Comparative Study on Production Performance of Different Oat (Avena sativa) Varieties and Soil Physicochemical Properties in Qaidam Basin
Abstract
1. Introduction
2. Results
2.1. The Phenological Stages of Different Oat Varieties
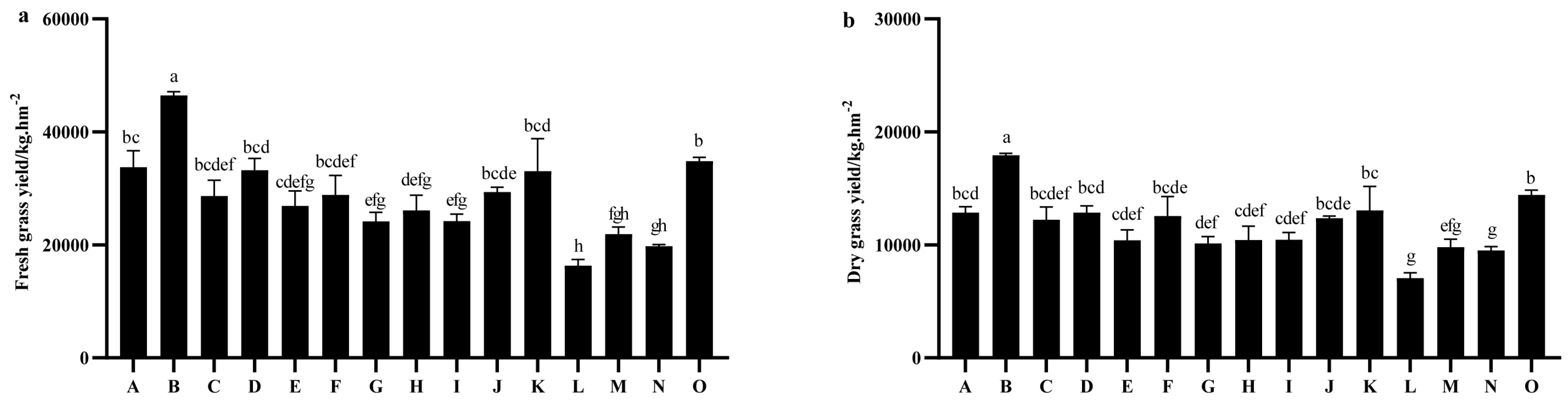
2.2. Comparison of Grass Yield Among Different Oat Varieties
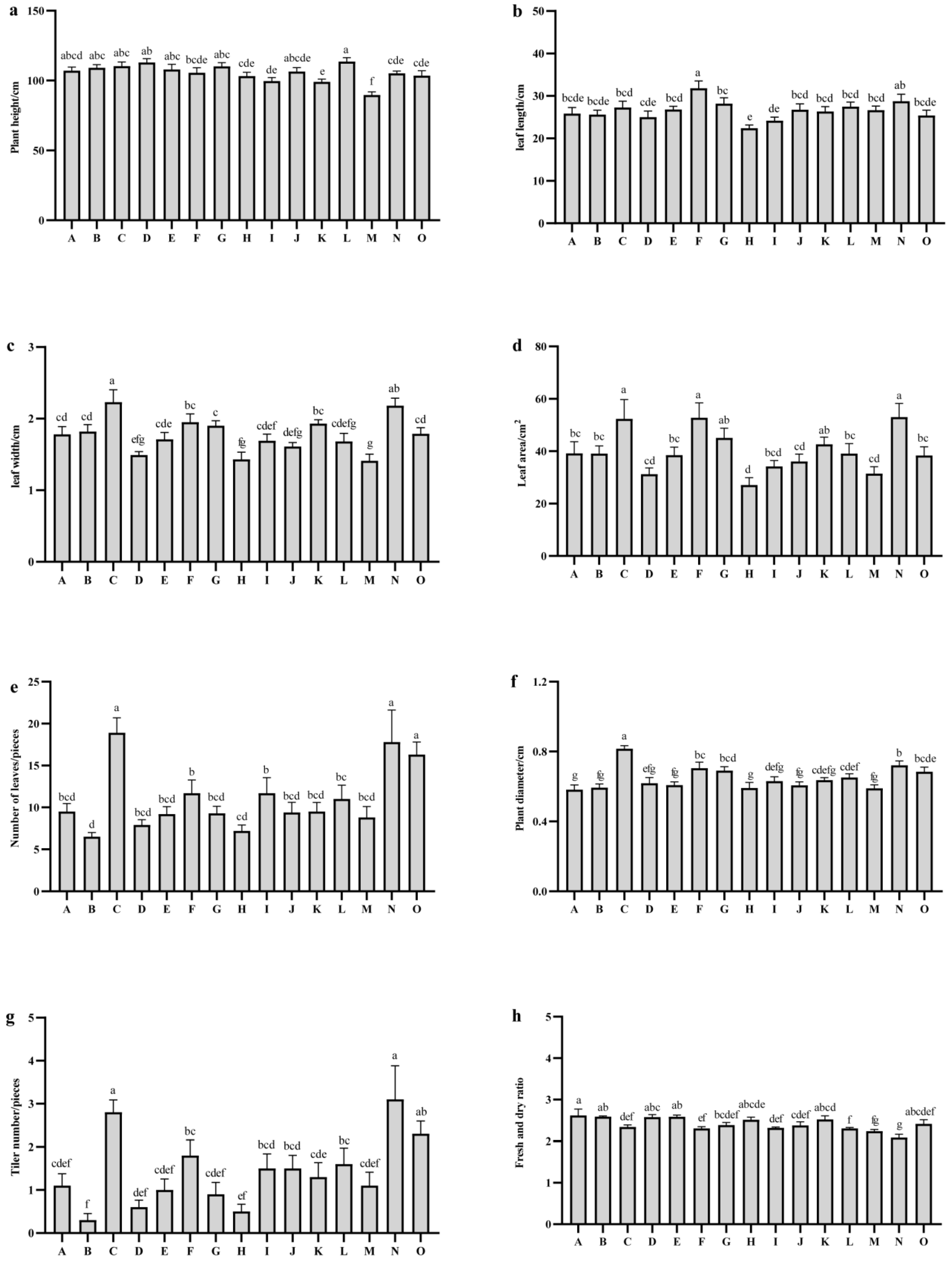
2.3. Comparison of Agronomic Traits Among Different Oat Varieties
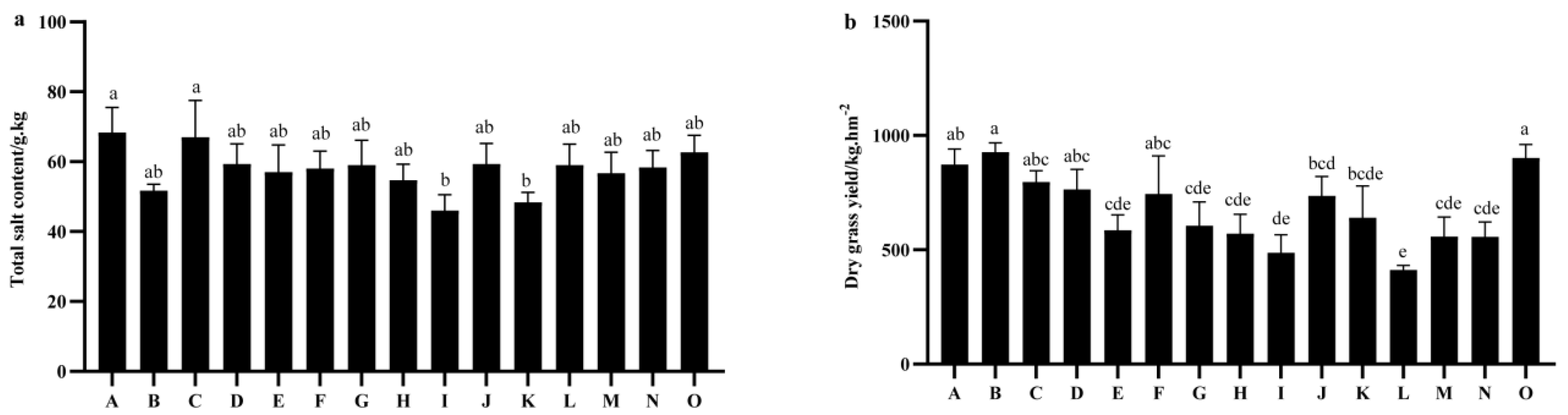
2.4. Comparison of Total Salt Content and Salt Accumulation
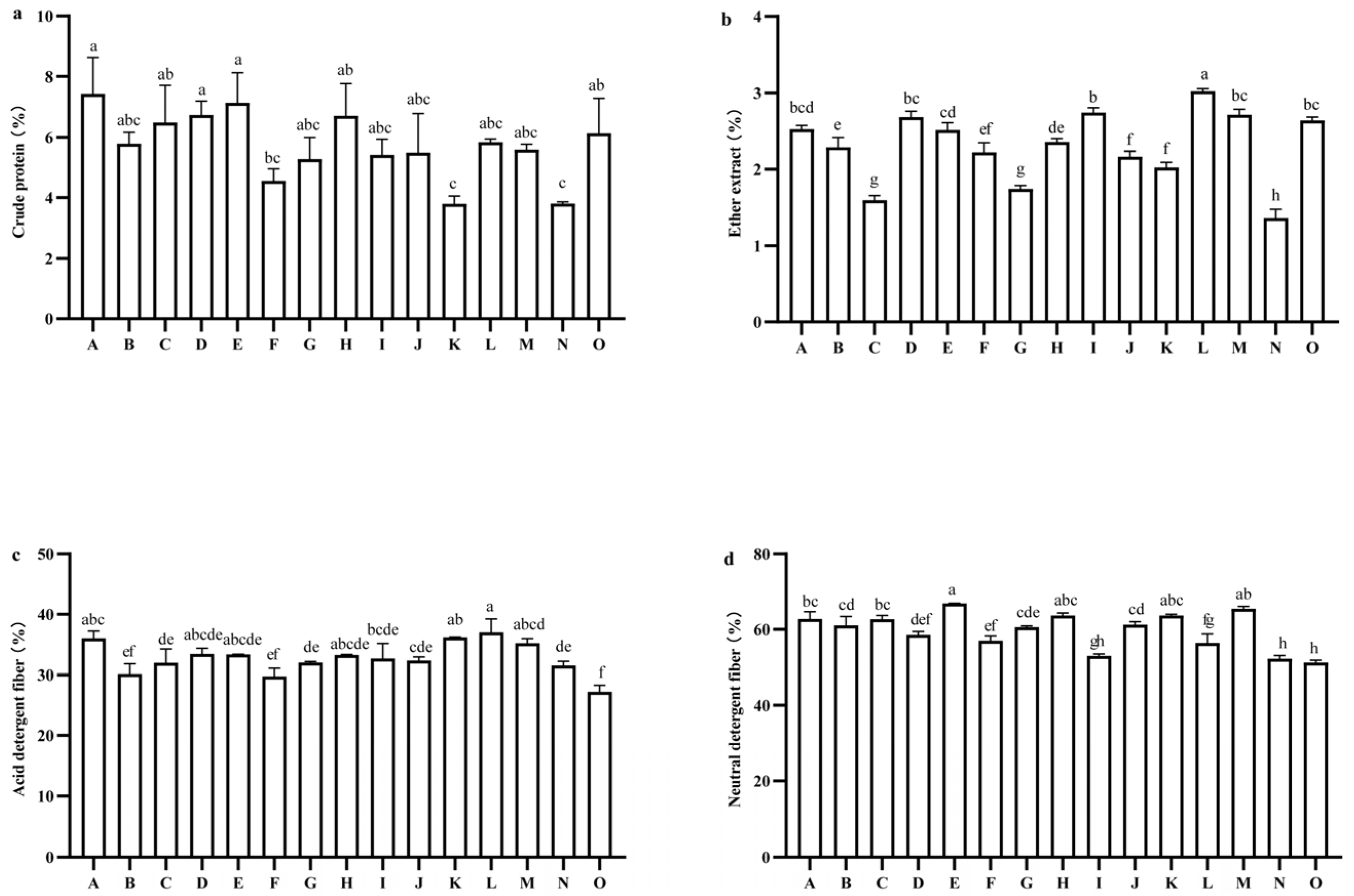
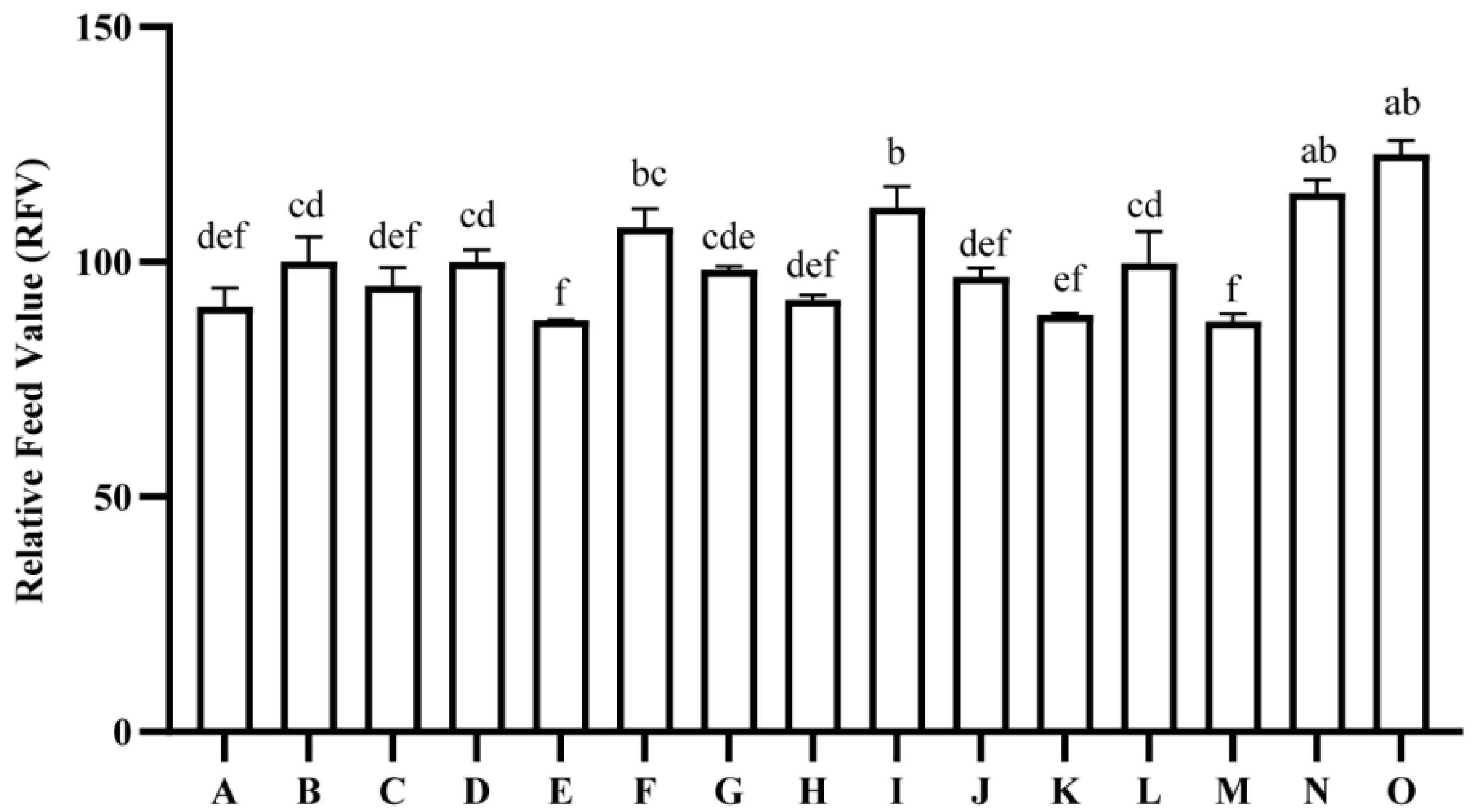
2.5. Comparison of Nutritional Quality and Relative Feed Value
2.6. Changes in Soil Physicochemical Properties of Different Oat Varieties
2.6.1. The Impact of Different Oat Varieties on Soil pH
2.6.2. The Impact of Different Oat Varieties on Soil EC
2.6.3. The Impact of Different Oat Varieties on Total Soil Salinity
2.6.4. The Impact of Different Oat Varieties on Soil Nutrient Characteristics
2.6.5. The Effects of Different Oat Varieties on Soil Base Ions
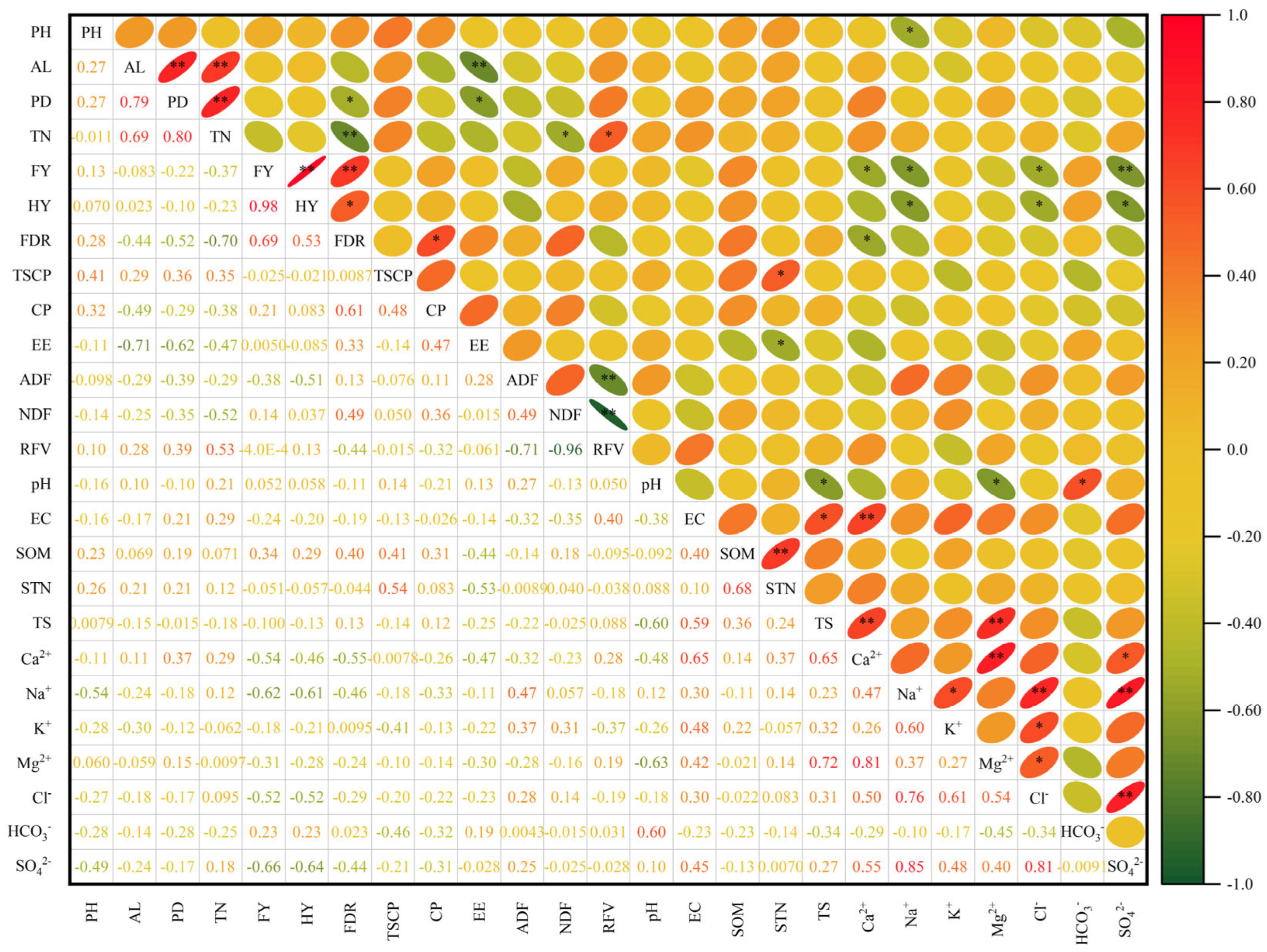
2.7. Correlation Analysis of Cultivated Oat Production Performance and Soil Physical and Chemical Properties
2.8. Comprehensive Evaluation of Production Performance and Soil Physicochemical Properties of Different Oat Varieties
2.8.1. PCA and Membership Function
2.8.2. Membership Function Values of Soil Physicochemical Properties
3. Discussion
4. Materials and Methods
4.1. Overview of the Experimental Site
4.2. Test Materials
4.3. Experimental Design and Methodology
4.4. Measurement Indicators and Methods
4.4.1. Phenological Stages and Agronomic Traits
4.4.2. Grass Yield and Total Salt Content in Plants
4.4.3. Nutritional Quality
4.4.4. Soil Measurement Indicators and Methods
4.5. Comprehensive Evaluation of Oat Variety Production Performance and Comprehensive Evaluation of Soil Physicochemical Properties
4.5.1. Membership Function Value of Comprehensive Indicators for Wheat Variety Production Performance
4.5.2. Comprehensive Indicator Weights for Oat Variety Production Performance
4.5.3. Comprehensive Evaluation of Production Performance in Oat Varieties
4.5.4. Comprehensive Evaluation of Soil Indicators for Oat Varieties
4.6. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CP | Crude protein |
| EE | Ether extract |
| ADF | Acid detergent fiber |
| NDF | Neutral detergent fiber |
| RFV | Relative feeding value |
| EC | Electrical conductivity |
References
- Zhu, G.; Xu, Z.; Xu, Y.; Lu, H.; Ji, Z.; Zhou, G. Different types of fertilizers enhanced salt resistance of oat and associated physiological mechanisms in saline soils. Agronomy 2022, 12, 317. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.-K. Understanding and improving salt tolerance in plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, J.; Cao, T.; Zhang, J.; Liu, H. Response to salt stresses and assessment of salt tolerability of soybean varieties in emergence and seedling stages. Acta Ecol. Sin. 2011, 59, 1471–1478. [Google Scholar]
- Zhang, H.; Han, B.; Wang, T.; Chen, S.; Li, H.; Zhang, Y.; Dai, S. Mechanisms of plant salt response: Insights from proteomics. J. Proteome Res. 2012, 11, 49–67. [Google Scholar] [CrossRef]
- Singh, A.K.; Gupta, K.J.; Singla-Pareek, S.L.; Foyer, C.H.; Pareek, A. Raising crops for dry and saline lands: Challenges and the way forward. Physiol. Plant. 2022, 174, e13730. [Google Scholar] [CrossRef]
- Liu, Z.; Shang, H.; Han, F.; Zhang, M.; Zhou, W. Improvement of nitrogen and phosphorus availability by Pseudoalteromonas sp. during salt-washing in saline-alkali soil. Appl. Soil Ecol. 2021, 168, 104117. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Zhang, T.; Yin, B.; Li, R.; Sheng, Z.; Li, S. Variations in soil microbial communities in different saline soils under typical Populus spp. vegetation in alpine region of the Qaidam Basin, NW China. Ecotoxicol. Environ. Saf. 2024, 282, 116747. [Google Scholar] [CrossRef]
- Wang, l.; Pu, X.; Wei, X.; Fu, Y.; Xu, C. Comprehensive evaluation of production performance and feed quality of 19 alfalfa varieties in Qaidam Basin. Acta Agrestia Sin. 2023, 31, 3136–3144. [Google Scholar]
- Liwei, J.; Yongjiang, L.; Weimin, L.; Sihua, Y.; Jinglian, Y.; Sanzhong, L.; Zhihong, L. Cenozoic double-layered structure in the western Qaidam Basin, northern Tibetan Plateau, China. J. Asian Earth Sci. 2022, 232, 105123. [Google Scholar] [CrossRef]
- Ponce, K.S.; Guo, L.; Leng, Y.; Meng, L.; Ye, G. Advances in sensing, response and regulation mechanism of salt tolerance in rice. Int. J. Mol. Sci. 2021, 22, 2254. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhu, G.; Zhou, G.; Younas, M.U.; Suliman, M.S.E.; Liu, J.; Zhu, Y.m.; Salih, E.G.I. Integrated approaches for increasing plant yield under salt stress. Front. Plant Sci. 2023, 14, 1215343. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M. Some important physiological selection criteria for salt tolerance in plants. Flora 2004, 199, 361–376. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant salt stress: Adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef]
- Puniran-Hartley, N.; Hartley, J.; Shabala, L.; Shabala, S. Salinity-induced accumulation of organic osmolytes in barley and wheat leaves correlates with increased oxidative stress tolerance: In planta evidence for cross-tolerance. Plant. Physiol. Biochem. 2014, 83, 32–39. [Google Scholar] [CrossRef]
- Tuteja, N. Mechanisms of High Salinity Tolerance in Plants. Methods Enzymol. 2007, 428, 419–438. [Google Scholar]
- Oliveira, D.M.D.; Mota, T.R.; Salatta, F.V.; Sinzker, R.C.; Santos, W.D.D.J.P.C. Cell wall remodeling under salt stress: Insights into changes in polysaccharides, feruloylation, lignification, and phenolic metabolism in maize. Plant Cell Environ. 2020, 43, 2172–2191. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef]
- Ayalew, H.; Anderson, J.D.; Kumssa, T.T.; Maulana, F.; Ma, X.F. Screening oat germplasm for better adaptation to cold stress in the Southern Great Plains of the United States. J. Agron. Crop Sci. 2018, 205, 213–219. [Google Scholar] [CrossRef]
- Jiao, J.J.; Zhang, X.; Liu, Y.; Kuang, X. Increased water storage in the Qaidam Basin, the North Tibet Plateau from GRACE Gravity data. PLoS ONE 2015, 10, e0141442. [Google Scholar] [CrossRef]
- Hui, R.; Tan, H.; Li, X.; Wang, B. Variation of soil physical-chemical characteristics in salt-affected soil in the Qarhan Salt Lake, Qaidam Basin. J. Arid Land 2022, 14, 341–355. [Google Scholar] [CrossRef]
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Raquel, P.-R.; Stephen, R.G.; Adolfo, P.-G.; José, A.P.-P.; Francisco, J.D.-P. Marginal quality waters: Adequate resources for sustainable forage production in saline soils? Agric. Water Manag. 2024, 305, 109142. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Ranjbar, G.; Akram, N.A.; Ghafar, M.A.; Panico, A. Forage potential of several halophytic species grown on saline soil in arid environments. Environ. Res. 2022, 219, 114954. [Google Scholar] [CrossRef]
- Sadaqat Shah, S.; Li, Z.; Yan, H.; Shi, L.; Zhou, B. Comparative study of the effects of salinity on growth, gas exchange, N accumulation and stable isotope signatures of forage oat (Avena sativa L.) genotypes. Plants 2020, 9, 1025. [Google Scholar] [CrossRef] [PubMed]
- Marshall, A.; Cowan, S.; Edwards, S.; Griffiths, I.; Howarth, C.; Langdon, T.; White, E. Crops that feed the world 9. Oats a cereal crop for human and livestock feed with industrial applications. Food Secur. 2013, 5, 13–33. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, J.; Xue, L.; Kamran, M.; Wang, Y.; Wei, X.; Zhao, G.; Li, C. The impact of bacterial leaf blight disease (Pantoea agglomerans) on grain yield and nutritional quality of oat. Microorganisms 2025, 13, 141. [Google Scholar] [CrossRef]
- Rafique, H.; Dong, R.; Wang, X.; Alim, A.; Aadil, R.M.; Li, L.; Zou, L.; Hu, X. Dietary-nutraceutical properties of oat protein and peptides. Front. Nutr. 2022, 9, 950400. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C.; Bao, Y.; Liu, F.; Wang, H.; Wang, Y. Oat: Current state and challenges in plant-based food applications. Trends Food Sci. Technol. 2023, 134, 56–71. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Z.; Zhao, B.; Yang, Y.; Mi, J.; Zhao, Z.; Liu, J. Physiological and proteomic analysis responsive mechanisms for salt stress in oat. Front. Plant Sci. 2022, 13, 891674. [Google Scholar] [CrossRef]
- Nabi, S.; Hussan, S.u.; Nabi, T.; Shikari, A.B.; Dar, Z.A.; Khuroo, N.S.; Lone, A.A.; Sofi, P.A.; Amin, R.; Wani, M.A. Bi-environmental evaluation of oat (Avena sativa L.) genotypes for yield and nutritional traits under cold stress conditions using multivariate analysis. Plant Genet. Resour. Charact. Util. 2024, 22, 295–303. [Google Scholar] [CrossRef]
- Su, S.; Wang, L.; Fu, S.; Zhao, J.; He, X.; Chen, Q.; Belobrajdic, D.P.; Yu, C.; Liu, H.; Wu, H.; et al. Effects of oat (Avena sativa L.) hay diet supplementation on the intestinal microbiome and metabolome of Small-tail Han sheep. Front. Microbiol. 2022, 13, 1032622. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Zhao, B.; Mi, J.; Xu, Z. Utilizing multi-omics analysis to elucidate the molecular mechanisms of oat responses to drought stress. Plants 2025, 14, 792. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, B.; Liu, H.; Wu, J.; Xu, H. Effects of salt stress on plasmalemma permeability, osmolyte accumulation and protective enzyme activities in oat plants. J. Food Agric. Environ. Res. 2013, 11, 696–701. [Google Scholar]
- Ahmed, S.; Patel, R.; Rana, M.; Kumar, N.; Indu, I.; Choudhary, M.; Chand, S.; Singh, A.K.; Ghosh, A.; Singhal, R.K. Effect of salt, alkali and combined stresses on root system architecture and ion profiling in a diverse panel of oat (Avena spp.). Funct. Plant Biol. 2024, 51, 1. [Google Scholar] [CrossRef]
- Bai, J.; Yan, W.; Wang, Y.; Yin, Q.; Liu, J.; Wight, C.; Ma, B. Screening oat genotypes for tolerance to salinity and alkalinity. Front. Plant Sci. 2018, 9, 1302. [Google Scholar] [CrossRef] [PubMed]
- Sarker, J.R.; Rolfe, J.; Ananda, J. Analysis of technical efficiency of dry season rice production in saline and non-saline areas of Bangladesh. J. Environ. Manag. 2022, 316, 115256. [Google Scholar] [CrossRef]
- Hai, X.; Mi, J.; Zhao, B.; Zhang, B.; Zhao, Z.; Liu, J. Foliar application of spermidine reduced the negative effects of salt stress on oat seedlings. Front. Plant Sci. 2022, 13, 846280. [Google Scholar] [CrossRef]
- Liang, X.-D.; Shalapy, M.; Zhao, S.-F.; Liu, J.-H.; Wang, J.-Y. A stress-responsive transcription factor PeNAC1 regulating beta-d-glucan biosynthetic genes enhances salt tolerance in oat. Planta 2021, 254, 130. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zheng, Y.; Zhao, T.; Tang, L.; Zhang, F.; Xie, W. The potential spatiotemporal distribution patterns of Avena nuda and Avena sativa from global perspective provide new insights for the cultivation of commonly cultivated oats. BMC Plant Biol. 2025, 25, 550. [Google Scholar] [CrossRef]
- Wu, W.; Xu, C.; Pu, X.; Zhao, Y.; Ge, R.; Wang, W. Comparative study on production performance of different oat varieties in mild saline-alkali land of Qaidam. Acta Agrestia Sin. 2025, 33, 1497–1509. [Google Scholar]
- Jin, X.; Liu, X.; Wang, J.; Chang, J.; Li, C.; Lu, G. Rhizosphere growth-promoting bacteria enhance oat growth by improving microbial stability and soil organic matter in the saline soil of the Qaidam Basin. Plants 2025, 14, 1926. [Google Scholar] [CrossRef]
- Lizhuo, G.; Wentao, C.; Gao, Y. Effects of amendments on soil salinization characteristics and biological salt removal of oat. J. Triticeae Crops 2024, 44, 1619–1629. [Google Scholar]
- Zhou, X.; Wang, M.; Yang, L.; Wang, W.; Zhang, Y.; Liu, L.; Chai, J.; Liu, H.; Zhao, G. Comparative physiological and transcriptomic analyses of oat (Avena sativa) seedlings under salt stress reveal salt tolerance mechanisms. Plants 2024, 13, 2238. [Google Scholar] [CrossRef]
- Fan, Y.; Ren, C.Z.; Li, P.F.; Ren, T.S. Oat growth and cation absorption characteristics under salt and alkali stress. J. Appl. Ecol. 2011, 22, 2875–2882. [Google Scholar]
- Ran, Z.; Zhen, X.; Jian, R.; Jianfan, T.; Li, L.; Xiangli, M. Evaluation of production performance and adaptability of 11 oat varieties in Diqing region. Chin. J. Grassl. 2023, 45, 32–40. [Google Scholar] [CrossRef]
- Hongfei, L.; Bangwei, Z.; Miao, Z.; Shunan, S.; Zhijian, L. Evaluation of introduction adaptability of different oat varieties in Hulunbuir region. Acta Pratacult. Sin. 2024, 33, 60–72. [Google Scholar]
- Pang, K.; Van Sambeek, J.W.; Navarrete-Tindall, N.E.; Lin, C.-H.; Jose, S.; Garrett, H.E. Responses of legumes and grasses to non-, moderate, and dense shade in Missouri, USA. II. Forage quality and its species-level plasticity. Agrofor. Syst. 2019, 93, 25–38. [Google Scholar] [CrossRef]
- Jing, L.; Ming, N.; Yanming, L.; Chengjun, Z.; Shenglan, R. Comprehensive evaluation of yield, quality and feeding performance of different oat varieties. Acta Agrestis Sin. 2023, 31, 1089–1098. [Google Scholar]
- Liu, A.; Qu, Z.; Nachshon, U. On the potential impact of root system size and density on salt distribution in the root zone. Agric. Water Manag. 2020, 234, 106118. [Google Scholar] [CrossRef]
- Outbakat, M.B.; El Mejahed, K.; El Gharous, M.; El Omari, K.; Beniaich, A. Effect of phosphogypsum on soil physical properties in moroccan salt-affected soils. Sustainability 2022, 14, 13087. [Google Scholar] [CrossRef]
- Trabelsi, L.; Gargouri, K.; Ben Hassena, A.; Mbadra, C.; Ghrab, M.; Ncube, B.; Van Staden, J.; Gargouri, R. Impact of drought and salinity on olive water status and physiological performance in an arid climate. Agric. Water Manag. 2019, 213. [Google Scholar] [CrossRef]
- Anaya-Romero, M.; Abd-Elmabod, S.K.; Muñoz-Rojas, M.; Castellano, G.; Ceacero, C.J.; Alvarez, S.; Méndez, M.; De la Rosa, D. Evaluating soil threats under climate change scenarios in the andalusia region, southern Spain. Land Degrad. Dev. 2015, 26. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Pires, I.S.; Negrão, S.; Oliveira, M.M.; Purugganan, M.D. Comprehensive phenotypic analysis of rice (Oryza sativa) response to salinity stress. Physiol. Plant. 2015, 155, 43–54. [Google Scholar] [CrossRef]
- Li, R.; Shi, F.; Fukuda, K.; Yang, Y. Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci. Plant Nutr. 2010, 56, 725–733. [Google Scholar] [CrossRef]
- Qi, Z.; Spalding, E.P. Protection of plasma membrane K+ transport by the salt overly sensitive1 Na+-H+ antiporter during salinity stress. Plant Physiol. 2004, 136, 2548–2555. [Google Scholar] [CrossRef]
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+ /Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Ali, A.; Khan, I.U.; Jan, M.; Khan, H.A.; Hussain, S.; Nisar, M.; Chung, W.S.; Yun, D.-J. The High-affinity potassium transporter EpHKT1;2 from the extremophile eutrema parvula mediates salt tolerance. Front. Plant Sci. 2018, 9, 1108. [Google Scholar] [CrossRef]
- Momayyezi, M.; McKown, A.D.; Bell, S.C.S.; Guy, R.D. Emerging roles for carbonic anhydrase in mesophyll conductance and photosynthesis. Plant J. 2020, 101, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Stavi, I.; Thevs, N.; Priori, S. Soil salinity and sodicity in drylands: A review of causes, effects, monitoring, and restoration measures. Front. Environ. Sci. 2021, 9, 712831. [Google Scholar] [CrossRef]
- Gao, K.; Yu, Y.F.; Xia, Z.T.; Yang, G.; Xing, Z.L.; Qi, L.T.; Ling, L.Z. Response of height, dry matter accumulation and partitioning of oat (Avena sativa L.) to planting density and nitrogen in Horqin Sandy Land. Sci. Rep. 2019, 9, 7961. [Google Scholar] [CrossRef]
- Liang, g.; Qin, y.; Wei, x.; Liu, y.; Liu, y.; Liu, w. Evaluation on productivity and quality of oat strain I-D in the alpine regions of the Qinghai Tibetan Plateau. Acta Agrestia Sin. 2018, 26, 917–927. [Google Scholar]
- Bilal, M.; Ayub, M.; Tariq, M.; Tahir, M.; Nadeem, M.A. Dry matter yield and forage quality traits of oat (Avena sativa L.) under integrative use of microbial and synthetic source of nitrogen. J. Saudi Soc. Agric. Sci. 2017, 16, 236–241. [Google Scholar] [CrossRef]
- Guo, C.; Xu, C.; Pu, X.; Zhao, Y.; Wang, J.; Fu, Y.; Wang, W. Oat and forage pea mixed sowing improves soil chemical fertility and fresh and dry mass yield in light saline–alkali land: Preliminary results. Agronomy 2025, 15, 297. [Google Scholar] [CrossRef]
- Opoku, A.; Ogunleye, A.M.; Solomon, J.K.Q.; Payne, W.A. Cover crop systems impact on biomass production, carbon-to-nitrogen ratio, forage quality, and soil health in a semi-arid environment. Heliyon 2024, 10, e39600. [Google Scholar] [CrossRef]
- Cardoso, R.; Drouinot, T.; de Freitas, S.C. Miniaturized device to measure urease activity in the soil interstitial fluid using wenner method. Biogeotechnics 2025, 3, 100120. [Google Scholar] [CrossRef]
- Ivezi, V.; Kraljevi, D.; Lonari, Z.; Engler, M.; Jovi, J. Organic matter determined by loss on ignition and potassium dichromate method. In Proceedings of the 51st Croatian and 11th International Symposium on Agriculture, Opatija, Croatia, 15–18 February 2016; pp. 36–40. [Google Scholar]
- Hausherr Lüder, R.-M.; Qin, R.; Richner, W.; Stamp, P.; Noulas, C. Spatial variability of selected soil properties and its impact on the grain yield of oats (Avena sativa L.) in small fields. J. Plant Nutr. 2018, 41, 2446–2469. [Google Scholar] [CrossRef]
- Carr, M.H.; Frank, H.A. Improved method for determination of calcium and magnesium in biologic fluids by EDTA titration. Am. J. Clin. Pathol. 1956, 26, 1157–1168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pätsch, R.; Midolo, G.; Dítě, Z.; Dítě, D.; Wagner, V.; Pavonič, M.; Danihelka, J.; Preislerová, Z.; Ćuk, M.; Stroh, H.G.; et al. Beyond salinity: Plants show divergent responses to soil ion composition. Glob. Ecol. Biogeogr. 2024, 33, e13821. [Google Scholar] [CrossRef]
- Siebers, R.W.; Maling, T.J. Flame photometry: A simple method and reference range for erythrocyte sodium and potassium. Med. Lab. Sci. 1988, 45, 270–272. [Google Scholar]
- Hussain, B.; Ma, Y.; Li, J.; Gao, J.; Ullah, A.; Tahir, N. Cadmium in rice Is affected by fertilizer-borne chloride and sulfate anions: Long term field versus pot experiments. Processes 2022, 10, 1253. [Google Scholar] [CrossRef]
- Han, L.; Liu, H.; Yu, S.; Wang, W.; Liu, J.J.E.E. Potential application of oat for phytoremediation of salt ions in coastal saline-alkali soil. Ecol. Eng. 2013, 61, 274–281. [Google Scholar] [CrossRef]
| Variety | Seedling Stage 0–15 cm | Harvest Period 0–15 cm | Seedling Stage 15–30 cm | Harvest Period 15–30 cm |
|---|---|---|---|---|
| CK | 8.81 ± 0.18 a | 8.92 ± 0.22 a | 8.91 ± 0.08 a | 9.16 ± 0.15 a |
| Qingtian No. 1 | 8.72 ± 0.17 abc | 8.50 ± 0.07 b | 8.66 ± 0.18 abcde | 8.63 ± 0.18 bc |
| Molasses | 8.52 ± 0.08 bcde | 8.47 ± 0.11 bc | 8.85 ± 0.13 ab | 8.44 ± 0.11 cd |
| Qinghai sweet oat | 8.30 ± 0.07 e | 8.14 ± 0.16 de | 8.73 ± 0.08 abcd | 8.03 ± 0.04 ef |
| Qingyin No. 1 | 8.46 ± 0.14 cde | 8.17 ± 0.21 de | 8.72 ± 0.08 abcd | 8.16 ± 0.14 de |
| Qingyin No. 2 | 8.32 ± 0.11 de | 8.24 ± 0.03 cd | 8.75 ± 0.18 abc | 8.04 ± 0.10 ef |
| Dafuweng | 8.64 ± 0.08 abc | 8.07 ± 0.06 def | 8.82 ± 0.18 ab | 7.95 ± 0.06 ef |
| Baiyan No. 7 | 8.62 ± 0.07 abc | 8.05 ± 0.11 def | 8.55 ± 0.13 cde | 8.00 ± 0.23 ef |
| Northwestern No. 1 | 8.68 ± 0.07 abc | 7.88 ± 0.08 f | 8.44 ± 0.08 e | 7.83 ± 0.08 f |
| Lena | 8.70 ± 0.01 abc | 7.99 ± 0.10 ef | 8.83 ± 0.10 ab | 7.90 ± 0.12 ef |
| Jiayan No. 2 | 8.48 ± 0.19 cde | 8.18 ± 0.08 de | 8.48 ± 0.12 de | 8.09 ± 0.11 ef |
| Mengshi No. 1 | 8.59 ± 0.11 abcd | 8.54 ± 0.03 b | 8.71 ± 0.10 abcde | 8.68 ± 0.16 bc |
| Qinghai No. 444 | 8.72 ± 0.12 abc | 8.52 ± 0.10 b | 8.79 ± 0.18 abc | 8.80 ± 0.22 b |
| Haymaker | 8.58 ± 0.11 abcd | 8.5 ± 0.09 b | 8.72 ± 0.12 abcd | 8.67 ± 0.22 bc |
| Gaoyan No. 1 | 8.78 ± 0.19 ab | 8.57 ± 0.02 b | 8.63 ± 0.13 bcde | 8.62 ± 0.04 bc |
| Baler | 8.81 ± 0.19 a | 8.60 ± 0.15 b | 8.83 ± 0.14 ab | 8.54 ± 0.22 bc |
| F Value | 2.87 ** | 11.27 ** | 2.09 * | 13.11 ** |
| Variety | Seedling Stage 0–15 cm Soil EC ms/m | Harvest Period 0–15 cm Soil Conductivity | Seedling Stage 15–30 cm Soil Conductivity | Harvest Period 15–30 cm Soil EC ms/m |
|---|---|---|---|---|
| CK | 0.70 ± 0.08 a | 0.77 ± 0.05 a | 0.80 ± 0.08 a | 0.83 ± 0.05 a |
| Qingtian No. 1 | 0.40 ± 0 de | 0.20 ± 0 fg | 0.37 ± 0.05 fgh | 0.17 ± 0.05 fg |
| Molasses | 0.23 ± 0.05 f | 0.20 ± 0 fg | 0.70 ± 0 ab | 0.13 ± 0.05 g |
| Qinghai sweet oat | 0.47 ± 0.05 cd | 0.43 ± 0.09 bc | 0.47 ± 0.05 def | 0.17 ± 0.05 fg |
| Qingyin No. 1 | 0.37 ± 0.05 de | 0.33 ± 0.05 de | 0.27 ± 0.05 h | 0.27 ± 0.05 cdef |
| Qingyin No. 2 | 0.53 ± 0.09 bc | 0.20 ± 0 fg | 0.33 ± 0.05 gh | 0.33 ± 0.05 cd |
| Dafuweng | 0.57 ± 0.05 bc | 0.20 ± 0 fg | 0.50 ± 0.08 cde | 0.30 ± 0 cde |
| Baiyan No. 7 | 0.63 ± 0.05 ab | 0.27 ± 0.05 ef | 0.77 ± 0.05 a | 0.20 ± 0 efg |
| Northwestern No. 1 | 0.47 ± 0.05 cd | 0.47 ± 0.05 b | 0.53 ± 0.05 cd | 0.47 ± 0.05 b |
| Lena | 0.47 ± 0.09 cd | 0.37 ± 0.05 cd | 0.33 ± 0.05 gh | 0.33 ± 0.05 cd |
| Jiayan No. 2 | 0.53 ± 0.05 bc | 0.17 ± 0.05 g | 0.53 ± 0.09 cd | 0.37 ± 0.05 bc |
| Mengshi No. 1 | 0.33 ± 0.05 ef | 0.27 ± 0.05 ef | 0.43 ± 0.09 defg | 0.27 ± 0.05 cdef |
| Qinghai No. 444 | 0.40 ± 0.08 de | 0.20 ± 0 fg | 0.33 ± 0.05 gh | 0.23 ± 0.05 defg |
| Haymaker | 0.40 ± 0.08 de | 0.20 ± 0 fg | 0.47 ± 0.05 def | 0.23 ± 0.05 defg |
| Gaoyan No. 1 | 0.37 ± 0.05 de | 0.37 ± 0.05 cd | 0.40 ± 0.08 efg | 0.37 ± 0.12 bc |
| Baler | 0.37 ± 0.05 de | 0.30 ± 0 de | 0.60 ± 0 bc | 0.47 ± 0.05 b |
| F Value | 7.66 ** | 30.93 ** | 13.72 ** | 20.51 ** |
| Variety | Seedling Stage 0–15 cm Total Salt Content | Harvest Period 0–15 cm Total Salt Content | Seedling Stage 15–30 cm Total Salt Content | Harvest Period 15–30 cm Total Salt Content |
|---|---|---|---|---|
| CK | 2.45 ± 0.06 a | 2.53 ± 0.17 a | 2.38 ± 0.03 a | 2.44 ± 0.05 a |
| Qingtian No. 1 | 1.06 ± 0.06 g | 0.9 ± 0.09 cd | 0.75 ± 0.04 i | 0.72 ± 0.09 fg |
| Molasses | 0.61 ± 0.07 h | 0.51 ± 0.03 e | 0.99 ± 0.06 h | 0.69 ± 0.06 fg |
| Qinghai sweet oat | 1.44 ± 0.07 f | 1.05 ± 0.05 c | 1.49 ± 0.04 e | 0.31 ± 0.07 i |
| Qingyin No. 1 | 1.34 ± 0.09 f | 0.91 ± 0.07 cd | 1.16 ± 0.09 fg | 0.31 ± 0.07 i |
| Qingyin No. 2 | 1.8 ± 0.08 d | 0.88 ± 0.06 d | 1.26 ± 0.09 f | 0.87 ± 0.07 de |
| Dafuweng | 1.68 ± 0.03 de | 0.76 ± 0.01 d | 1.84 ± 0.05 d | 0.6 ± 0.04 g |
| Baiyan No. 7 | 2.06 ± 0.08 c | 1.26 ± 0.08 b | 1.87 ± 0.07 d | 1.49 ± 0.05 c |
| Northwestern No. 1 | 1.58 ± 0.05 e | 1.36 ± 0.05 b | 1.81 ± 0.05 d | 1.73 ± 0.04 b |
| Lena | 1.33 ± 0.05 f | 1.31 ± 0.08 b | 1.13 ± 0.06 g | 0.85 ± 0.03 de |
| Jiayan No. 2 | 1.78 ± 0.08 d | 0.41 ± 0.02 e | 1.91 ± 0.03 cd | 0.96 ± 0.05 d |
| Mengshi No. 1 | 2.06 ± 0.02 c | 0.77 ± 0.04 d | 2.02 ± 0.04 c | 0.61 ± 0.06 g |
| Qinghai No. 444 | 1.72 ± 0.06 d | 0.38 ± 0.07 e | 0.97 ± 0.06 h | 0.46 ± 0.08 h |
| Haymaker | 2.27 ± 0.02 b | 0.36 ± 0.07 e | 1.57 ± 0.04 e | 0.46 ± 0.02 h |
| Gaoyan No. 1 | 1.37 ± 0.03 f | 0.88 ± 0.09 d | 2.18 ± 0.09 b | 0.79 ± 0.06 ef |
| Baler | 1.01 ± 0.07 g | 0.87 ± 0.06 d | 1.24 ± 0.02 fg | 0.9 ± 0.02 de |
| F Value | 119.95 ** | 91.82 ** | 147.09 ** | 182.29 ** |
| Index | Principal Component 1 | Principal Component 2 | Principal Component 3 | Principal Component 4 |
|---|---|---|---|---|
| Plant height | −0.035 | 0.240 | 0.373 | 0.293 |
| Flag leaf area | −0.358 | 0.106 | 0.247 | −0.253 |
| Stem thickness | −0.384 | 0.079 | 0.257 | −0.043 |
| Tillering number | −0.410 | −0.039 | 0.105 | 0.056 |
| Fresh grass yield | 0.166 | 0.528 | −0.081 | −0.192 |
| Hay yield | 0.091 | 0.540 | −0.125 | −0.219 |
| Fresh-to-dry ratio | 0.364 | 0.293 | 0.118 | −0.005 |
| Total salt content | −0.083 | 0.126 | 0.528 | 0.289 |
| Crude Protein | 0.271 | 0.127 | 0.310 | 0.420 |
| Crude Fat | 0.270 | −0.094 | −0.228 | 0.477 |
| Acid Detergent Fiber | 0.212 | −0.417 | 0.194 | −0.073 |
| Neutral Detergent Fiber | 0.304 | −0.098 | 0.331 | −0.389 |
| Relative Feeding Value. | −0.311 | 0.205 | −0.337 | 0.341 |
| Eigenvalue | 4.744 | 2.742 | 2.036 | 1.598 |
| Contribution rate/% | 36.496 | 21.094 | 15.664 | 12.290 |
| Cumulative contribution rate/% | 36.496 | 57.590 | 73.254 | 85.544 |
| Variety | Principal Component | Subordinate Function Values | D Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | U(X1) | U(X2) | U(X3) | U(X4) | ||
| Qingtian No. 1 | 2.359 | 0.627 | 1.829 | 0.646 | 0.998 | 0.562 | 0.784 | 0.702 | 0.809 |
| Molasses | 1.918 | 3.359 | −1.197 | −1.151 | 0.938 | 1.000 | 0.266 | 0.345 | 0.745 |
| Qinghai sweet oat | −2.552 | 0.873 | 3.095 | −0.588 | 0.328 | 0.601 | 1.000 | 0.457 | 0.537 |
| Qingyin No. 1 | 1.885 | 1.084 | 0.167 | 1.308 | 0.933 | 0.635 | 0.500 | 0.834 | 0.766 |
| Qingyin No. 2 | 2.147 | −0.367 | 1.244 | 0.067 | 0.969 | 0.402 | 0.684 | 0.587 | 0.722 |
| Dafuweng | −2.269 | 0.794 | −0.409 | −0.460 | 0.367 | 0.589 | 0.401 | 0.482 | 0.444 |
| Baiyan No. 7 | −0.909 | −0.224 | 0.928 | −0.519 | 0.552 | 0.425 | 0.630 | 0.470 | 0.523 |
| Northwestern No. 1 | 2.376 | −0.801 | −0.202 | 0.148 | 1.000 | 0.333 | 0.437 | 0.603 | 0.675 |
| Lena | −0.458 | −1.075 | −2.756 | 0.833 | 0.613 | 0.289 | 0.000 | 0.740 | 0.439 |
| Jiayan No. 2 | 0.281 | 0.086 | 0.011 | −0.236 | 0.714 | 0.475 | 0.473 | 0.527 | 0.584 |
| Mengshi No. 1 | 0.759 | −0.597 | −0.770 | −2.880 | 0.779 | 0.366 | 0.339 | 0.000 | 0.485 |
| Qinghai No. 444 | −0.252 | −2.485 | 0.647 | 2.140 | 0.642 | 0.063 | 0.582 | 1.000 | 0.539 |
| Haymaker | 1.470 | −2.876 | −0.847 | −0.741 | 0.876 | 0.000 | 0.326 | 0.426 | 0.495 |
| Gaoyan No. 1 | −4.957 | −0.941 | −0.285 | −0.481 | 0.000 | 0.310 | 0.422 | 0.478 | 0.223 |
| Baler | −1.799 | 2.544 | −1.454 | 1.914 | 0.431 | 0.869 | 0.223 | 0.955 | 0.576 |
| Weight | 0.427 | 0.247 | 0.183 | 0.1434 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Ge, R.; Wang, J.; Wei, X.; Zhao, Y.; Pu, X.; Xu, C. Comparative Study on Production Performance of Different Oat (Avena sativa) Varieties and Soil Physicochemical Properties in Qaidam Basin. Plants 2025, 14, 1978. https://doi.org/10.3390/plants14131978
Wu W, Ge R, Wang J, Wei X, Zhao Y, Pu X, Xu C. Comparative Study on Production Performance of Different Oat (Avena sativa) Varieties and Soil Physicochemical Properties in Qaidam Basin. Plants. 2025; 14(13):1978. https://doi.org/10.3390/plants14131978
Chicago/Turabian StyleWu, Wenqi, Ronglin Ge, Jie Wang, Xiaoli Wei, Yuanyuan Zhao, Xiaojian Pu, and Chengti Xu. 2025. "Comparative Study on Production Performance of Different Oat (Avena sativa) Varieties and Soil Physicochemical Properties in Qaidam Basin" Plants 14, no. 13: 1978. https://doi.org/10.3390/plants14131978
APA StyleWu, W., Ge, R., Wang, J., Wei, X., Zhao, Y., Pu, X., & Xu, C. (2025). Comparative Study on Production Performance of Different Oat (Avena sativa) Varieties and Soil Physicochemical Properties in Qaidam Basin. Plants, 14(13), 1978. https://doi.org/10.3390/plants14131978






