Revisiting Traditional Medicinal Plants: Integrating Multiomics, In Vitro Culture, and Elicitation to Unlock Bioactive Potential
Abstract
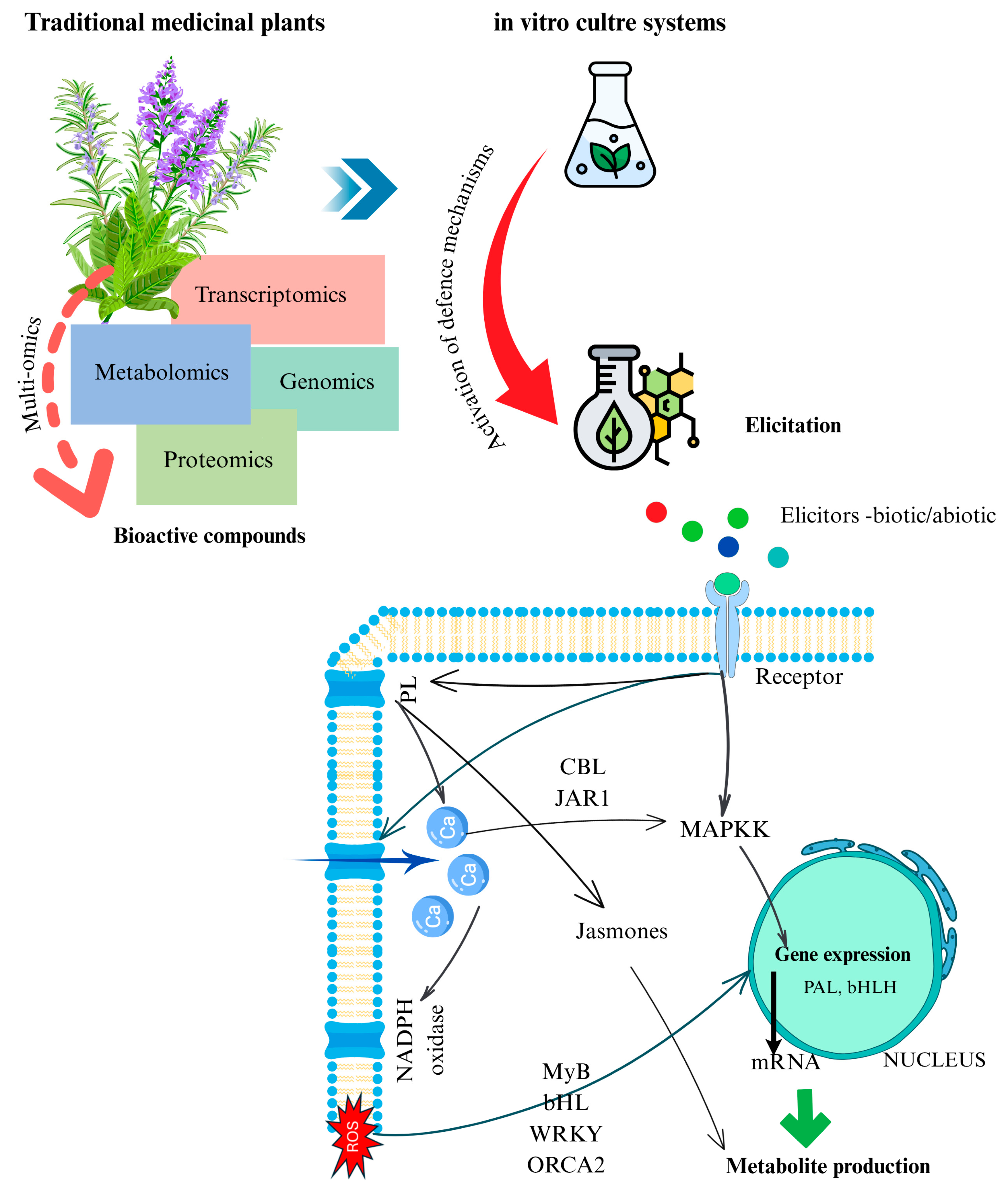
1. Introduction
2. Metabolomics: Chemical Profiling of Bioactive Compounds
3. Proteomics: Insights into Pathways and Mechanisms
4. Integrating Phytochemical Profiles with Biological Activity Assays
4.1. Antioxidant Activity Assays
4.2. Antimicrobial Activity Assays
4.3. Integrative Analysis: Linking Metabolites, Proteins, and Bioactivity
5. In Vitro Culture Systems as Platforms for Bioactive Compound Production
- Callus cultures: Masses of undifferentiated plant cells induced from explants on agar media. Callus can be proliferated indefinitely and later induced to form other structures or specialized cells;
- Cell suspension cultures: Cells from callus that are dispersed and grown in liquid shake flasks or bioreactors. These are particularly useful for metabolite production as they can be scaled up and are amenable to elicitor addition in a uniform way;
- Hairy root cultures: Fast-growing, genetically stable root cultures obtained by infecting a plant with Agrobacterium rhizogenes. Hairy roots often exhibit high productivity of root-derived secondary metabolites (e.g., alkaloids in Catharanthus or ginsenosides in Panax);
- Organ cultures (shoots, somatic embryos, etc.): Sometimes specific plant organs are cultured to exploit organ-specific pathways (for instance, shoot cultures of sweet sagewort (Artemisia annua) to produce artemisinin, since leaves produce this compound);
5.1. Elicitation Strategies: Awakening the Phytochemical Defense
- Biotic elicitors include molecules associated with pathogens or beneficial microbes (e.g., fungal cell wall fragments like chitosan or glucans, bacterial lysates, yeast extract, and even whole microbial cells). These mimic an attack, causing the plant cells to display a defense response [64]. Some biotic elicitors can also be endogenous, meaning produced by the plant itself in response to stress, such as plant hormone signals;
- Abiotic elicitors include physical factors like light (UV radiation), temperature extremes, osmotic stress (high salt or drought simulation), and chemical factors like heavy metals or plant hormones that are not directly of microbial origin. These factors also induce stress responses that converge on secondary metabolism upregulation [65];
5.1.1. Biotic Elicitors: Harnessing Biotic Signals to Boost Secondary Metabolite Production
5.1.2. Abiotic Elicitors: Methyl Jasmonate and Salicylic Acid
5.2. Mechanisms of Elicitor Action and Multiomics Linkages
5.3. Multiomics Integration: Linking Elicitation to Metabolic Pathways
6. Gaps in Current Knowledge and Challenges
7. Future Directions: Integrating Multiomics with Synthetic Biology and Metabolic Engineering
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| API | Atmospheric Pressure Ionization |
| BA | 6-Benzyladenine |
| CI | Chemical Ionization |
| CMAA | Cellular Metabolomics Antioxidant Activity |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| EI | Electron Ionization |
| ESI | Electrospray Ionization |
| FRAP | Ferric Reducing Antioxidant Power |
| GC–MS | Gas Chromatography–Mass Spectrometry |
| HPLC | High-Performance Liquid Chromatography |
| KIN | Kinetin |
| LC–MS | Liquid Chromatography–Mass Spectrometry |
| LC–MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| MALDI | Matrix-Assisted Laser Desorption/Ionization |
| MAPK | Mitogen-Activated Protein Kinase |
| MeJA | Methyl Jasmonate |
| MIC | Minimum Inhibitory Concentration |
| MS | Mass Spectrometry |
| NMR | Nuclear Magnetic Resonance |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| PAL | Phenylalanine Ammonia-Lyase |
| PCA | Principal Component Analysis |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| PR Proteins | Pathogenesis-Related Proteins |
| Quorum Quenching | |
| QS | Quorum Sensing |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| SA | Salicylic Acid |
| TOF-MS | Time-of-Flight Mass Spectrometry |
| TF | Transcription Factor |
| WEPs | Wild Edible Plants |
| WGCNA | Weighted Gene Co-expression Network Analysis |
| ZEA | Zeatin |
References
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109 (Suppl. S1), 69. [Google Scholar] [CrossRef] [PubMed]
- Karalija, E.; Dahija, S.; Demir, A.; Bešta-Gajević, R.; Zeljković, S.Ć.; Tarkowski, P. Exploring new sources of bioactive phenolic compounds from Western Balkan mountains. Plants 2022, 11, 1002. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Lange, D. Europe’s Medicinal and Aromatic Plants: Their Use, Trade and Conservation; TRAFFIC International: Cambridge, UK, 1998. [Google Scholar]
- Lange, D. Trade in medicinal and aromatic plants: A financial instrument for nature conservation in Eastern and Southeast Europe. In Financial Instruments for Nature Conservation in Central and Eastern Europe; Heinze, B., Bäurle, G., Stolpe, G., Eds.; BfN—German Federal Agency for Nature Conservation: Bonn, Germany, 2001; pp. 157–171. [Google Scholar]
- Lange, D. Medicinal and aromatic plants: Trade, production, and management of botanical resources. Acta Hortic. 2004, 629, 177–197. [Google Scholar] [CrossRef]
- Groombridge, B. Centres of species diversity. In Global Biodiversity: Status of the Earth’s Living Resources; Groombridge, B., Ed.; Springer: Dordrecht, The Netherlands, 1992; pp. 154–191. [Google Scholar] [CrossRef]
- Heinrich, M.; Booker, A. Can there be an ethnopharmacology of inflammation? In Ethnopharmacol; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 159–168. [Google Scholar] [CrossRef]
- Bhatia, H.; Sharma, Y.P.; Manhas, R.K.; Kumar, K. Traditionally used wild edible plants of district Udhampur, J&K, India. J. Ethnobiol. Ethnomed. 2018, 14, 73. [Google Scholar] [CrossRef]
- Awoke, A.; Siyum, Y.; Awoke, D.; Gebremedhin, H.; Tadesse, A. Ethnobotanical study of medicinal plants and their threats in Yeki district, Southwestern Ethiopia. J. Ethnobiol. Ethnomed. 2024, 20, 107. [Google Scholar] [CrossRef] [PubMed]
- Macanović, A. Evaluation of the State of Ecosystem Services and Traditional Knowledge of Biodiversity in the Federation of Bosnia and Herzegovina. Doctoral Thesis, Faculty of Science, University of Sarajevo, Sarajevo, Bosnia and Herzegovina, 2019. [Google Scholar]
- Karalija, E.; Šamec, D. Amentoflavone: Structure, Resources, Bioactivity and Pharmacology. In Handbook of Dietary Flavonoids; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–35. [Google Scholar] [CrossRef]
- Šamec, D.; Jurčević-Šangut, I.; Karalija, E. Ginkgetin. Advances on resources, bioactivity, and pharmacology. In Handbook of Dietary Flavonoids; International Publishing: Cham, Switzerland, 2023; pp. 1–26. [Google Scholar] [CrossRef]
- Redžić, S.; Tuka, M.; Pajević, A. Research into microscopic structure and essential oils of endemic medicinal plant species Satureja subspicata Bartl. ex Vis. (Lamiaceae). Bosn. J. Basic Med. Sci. 2006, 6, 25. [Google Scholar] [CrossRef]
- Osmić, N.; Ćulum, D.; Ibragić, Š. Catechins and other phenolic compounds in herb of eight Ephedra species in comparison to Camellia sinensis. Nat. Prod. Res. 2024, 38, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Ibragić, Š.; Barbini, S.; Oberlerchner, J.T.; Potthast, A.; Rosenau, T.; Böhmdorfer, S. Antioxidant properties and qualitative analysis of phenolic constituents in Ephedra spp. by HPTLC together with injection port derivatization GC–MS. J. Chromatogr. B 2021, 1180, 122877. [Google Scholar] [CrossRef]
- Ibragić, Š.; Mesinović, A.; Arnaut, S.; Delić, E.; Bešta-Gajević, R.; Dahija, S.; Dizdar, M.; Karalija, E. Phytochemical and bioactive profile of medicinal plants used traditionally in Bosnia and Herzegovina. Nat. Prod. J. 2024, 14, 80–89. [Google Scholar] [CrossRef]
- Ibragić, Š.; Dahija, S.; Bešta-Gajević, R.; Durak, S.; Garbo, H.; Karalija, E. Phytochemicals and antimicrobial properties of traditional medicinal plants in Bosnia and Herzegovina. J. Res. Pharm. 2025, 29, 722–741. [Google Scholar] [CrossRef]
- Scossa, F.; Roda, F.; Tohge, T.; Georgiev, M.I.; Fernie, A.R. The hot and the colorful: Understanding the metabolism, genetics and evolution of consumer preferred metabolic traits in pepper and related species. Crit. Rev. Plant Sci. 2019, 38, 339–381. [Google Scholar] [CrossRef]
- Liu, F.; Meng, Y.; He, K.; Song, F.; Cheng, J.; Wang, H.; Huang, Z.; Luo, Z.; Yan, X. Comparative analysis of proteomic and metabolomic profiles of different species of Paris. J. Proteom. 2019, 200, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Singh, C.; Singh, A.; Singh, M.P.; Singh, B.K. Mitochondrial dysfunction: A potential therapeutic target to treat Alzheimer’s disease. Mol. Neurobiol. 2020, 57, 3075–3088. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K. Exploring the biochemical profiles of medicinal plants cultivated under stressful environmental conditions. Curr. Agric. Res. J. 2024, 12, 81–103. [Google Scholar] [CrossRef]
- Rai, K.K.; Rai, N.; Pandey-Rai, S. Unlocking pharmacological and therapeutic potential of hyacinth bean (Lablab purpureus L.): Role of OMICS based biology, biotic and abiotic elicitors. In Legumes Research—Volume 2; IntechOpen: London, UK, 2022; pp. 1–25. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Stasińska-Jakubas, M.; Dresler, S.; Strzemski, M.; Rubinowska, K.; Hawrylak-Nowak, B. Differentiated response of Hypericum perforatum to foliar application of selected metabolic modulators: Elicitation potential of chitosan, selenium, and salicylic acid mediated by redox imbalance. Phytochemistry 2024, 227, 114231. [Google Scholar] [CrossRef]
- Anadozie, S.O.; Adewale, O.B.; Sibuyi, N.R.; Fadaka, A.O.; Isitua, C.C.; Davids, H.; Roux, S. One-pot synthesis, characterisation and biological activities of gold nanoparticles prepared using aqueous seed extract of Garcinia kola. Process Biochem. 2023, 128, 49–57. [Google Scholar] [CrossRef]
- Waris, M.; Koçak, E.; Gonulalan, E.M.; Demirezer, L.O.; Kır, S.; Nemutlu, E. Metabolomics analysis insight into medicinal plant science. TrAC Trends Anal. Chem. 2022, 157, 116795. [Google Scholar] [CrossRef]
- Bao, L.; Liu, X. Pan-metabolomics and its applications. In Pan-Genomics: Applications, Challenges, and Future Prospects; Barh, D., Azevedo, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 371–395. [Google Scholar] [CrossRef]
- Manickam, S.; Rajagopalan, V.R.; Kambale, R.; Rajasekaran, R.; Kanagarajan, S.; Muthurajan, R. Plant metabolomics: Current initiatives and future prospects. Curr. Issues Mol. Biol. 2023, 45, 8894–8906. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Z.; Luo, E.; Yang, J.; Wang, S. Plant metabolomics: Applications and challenges in the era of multi-omics big data. aBIOTECH 2025, 6, 116–132. [Google Scholar] [CrossRef]
- Graczyk, F.; Strzemski, M.; Balcerek, M.; Kozłowska, W.; Mazurek, B.; Karakuła, M.; Sowa, I.; Ptaszyńska, A.A.; Załuski, D. Pharmacognostic evaluation and HPLC–PDA and HS–SPME/GC–MS metabolomic profiling of Eleutherococcus senticosus fruits. Molecules 2021, 26, 1969. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.K.; Pandey, S.; Kumar, M.; Haque, M.I.; Pal, S.; Yadav, N.S. Plants metabolome study: Emerging tools and techniques. Plants 2021, 10, 2409. [Google Scholar] [CrossRef]
- Kiani, H.S.; Ali, B.; Al-Sadoon, M.K.; Al-Otaibi, H.S.; Ali, A. LC-MS/MS and GC-MS identification of metabolites from the selected herbs and spices, their antioxidant, anti-diabetic potential, and chemometric analysis. Processes 2023, 11, 2721. [Google Scholar] [CrossRef]
- Okazaki, Y.; Saito, K. Recent advances of metabolomics in plant biotechnology. Plant Biotechnol. Rep. 2012, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hoerr, V.; Duggan, G.E.; Zbytnuik, L.; Poon, K.K.; Große, C.; Neugebauer, U.; Methling, K.; Löffler, B.; Vogel, H.J. Characterization and prediction of the mechanism of action of antibiotics through NMR metabolomics. BMC Microbiol. 2016, 16, 82. [Google Scholar] [CrossRef]
- Seshadri, K.; Abad, A.N.; Nagasawa, K.K.; Yost, K.M.; Johnson, C.W.; Dror, M.J.; Tang, Y. Synthetic biology in natural product biosynthesis. Chem. Rev. 2025, 125, 3814–3931. [Google Scholar] [CrossRef]
- Li, C.; Yan, X.; Xu, Z.; Wang, Y.; Shen, X.; Zhang, L.; Zhou, Z.; Wang, P. Pathway elucidation of bioactive rhamnosylated ginsenosides in Panax ginseng and their de novo high-level production by engineered Saccharomyces cerevisiae. Commun. Biol. 2022, 5, 775. [Google Scholar] [CrossRef] [PubMed]
- Cusidó, R.M.; Palazón, J.; Navia-Osorio, A.; Mallol, A.; Bonfill, M.; Morales, C.; Piñol, M.T. Production of Taxol® and baccatin III by a selected Taxus baccata callus line and its derived cell suspension culture. Plant Sci. 1999, 146, 101–107. [Google Scholar] [CrossRef]
- Karami, M.; Naghavi, M.R.; Nasiri, J.; Farzin, N.; Ignea, C. Enhanced production of withaferin A from the hairy root culture of Withania somnifera via synergistic effect of methyl jasmonate and β-cyclodextrin. Plant Physiol. Biochem. 2024, 208, 108440. [Google Scholar] [CrossRef]
- Belchí-Navarro, S.; Almagro, L.; Sabater-Jara, A.B.; Fernández-Pérez, F.; Bru, R.; Pedreño, M.A. Early signaling events in grapevine cells elicited with cyclodextrins and methyl jasmonate. Plant Physiol. Biochem. 2013, 62, 107–110. [Google Scholar] [CrossRef]
- Banadka, A.; Narasimha, S.W.; Dandin, V.S.; Naik, P.M.; Vennapusa, A.R.; Melmaiee, K.; Nagella, P. Biotechnological approaches for the production of camptothecin. Appl. Microbiol. Biotechnol. 2024, 108, 382. [Google Scholar] [CrossRef] [PubMed]
- Karalija, E.; Zeljković, S.Ć.; Parić, A. Harvest time–related changes in biomass, phenolics and antioxidant potential in Knautia sarajevensis shoot cultures after elicitation with salicylic acid and yeast. In Vitro Cell. Dev. Biol.–Plant 2020, 56, 177–183. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, S.; Zhang, Q.; Wang, N.; Yang, Q.; Hao, J. Development and standardization of spectrophotometric assay for quantification of thermal hydrolysis-origin melanoidins and its implication in antioxidant activity evaluation. J. Hazard. Mater. 2024, 476, 135021. [Google Scholar] [CrossRef]
- Pan, L.; Yang, N.; Sui, Y.; Li, Y.; Zhao, W.; Zhang, L.; Tang, Z. Altitudinal variation on metabolites, elements, and antioxidant activities of medicinal plant Asarum. Metabolites 2023, 13, 1193. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Assessment of the antioxidant activity of catechin in nutraceuticals: Comparison between a newly developed electrochemical method and spectrophotometric methods. Int. J. Mol. Sci. 2022, 23, 8110. [Google Scholar] [CrossRef] [PubMed]
- Gaber, N.B.; El-Dahy, S.I.; Shalaby, E.A. Comparison of ABTS, DPPH, permanganate, and methylene blue assays for determining antioxidant potential of successive extracts from pomegranate and guava residues. Biomass Convers. Biorefin. 2023, 13, 4011–4020. [Google Scholar] [CrossRef]
- Fu, N.; Yao, H.; Nan, Y.; Qiao, L. Role of oxidative stress in hepatitis C virus induced hepatocellular carcinoma. Curr. Cancer Drug Targets 2017, 17, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Kok, M.; Maton, L.; van der Peet, M.; Hankemeier, T.; van Hasselt, J.C. Unraveling antimicrobial resistance using metabolomics. Drug Discov. Today 2022, 27, 1774–1783. [Google Scholar] [CrossRef]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K.J.A.M. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef]
- Kilibarda, S.; Jović, M.D.; Milinčić, D.D.; Vuković, S.; Trifković, J.Đ.; Pešić, M.B.; Kostić, A.Ž. Phytochemical Profile and Biological Activities of Rtanj’s Hypericum perforatum Infusion Tea and Methanolic Extracts: Insights from LC-MS/MS and HPTLC–Bioautography. Plants 2025, 14, 1377. [Google Scholar] [CrossRef]
- Rahim, N.; Derabli, C.; Bramki, A.; Mahdjoub, S.; Rup-Jacques, S.; Barboucha, G.; Boulebd, H. Evaluating the multifaceted bioactivity of Syzygium aromaticum essential oil: The central role of eugenol. Turk. J. Biol. 2025, 49, 102–117. [Google Scholar] [CrossRef]
- Halder, M.; Majumder, A.; Ray, S.; Jha, S. Medicinal plant research at crossroads: Biotechnological approaches for conservation, production and stability in tissue cultures and regenerated plants. In Medicinal Plants: Domestication, Biotechnology and Regional Importance; Sharma, A., Ed.; Springer: Singapore, 2021; pp. 459–544. [Google Scholar] [CrossRef]
- Barredo, J.L. (Ed.) Microbial Enzymes and Biotransformations; Humana Press: New York, NY, USA, 2005; pp. 1–319. [Google Scholar]
- Leitner, M.; Kaiser, R.; Rasmussen, M.O.; Driguez, H.; Boland, W.; Mithöfer, A. Microbial oligosaccharides differentially induce volatiles and signalling components in Medicago truncatula. Phytochemistry 2008, 69, 2029–2040. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Sukhikh, S.; Pungin, A.; Ivanova, S.; Asyakina, L.; Prosekov, A. Modern trends in the in vitro production and use of callus, suspension cells and root cultures of medicinal plants. Molecules 2020, 25, 5805. [Google Scholar] [CrossRef]
- Kang, S.M.; Min, J.Y.; Kim, Y.D.; Kang, Y.M.; Park, D.J.; Jung, H.N.; Choi, M.S. Effects of methyl jasmonate and salicylic acid on the production of bilobalide and ginkgolides in cell cultures of Ginkgo biloba. In Vitro Cell. Dev. Biol. Plant 2006, 42, 44–49. [Google Scholar] [CrossRef]
- Merritt, C.D. Use of Green Fluorescent Protein as a Reporter of Induced Biochemical Production in Hyoscyamus muticus Root Cultures. Ph.D. Thesis, The Pennsylvania State University, University Park, PA, USA, 2001. [Google Scholar]
- Farkya, S.; Bisaria, V.S. Exogenous hormones affecting morphology and biosynthetic potential of hairy root line (LYR2i) of Linum album. J. Biosci. Bioeng. 2008, 105, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Kumar, S.R.; Dwivedi, V.; Rai, A.; Shukla, A.K.; Shanker, K.; Nagegowda, D.A. Precursor feeding studies and molecular characterization of geraniol synthase establish the limiting role of geraniol in monoterpene indole alkaloid biosynthesis in Catharanthus roseus leaves. Plant Sci. 2015, 239, 56–66. [Google Scholar] [CrossRef]
- Srivastava, S. Mycorrhizal Elicitation of Secondary Metabolites in Hairy Roots of Ocimum basilicum. Doctoral Dissertation, Deakin University, Geelong, Australia, 2015. [Google Scholar]
- Alvarado, A.M.; Aguirre-Becerra, H.; Vázquez-Hernández, M.C.; Magaña-Lopez, E.; Parola-Contreras, I.; Caicedo-Lopez, L.H.; Contreras-Medina, L.M.; Garcia-Trejo, J.F.; Guevara-Gonzalez, R.G.; Feregrino-Perez, A.A. Influence of Elicitors and Eustressors on the Production of Plant Secondary Metabolites. In Natural Bio-Active Compounds: Volume 1: Production and Applications; Feregrino-Perez, A.A., Ed.; Springer: Cham, Switzerland, 2019; pp. 333–388. [Google Scholar] [CrossRef]
- Yoshikawa, M. Diverse Modes of Action of Biotic and Abiotic Phytoalexin Elicitors. Nature 1978, 275, 546–547. [Google Scholar] [CrossRef]
- Meena, M.; Yadav, G.; Sonigra, P.; Nagda, A.; Mehta, T.; Zehra, A.; Swapnil, P. Role of Microbial Bioagents as Elicitors in Plant Defense Regulation. In Transcription Factors for Biotic Stress Tolerance in Plants; Springer International Publishing: Cham, Switzerland, 2022; pp. 103–128. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitor Signal Transduction Leading to Production of Plant Secondary Metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar] [CrossRef]
- Kumari, N.; Varghese, B.A.; Khan, M.A.; Jangra, S.; Kumar, A. Biotic Elicitation: A Tool for Producing Bioactive Compounds. Plant Arch. 2020, 20, 3385–3391. [Google Scholar]
- El Gizawy, H.A.; El Zanaty, S.A.; El Ghaly, E.M.; Seif-Eldein, N.A. Thevetia peruviana leaves, HPLC profile, isolation, and in vitro cytotoxicity. RSC Adv. 2023, 13, 12072–12079. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Islam, M.R. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. Biomed. Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants, 2nd ed.; Joshee, N., Dhekney, S., Parajuli, P., Eds.; Springer: Cham, Switzerland, 2019; Volume 3, pp. 154–196. [Google Scholar] [CrossRef]
- Twaij, B.M.; Hasan, M.N. Bioactive secondary metabolites from plant sources: Types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Hilal, B.; Khan, M.M.; Fariduddin, Q. Recent advancements in deciphering the therapeutic properties of plant secondary metabolites: Phenolics, terpenes, and alkaloids. Plant Physiol. Biochem. 2024, 108674. [Google Scholar] [CrossRef]
- Tusevski, O.; Petreska Stanoeva, J.; Stefova, M.; Gadzovska Simic, S. Agrobacterium Enhances Xanthone Production in Hypericum perforatum Cell Suspensions. Plant Growth Regul. 2015, 76, 199–210. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, S.; Zhang, J.; Ma, P.; Duan, J.; Liang, Z. Effect and Mechanism of Endophytic Bacteria on Growth and Secondary Metabolite Synthesis in Salvia miltiorrhiza Hairy Roots. Acta Physiol. Plant. 2014, 36, 1095–1105. [Google Scholar] [CrossRef]
- Roychowdhury, R.; Das, S.P.; Gupta, A.; Parihar, P.; Chandrasekhar, K.; Sarker, U.; Sudhakar, C. Multi-Omics Pipeline and Omics-Integration Approach to Decipher Plant’s Abiotic Stress Tolerance Responses. Genes 2023, 14, 1281. [Google Scholar] [CrossRef]
- Karalija, E.; Ćavar Zeljković, S.; Tarkowski, P.; Muratović, E.; Parić, A. The Effect of Cytokinins on Growth, Phenolics, Antioxidant and Antimicrobial Potential in Liquid Agitated Shoot Cultures of Knautia sarajevensis. Plant Cell Tissue Organ Cult. 2017, 131, 347–357. [Google Scholar] [CrossRef]
- Jain, D.; Bisht, S.; Parvez, A.; Singh, K.; Bhaskar, P.; Koubouris, G. Effective Biotic Elicitors for Augmentation of Secondary Metabolite Production in Medicinal Plants. Agriculture 2024, 14, 796. [Google Scholar] [CrossRef]
- Patil, R.A. Molecular and Population Level Approaches to Understand Taxus Metabolism in Cell Suspension Cultures. Doctoral Dissertation, University of Massachusetts Amherst, Amherst, MA, USA, 2013. [Google Scholar]
- Shi, J.; Wang, J.; Lv, H.; Peng, Q.; Schreiner, M.; Baldermann, S.; Lin, Z. Integrated proteomic and metabolomic analyses reveal the importance of aroma precursor accumulation and storage in methyl jasmonate-primed tea leaves. Hortic. Res. 2021, 8, 123–134. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Moghaddam, M. Hydroponics and elicitation, a combined approach to enhance the production of bioactive compound from medicinal plants. In Hydroponics: The Future of Sustainable Farming; Springer: New York, NY, USA, 2024; pp. 87–100. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, R.; Brunetti, C.; Cammareri, M.; Caretto, S.; Cavallaro, V.; Cominelli, E.; De Palma, M.; Docimo, T.; Giovinazzo, G.; Grandillo, S.; et al. Strategies to Modulate Specialized Metabolism in Mediterranean Crops: From Molecular Aspects to Field. Int. J. Mol. Sci. 2021, 22, 2887. [Google Scholar] [CrossRef]
- Martínez-Esteso, M.J.; Morante-Carriel, J.; Samper-Herrero, A.; Martínez-Márquez, A.; Sellés-Marchart, S.; Nájera, H.; Bru-Martínez, R. Proteomics: An Essential Tool to Study Plant-Specialized Metabolism. Biomolecules 2024, 14, 1539. [Google Scholar] [CrossRef]
- Rong, T.; Chunchun, Z.; Wei, G.; Yuchen, G.; Fei, X.; Tao, L.; Yuanyuan, J.; Chenbin, W.; Wenda, X.; Wenqing, W. Proteomic insights into protostane triterpene biosynthesis regulatory mechanism after MeJA treatment in Alisma orientale (Sam.) Juz. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140671. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T. Elicitor induced increased rosmarinic acid content of in vitro root cultures of Ocimum basilicum L. (Sweet Basil). Plant Sci. Today 2020, 7, 157–163. [Google Scholar] [CrossRef]
- Thakur, S.; Ghosh, S.; Dasgupta, M. Implementation of WGCNA for Identifying Regulatory Modules in Biological Networks. In Next-Generation Sequencing; CRC Press: Boca Raton, FL, USA, 2025; pp. 80–97. [Google Scholar] [CrossRef]
- Chavali, A.K.; Rhee, S.Y. Bioinformatics tools for the identification of gene clusters that biosynthesize specialized metabolites. Brief. Bioinform. 2018, 19, 1022–1034. [Google Scholar] [CrossRef]
- Tong, L.; Shi, W.; Isgut, M.; Zhong, Y.; Lais, P.; Gloster, L.; Wang, M.D. Integrating multi-omics data with EHR for precision medicine using advanced artificial intelligence. IEEE Rev. Biomed. Eng. 2023, 17, 80–97. [Google Scholar] [CrossRef]
- Wang, M.; Lin, H.; Lin, H.; Du, P.; Zhang, S. From species to varieties: How modern sequencing technologies are shaping Medicinal Plant Identification. Genes 2024, 16, 16. [Google Scholar] [CrossRef]
- Mirveis, Z.; Howe, O.; Cahill, P.; Patil, N.; Byrne, H.J. Monitoring and modelling the glutamine metabolic pathway: A review and future perspectives. Metabolomics 2023, 19, 67. [Google Scholar] [CrossRef]
- Wang, Y. Bioinformatics Tool Development and Application for Herbal Therapeutic Investigations. Ph.D. Thesis, National University of Singapore, Singapore, 2021. [Google Scholar]
- Kress, W.J.; Soltis, D.E.; Kersey, P.J.; Wegrzyn, J.L.; Leebens-Mack, J.H.; Gostel, M.R.; Soltis, P.S. Green plant genomes: What we know in an era of rapidly expanding opportunities. Proc. Natl. Acad. Sci. USA 2022, 119, e2115640118. [Google Scholar] [CrossRef]
- Hesami, M.; Jones, A.M.P. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Appl. Microbiol. Biotechnol. 2020, 104, 9449–9485. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, Y.; Dong, Z.; Guo, Y.; Luo, J.; Wang, F.; Zou, X. Endophytic Fungal Diversity and Its Interaction Mechanism with Medicinal Plants. Molecules 2025, 30, 1028. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Liu, F.; Zhang, C.; Deng, R.; Fernie, A.R.; Zhang, Y. Systems and synthetic biology for plant natural product pathway elucidation. Cell Rep. 2025, 44, 6. [Google Scholar] [CrossRef] [PubMed]

| Plant Species | Elicitor | Targeted Pathway | Upregulated Proteins/Enzymes |
|---|---|---|---|
| St. John’s wort (Hypericum perforatum) [24,30] | Agrobacterium sp. | Xanthone/flavonoid | Xanthone synthase, PAL, PR proteins, glutaredoxins |
| Asian ginseng (Panax ginseng) [24,37] | Methyl jasmonate (MeJA) | Ginsenoside | Oxidosqualene cyclase (OSC), HSPs, SAMS |
| English jew (Taxus baccata) [24,38] | Methyl jasmonate (MeJA) | Taxol biosynthesis | 10-deacetylbaccatin III-10-O-acetyltransferase, T5αH, PR proteins |
| Indian ginseng (Withania somnifera) [24,39] | Salicylic acid (SA) | Withanolide synthesis | HSP70, CYP450, SQS, SGT |
| Purple coneflower (Echinacea purpurea) [24,40] | Yeast extract | Caffeic acid derivatives | Caffeoyl-CoA O-methyltransferase, PAL |
| Liquorice (Glycyrrhiza glabra) [24,41] | Methyl jasmonate (MeJA) | Glycyrrhizin biosynthesis | β-amyrin synthase, CYP88D6, UGT73C11 |
| Asiatic pennywort (Centella asiatica) [24,42] | Methyl jasmonate (MeJA) | Triterpenoid saponin | β-amyrin synthase, OSC, UGTs |
| Culture Type | Plant Species | Bioactive Compound(s) | Elicitors Used |
|---|---|---|---|
| Callus cultures | Indian ginseng Withania somnifera [39] | Withanolides | Induced from leaf explants; MeJA and SA enhanced production |
| Happy tree Camptotheca acuminata [41] | Camptothecin | Callus derived from leaf explants; responsive to elicitation | |
| English jew Taxus baccata [38] | Taxanes (e.g., paclitaxel) | Callus used as starting point for suspension cultures | |
| Cell suspension cultures | Common grape wine Vitis vinifera [40] | Resveratrol, stilbenes | Elicited with MeJA + cyclodextrins |
| Madagascar periwinkle Catharanthus roseus [33] | Ajmalicine, serpentine | Scalable production in bioreactors; MeJA-induced | |
| Maidenhair tree Ginkgo biloba [57] | Ginkgolides, bilobalide | Treated with salicylic acid to enhance yields | |
| Hairy root cultures | Asian ginseng Panax ginseng [37] | Ginsenosides | Stable high-yield system; strong response to MeJA |
| Egyptian henbane Hyoscyamus muticus [58] | Tropane alkaloids (e.g., scopolamine) | Induced with A. rhizogenes; SA and yeast extract effective | |
| White flax Linum album [59] | Podophyllotoxin | Hairy roots show higher yields than callus or suspension | |
| Organ cultures | Sweet sagewort Artemisia annua (shoots) [60] | Artemisinin | Shoot cultures retain leaf-specific biosynthesis |
| Sarajevos’ widow flower Knautia sarajevensis (shoots) [42] | Phenolics, flavonoids | SA elicitation enhanced antioxidant compounds | |
| Red sage Salvia miltiorrhiza (roots) [61] | Tanshinones | Root-derived compounds induced by bacterial co-culture |
| Elicitor Type | Elicitor | Mode of Action/Induced Response | Examples |
|---|---|---|---|
| Biotic | Yeast extract [42] | Induces phenylpropanoid pathway, increases phenolic/flavonoid content and antioxidant capacity | Sarajevos widow flower (Knautia sarajevensis): phenolics; Purple coneflower (Echinacea purpurea) |
| Agrobacterium rhizogenes [72] | Triggers defense responses and secondary metabolite biosynthesis via T-DNA integration and transformation | St. John’s wort (Hypericum perforatum): xanthones, flavonoids | |
| Fungal cell wall fragments (e.g., chitin, glucans) [24,64] | Elicits PR proteins and oxidative stress-related secondary metabolites | General in vitro applications; Madagascar periwinkle (Catharanthus roseus) | |
| Bacterial co-culture (e.g., Bacillus sp.) [73] | Activates multiple stress-responsive biosynthetic pathways, enhancing compounds like tanshinones | Red sage (Salvia miltiorrhiza): tanshinones | |
| Abiotic | Methyl jasmonate (MeJA) [24,37,77] | Activates jasmonate signaling cascade, boosting alkaloids, terpenoids, and phenolics | Asian ginseng (Panax ginseng): ginsenosides, English jew (Taxus baccata): paclitaxel, Asiatic pennywort (Centella asiatica): triterpenoids |
| Salicylic acid (SA) [24,42,57] | Induces systemic acquired resistance (SAR), increases pathogenesis-related proteins and phenolic production | Maidenhair tree (Ginkgo biloba): bilobalide, Knautia sarajevensis | |
| Cytokinins (e.g., zeatin, BA, kinetin) [75] | Stimulates organogenesis and enhances biosynthesis of selected phenolics and phytohormones | Knautia sarajevensis: rosmarinic acid, flavonoids | |
| Nanoparticles (e.g., gold NPs, silver NPs) [26] | Enhances cytotoxicity and metabolite accumulation through ROS generation and enzyme activation | Bitter kola (Garcinia kola): gold NPs; general examples in callus cultures | |
| Heavy metals (low dose Cd, etc.) [25] | Mimics abiotic stress signals and induces oxidative defense-related pathways | Hypericum perforatum; general use in priming | |
| Physical stress (UV, temperature, osmotic) [79] | Triggers reactive oxygen species (ROS), calcium influx, MAPK signaling, and defense gene activation | Santa Catalina indian Paintbrush (Castilleja tenuiflora); lavender (Lavandula angustifolia); various in vitro systems |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karalija, E.; Macanović, A.; Ibragić, S. Revisiting Traditional Medicinal Plants: Integrating Multiomics, In Vitro Culture, and Elicitation to Unlock Bioactive Potential. Plants 2025, 14, 2029. https://doi.org/10.3390/plants14132029
Karalija E, Macanović A, Ibragić S. Revisiting Traditional Medicinal Plants: Integrating Multiomics, In Vitro Culture, and Elicitation to Unlock Bioactive Potential. Plants. 2025; 14(13):2029. https://doi.org/10.3390/plants14132029
Chicago/Turabian StyleKaralija, Erna, Armin Macanović, and Saida Ibragić. 2025. "Revisiting Traditional Medicinal Plants: Integrating Multiomics, In Vitro Culture, and Elicitation to Unlock Bioactive Potential" Plants 14, no. 13: 2029. https://doi.org/10.3390/plants14132029
APA StyleKaralija, E., Macanović, A., & Ibragić, S. (2025). Revisiting Traditional Medicinal Plants: Integrating Multiomics, In Vitro Culture, and Elicitation to Unlock Bioactive Potential. Plants, 14(13), 2029. https://doi.org/10.3390/plants14132029










