Plant-Pollinator and Plant-Florivore Interactions in Two Savanna Species of Malpighiaceae
Abstract
1. Introduction


| Peixotoa tomentosa | Byrsonima intermedia | |||
|---|---|---|---|---|
| Treatment | N Flowers/N Fruits | Fruit Set (%) | N Flowers/N Fruits | Fruit Set (%) |
| Agamospermy | 46/16 | 34.78 | 29/1 | 3.44 |
| Spontaneous Self-pollination | 46/5 | 10.86 | 30/0 | 0 |
| Manual Self-pollination | 41/9 | 21.95 | 28/0 | 0 |
| Cross-pollination | 31/5 | 16.12 | 30/24 | 80 |
| Control | 50/5 | 10.0 | 30/17 | 56.6 |
| ISI | 21.95/16.12 = 1.36 | 0/80 = 0 | ||
| IRE | 10/16.12 = 0.62 | 56.6/80 = 0.71 | ||
| Floral Visitor Tribe, Species | Peixotoa tomentosa | Byrsonima intermedia | Resource Collected | ||
|---|---|---|---|---|---|
| High Density | Low Density | High Density | Low Density | ||
| Apini | |||||
| Apis mellifera | - | 1 | - | - | PS |
| Centridini | |||||
| Centris flavifrons | - | - | 1 | - | O * |
| Centris sp. | - | - | 1 | 1 | PV */O * |
| Epicharis bicolor | - | - | 10 | 4 | PV */O * |
| Epicharis sp. | - | - | 13 | 3 | PV */O * |
| Exomalopsini | |||||
| Exomalopsis analis | - | - | 4 | - | O * |
| Halictini | |||||
| Agapostemon sp. | - | - | 2 | 3 | PS |
| Dialictus sp. | - | - | - | 1 | PS |
| Meliponini | |||||
| Paratrigona lineata | 1 | 1 | 5 | 12 | PS |
| Plebeia saiqui | - | - | 3 | - | O |
| Tetragona clavipes | - | - | 1 | - | O |
| Tetragonisca angustula | 2 | 3 | 3 | 1 | PS/O |
| Trigona spinipes | 1 | 4 | 2 | 5 | PS/O |
| Trigonisca sp. | - | - | - | 1 | PS |
| Total Bees | 4 | 9 | 46 | 31 | |
2. Results
2.1. Fruiting as a Function of the Reproductive System and Density
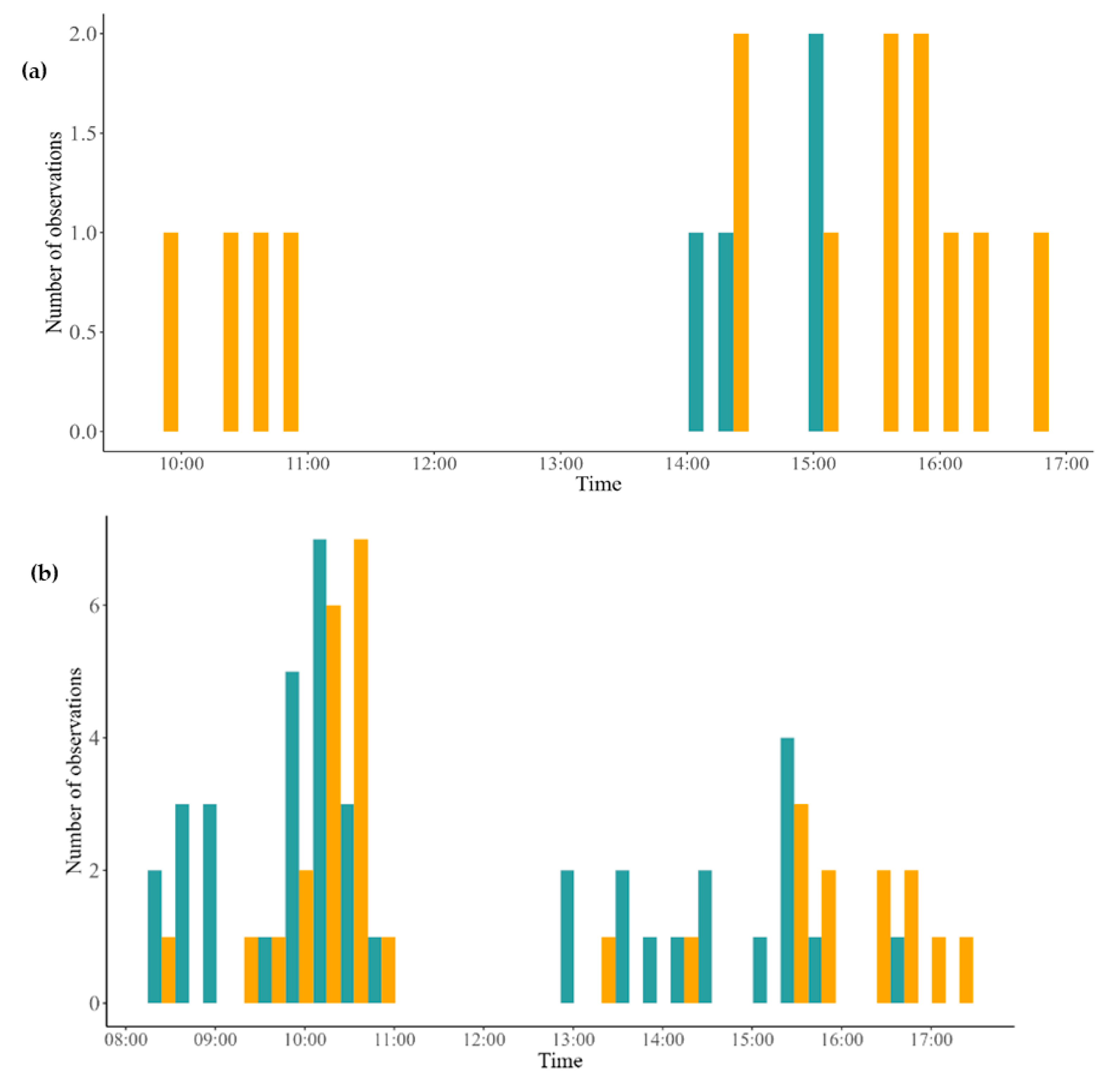
2.2. Floral Visitation Due to Density
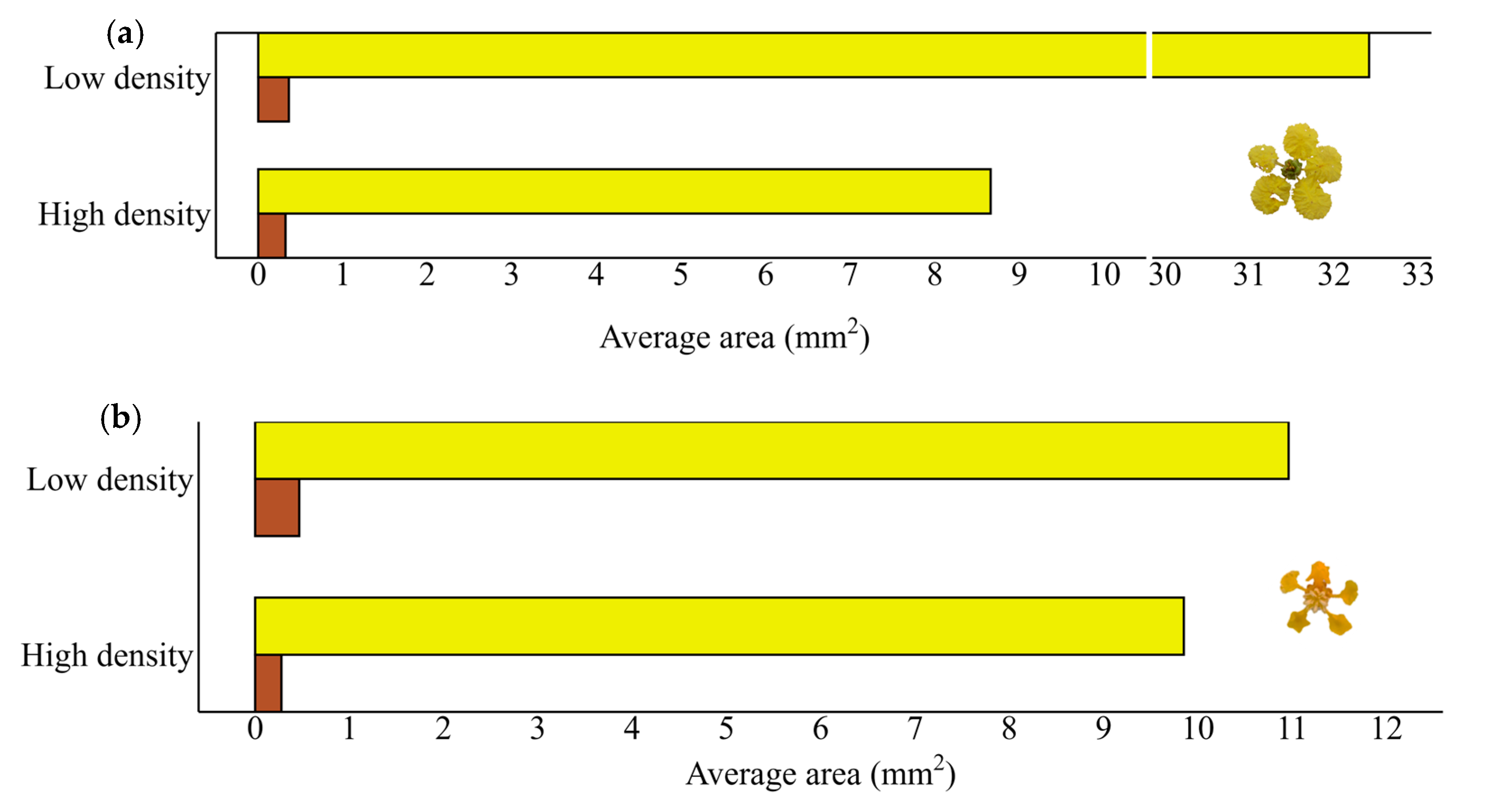
2.3. Investigating Florivory as a Function of Density
3. Discussion
4. Materials and Methods
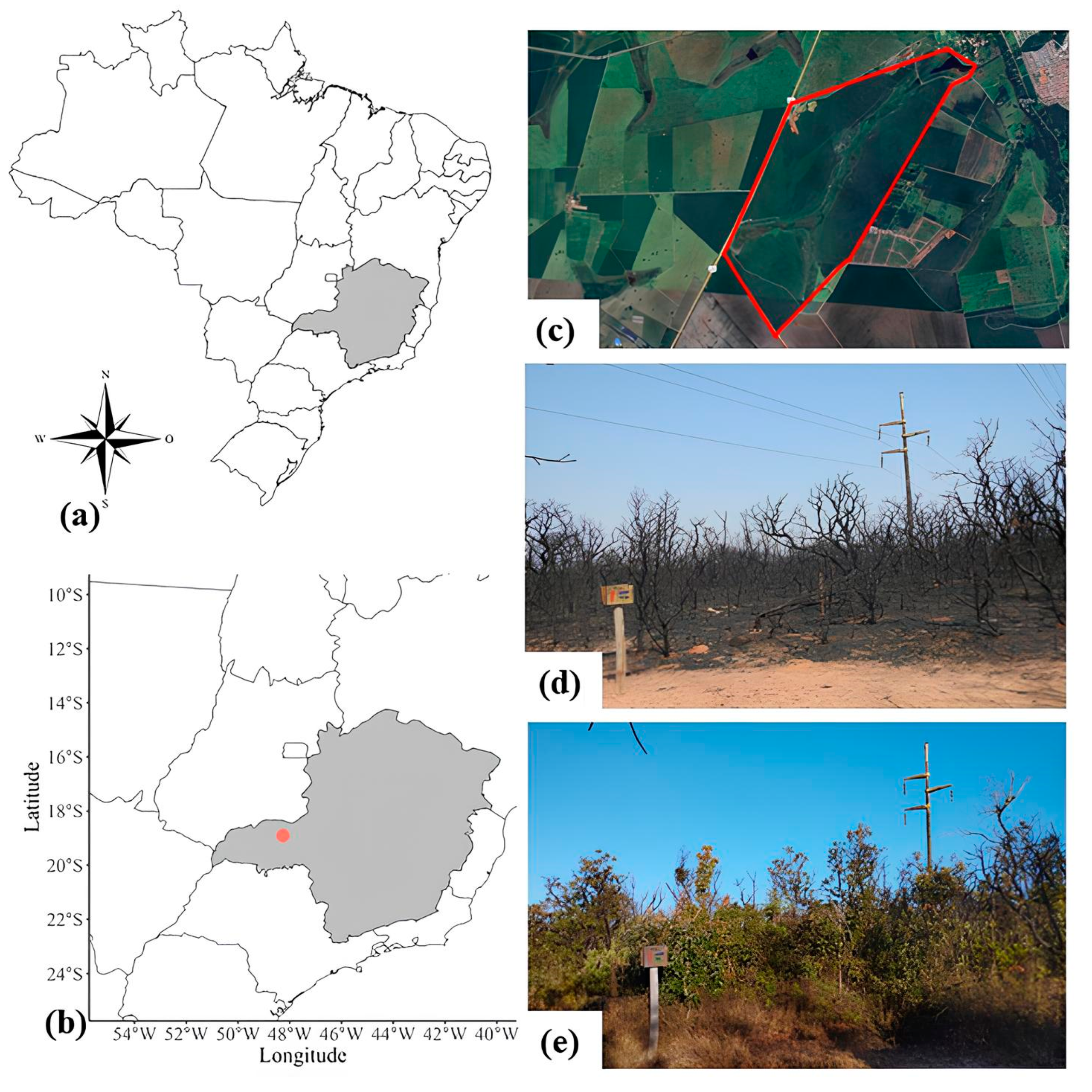
4.1. Area and Period of Study
4.2. Focal Species
4.3. Fruiting as a Function of Reproductive System and Density
4.4. Visitation as a Function of Density and Visitor’s Function
4.5. Florivory as a Function of Density
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janzen, D.H. Herbivores and the Number of Tree Species in Tropical Forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs: High Diversity of Trees and Corals Is Maintained Only in a Nonequilibrium State. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Clark, D.B. Spacing Dynamics of a Tropical Rain Forest Tree: Evaluation of the Janzen-Connell Model. Am. Nat. 1984, 124, 769–788. [Google Scholar] [CrossRef]
- Hülsmann, L.; Chisholm, R.A.; Hartig, F. Is Variation in Conspecific Negative Density Dependence Driving Tree Diversity Patterns at Large Scales? Trends Ecol. Evol. 2021, 36, 151–163. [Google Scholar] [CrossRef]
- Schupp, E.W. The Janzen-Connell Model for Tropical Tree Diversity: Population Implications and the Importance of Spatial Scale. Am. Nat. 1992, 140, 526–530. [Google Scholar] [CrossRef]
- Boaventura, M.G.; Villamil, N.; Teixido, A.L.; Tito, R.; Vasconcelos, H.L.; Silveira, F.A.O.; Cornelissen, T. Revisiting Florivory: An Integrative Review and Global Patterns of a Neglected Interaction. New Phytol. 2022, 233, 132–144. [Google Scholar] [CrossRef]
- Canela, M.B.F.; Sazima, M. Aechmea Pectinata: A Hummingbird-Dependent Bromeliad with Inconspicuous Flowers from the Rainforest in South-Eastern Brazil. Ann. Bot. 2003, 92, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.A.; Torezan-Silingardi, H.M. Implications of the Floral Herbivory on Malpighiacea Plant Fitness: Visual Aspect of the Flower Affects the Attractiveness to Pollinators. Sociobiology 2013, 60, 323–328. [Google Scholar] [CrossRef]
- McCall, A.C.; Irwin, R.E. Florivory: The Intersection of Pollination and Herbivory. Ecol. Lett. 2006, 9, 1351–1365. [Google Scholar] [CrossRef]
- Moreira, X.; Castagneyrol, B.; Abdala-Roberts, L.; Traveset, A. A Meta-analysis of Herbivore Effects on Plant Attractiveness to Pollinators. Ecology 2019, 100, e02707. [Google Scholar] [CrossRef]
- Bergamo, P.J.; Susin Streher, N.; Traveset, A.; Wolowski, M.; Sazima, M. Pollination Outcomes Reveal Negative Density-dependence Coupled with Interspecific Facilitation among Plants. Ecol. Lett. 2020, 23, 129–139. [Google Scholar] [CrossRef]
- Tong, Z.-Y.; Wu, L.-Y.; Feng, H.-H.; Zhang, M.; Armbruster, W.S.; Renner, S.S.; Huang, S.-Q. New Calculations Indicate That 90% of Flowering Plant Species Are Animal-Pollinated. Natl. Sci. Rev. 2023, 10, nwad219. [Google Scholar] [CrossRef]
- Ackerman, J.D. Abiotic Pollen and Pollination: Ecological, Functional, and Evolutionary Perspectives. In Pollen and Pollination; Dafni, A., Hesse, M., Pacini, E., Eds.; Springer: Vienna, Austria, 2000; pp. 167–185. ISBN 978-3-7091-7248-3. [Google Scholar]
- Rech, A.R.; Dalsgaard, B.; Sandel, B.; Sonne, J.; Svenning, J.-C.; Holmes, N.; Ollerton, J. The Macroecology of Animal versus Wind Pollination: Ecological Factors Are More Important than Historical Climate Stability. Plant Ecol. Divers. 2016, 9, 253–262. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How Many Flowering Plants Are Pollinated by Animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Da Silva, C.I.; Aleixo, K.P.; Nunes-Silva, B.; Freitas, B.M.; Imperatriz-Fonseca, V.L. Guia Ilustrado de Abelhas Polinizadoras no Brasil; Cartilhas; Instituto De Estudos Avançados—Iea: Paris, France, 2014; ISBN 978-85-63007-07-0. [Google Scholar]
- Silberbauer-Gottsberger, I.; Gottsberger, G. A Polinização de Plantas Do Cerrado. Revs. Brasil. Biol 1987, 48, 651–663. [Google Scholar]
- Faegri, K.; van der Pijl, L. The Principles of Pollination Ecology; Elsevier: Amsterdam, The Netherlands, 1979. [Google Scholar]
- Del-Claro, K.; Torezan-Silingardi, H. Plant-Animal Interactions Source of Biodiversity; Springer: Berlin/Heidelberg, Germany, 2021; ISBN 978-3-030-66876-1. [Google Scholar]
- Nunes, C.E.P.; Peñaflor, M.F.G.V.; Bento, J.M.S.; Salvador, M.J.; Sazima, M. The Dilemma of Being a Fragrant Flower: The Major Floral Volatile Attracts Pollinators and Florivores in the Euglossine-Pollinated Orchid Dichaea pendula. Oecologia 2016, 182, 933–946. [Google Scholar] [CrossRef]
- Grindeland, J.M.; Sletvold, N.; Ims, R.A. Effects of Floral Display Size and Plant Density on Pollinator Visitation Rate in a Natural Population of Digitalis purpurea. Funct. Ecol. 2005, 19, 383–390. [Google Scholar] [CrossRef]
- Essenberg, C.J. Explaining Variation in the Effect of Floral Density on Pollinator Visitation. Am. Nat. 2012, 180, 153–166. [Google Scholar] [CrossRef]
- Allee, W.C. Animal Aggregations. Q. Rev. Biol. 1927, 2, 367–398. [Google Scholar] [CrossRef]
- Courchamp, F.; Clutton-Brock, T.; Grenfell, B. Inverse Density Dependence and the Allee Effect. Trends Ecol. Evol. 1999, 14, 405–410. [Google Scholar] [CrossRef]
- Stephens, P.A.; Sutherland, W.J.; Freckleton, R.P. What Is the Allee Effect? Oikos 1999, 87, 185. [Google Scholar] [CrossRef]
- Johnson, S.D.; Torninger, E.; Ågren, J. Relationships between Population Size and Pollen Fates in a Moth-Pollinated Orchid. Biol. Lett. 2009, 5, 282–285. [Google Scholar] [CrossRef]
- Cheptou, P.; Avendaño, V.L.G. Pollination Processes and the Allee Effect in Highly Fragmented Populations: Consequences for the Mating System in Urban Environments. New Phytol. 2006, 172, 774–783. [Google Scholar] [CrossRef]
- Pettersson, M.W.; Sjödin, E. Effects of Experimental Plant Density Reductions on Plant Choice and Foraging Behaviour of Bees (Hymenoptera:Apoidea). Acta Agric. Scand. Sect. B—Soil Plant Sci. 2000, 50, 40–46. [Google Scholar] [CrossRef]
- Ghazoul, J. Floral Diversity and the Facilitation of Pollination. J. Ecol. 2006, 94, 295–304. [Google Scholar] [CrossRef]
- Bruninga-Socolar, B.; Winfree, R.; Crone, E.E. The Contribution of Plant Spatial Arrangement to Bumble Bee Flower Constancy. Oecologia 2022, 198, 471–481. [Google Scholar] [CrossRef]
- Ashman, T.-L.; Knight, T.M.; Steets, J.A.; Amarasekare, P.; Burd, M.; Campbell, D.R.; Dudash, M.R.; Johnston, M.O.; Mazer, S.J.; Mitchell, R.J.; et al. Pollen Limitation of Plant Reproduction: Ecological and Evolutionary Causes and Consequences. Ecology 2004, 85, 2408–2421. [Google Scholar] [CrossRef]
- Rodger, J.G.; Van Kleunen, M.; Johnson, S.D. Pollinators, Mates and Allee Effects: The Importance of Self-pollination for Fecundity in an Invasive Lily. Funct. Ecol. 2013, 27, 1023–1033. [Google Scholar] [CrossRef]
- Cheptou, P.-O. Allee Effect and Self-Fertilization in Hermaphrodites: Reproductive Assurance in Demographically Stable Populations. Evolution 2004, 58, 2613–2621. [Google Scholar] [CrossRef]
- Morgan, M.T.; Wilson, W.G.; Knight, T.M. Plant Population Dynamics, Pollinator Foraging, and the Selection of Self-Fertilization. Am. Nat. 2005, 166, 169–183. [Google Scholar] [CrossRef]
- Hörandl, E. Apomixis and the Paradox of Sex in Plants. Ann. Bot. 2024, 134, 1–18. [Google Scholar] [CrossRef]
- Rivest, S. Interspecific Variation in Plant Abundance Can Contribute to the Diversity and Evolutionary Assembly of Floral Communities. Oikos 2023, 2023, e09532. [Google Scholar] [CrossRef]
- Porto, G.F.; Carvalho-Leite, L.J.; Pezzonia, J.H.; Santos, J.L.S.; Del-Claro, K. The Effects of Frost and Fire on the Characteristics, Resources and Floral Visitors of Byrsonima intermedia A. Juss., and Their Impact on the Plant-Visitor Interaction Network and Fruit Formation. Plants 2025, 14, 1977. [Google Scholar] [CrossRef]
- Torezan-Silingardi, H.M. A Influência dos Herbívoros Florais, dos Polinizadores e das Características Fenológicas Sobre a Frutificação de Espécies da Família Malpighiaceae em um Cerrado de Minas Gerais. Ph.D. Thesis, Universidade de São Paulo: Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, São Paulo, Brazil, 2007. [Google Scholar]
- Barônio, G.J.; Haleem, M.A.; Marsaioli, A.J.; Torezan-Silingardi, H.M. Characterization of Malpighiaceae Flower-Visitor Interactions in a Brazilian Savannah: How Do Floral Resources and Visitor Abundance Change over Time. Flora 2017, 234, 126–134. [Google Scholar] [CrossRef]
- Vilela, A.A.; Del Claro, V.T.S.; Torezan-Silingardi, H.M.; Del-Claro, K. Climate Changes Affecting Biotic Interactions, Phenology, and Reproductive Success in a Savanna Community over a 10-Year Period. Arthropod-Plant Interact. 2018, 12, 215–227. [Google Scholar] [CrossRef]
- Anderson, C. A Monografph of the Genus Peixotoa (Malpighiaceae). Contr. Univ. Mich. Herb. 1982, 15, 1–92. [Google Scholar]
- Cappellari, S.C. Evolutionary Ecology of Malpighiaceae Pollination at the Species and Community Levels. Ph.D. Thesis, The University of Texas at Austin: Faculty of the Graduate School, Austin, TX, USA, 2011. [Google Scholar]
- Sigrist, M.R.; Sazima, M. Pollination and Reproductive Biology of Twelve Species of Neotropical Malpighiaceae: Stigma Morphology and Its Implications for the Breeding System. Ann. Bot. 2004, 94, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Possobom, C.C.F.; Machado, S.R.; Guimarães, E. Reproductive System of Diplopterys pubipetala (Malpighiaceae) Plants from a Savanna Ecosystem. Int. J. Plant Reprod. Biol. 2016, 8, 1–6. [Google Scholar]
- Mendes, F.N.; Rêgo, M.M.C.; Albuquerque, P.M.C.D. Fenologia e biologia reprodutiva de duas espécies de Byrsonima Rich. (Malpighiaceae) em área de Cerrado no Nordeste do Brasil. Biota Neotrop. 2011, 11, 103–115. [Google Scholar] [CrossRef]
- Streher, N.S.; Bergamo, P.J.; Ashman, T.-L.; Wolowski, M.; Sazima, M. Effect of Heterospecific Pollen Deposition on Pollen Tube Growth Depends on the Phylogenetic Relatedness between Donor and Recipient. AoB PLANTS 2020, 12, plaa016. [Google Scholar] [CrossRef]
- Reposi, S.D.; Avalos, A.A.; Gotelli, M.M.; Aliscioni, S.S.; Torretta, J.P. Reproductive Biology of Malpighiaceae: How Much Do We Know? Plant Syst. Evol. 2023, 309, 25. [Google Scholar] [CrossRef]
- Cappellari, S.C.; Haleem, M.A.; Marsaioli, A.J.; Tidon, R.; Simpson, B.B. Pterandra Pyroidea: A Case of Pollination Shift within Neotropical Malpighiaceae. Ann. Bot. 2011, 107, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.F.; de Oliveira, D.; Ohashi, O.S. Pollination and Pollen Vectors in Acerola Malpighia punicifolia. Acta. Hortic. 1997, 437, 419–424. [Google Scholar] [CrossRef]
- Batista, J.; Pacheco, J.F.M.; Santos, L.M. Biologia Reprodutiva de Três Espécies de Byrsonima Rich Ex Kunth (Malpighiaceae) Em Um Cerrado Sensu Stricto No Campus Da Universidade Estadual de Goiás. Rev. De Biol. Neotrop. 2005, 2, 109–122. [Google Scholar]
- Benezar, R.M.C.; Pessoni, L.A. Biologia floral e sistema reprodutivo de Byrsonima coccolobifolia (Kunth) em uma savana amazônica. Acta Amaz. 2006, 36, 159–168. [Google Scholar] [CrossRef]
- Amorim, M.; De Marco, P. Pollination of Byrsonima coccolobifolia: Short-Distance Isolation and Possible Causes for Low Fruit Production. Braz. J. Biol. 2011, 71, 709–717. [Google Scholar] [CrossRef]
- Bachiega, M.I.; Carvalho, L. Sistema reprodutivo e polinização de Byrsonima intermedia A. Juss. (Malpighiaceae) em Mato Grosso do Sul, Brasil. Rev. Bras. Biociências 2007, 5, 756–758. [Google Scholar]
- Melo, L.R.F.; Guimarães, B.M.D.C.; Barônio, G.J.; Oliveira, L.C.D.; Cardoso, R.K.D.O.A.; Araújo, T.N.; Telles, F.J. Como as abelhas percebem as flores e por que isto é importante? Oecol. Aust. 2018, 22, 362–389. [Google Scholar] [CrossRef]
- Underwood, N.; Hambäck, P.A.; Inouye, B.D. Pollinators, Herbivores, and Plant Neighborhood Effects. Q. Rev. Biol. 2020, 95, 37–57. [Google Scholar] [CrossRef]
- Torezan-Silingardi, H.M.; Ilse, S.-G.; Gottsberger, G. Pollination Ecology: Natural History, Perspectives and Future Directions. In Plant-Animal Interactions Source of Biodiversity; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Makino, T.T.; Ohashi, K.; Sakai, S. How Do Floral Display Size and the Density of Surrounding Flowers Influence the Likelihood of Bumble Bee Revisitation to a Plant? Funct. Ecol. 2007, 21, 87–95. [Google Scholar] [CrossRef]
- Agren, J. Population Size, Pollinator Limitation, and Seed Set in the Self- Incompatible Herb Lythrum salicaria. Ecology 1996, 77, 1779–1790. [Google Scholar] [CrossRef]
- Bruninga-Socolar, B.; Crone, E.E.; Winfree, R. The Role of Floral Density in Determining Bee Foraging Behavior: A Natural Experiment. Nat. Areas J. 2016, 36, 392–399. [Google Scholar] [CrossRef]
- Chittka, L.; Thomsom, J.D. Cognitive Ecology of Pollination; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Barley, T.A.; Martinez Algarin, M.G.; Bauer, J.T. The Effects of Flower Patch Density on Pollinator Visitation. Environ. Entomol. 2022, 51, 482–491. [Google Scholar] [CrossRef]
- Hurtado, M.; Godoy, O.; Bartomeus, I. Plant Spatial Aggregation Modulates the Interplay between Plant Competition and Pollinator Attraction with Contrasting Outcomes of Plant Fitness. Web Ecol. 2023, 23, 51–69. [Google Scholar] [CrossRef]
- Barônio, G.J.; Torezan-Silingardi, H.M. Temporal Niche Overlap and Distinct Bee Ability to Collect Floral Resources on Three Species of Brazilian Malpighiaceae. Apidologie 2017, 48, 168–180. [Google Scholar] [CrossRef]
- Vogel, S. History in Malpighiaceae. Mem. N. Y. Bot. Gard. 1990, 55, 130–142. [Google Scholar]
- Alves-dos-Santos, I.; Machado, I.C.; Gaglianone, M.C. História natural das abelhas coletoras de óleo. Oeco. Bras. 2007, 11, 544–557. [Google Scholar] [CrossRef][Green Version]
- Michener, C.D. The Bees of the World; JHU Press: Baltimore, MD, USA, 2000; Volume 1. [Google Scholar]
- De Oliveira Dias, K.P.; Stefani, V. Spider–Plant Interaction: The Role of Extrafloral Nectaries in Spider Attraction and Their Influence on Plant Herbivory and Reproduction. Plants 2024, 13, 368. [Google Scholar] [CrossRef]
- Sazan, M.S.; Bezerra, A.D.M.; Freitas, B.M. Oil Collecting Bees and Byrsonima cydoniifolia A. Juss. (Malpighiaceae) Interactions: The Prevalence of Long-Distance Cross Pollination Driving Reproductive Success. An. Acad. Bras. Ciênc. 2014, 86, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, L.S.; Bastos, E.M.A.F.; Augusto, S.C. Food Niche of Exomalopsis (Exomalopsis) fulvofasciata Smith (Hymenoptera: Apidae) in Brazilian Savannah: The Importance of Oil-Producing Plant Species as Pollen Sources. J. Nat. Hist. 2016, 50, 1859–1873. [Google Scholar] [CrossRef]
- Comita, L.S.; Stump, S.M. Natural Enemies and the Maintenance of Tropical Tree Diversity: Recent Insights and Implications for the Future of Biodiversity in a Changing World. Annals 2020, 105, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Feeny, P. Plant Apparency and Chemical Defense. In Biochemical Interaction Between Plants and Insects; Wallace, J.W., Mansell, R.L., Eds.; Springer: Boston, MA, USA, 1976; pp. 1–40. ISBN 978-1-4684-2648-9. [Google Scholar]
- Menge, E.O.; Greenfield, M.L.; McConchie, C.A.; Bellairs, S.M.; Lawes, M.J. Density-dependent Reproduction and Pollen Limitation in an Invasive Milkweed, Calotropis procera (Ait.) R. Br. (Apocynaceae). Austral Ecol. 2017, 42, 61–71. [Google Scholar] [CrossRef]
- Rusman, Q.; Poelman, E.H.; Nowrin, F.; Polder, G.; Lucas-Barbosa, D. Floral Plasticity: Herbivore-species-specific-induced Changes in Flower Traits with Contrasting Effects on Pollinator Visitation. Plant Cell Environ. 2019, 42, 1882–1896. [Google Scholar] [CrossRef] [PubMed]
- Rusman, Q.; Lucas-Barbosa, D.; Poelman, E.H.; Dicke, M. Ecology of Plastic Flowers. Trends Plant Sci. 2019, 24, 725–740. [Google Scholar] [CrossRef]
- Aguirrebengoa, M.; Müller, C.; Hambäck, P.A.; González-Megías, A. Density-Dependent Effects of Simultaneous Root and Floral Herbivory on Plant Fitness and Defense. Plants 2023, 12, 283. [Google Scholar] [CrossRef]
- Gélvez-Zúñiga, I.; Teixido, A.L.; Neves, A.C.O.; Fernandes, G.W. Floral Antagonists Counteract Pollinator-mediated Selection on Attractiveness Traits in the Hummingbird-pollinated Collaea cipoensis (Fabaceae). Biotropica 2018, 50, 797–804. [Google Scholar] [CrossRef]
- Réu-Júnior, W.F.; Del-Claro, K. Interações Formigas-Malpighiaceae, Byrsonima intermedia (A. Juss.) e Heteropterys pteropetala (H.B.K.), No Cerrado: Atratividade Ligada aos Nectários Extraflorais e Defesa Biológica à Planta. Ph.D. Thesis, Universidade de São Paulo: Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, São Paulo, Brazil, 2005. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Metz 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Almeida, R.F. Peixotoa in Flora Do Brasil 2020. Jardim Botânico Do Rio de Janeiro. 2020. Available online: https://floradobrasil2020.jbrj.gov.br/reflora/floradobrasil/FB19480 (accessed on 8 March 2023).
- Torezan-Silingardi, H.M. Predatory Behavior of Pachodynerus brevithorax (Hymenoptera: Vespidae, Eumeninae) on Endophytic Herbivore Beetles in the Brazilian Tropical Savanna. Sociobiology 2011, 57, 181. [Google Scholar]
- Mendes-Silva, I.; Queiroga, D.; Calixto, E.; Torezan-Silingardi, H.; Del-Claro, K. Multiple Cues Guarantee Successful Predation by a Neotropical Wasp. Behaviour 2021. [Google Scholar] [CrossRef]
- Barroso, G.M.; Morim, M.P.; Peixoto, A.L.; Ichaso, C.L.F. Frutos e Sementes: Morfologia Aplicada à Sistemática de Dicotiledôneas; UFV: Viçosa, Brazil, 1999. [Google Scholar]
- Novaes, L.R.; Calixto, E.S.; Alves-de-Lima, L.; de Oliveira, M.L.; Del-Claro, K.; Torezan-Silingardi, H.M. Testing Direct and Indirect Road Edge Effects on Reproductive Components of Anemochoric Plants. Landsc. Urban Plan. 2022, 218, 104291. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Harting, F.; Lohse, L.; Leite, M.d.S. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, CRAN: Contributed Packages. 2022. [CrossRef]
- Ludecke, D.; Ben-Shachar, M.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Da Silva, F.R.; Gonçalves-Souza, T.; Paterno, G.B.; Provete, D.B.; Vancine, M.H. Análises Ecológicas No R.; Nupeea: Recife, Brazil, 2022; ISBN 978-85-7917-564-0. [Google Scholar]
- Bullock, S.H. Breeding Systems in the Flora of a Tropical Deciduous Forest in Mexico. Biotropica 1985, 17, 287. [Google Scholar] [CrossRef]
- Zapata, T.R.; Arroyo, M.T.K. Plant Reproductive Ecology of a Secondary Deciduous Tropical Forest in Venezuela. Biotropica 1978, 10, 221. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.; Christensen, R. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]

| Secondary Hypotheses and Specific Objectives | Prediction | Statistical Analysis | Results |
|---|---|---|---|
| H1. Fruiting is differently affected by population density depending on the plant’s reproductive system. O1. Determine the type of reproductive system of two plant species. O2. Evaluate fruiting under the influence of population density. | Autogamous and agamospermous plants will present more similar fruiting rates regardless of density, unlike allogamous plants. | GLM’s, Self-Incompatibility Index and Reproductive Efficacy Index | Table 2 Figure 1 |
| H2. The visitation rate is dependent on the degree of isolation of the plants. O3. Check the number of floral visits in populations with different plant densities. O4. Identify floral visitors as pollinators or pillagers. | Plants close to their conspecifics will be visited more than isolated plants. | GLM T-test Randomized block ANOVA | Table 3 Figure 2 |
| H3. The florivory rate is dependent on the degree of insulation of the plants. O5. Determine the number of flowers damaged by florivores and the area consumed by florivores, considering the density of conspecific plants. | Florivores will affect grouped plants in greater quantity and intensity than isolated plants. | GLM Randomized block ANOVA | Figure 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho-Leite, L.J.; Torezan-Silingardi, H.M. Plant-Pollinator and Plant-Florivore Interactions in Two Savanna Species of Malpighiaceae. Plants 2025, 14, 2519. https://doi.org/10.3390/plants14162519
Carvalho-Leite LJ, Torezan-Silingardi HM. Plant-Pollinator and Plant-Florivore Interactions in Two Savanna Species of Malpighiaceae. Plants. 2025; 14(16):2519. https://doi.org/10.3390/plants14162519
Chicago/Turabian StyleCarvalho-Leite, Ludimila Juliele, and Helena Maura Torezan-Silingardi. 2025. "Plant-Pollinator and Plant-Florivore Interactions in Two Savanna Species of Malpighiaceae" Plants 14, no. 16: 2519. https://doi.org/10.3390/plants14162519
APA StyleCarvalho-Leite, L. J., & Torezan-Silingardi, H. M. (2025). Plant-Pollinator and Plant-Florivore Interactions in Two Savanna Species of Malpighiaceae. Plants, 14(16), 2519. https://doi.org/10.3390/plants14162519






