Adamsen, F.J. Sorption–Desorption of Carbamazepine from Irrigated Soils. J. Environ. Qual. 2006, 35, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Clara, M.; Strenn, B.; Kreuzinger, N. Carbamazepine as a Possible Anthropogenic Marker in the Aquatic Environment: Investigations on the Behaviour of Carbamazepine in Wastewater Treatment and during Groundwater Infiltration. Water Res. 2004, 38, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Galbán-Malagón, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A.; et al. Pharmaceutical Pollution of the World’s Rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef]
- Santos, M.E.S.; Horký, P.; Grabicová, K.; Hubená, P.; Slavík, O.; Grabic, R.; Douda, K.; Randák, T. Traces of Tramadol in Water Impact Behaviour in a Native European Fish. Ecotoxicol. Environ. Saf. 2021, 212, 111999. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, K.; Du, W.; Cai, M.; Zhang, Z.; Li, X. Diluted Concentrations of Methamphetamine in Surface Water Induce Behavior Disorder, Transgenerational Toxicity, and Ecosystem-Level Consequences of Fish. Water Res. 2020, 184, 116164. [Google Scholar] [CrossRef]
- Bahlmann, A.; Carvalho, J.J.; Weller, M.G.; Panne, U.; Schneider, R.J. Immunoassays as High-Throughput Tools: Monitoring Spatial and Temporal Variations of Carbamazepine, Caffeine and Cetirizine in Surface and Wastewaters. Chemosphere 2012, 89, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Brezina, E.; Prasse, C.; Meyer, J.; Mückter, H.; Ternes, T.A. Investigation and Risk Evaluation of the Occurrence of Carbamazepine, Oxcarbazepine, Their Human Metabolites and Transformation Products in the Urban Water Cycle. Environ. Pollut. 2017, 225, 261–269. [Google Scholar] [CrossRef]
- Guo, Y.C.; Krasner, S.W. Occurrence of Primidone, Carbamazepine, Caffeine, and Precursors for N-Nitrosodimethylamine in Drinking Water Sources Impacted by Wastewater1. J. Am. Water Resour. Assoc. 2009, 45, 58–67. [Google Scholar] [CrossRef]
- Regmi, A.; Rueda-Kunz, D.; Liu, H.; Trevino, J.; Kathi, S.; Simpson, C. Comparing Resource Use Efficiencies in Hydroponic and Aeroponic Production Systems. Technol. Hortic. 2024, 4, e005. [Google Scholar] [CrossRef]
- coRagaveena, S.; Shirly Edward, A.; Surendran, U. Smart Controlled Environment Agriculture Methods: A Holistic Review. Rev. Environ. Sci. Biotechnol. 2021, 20, 887–913. [Google Scholar] [CrossRef]
- Nguyen, N.T.; McInturf, S.A.; Mendoza-Cózatl, D.G. Hydroponics: A Versatile System to Study Nutrient Allocation and Plant Responses to Nutrient Availability and Exposure to Toxic Elements. J. VIS. Exp. 2016, 113, e54317. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.; Syed, T.N.; Zhang, C.; Shaikh, S.A.; Rakibuzzaman, M.; Vistro, R.B. Soilless Agricultural Systems: Opportunities, Challenges, and Applications for Enhancing Horticultural Resilience to Climate Change and Urbanization. Horticulturae 2025, 11, 568. [Google Scholar] [CrossRef]
- Herklotz, P.A.; Gurung, P.; Vanden Heuvel, B.; Kinney, C.A. Uptake of Human Pharmaceuticals by Plants Grown under Hydroponic Conditions. Chemosphere 2010, 78, 1416–1421. [Google Scholar] [CrossRef]
- García-Valcárcel, A.I.; Loureiro, I.; Escorial, C.; Molero, E.; Tadeo, J.L. Uptake of Azoles by Lamb’s Lettuce (Valerianella Locusta L.) Grown in Hydroponic Conditions. Ecotoxicol. Environ. Saf. 2016, 124, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, L.; Duenk, P.; Bonte-Gelok, S.; Payne, M.; Lapen, D.R.; Topp, E. Uptake of Pharmaceuticals, Hormones and Parabens into Vegetables Grown in Soil Fertilized with Municipal Biosolids. Sci. Total Environ. 2012, 431, 233–236. [Google Scholar] [CrossRef]
- Williams, M.; Martin, S.; Kookana, R.S. Sorption and Plant Uptake of Pharmaceuticals from an Artificially Contaminated Soil Amended with Biochars. Plant Soil. 2015, 395, 75–86. [Google Scholar] [CrossRef]
- Wu, C.; Spongberg, A.L.; Witter, J.D.; Fang, M.; Czajkowski, K.P. Uptake of Pharmaceutical and Personal Care Products by Soybean Plants from Soils Applied with Biosolids and Irrigated with Contaminated Water. Environ. Sci. Technol. 2010, 44, 6157–6161. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, P.E.E. Plant Physiology, 5th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2010; ISBN 0-87893-866-4. [Google Scholar]
- Chuang, Y.-H.; Liu, C.-H.; Sallach, J.B.; Hammerschmidt, R.; Zhang, W.; Boyd, S.A.; Li, H. Mechanistic Study on Uptake and Transport of Pharmaceuticals in Lettuce from Water. Environ. Int. 2019, 131, 104976. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Spongberg, A.L.; Witter, J.D.; Sridhar, B.B.M. Transfer of Wastewater Associated Pharmaceuticals and Personal Care Products to Crop Plants from Biosolids Treated Soil. Ecotoxicol. Environ. Saf. 2012, 85, 104–109. [Google Scholar] [CrossRef]
- Solanki, A.; Boyer, T.H. Pharmaceutical Removal in Synthetic Human Urine Using Biochar. Environ. Sci. Water Res. Technol. 2017, 3, 553–565. [Google Scholar] [CrossRef]
- Calderón-Preciado, D.; Matamoros, V.; Bayona, J.M. Occurrence and Potential Crop Uptake of Emerging Contaminants and Related Compounds in an Agricultural Irrigation Network. Sci. Total Environ. 2011, 412–413, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Boland, A.T.; DeViney, C.K.; Justice, J.R.; Arce, E.D.P.; Wiele, E.C.; Wiens, N.J.; Louis, G.E. Hydroponic Crop Cultivation as a Strategy for Reducing Food Insecurity. In Proceedings of the 2022 Systems and Information Engineering Design Symposium (SIEDS), Charlottesville, VA, USA, 28 April 2022; pp. 202–206. [Google Scholar]
- Rajaseger, G.; Chan, K.L. Hydroponics: Current Trends in Sustainable Crop Production. Bioinformation 2023, 19, 925–938. [Google Scholar] [CrossRef]
- Rajendran, S.; Domalachenpa, T.; Arora, H.; Li, P.; Sharma, A.; Rajauria, G. Hydroponics: Exploring Innovative Sustainable Technologies and Applications across Crop Production, with Emphasis on Potato Mini-Tuber Cultivation. Heliyon 2024, 10, e26823. [Google Scholar] [CrossRef]
- Monnet, F.; Vaillant, N.; Hitmi, A.; Vernay, P.; Coudret, A.; Sallanon, H. Treatment of Domestic Wastewater Using the Nutrient Film Technique (NFT) to Produce Horticultural Roses. Water Res. 2002, 36, 3489–3496. [Google Scholar] [CrossRef]
- Brechner, M.; Both, A.J. Hydroponic Lettuce Handbook; Cornell Controlled Environment Agriculture: Ithaca, NY, USA, 2013. [Google Scholar]
- Winker, M.; Clemens, J.; Reich, M.; Gulyas, H.; Otterpohl, R. Ryegrass Uptake of Carbamazepine and Ibuprofen Applied by Urine Fertilization. Sci. Total Environ. 2010, 408, 1902–1908. [Google Scholar] [CrossRef]
- Seltsam, L.; Owen, W.G. Photosynthetic Daily Light Integral Influences Growth, Morphology, Physiology, and Quality of Swordfern Cultivars. HortScience 2022, 57, 1564–1571. [Google Scholar] [CrossRef]
- Stanghellini, C. Greenhouse Horticulture: Technology for Optimal Crop Production, 2nd ed.; Wageningen Academic Publisher: Wageningen, The Netherlands, 2024; ISBN 90-04-69703-9. [Google Scholar]
- Ahmed, H.A.; Yu-Xin, T.; Qi-Chang, Y. Optimal Control of Environmental Conditions Affecting Lettuce Plant Growth in a Controlled Environment with Artificial Lighting: A Review. South Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Choi, K.Y.; Paek, K.Y.; Lee, Y.B. Effect of Air Temperature on Tipburn Incidence of Butterhead and Leaf Lettuce in a Plant Factory. In Transplant Production in the 21st Century; Kubota, C., Chun, C., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 166–171. ISBN 978-90-481-5570-5. [Google Scholar]
- Hao, X.; Jia, J.; Gao, W.; Guo, X.; Zhang, W.; Zheng, L.; Wang, M. MFC-CNN: An Automatic Grading Scheme for Light Stress Levels of Lettuce (Lactuca sativa L.) Leaves. Comput. Electron. Agric. 2020, 179, 105847. [Google Scholar] [CrossRef]
- Lopez, R.G.; Runkle, E. Light Management in Controlled Environments; CreateSpace Independent Publishing Platform: North Charleston, SA, USA, 2017; ISBN 1-5442-5449-0. [Google Scholar]
- van Weel, P.; Geelen, P.; Jan Voogt, P.A. Plant Empowerment: The Basic Principles; LetsGrow: Vlaardingen, The Netherlands, 2018; ISBN 978-908-290-35-46. [Google Scholar]
- Zhao, X.; Sui, X.; Zhao, L.; Gao, X.; Wang, J.; Wen, X.; Li, Y. Morphological and Physiological Response Mechanism of Lettuce (Lactuca sativa L.) to Consecutive Heat Stress. Sci. Hortic. 2022, 301, 111112. [Google Scholar] [CrossRef]
- Tarr, S.T.; Valle De Souza, S.; Lopez, R.G. Influence of Day and Night Temperature and Radiation Intensity on Growth, Quality, and Economics of Indoor Green Butterhead and Red Oakleaf Lettuce Production. Sustainability 2023, 15, 829. [Google Scholar] [CrossRef]
- Shibata, T.; Iwao, T.; Takano, T. Effect of Vertical Air Flowing on Lettuce Growing in a Plant Factory. Acta Hortic. 1995, 175–182. [Google Scholar] [CrossRef]
- Carvalho, D.R.A.; Torre, S.; Kraniotis, D.; Almeida, D.P.F.; Heuvelink, E.; Carvalho, S.M.P. Elevated Air Movement Enhances Stomatal Sensitivity to Abscisic Acid in Leaves Developed at High Relative Air Humidity. Front. Plant Sci. 2015, 6, 383. [Google Scholar] [CrossRef] [PubMed]
- Frantz, J.M.; Ritchie, G.; Cometti, N.N.; Robinson, J.; Bugbee, B. Exploring the Limits of Crop Productivity: Beyond the Limits of Tipburn in Lettuce. J. Am. Soc. Hortic. Sci. 2004, 129, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Nishat, A.; Yusuf, M.; Qadir, A.; Ezaier, Y.; Vambol, V.; Ijaz Khan, M.; Ben Moussa, S.; Kamyab, H.; Sehgal, S.S.; Prakash, C.; et al. Wastewater Treatment: A Short Assessment on Available Techniques. Alex. Eng. J. 2023, 76, 505–516. [Google Scholar] [CrossRef]




| Concentration of CBZ | Dose of CBZ Added (mg) | Amount of CBZ in Solution (mg) | |||
|---|---|---|---|---|---|
| Weekly | Total | Weekly | Total | ||
| Trial 1 (µg L−1) | 0 | - | - | - | - |
| 12.5 | 0.0125 | 0.075 | 0.09 | 5.4 | |
| 25 | 0.025 | 0.15 | 0.18 | 10.8 | |
| 50 | 0.05 | 0.3 | 0.36 | 21.6 | |
| Trial 2 (mg L−1) | 0 | - | - | - | - |
| 21 | 21 | 126 | 151.2 | 907.2 | |
| 43 | 43 | 258 | 309.6 | 1857.6 | |
| 82 | 82 | 492 | 590.4 | 3542.4 | |
| Trial 1 | |||||
|---|---|---|---|---|---|
| Dose Concentration of CBZ (μg L−1) | Root Weight (g) | Inner Leaves (g) | Outer Leaves (g) | Total Head Weight (g) | Total Weight (g) |
| 0 | 26.38 | 206.10 | 202.96 | 409.06 | 435.44 |
| 12.5 | 32.52 | 135.98 | 71.10 | 207.08 | 239.60 |
| 25 | 34.44 | 212.82 | 236.60 | 449.42 | 483.86 |
| 50 | 34.60 | 194.34 | 113.80 | 308.14 | 342.74 |
| p-value | 0.80 | 0.38 | 0.195 | 0.072 | 0.325 |
| Final Harvest * | ||||
|---|---|---|---|---|
| Dose of CBZ (μg L−1) | Total Concentration (µg L−1) | Inner Leaves Concentration (µg L−1) | Outer Leaves Concentration (µg L−1) | Root Concentration (µg L−1) |
| 0 | 0.27 b | 0.00 | 0.14 | 0.17 |
| 12.5 | 10.17 b | 3.31 | 4.47 | 2.38 |
| 25 | 8.62 b | 6.18 | 1.93 | 2.13 |
| 50 | 73.23 a | 24.68 | 22.80 | 25.75 |
| p concentration | <0.0001 | 0.0001 | ||
| p plant part | N/A | 0.725 | ||
| p concentration × part | N/A | 0.819 | ||
| OL | W | DOL | SR | PPFD | |
|---|---|---|---|---|---|
| OL | 1.000 | ||||
| W | 0.404 | 1.000 | |||
| DOL | 0.605 | 0.427 | 1.000 | ||
| SR | −0.340 | 0.327 | −0.352 | 1.000 | |
| PPFD | −0.030 | 0.080 | 0.090 | 0.241 | 1.000 |
| Trial 2 | |||||
|---|---|---|---|---|---|
| Concentration (mg L−1) | Root Weight (g) | Inner Leaves (g) | Outer Leaves (g) | Total Head Weight (g) | Total Weight (g) |
| 0 | 18.41 ab | 90.40 | 71 ab | 161.4 ab | 179.81 ab |
| 21 | 39.97 a | 109.80 | 101.6 a | 211.4 a | 251.37 a |
| 41 | 21.73 ab | 71.60 | 39 b | 110.6 ab | 132.33 b |
| 83 | 10.57 b | 27.36 | 25.5 b | 52.86 b | 63.43 b |
| p-value | 0.036 | 0.097 | 0.010 | 0.025 | 0.008 |
| Harvest | Treatment Concentration (mg L−1) | Total Concentration (mg L−1) | Inner Leaves Concentration (mg L−1) | Outer Leaves Concentration (mg L−1) | Root Concentration (mg L−1) |
|---|---|---|---|---|---|
| 1–3 * | 0 | 8.59 c | 5.28 D | 2.36 D | 3.60 D |
| 21 | 29.43 bc | 13.98 CD | 21.53 CD | 6.61 D | |
| 41 | 46.15 b | 19.62 CD | 47.09 AB | 5.34 CD | |
| 83 | 82.32 a | 33.71 BC | 60.66 a | 19.62 B | |
| p concentration | 0.0001 | 0.0001 | |||
| p part | na | 0.0001 | |||
| p conc. × part | na | 0.002 | |||
| 4 | 0 | 9.16 d | 1.30 B | 5.70 B | 2.16 B |
| 21 | 43.76 c | 11.04 B | 27.68 B | 15.95 B | |
| 41 | 110.67 b | 25.62 B | 69.71 A | 19.17 B | |
| 83 | 187.79 a | 77.32 A | 95.10 A | 15.37 B | |
| p concentration | 0.0001 | 0.0001 | |||
| p part | na | ||||
| p conc. × part | na | 0.0003 | |||
| 5 | 0 | 1.76 c | 0.30 C | 0.93 C | 0.54 C |
| 21 | 66.28 b | 34.15 ABC | 24.61 BC | 7.51 C | |
| 41 | 107.03 ab | 23.21 BC | 60.79 AB | 23.02 BC | |
| 83 | 111.47 a | 35.45 ABC | 78.70 A | 24.20 BC | |
| p concentration | 0.0014 | 0.0001 | |||
| p part | na | 0.0003 | |||
| p conc. × part | na | 0.0117 | |||
| 6 | 0 | 2.56 c | 0.62 | 0.67 | 1.28 |
| 21 | 44.08 bc | 9.40 | 29.74 | 4.93 | |
| 41 | 87.66 b | 28.89 | 41.81 | 21.20 | |
| 83 | 153.06 a | 54.35 | 63.36 | 35.35 | |
| p concentration | 0.0004 | 0.0001 | |||
| p part | na | 0.0114 | |||
| p conc. × part | na | 0.59 | |||
| Dose Concentration of CBZ (mg L−1) | Total Average Concentration (mg L−1) | Inner Leaves Average Concentration (mg L−1) | Outer Leaves Average Concentration (mg L−1) | Root Average Concentration (mg L−1) |
|---|---|---|---|---|
| 0 | 6.54 d | 3.01 F | 2.41 EF | 2.23 EF |
| 21 | 40.40 c | 16.09 DE | 25.02 CD | 8.04 EF |
| 41 | 73.97 b | 22.76 D | 53.30 AB | 14.81 DEF |
| 83 | 116.54 a | 44.71 BC | 71.70 A | 22.83 CD |
| p concentration | 0.0001 | 0.0001 | ||
| p part | na | 0.0001 | ||
| p conc. × part | na | 0.0001 | ||
| HE | DR | DIL | SW | T | RH | TC | |
|---|---|---|---|---|---|---|---|
| HE | 1.000 | ||||||
| DR | 0.072 | 1.000 | |||||
| DIL | 0.689 | 0.145 | 1.000 | ||||
| SW | −0.325 | 0.150 | −0.100 | 1.000 | |||
| T | −0.172 | 0.192 | −0.197 | 0.225 | 1.000 | ||
| RH | 0.106 | 0.126 | −0.061 | −0.154 | 0.580 | 1.000 | |
| TC | 0.823 | 0.029 | 0.052 | −0.244 | 0.004 | 0.090 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamm, E.R.; Coldren, C.; Williams, C.; Simpson, C. Uptake, Partitioning, and Accumulation of High and Low Rates of Carbamazepine in Hydroponically Grown Lettuce (Lactuca sativa var. capitata). Plants 2025, 14, 2165. https://doi.org/10.3390/plants14142165
Stamm ER, Coldren C, Williams C, Simpson C. Uptake, Partitioning, and Accumulation of High and Low Rates of Carbamazepine in Hydroponically Grown Lettuce (Lactuca sativa var. capitata). Plants. 2025; 14(14):2165. https://doi.org/10.3390/plants14142165
Chicago/Turabian StyleStamm, Emily R., Cade Coldren, Clinton Williams, and Catherine Simpson. 2025. "Uptake, Partitioning, and Accumulation of High and Low Rates of Carbamazepine in Hydroponically Grown Lettuce (Lactuca sativa var. capitata)" Plants 14, no. 14: 2165. https://doi.org/10.3390/plants14142165
APA StyleStamm, E. R., Coldren, C., Williams, C., & Simpson, C. (2025). Uptake, Partitioning, and Accumulation of High and Low Rates of Carbamazepine in Hydroponically Grown Lettuce (Lactuca sativa var. capitata). Plants, 14(14), 2165. https://doi.org/10.3390/plants14142165







