The Effects of Frost and Fire on the Traits, Resources, and Floral Visitors of a Cerrado Plant, and Their Impact on the Plant–Visitor Interaction Network and Fruit Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study System
2.3. Measuring Richness, Abundance, and Frequency of Floral Visitors
2.4. Measuring Traits and Resources of B. Intermedia
2.5. Measuring Formed Fruits
2.6. Plant–Pollinator Interaction Network
2.7. Data Analysis
3. Results
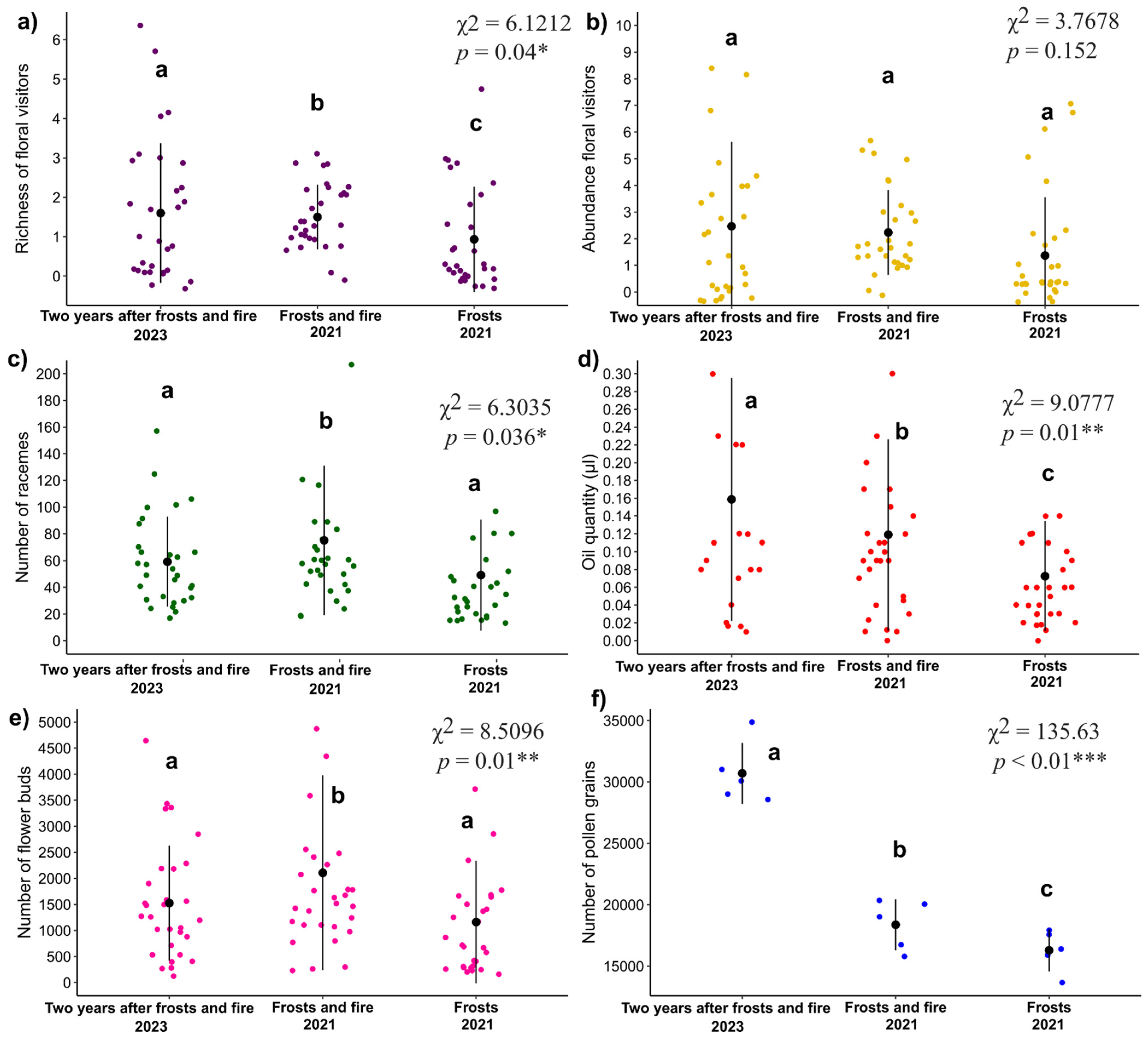
3.1. Richness and Abundance of Floral Visitors
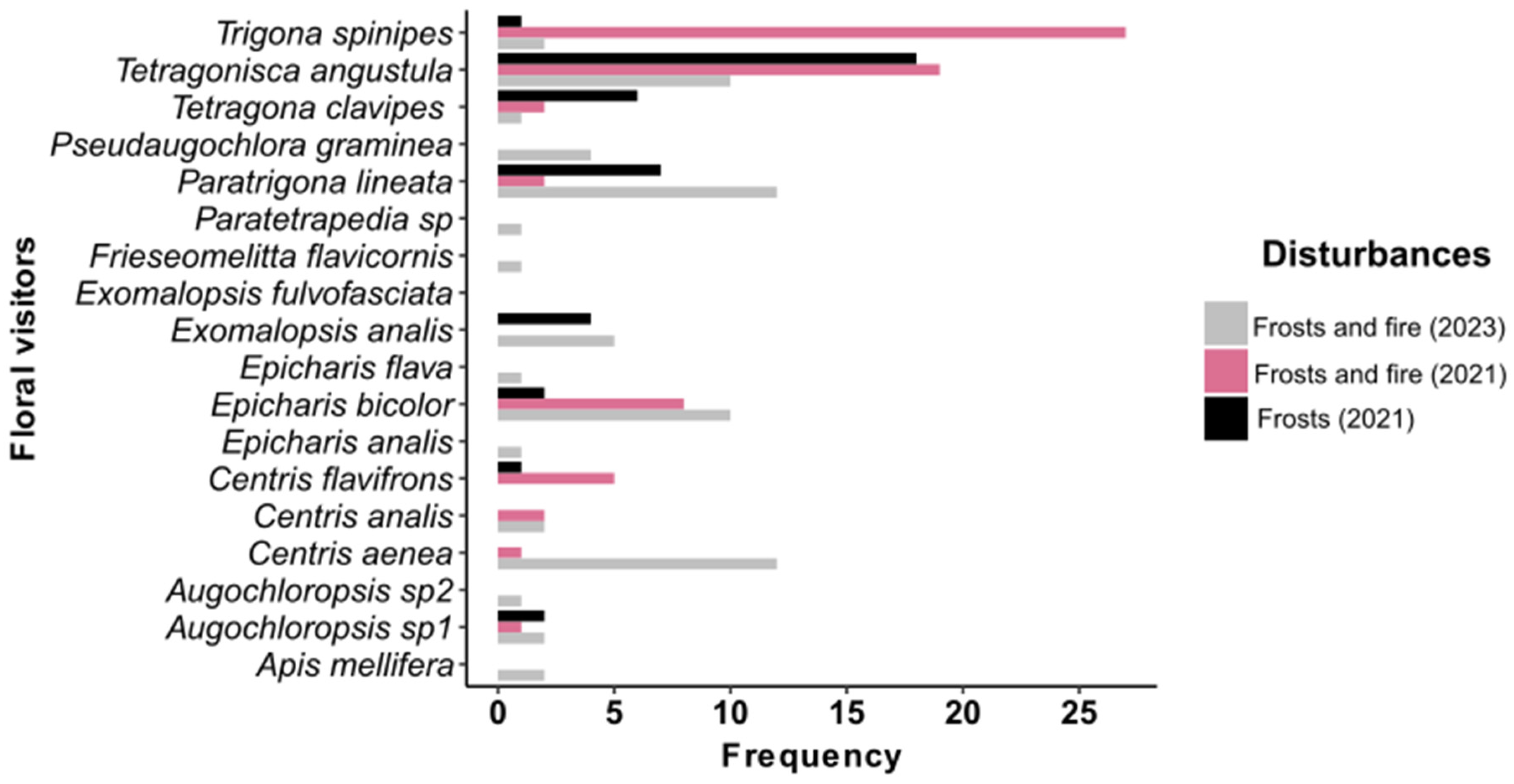
3.2. Frequency of Floral Visitors
3.3. Traits and Resources of B. Intermedia
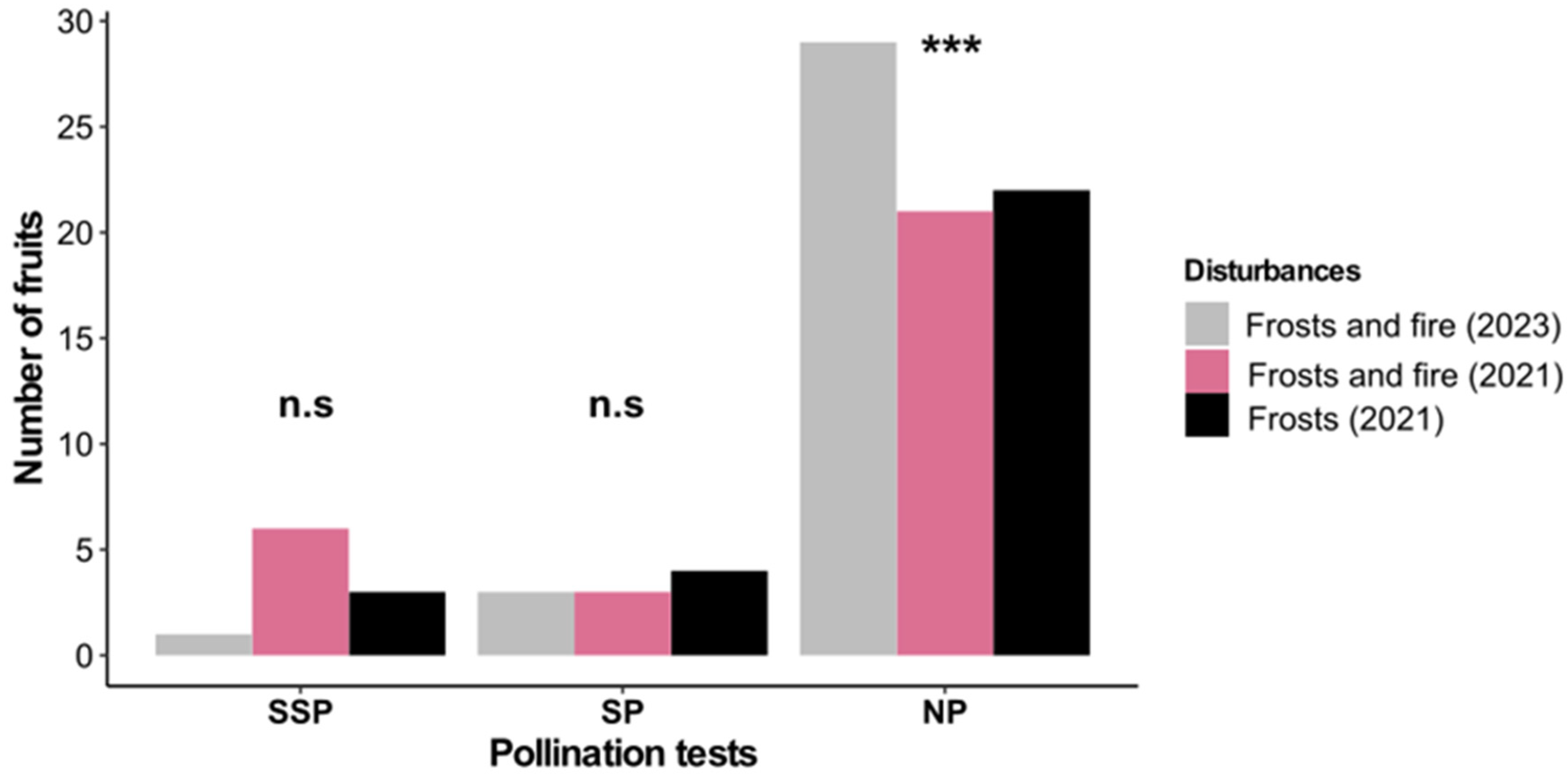
3.4. Fruit Production
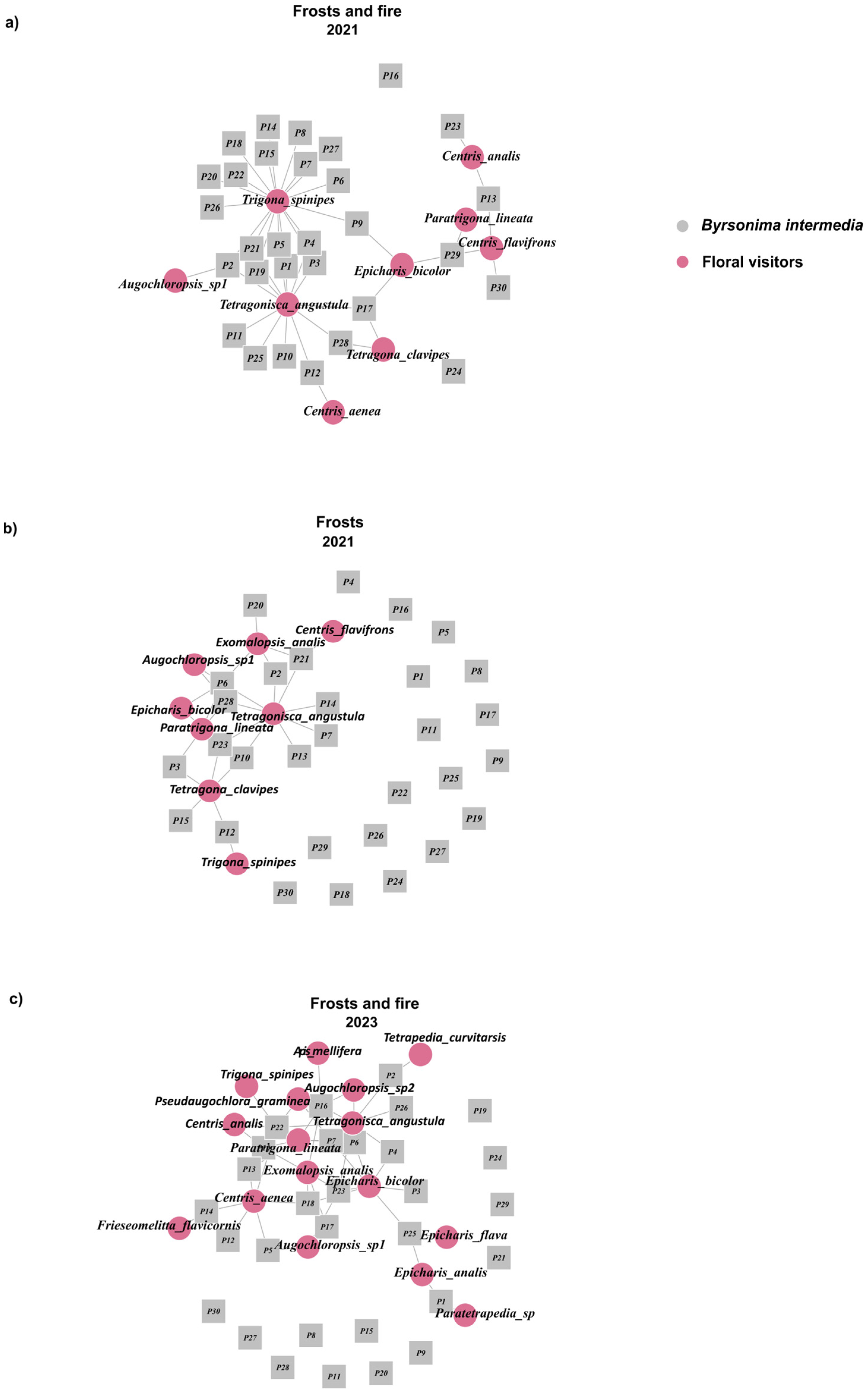
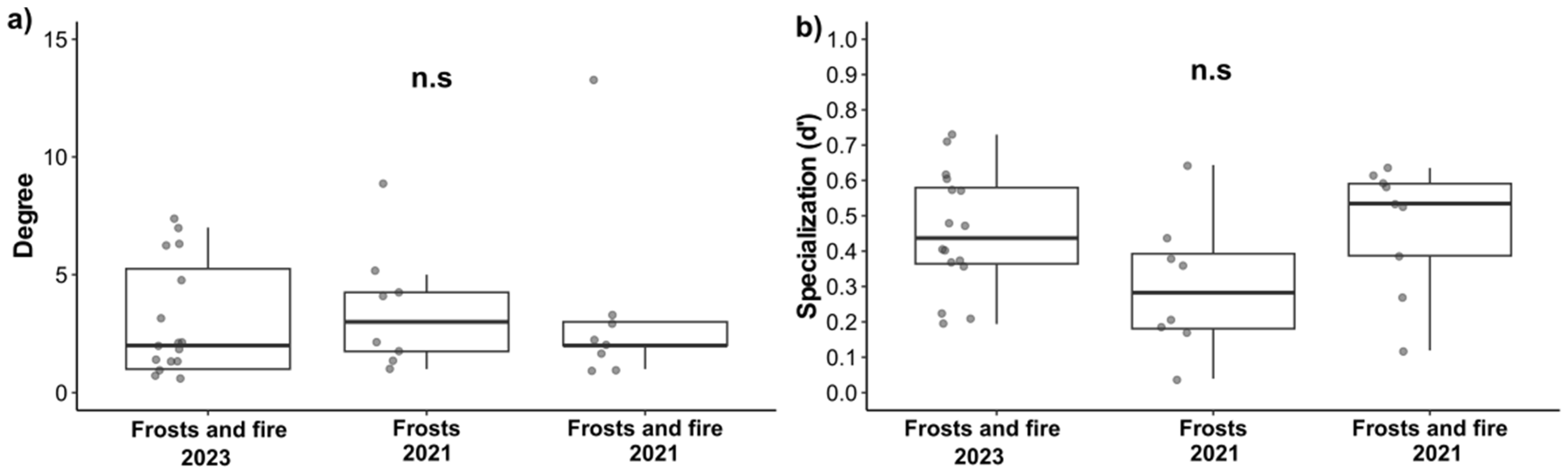
3.5. Plant–Visitor Interaction Network
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, M.F.; Grether, R.; Queiroz, L.P.; Skema, C.; Pennington, R.T.; Hughes, C.E. Recent Assembly of the Cerrado, a Neotropical Plant Diversity Hotspot, by In Situ Evolution of Adaptations to Fire. Proc. Natl. Acad. Sci. USA 2009, 106, 20359–20364. [Google Scholar] [CrossRef] [PubMed]
- Pivello, V.R. The Use of Fire in the Cerrado and Amazonian Rainforests of Brazil: Past and Present. Fire Ecol. 2011, 7, 24–39. [Google Scholar] [CrossRef]
- February, E.C.; Coetsee, C.; Cook, G.D. Physiological Traits of Savanna Woody Species. In Savanna Woody Plants and Large Herbivores; Blackwell Publishing Ltd.: Oxford, UK, 2019; pp. 309–329. [Google Scholar] [CrossRef]
- Carbone, L.M.; Tavella, J.; Marquez, V.; Ashworth, L.; Pausas, J.G.; Aguilar, R. Fire effects on pollination and plant reproduction: A quantitative review. Ann. Bot. 2025, 135, 43–56. [Google Scholar] [CrossRef]
- Lazarina, M.; Sgardelis, S.P.; Tscheulin, T.; Kallimanis, A.S.; Devalez, J.; Petanidou, T. Bee Response to Fire Regimes in Mediterranean Pine Forests: The Role of Nesting Preference, Trophic Specialization, and Body Size. Basic Appl. Ecol. 2016, 17, 308–320. [Google Scholar] [CrossRef]
- Antonio, A.C.; Scalon, M.C.M.; Rossatto, D.R. The Role of Bud Protection and Bark Density in Frost Resistance of Savanna Trees. Plant Biol. 2019, 22, 55–61. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Flake, S.W.; Abreu, R.C.; Pilon, N.A.; Rossatto, D.R.; Durigan, G. Rare frost events reinforce tropical savanna—Forest boundaries. J. Ecol. 2019, 107, 468–477. [Google Scholar] [CrossRef]
- Porto, G.F.; Pezzonia, J.H.; Del-Claro, K. Extrafloral Nectary-Bearing Plants Recover Ant Association Benefits Faster and More Effectively after Frost-Fire Events Than after Frost Alone. Plants 2023, 12, 3592. [Google Scholar] [CrossRef]
- Ollerton, J. Pollinator Diversity: Distribution, Ecological Function, and Conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P. Plant-Animal Mutualistic Networks: The Architecture of Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 567–593. [Google Scholar] [CrossRef]
- Torezan-Silingardi, H.M.; Silberbauer-Gottsberger, I.; Gottsberger, G. Pollination ecology: Natural history, perspectives and future directions. In Plant-Animal Interactions; Springer: Cham, Switzerland, 2021; pp. 119–174. [Google Scholar]
- Morton, E.M.; Rafferty, N.E. Plant–Pollinator Interactions under Climate Change: The Use of Spatial and Temporal Transplants. Appl. Plant Sci. 2017, 5, 1600133. [Google Scholar] [CrossRef]
- Caruso, C.M.; Eisen, K.; Martin, R.A.; Sletvold, N. A meta-analysis of the agents of selection on floral traits. Evolution 2018, 73, 4–14. [Google Scholar] [CrossRef]
- Thompson, J.N. The coevolving web of life. Am. Nat. 2009, 173, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Morente-López, J.; Lara-Romero, C.; Ornosa, C.; Iriondo, J.M. Phenology Drives Species Interactions and Modularity in a Plant—Flower Visitor Network. Sci. Rep. 2018, 8, 9386. [Google Scholar] [CrossRef]
- Suárez-Mariño, A.; Arceo-Gómez, G.; Albor, C.; Parra-Tabla, V. Co-flowering modularity and floral trait similarity help explain temporal changes in plant–pollinator network structure. Plant Ecol. 2022, 223, 1289–1304. [Google Scholar] [CrossRef]
- Layek, U.; Das, U.; Karmakar, P. The Pollination Efficiency of a Pollinator Depends on Its Foraging Strategy, Flowering Phenology, and the Flower Characteristics of a Plant Species. J. Asia-Pac. Entomol. 2022, 25, 101882. [Google Scholar] [CrossRef]
- Peralta, G.; CaraDonna, P.J.; Rakosy, D.; Fründ, J.; Tudanca, M.P.P.; Dormann, C.F.; Burkle, L.A.; Kaiser-Bunbury, C.N.; Knight, T.M.; Resasco, J.; et al. Predicting Plant–Pollinator Interactions: Concepts, Methods, and Challenges. Trends Ecol. Evol. 2024, 39, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, L.G.; Biesmeijer, J.C.; Benadi, G.; Fründ, J.; Stang, M.; Bartomeus, I.; Kaiser-Bunbury, C.N.; Baude, M.; Gomes, S.I.F.; Merckx, V.; et al. The potential for indirect effects between co-flowering plants via shared pollinators depends on resource abundance, accessibility and relatedness. Ecol. Lett. 2014, 17, 1389–1399. [Google Scholar] [CrossRef]
- Welti, E.A.R.; Joern, A. Fire and Grazing Modulate the Structure and Resistance of Plant–Floral Visitor Networks in a Tallgrass Prairie. Oecologia 2018, 186, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Teixido, A.L.; Souza, C.S.; Barônio, G.J.; Torezan, J.M.D.; Fidelis, A. Post-Fire Temporal Dynamics of Plant-Pollinator Communities in a Tropical Savanna. Oecologia 2024, 206, 199–210. [Google Scholar] [CrossRef]
- Pires, E.P.; Faria, L.D.B.; Monteiro, A.B.; Domingos, D.Q.; Mansanares, M.E.; Hermes, M.G. Insect Sociality Plays a Major Role in a Highly Complex Flower-Visiting Network in the Neotropical Savanna. Apidologie 2022, 53, 14. [Google Scholar] [CrossRef]
- Zografou, K.; Swartz, M.T.; Tilden, V.P.; McKinney, E.N.; Eckenrode, J.A.; Sewall, B.J. Stable Generalist Species Anchor a Dynamic Pollination Network. Bull. Ecol. Soc. Am. 2020, 101, e01779. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide Decline of Specialist Species: Toward a Global Functional Homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Bugmann, H.; Seidl, R.; Hartig, F.; Bohn, F.; Brůna, J.; Cailleret, M.; François, L.; Heinke, J.; Henrot, A.J.; Hickler, T.; et al. Tree mortality submodels drive simulated long-term forest dynamics: Assessing 15 models from the stand to global scale. Ecosphere 2019, 10, e02616. [Google Scholar] [CrossRef] [PubMed]
- de Deus, F.F.; Oliveira, P.E. Changes in Floristic Composition and Pollination Systems in a “Cerrado” Community after 20 Years of Fire Suppression. Braz. J. Bot. 2016, 39, 1051–1063. [Google Scholar] [CrossRef]
- Aizen, M.A.; Sabatino, M.; Tylianakis, J.M. Specialization and Rarity Predict Nonrandom Loss of Interactions from Mutualist Networks. Science 2012, 335, 1486–1489. [Google Scholar] [CrossRef] [PubMed]
- Peralta, G.; Stevani, E.L.; Chacoff, N.P.; Dorado, J.; Vázquez, D.P. Fire influences the structure of plant–bee networks. J. Anim. Ecol. 2017, 86, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Maxwell, S.L.; Butt, N.; Maron, M.; McAlpine, C.A.; Chapman, S.; Ullmann, A.; Segan, D.B.; Watson, J.E.M. Conservation Implications of Ecological Responses to Extreme Weather and Climate Events. Divers. Distrib. 2019, 25, 613–625. [Google Scholar] [CrossRef]
- Hardesty, J.; Myers, R.; Fulks, W. January. Fire, ecosystems, and people: A preliminary assessment of fire as a global conservation issue. Georg. Wright Forum 2005, 22, 78–87. [Google Scholar]
- Simon, M.F.; Pennington, R.T. Evidence for Adaptation to Fire Regimes in the Tropical Savannas of the Brazilian Cerrado. Int. J. Plant Sci. 2012, 173, 711–723. [Google Scholar] [CrossRef]
- Pilon, N.A.L.; Cava, M.G.B.; Hoffmann, W.A.; Abreu, R.C.R.; Fidelis, A.; Durigan, G. The Diversity of Post-Fire Regeneration Strategies in the Cerrado Ground Layer. J. Veg. Sci. 2018, 29, 781–792. [Google Scholar] [CrossRef]
- da Silva Goldas, C.; Podgaiski, L.R.; Veronese Corrêa da Silva, C.; Abreu Ferreira, P.M.; Vizentin-Bugoni, J.; de Souza Mendonça, M., Jr. Structural Resilience and High Interaction Dissimilarity of Plant–Pollinator Interaction Networks in Fire-Prone Grasslands. Oecologia 2022, 198, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, P.K.; Bosenbecker, C.; Cardoso, J.C.F.; Sonne, J.; Ballarin, C.S.; Souza, C.S.; Leguizamón, J.; Lopes, A.V.; Maglianesi, M.A.; Otárola, M.F.; et al. Urban Environments Increase Generalization of Hummingbird–Plant Networks across Climate Gradients. Proc. Natl. Acad. Sci. USA 2024, 121, e2322347121. [Google Scholar] [CrossRef] [PubMed]
- Velasque, M.; Del-Claro, K. Host plant phenology may determine the abundance of an ecosystem engineering herbivore in a tropical savanna. Ecol. Entomol. 2016, 41, 421–430. [Google Scholar] [CrossRef]
- Fagundes, R.; Lange, D.; Anjos, D.V.; de Lima, F.P.; Nahas, L.; Corro, E.J.; Silva, P.B.G.; Del-Claro, K.; Ribeiro, S.P.; Dáttilo, W. Limited effects of fire disturbances on the species diversity and structure of ant-plant interaction networks in Brazilian Cerrado. Acta Oecol. 2018, 93, 65–73. [Google Scholar] [CrossRef]
- Sannomia, M.; Cardoso, C.R.P.; Figueiredo, M.E.; Rodrigues, C.M.; Santos, L.C.S.; dos Santos, F.V.; Serpeloni, J.M.; Cólus, I.M.; Vilegas, W.; Varanda, E.A. Mutagenic evaluation and chemical investigation of Byrsonima intermedia A. Juss. leaf extracts. J. Ethnopharmacol. 2007, 112, 319–326. [Google Scholar] [CrossRef]
- Oliveira, M.I.B.; Polido, C.A.; Costa, L.C.; Fava, W.S. Sistema reprodutivo e polinização de Byrsonima intermedia A. Juss. (Malpighiaceae) em Mato Grosso do Sul, Brasil. Rev. Bras. Biociênc. 2007, 5, 756–758. [Google Scholar]
- Filho, L.C.R.; Lomônaco, C. Variações fenotípicas em subpopulações de Davilla elliptica A. St.-Hil. (Dilleniaceae) e Byrsonima intermedia A. Juss. (Malpighiaceae) em uma área de transição cerrado-vereda. Acta Bot. Bras. 2006, 20, 719–725. [Google Scholar] [CrossRef]
- Boas, J.C.V.; Fava, W.S.; Laroca, S.; Sigrist, M.R. Two sympatric Byrsonima species (Malpighiaceae) differ in phenological and reproductive patterns. Flora 2013, 208, 360–369. [Google Scholar] [CrossRef]
- Balestra, C.L.; Fachardo, A.L.S.; Soares, M.P.; Reys, P.; Silva, F.G. Reproductive biology and pollination of two species of Byrsonima Kunth in a Cerrado fragment in Central Brazil. Rev. Bioci. 2014, 20, 71–81. [Google Scholar]
- Silveira, F.A.; Melo, G.A.R.; Almeida, E.A.B. Abelhas Brasileiras: Sistemática e Identificação; Fundação Araucária: Belo Horizonte, Brazil, 2002. [Google Scholar]
- Oliveira, F.F.; Richers, B.T.T.; Silva, J.R.; Farias, R.C.; Matos, T.A.L. Guia Ilustrado das Abelhas Sem-Ferrão das Reservas Amanã e Mamirauá, Brasil (Hymenoptera, Apidae, Meliponini); IDSN: Manaus, Brazil, 2013. [Google Scholar]
- Kearns, C.A.; Inouye, D. Techniques for Pollination Biologists; University Press of Colorado: Niwot, CO, USA, 1993. [Google Scholar]
- Dormann, C.F.; Gruber, B.; Fründ, J. Introducing the bipartite package: Analysing ecological networks. R News 2008, 8, 8–11. [Google Scholar]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, graphs and null models: Analyzing bipartite ecological networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Blüthgen, N.; Fründ, J.; Vázquez, D.P.; Menzel, F. What do interaction network metrics tell us about specialization and biological traits? Ecology 2008, 89, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Patefield, W.M. Algorithm AS 159: An efficient method of generating random R × C tables with given row and column totals. J. R. Stat. Soc. C (Appl. Stat.) 1981, 30, 91–97. [Google Scholar] [CrossRef]
- Jordano, P.; Bascompte, J.; Olesen, J.M. Invariant properties in coevolutionary networks of plant–animal interactions. Ecol. Lett. 2003, 6, 69–81. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models (Version 0.4.1) [R Package]. Available online: https://cran.r-project.org/package=DHARMa (accessed on 24 June 2024).
- Lenth, R. Estimated Marginal Means, Aka Least-Squares Means (emmeans). Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 24 June 2024).
- Teixeira, L.A.G.; Machado, I.C. Sistema de polinização e reprodução de Byrsonima sericea DC (Malpighiaceae). Acta Bot. Bras. 2000, 14, 347–357. [Google Scholar] [CrossRef]
- McCallum, K.P.; McDougall, F.O.; Seymour, R.S. A review of the energetics of pollination biology. J. Comp. Physiol. B 2013, 183, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Classen, A.; Peters, M.K.; Kindeketa, W.J.; Appelhans, T.; Eardley, C.D.; Gikungu, M.W.; Hemp, A.; Nauss, T.; Steffan-Dewenter, I. Temperature versus resource constraints: Which factors determine bee diversity on Mount Kilimanjaro, Tanzania? Glob. Ecol. Biogeogr. 2015, 24, 642–652. [Google Scholar] [CrossRef]
- Li, D.; Belitz, M.; Campbell, L.; Guralnick, R. Extreme Weather Events Have Strong but Different Impacts on Plant and Insect Phenology. Nat. Clim. Change 2025, 15, 321–328. [Google Scholar] [CrossRef]
- Ohnishi, S.; Miyoshi, T.; Shirai, S. Low temperature stress at different flower developmental stages affects pollen development, pollination, and pod set in soybean. Environ. Exp. Bot. 2010, 69, 56–62. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Chiminazzo, M.A.; Bombo, A.B.; Charles-Dominique, T.; Fidelis, A. To protect or to hide: Why not both? An investigation of fire-related strategies in Cerrado woody species. Flora 2023, 306, 152350. [Google Scholar] [CrossRef]
- de Antonio, A.C.; Scalon, M.C.; Rossatto, D.R. Leaf Size and Thickness Are Related to Frost Damage in Ground Layer Species of Neotropical Savannas. Flora 2023, 299, 152208. [Google Scholar] [CrossRef]
- Pearce, R. Plant freezing and damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Muller, K.; O’Connor, T.G.; Henschel, J.R. Impact of a Severe Frost Event in 2014 on Woody Vegetation within the Nama-Karoo and Semi-Arid Savanna Biomes of South Africa. J. Arid Environ. 2016, 133, 112–121. [Google Scholar] [CrossRef]
- Whitecross, M.A.; Archibald, S.A.; Witkowski, E.T.F. Do Freeze Events Create a Demographic Bottleneck for Colophospermum mopane? S. Afr. J. Bot. 2012, 83, 9–18. [Google Scholar] [CrossRef]
- Pardee, G.L.; Inouye, D.W.; Irwin, R.E. Direct and indirect effects of episodic frost on plant growth and reproduction in subalpine wildflowers. Glob. Change Biol. 2018, 24, 848–857. [Google Scholar] [CrossRef]
- e Silva, S.C.O.; Souza, C.S.; de Araújo, W.S. Individual-based plant-visitor networks in Brazilian palm swamps under different dryness levels. Plant Ecol. 2024, 225, 543–554. [Google Scholar] [CrossRef]
- Roel, A.R.; Peruca, R.D.; Lima, F.V.O.; Cheung, K.C.; Neto, A.A.; Da Silva, L.V.; Soares, S. Diversity of Meliponini and Other Apiformes (Apidae sensu lato) in a Cerrado Fragment and Its Surroundings, Campo Grande, MS. Biota Neotropica 2019, 19, e20170333. [Google Scholar] [CrossRef]
- Giannini, T.C.; Garibaldi, L.A.; Acosta, A.L.; Silva, J.S.; Maia, K.P.; Saraiva, A.M.; Guimarães, P.R.; Kleinert, A.M.P.; Blenau, W. Native and non-native supergeneralist bee species have different effects on plant-bee networks. PLoS ONE 2015, 10, e0137198. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P.; Melián, C.J.; Olesen, J.M. The nested assembly of plant–animal mutualistic networks. Proc. Natl. Acad. Sci. USA 2003, 100, 9383–9387. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.; Villalobos, S.; Vamosi, J.C. When less is more: Visitation by generalist pollinators can have neutral or negative effects on plant reproduction. Front. Ecol. Evol. 2022, 10, 1012809. [Google Scholar] [CrossRef]
- Tunes, P.; Alves, V.N.; Valentin-Silva, A.; Batalha, M.A.; Guimarães, E. Does fire affect the temporal pattern of trophic resource supply to pollinators and seed-dispersing frugivores in a Brazilian savanna community? Plant Ecol. 2017, 218, 345–357. [Google Scholar] [CrossRef]
- Potts, S.G.; Vulliamy, B.; Dafni, A.; Ne’eman, G.; Willmer, P.G. Linking bees and flowers: How do floral communities structure pollinator communities? Ecology 2003, 84, 2628–2642. [Google Scholar] [CrossRef]
- Barônio, G.J.; Souza, C.S.; Maruyama, P.K.; Raizer, J.; Sigrist, M.R.; Aoki, C. Natural fire does not affect the structure and beta diversity of plant–pollinator networks, but diminishes floral-visitor specialization in Cerrado. Flora 2021, 281, 151869. [Google Scholar] [CrossRef]
- Aguiar, L.M.S.; Diniz, U.M.; Bueno-Rocha, I.D.; Filomeno, L.R.A.; Aguiar-Machado, L.S.; Gomes, P.A.; Togni, P.H.B. Untangling Biodiversity Interactions: A Meta-Network on Pollination in Earth’s Most Diverse Tropical Savanna. Ecol. Evol. 2024, 14, e11094. [Google Scholar] [CrossRef]
- Brown, J.; York, A.; Christie, F.; McCarthy, M. Effects of Fire on Pollinators and Pollination. J. Appl. Ecol. 2016, 53, 313–322. [Google Scholar] [CrossRef]
- Carbone, M.; Aguilar, R. Fire frequency effects on soil and pollinators: What shapes sexual plant reproduction? Plant Ecol. 2017, 218, 1283–1297. [Google Scholar] [CrossRef]
- García, Y.; Castellanos, M.C.; Pausas, J.G. Fires can benefit plants by disrupting antagonistic interactions. Oecologia 2016, 182, 1165–1173. [Google Scholar] [CrossRef]
- Chiminazzo, M.A.; Charles-Dominique, T.; Andrade, R.S.; Bombo, A.B.; Fidelis, A. How Do Plants Survive in the Starving, Burning, and Hiding Vegetation Realms Generated by Novel Fire Regimes? Perspect. Plant Ecol. Evol. Syst. 2025, 68, 125885. [Google Scholar] [CrossRef]
- Aguiar, J.C.; Melo, G.A.R. Revision and phylogeny of the bee genus Paratetrapedia Moure, with description of a new genus from the Andean Cordillera (Hymenoptera, Apidae, Tapinotaspidini). Zool. J. Linn. Soc. 2011, 162, 351–442. [Google Scholar] [CrossRef]
- Jones, M.W.; Abatzoglou, J.T.; Veraverbeke, S.; Andela, N.; Lasslop, G.; Forkel, M.; Smith, A.J.P.; Burton, C.; Betts, R.A.; van der Werf, G.R.; et al. Global and Regional Trends and Drivers of Fire Under Climate Change. Rev. Geophys. 2022, 60, e2020RG000726. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto, G.F.; Pezzonia, J.H.; Leite, L.J.C.; Sousa Santos, J.L.; Del-Claro, K. The Effects of Frost and Fire on the Traits, Resources, and Floral Visitors of a Cerrado Plant, and Their Impact on the Plant–Visitor Interaction Network and Fruit Formation. Plants 2025, 14, 1977. https://doi.org/10.3390/plants14131977
Porto GF, Pezzonia JH, Leite LJC, Sousa Santos JL, Del-Claro K. The Effects of Frost and Fire on the Traits, Resources, and Floral Visitors of a Cerrado Plant, and Their Impact on the Plant–Visitor Interaction Network and Fruit Formation. Plants. 2025; 14(13):1977. https://doi.org/10.3390/plants14131977
Chicago/Turabian StylePorto, Gabriela Fraga, José Henrique Pezzonia, Ludimila Juliele Carvalho Leite, Jordanny Luiza Sousa Santos, and Kleber Del-Claro. 2025. "The Effects of Frost and Fire on the Traits, Resources, and Floral Visitors of a Cerrado Plant, and Their Impact on the Plant–Visitor Interaction Network and Fruit Formation" Plants 14, no. 13: 1977. https://doi.org/10.3390/plants14131977
APA StylePorto, G. F., Pezzonia, J. H., Leite, L. J. C., Sousa Santos, J. L., & Del-Claro, K. (2025). The Effects of Frost and Fire on the Traits, Resources, and Floral Visitors of a Cerrado Plant, and Their Impact on the Plant–Visitor Interaction Network and Fruit Formation. Plants, 14(13), 1977. https://doi.org/10.3390/plants14131977






