Xylem Sap Mycobiota in Grapevine Naturally Infected with Xylella fastidiosa: A Case Study: Interaction of Xylella fastidiosa with Sclerotinia sclerotiorum
Abstract
1. Introduction
2. Results
2.1. Xylella fastidiosa subsp. Fastidiosa PCR Identification
2.2. Fungal Microbial Diversity Recovered from the Sap of Grapevine Plants
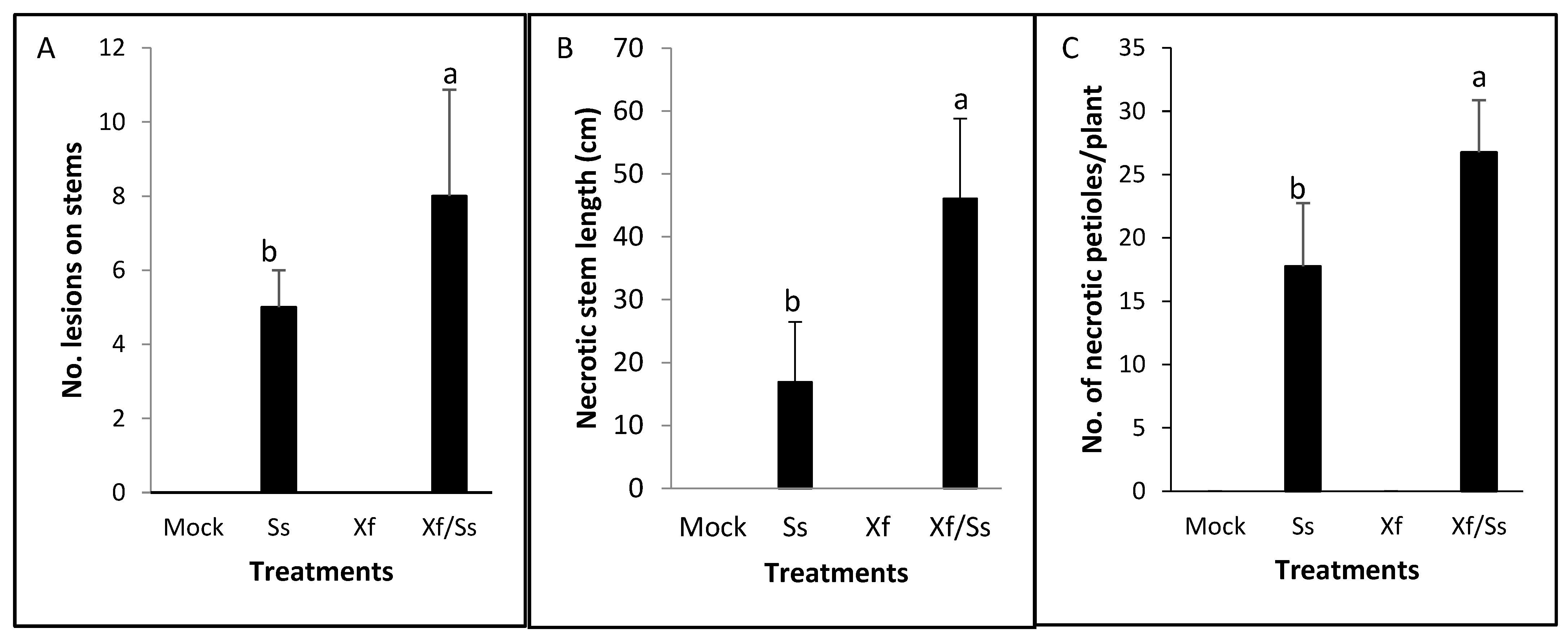
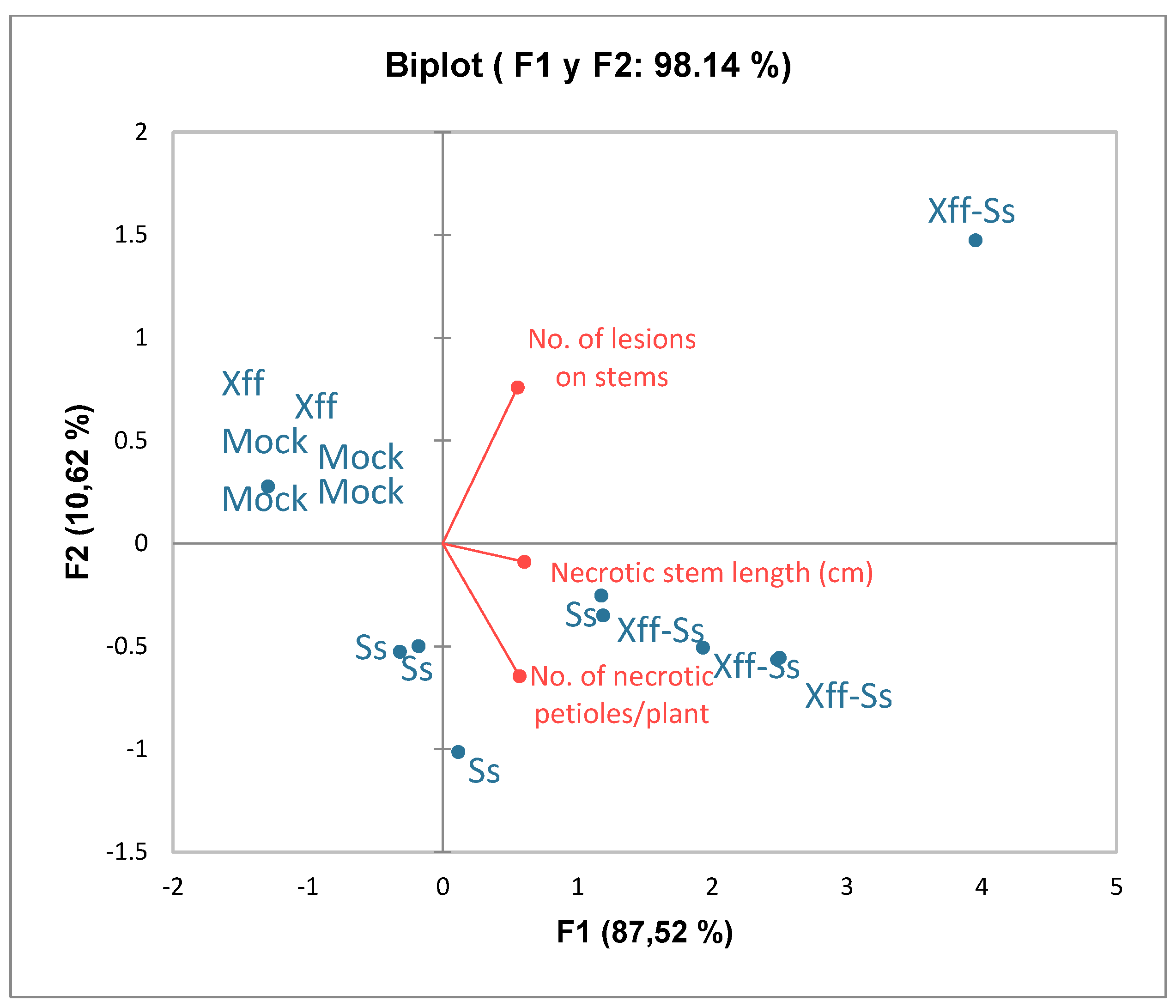
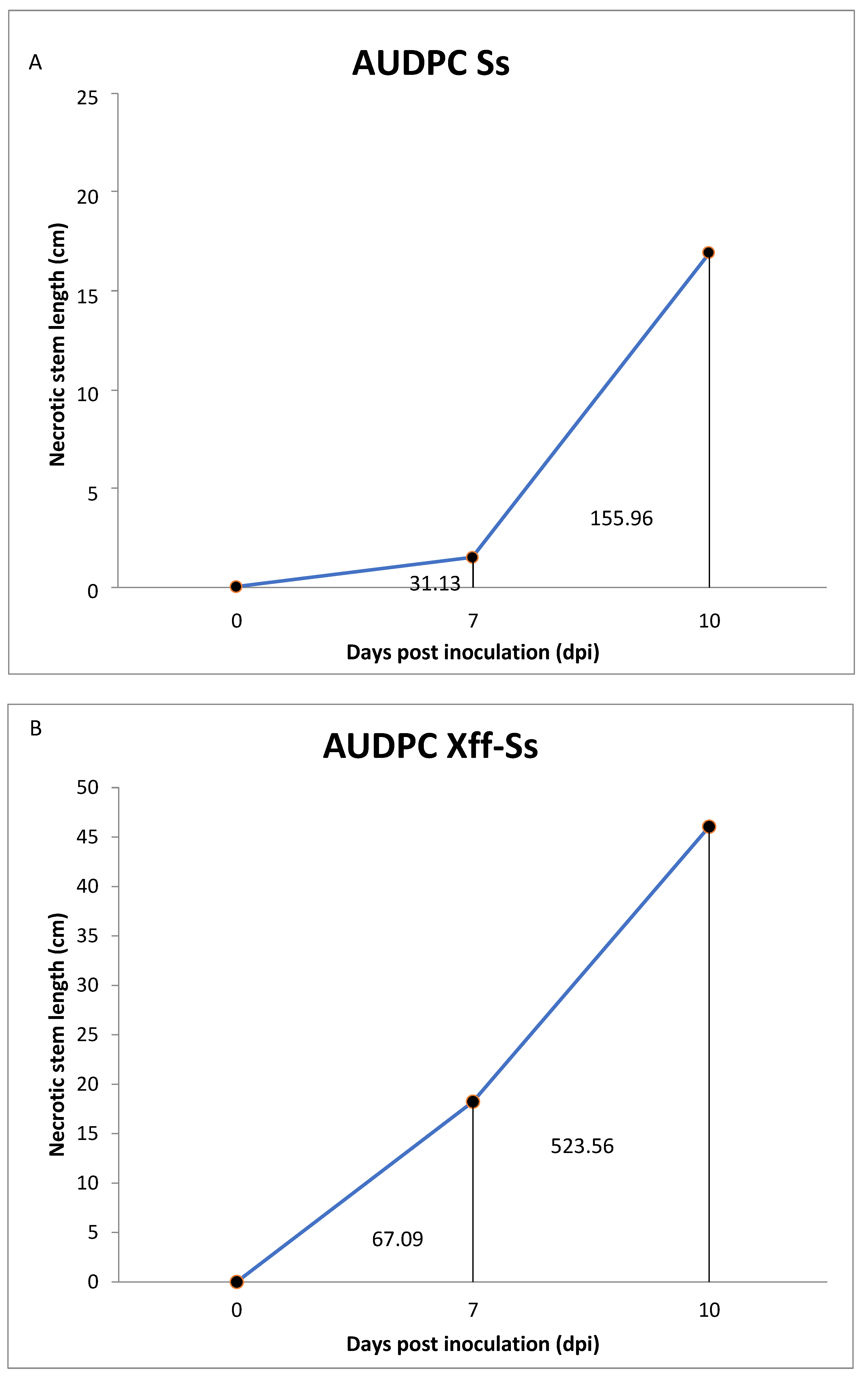
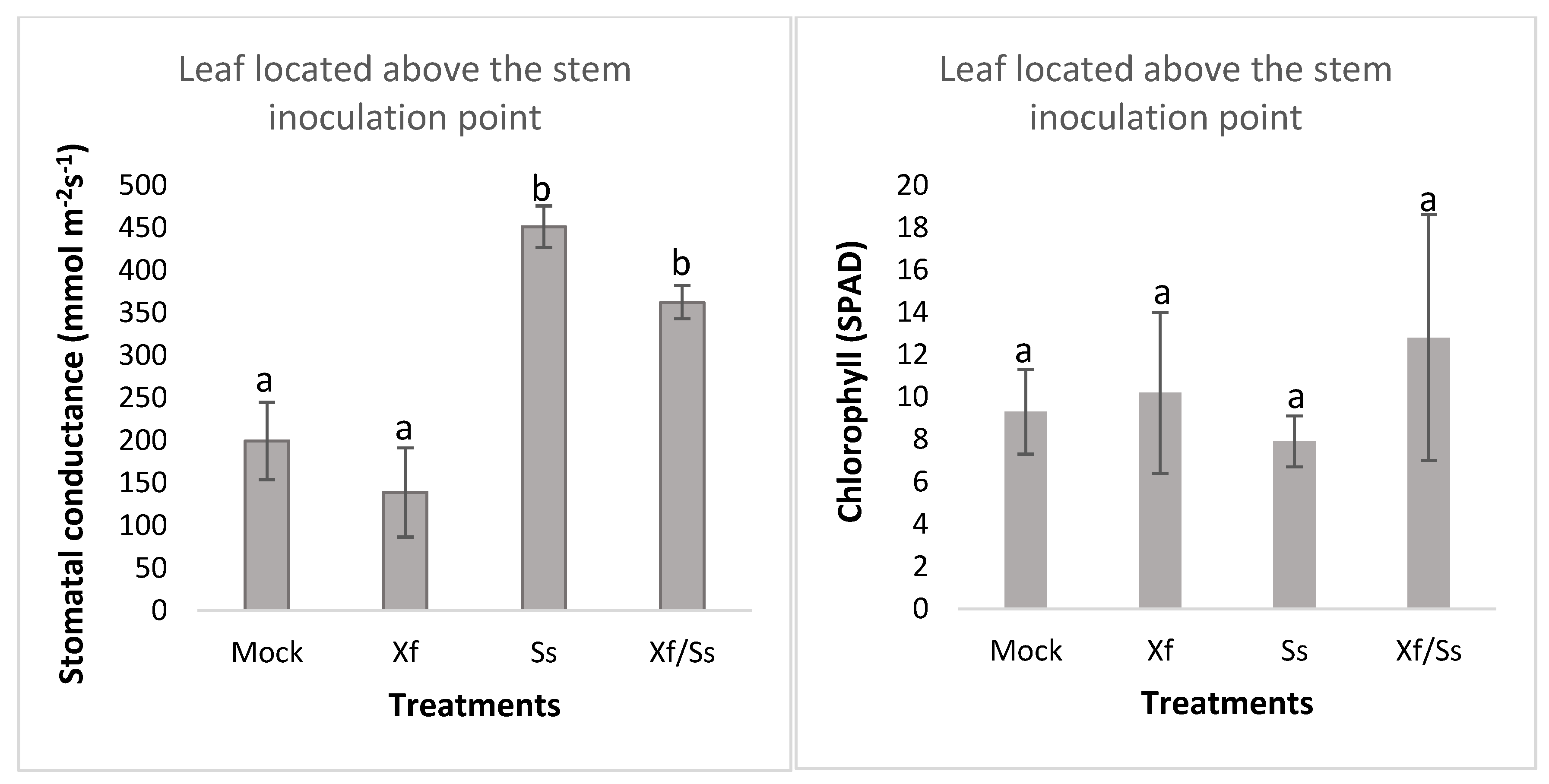
2.3. Interaction Between Sclerotinia sclerotiorum (Ss) and Xylella fastidiosa (Xff) on Grapevine Plants Under Greenhouse-Controlled Conditions
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. PCR Assays to Test the Presence of Xff
4.3. Sap Collection
4.4. Fungal Identification
4.5. Co-Inoculation Assays to Test the Interaction Xylella fastidiosa subsp. fastidiosa (Xff) with Sclerotinia sclerotiorum (Ss)
4.6. Chlorophyll SPAD and Stomatal Conductance
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landa, B.B.; Saponari, M.; Feitosa-Junior, O.R.; Giampetruzzi, A.; Vieira, F.J.D.; Mor, E.; Robatzek, S. Xylella fastidiosa’s relationships: The bacterium, the host plants, and the plant microbiome. New Phytol. 2022, 234, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Raju, B.C.; Hung, H.Y.; Weisburg, W.G.; Mandelco-Paul, L.; Brenner, D.J. Xylella fastidiosa gen. nov, sp. nov: Gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas subsp. Int. J. Syst. Bacteriol. 1987, 37, 136–143. [Google Scholar] [CrossRef]
- Kahn, A.K.; Sicard, A.; Cooper, M.L.; Matthew, P.; Daugherty, P.; Donegan, M.A.; Almeida, P.P. Progression of Xylella fastidiosa infection in grapevines under field conditions. Phytopathology 2023, 113, 1465–1473. [Google Scholar] [CrossRef]
- Chatterjee, S.; Almeida, R.; Lindow, S. Living in two Worlds: The Plant and Insect Lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 2008, 46, 243–271. [Google Scholar] [CrossRef]
- Wistrom, C.M.; Purcell, A. The Fate of Xylella fastidiosa in Vineyard Weeds and Other Alternate Hosts in California. Plant Dis. 2005, 89, 994–999. [Google Scholar] [CrossRef]
- Delbianco, A.; Czwienczek, E.; Pautasso, M.; Kozelska, S.; Monguidi, M.; Stancanelli, G. A new resource for research and risk analysis: The updated European food safety authority database of Xylella spp. host plant species. Phytopathology 2019, 109, 213–215. [Google Scholar] [CrossRef]
- Gómez, P.; Báidez, A.G.; Ortuño, A.; Del Río, J.A. Grapevine xylem response to fungi involved in trunk diseases. Ann. Appl. Biol 2016, 169, 116–124. [Google Scholar] [CrossRef]
- Deyett, E.; Roper, M.C.; Ruegger, P.; Yang, J.I.; Borneman, J.; Rolshausen, P.E. Microbial landscape of the grapevine endosphere in the context of Pierce’s disease. Phytobiomes 2017, 1, 138–149. [Google Scholar] [CrossRef]
- Araujo, W.L.; Marcon, J.; Maccheroni, W.; van Elsas, J.D.; van Vuurde, J.W.L.; Acevedo, J.L. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl. Environ. Microb. 2002, 68, 4906–4914. [Google Scholar] [CrossRef]
- Sarahan, G.; Mehta, N. Sclerotinia Diseases of Crop Plants: Biology, Ecology and Disease Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Guilger-Casagrande, M.; Germano-Costa, T.; Pasquoto-Stigliani, L.; Fraceto, F.; Lima, R.D. Biosynthesis of silver nanoparticles employing Trichoderma harzianum with enzymatic stimulation for the control of Sclerotinia sclerotiorum. Sci. Rep. 2019, 9, 14351. [Google Scholar] [CrossRef]
- Smolińska Uand, B.; Kowalska, B. Biological control of the soil-borne fungal pathogen Sclerotinia sclerotiorum—A review. J. Plant Pathol. 2018, 100, 1–12. [Google Scholar] [CrossRef]
- Aldrich-Wolfe, L.; Travers, S.; Nelson, B.D., Jr. Genetic variation of Sclerotinia sclerotiorum from multiple crops in the North Central United States. PLoS ONE 2015, 10, e0139188. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.; Denton-Giles, M.; Derbyshire, M.; Kamphuis, L.G. Abiotic conditions governing the myceliogenic germination of Sclerotinia sclerotiorum allowing the basal infection of Brassica napus. Australas. Plant Pathol. 2019, 48, 85–91. [Google Scholar] [CrossRef]
- Latorre, B.A.; Guerrero, M.J. First Report of Shoot Blight of Grapevine Caused by Sclerotinia sclerotiorum in Chile. Plant Dis. 2001, 85, 1122. [Google Scholar] [CrossRef]
- Park, J.-H.; Han, K.-S.; Han, Y.-K.; Lee, J.-S.; Kim, D.-H.; Hwang, J.-H. Sclerotinia Shoot Rot of Grapevine (Vitis spp.) Caused by Sclerotinia sclerotiorum in Korea. Res. Plant Dis. 2009, 15, 259–261. [Google Scholar] [CrossRef]
- Boland, G.J.; Hall, R. Index of Plant Hosts of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 1994, 16, 93–108. [Google Scholar] [CrossRef]
- Hall, B.H.; McMahon, R.L.; Wicks, T.J. First report of Sclerotinia sclerotiorum on grape (Vitis vinifera) in South Australia. Australas. Plant Pathol. 2002, 31, 417–418. [Google Scholar] [CrossRef]
- Gonzalez, V.; Tello, M.L. The endophytic mycota associated with Vitis vinifera in central Spain. Fungal Divers. 2011, 47, 29–42. [Google Scholar] [CrossRef]
- Perelló, A.; Olmo, D.; Busquets, A.; Romero Munar, A.; Quetglas, B.; Gost, P.A.; Berbegal, M.; Armengol, J. First report of shoot blight of grapevine caused by Sclerotinia sclerotiorum in Illes Balears, Mallorca, Spain. Plant Dis. 2024, 108, 833–1124. [Google Scholar] [CrossRef]
- Francis, M.; Civerolo, E.L.; Bruening, G. Improved Bioassay of Xylella fastidiosa Using Nicotiana tabacum Cultivar SR1. Plant Dis. 2008, 92, 14. [Google Scholar] [CrossRef]
- Fagorzi, C.; Mengoni, A. Endophytes: Improving Plant Performance. Microorganisms 2022, 10, 1777. [Google Scholar] [CrossRef]
- Almeida, A.B.; Concas, J.; Campos, M.D.; Materatski, P.; Varanda, C.; Patanita, M.; Murolo, S.; Romanazzi, G.; Félix, M.D.R. Endophytic Fungi as Potential Biological Control Agents against Grapevine Trunk Diseases in Alentejo Region. Biology 2020, 9, 420. [Google Scholar] [CrossRef]
- Pinto, C.; Pinho, D.; Sousa, S.; Pinheiro, M.; Egas, C.; Gomes, A.C. Unravelling the diversity of grapevine microbiome. PLoS ONE 2014, 9, e85622. [Google Scholar] [CrossRef]
- Pancher, M.; Ceol, M.; Corneo, P.E.; Longa, C.M.; Yousaf, S.; Pertot, I.; Campisano, A. Fungal endophytic communities in grapevines (Vitis vinifera L.) respond to crop management. Appl. Environ. Microbiol. 2012, 78, 4308–4317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pacifico, D.; Squartini, A.; Crucitti, D.; Barizza, E.; Lo Schiavo, F.; Muresu, R.; Francesco Carimi, F.; Michela Zottini, M. The Role of the Endophytic Microbiome in the Grapevine Response to Environmental Triggers. Front. Plant Sci. 2019, 10, 1256. [Google Scholar] [CrossRef]
- Belda, I.; Zarraonaindia, I.; Perisin, M.; Palacios, A.; Acedo, A. From Vineyard Soil to Wine Fermentation: Microbiome Approximations to Explain the “terroir” Concept. Front. Microbiol. 2017, 8, 821. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A.; Lindow, S.E. Associations among wine grape microbiome, metabolome, and fermentation behavior suggest microbial contribution to regional wine characteristics. mBio 2016, 7, e00631-16. [Google Scholar] [CrossRef]
- Varela, C.; Borneman, A.R. Yeast found in vineyards and wineries. Yeast 2016, 34, 111–128. [Google Scholar] [CrossRef]
- Rathnayake, R.; Savocchia, S.; Schmidtke, L.; Steel, C. Characterisation of Aureobasidium pullulans isolates from Vitis vinifera and potential biocontrol activity for the management of bitter rot of grapes. Eur. J. Plant Pathol. 2018, 151, 593–611. [Google Scholar] [CrossRef]
- Yurchenko, E.; Karpova, D.; Burovinskaya, M.; Vinogradova, S. Leaf Spot Caused by Alternaria spp. Is a New Disease of Grapevine. Plants 2024, 13, 3335. [Google Scholar] [CrossRef]
- Paradiso, G.; Spada, A.; Nerva, L.; Chitarra, W. First report of Alternaria alternata complex causing leaf spot on Vitis vinifera in Italy. Plant Dis. 2024, 108, 2945–3207. [Google Scholar] [CrossRef] [PubMed]
- Granata, G.; Refatti, E. Decline and death of young grapevines by infection of Phoma glomerata on the rootstock. VITIS—J. Grapevine Res. 2016, 20, 341. [Google Scholar]
- Machowicz-Stefanik, Z.; Krol, E. Characterization of Phoma negriana, a new species from grapevine canes in Poland. Acta Mycol. 2013, 42, 113–117. [Google Scholar] [CrossRef]
- Jayawardena, R.; Purahong, P.; Zhang, W.; Tesfaye, W.; Li, X.; Liu, M.; Zhao, W.; Hyde, K.D.; Liu, J. Biodiversity of fungi on Vitis vinifera L. revealed by traditional and high-resolution culture-independent approaches. Fungal Divers. 2018, 90, 1–84. [Google Scholar] [CrossRef]
- Muntean, M.D.; Drăgulinescu, A.M.; Tomoiagă, L.L.; Comșa, M.; Răcoare, H.S.; Sîrbu, A.D.; Chedea, V.S. Fungal Grapevine Trunk Diseases in Romanian Vineyards in the Context of the International Situation. Pathogens 2022, 11, 1006. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Venturi, V. Synergisms between microbial pathogens in plant disease complexes: A growing trend. Front. Plant Sci. 2015, 6, 385. [Google Scholar] [CrossRef]
- Abdullah, A.S.; Moffat, C.S.; Lopez-Ruiz, F.J.; Gibberd, M.R.; Hamblin, J.; Zerihun, A. Host-multipathogen warfare: Pathogen interaction in co-infected plants. Front. Plant Sci. 2017, 8, 1806. [Google Scholar] [CrossRef]
- Roper, C.; Castro, C.; Ingel, B. Xylella fastidiosa: Bacterial parasitism with hallmarks of commensalism. Curr. Opin. Plant Biol. 2019, 50, 140–147. [Google Scholar] [CrossRef]
- Choi, H.K.; Landolino, A.; Goes da Silva, F.; Cook, D.R.B. Water deficit modulates the response of Vitis vinifera to Pierce’s disease pathogen Xylella fastidiosa. Mol. Plant Microbe Interact. 2013, 26, 643–657. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Castro, C.; DiSalvo, B.; Roper, M.C. Xylella fastidiosa: A reemerging plant pathogen that threatens crops globally. PLoS Pathog. 2021, 17, e1009813. [Google Scholar] [CrossRef] [PubMed]
- Camino, C.K.; Araño, J.A.; Berni, H.; Dierkes, J.L.; Trapero-Casas, G.; León-Ropero, M.; Montes-Borrego, M.; Roman-Écija, M.; Velasco-Amo, M.; Landa, B.; et al. Detecting Xylella fastidiosa in a machine learning framework using Vcmax and leaf biochemistry quantified with airborne hyperspectral imagery. Remote Sens. Environ. 2022, 282, 113281. [Google Scholar] [CrossRef]
- Guimarães, R.L.; Stotz, H.U. Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol. 2004, 136, 3703–3711. [Google Scholar] [CrossRef]
- EPPO. PM 7/24 (2) Xylella fastidiosa. EPPO Bull. 2016, 46, 463–500. [Google Scholar] [CrossRef]
- Harper, S.J.; Ward, L.I.; Clover, G.R. Development of LAMP and real-time PCR methods for the rapid detection of Xylella fastidiosa for quarantine and field applications. Phytopathology 2010, 100, 1282–1288. [Google Scholar] [CrossRef]
- Index Fungorum. 2018. Available online: https://indexfungorum.org/Names/Names.asp (accessed on 14 May 2023).
- Madden, L.V.; Hughes, G.; van den Bosch, F. The Study of Plant Disease Epidemics; APS Press: St. Paul, MN, USA, 2007. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2018; Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba: Córdoba, Argentina, 2018. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perelló, A.; Romero-Munar, A.; Martinez, S.I.; Busquets, A.; Cañellas, M.; Quetglas, B.M.; Bosch, R.; Vadell, J.; Cabot, C.; Gomila, M. Xylem Sap Mycobiota in Grapevine Naturally Infected with Xylella fastidiosa: A Case Study: Interaction of Xylella fastidiosa with Sclerotinia sclerotiorum. Plants 2025, 14, 1976. https://doi.org/10.3390/plants14131976
Perelló A, Romero-Munar A, Martinez SI, Busquets A, Cañellas M, Quetglas BM, Bosch R, Vadell J, Cabot C, Gomila M. Xylem Sap Mycobiota in Grapevine Naturally Infected with Xylella fastidiosa: A Case Study: Interaction of Xylella fastidiosa with Sclerotinia sclerotiorum. Plants. 2025; 14(13):1976. https://doi.org/10.3390/plants14131976
Chicago/Turabian StylePerelló, Analía, Antonia Romero-Munar, Sergio I. Martinez, Antonio Busquets, María Cañellas, Bárbara M. Quetglas, Rafael Bosch, Jaume Vadell, Catalina Cabot, and Marga Gomila. 2025. "Xylem Sap Mycobiota in Grapevine Naturally Infected with Xylella fastidiosa: A Case Study: Interaction of Xylella fastidiosa with Sclerotinia sclerotiorum" Plants 14, no. 13: 1976. https://doi.org/10.3390/plants14131976
APA StylePerelló, A., Romero-Munar, A., Martinez, S. I., Busquets, A., Cañellas, M., Quetglas, B. M., Bosch, R., Vadell, J., Cabot, C., & Gomila, M. (2025). Xylem Sap Mycobiota in Grapevine Naturally Infected with Xylella fastidiosa: A Case Study: Interaction of Xylella fastidiosa with Sclerotinia sclerotiorum. Plants, 14(13), 1976. https://doi.org/10.3390/plants14131976





