Genome-Wide Characterization of the ANN Gene Family in Corydalis saxicola Bunting and the Role of CsANN1 in Dehydrocavidine Biosynthesis
Abstract
1. Introduction
2. Results
2.1. Identification and Physicochemical Analysis of CsANNs
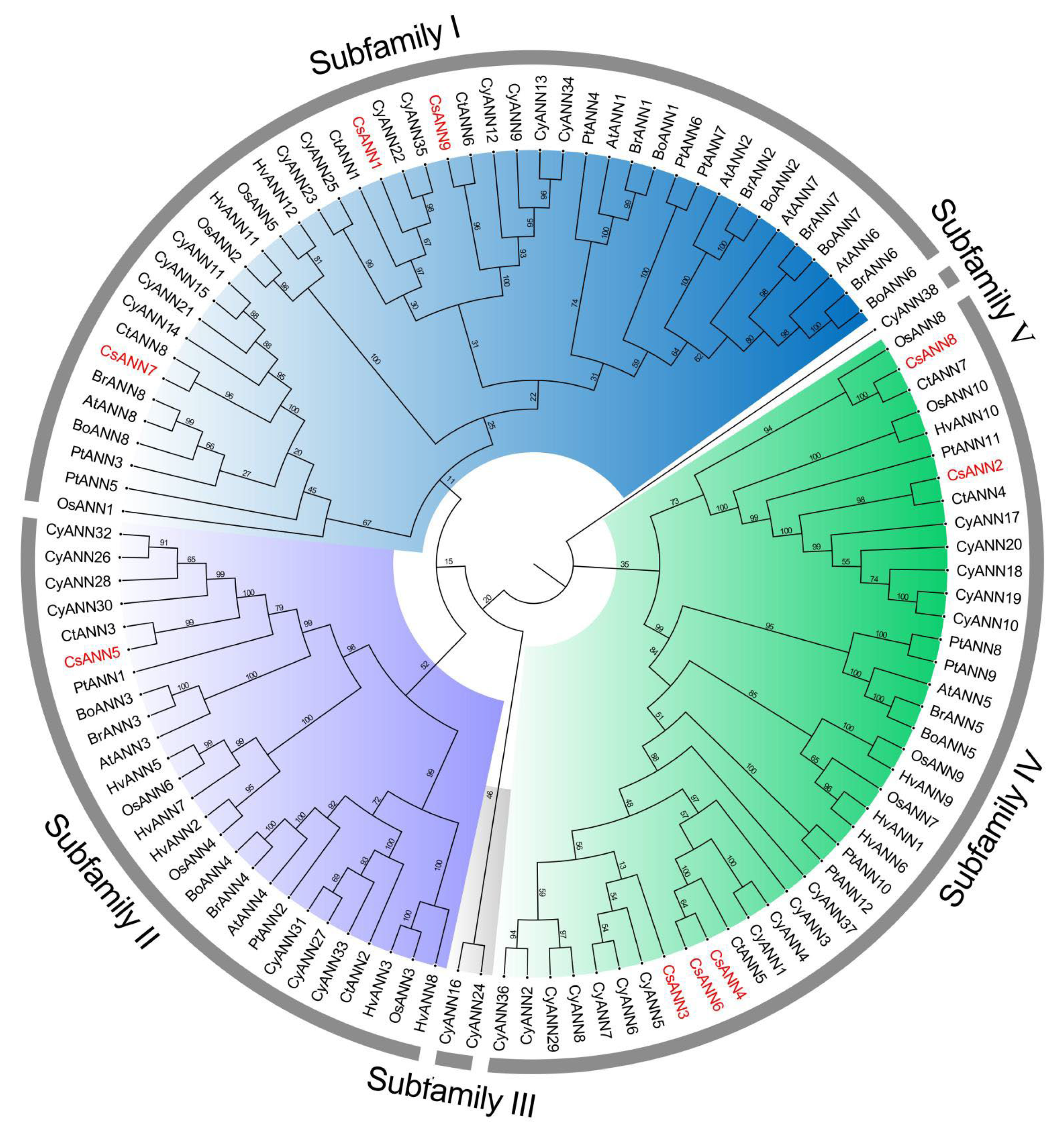
2.2. Sequences Alignment and Phylogenetic Analysis of ANNs
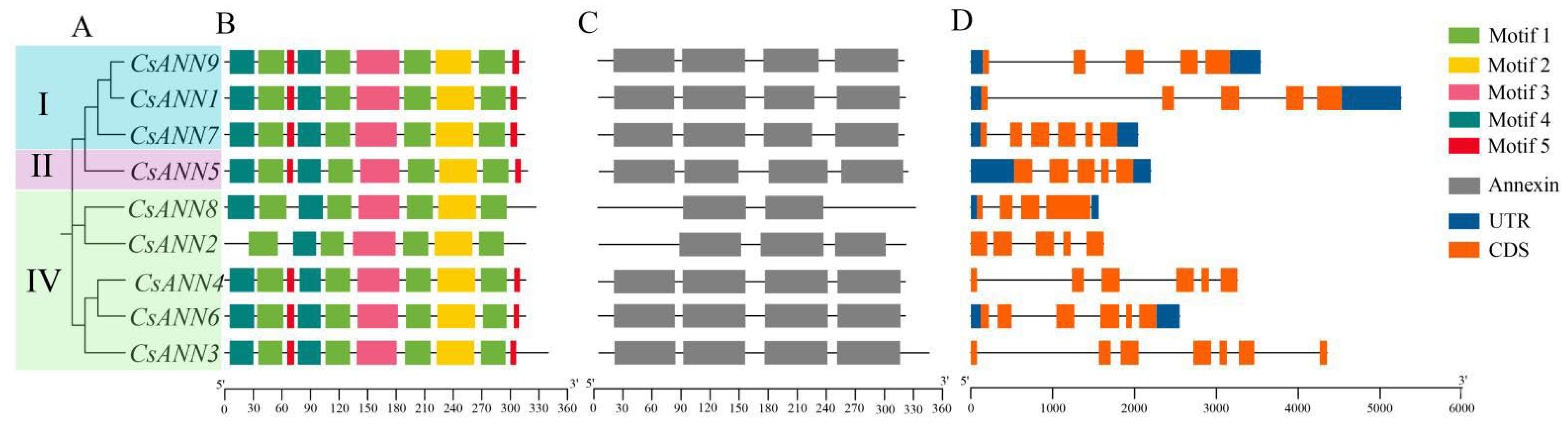
2.3. Conserved Motifs and Gene Structure of CsANNs
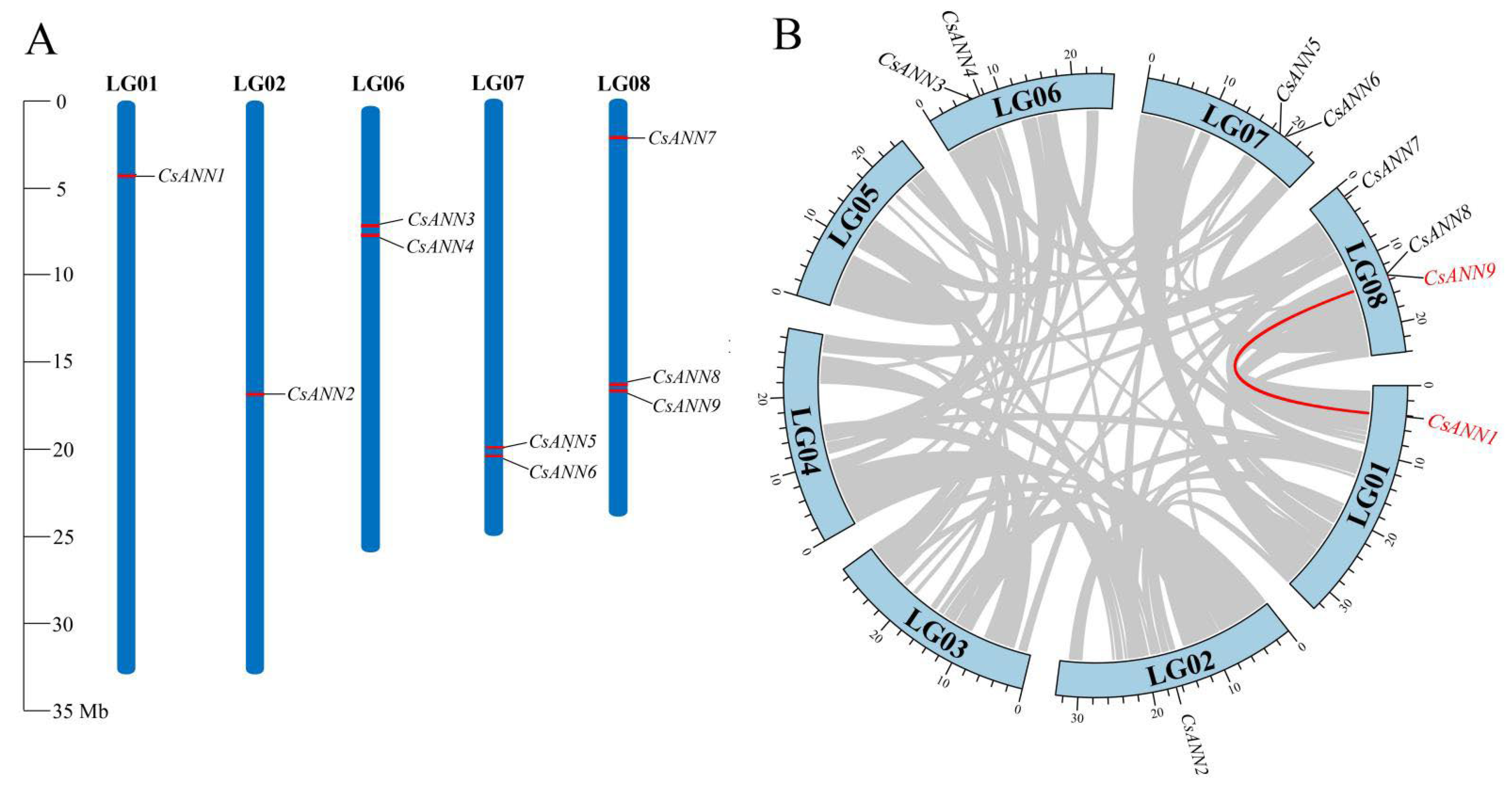
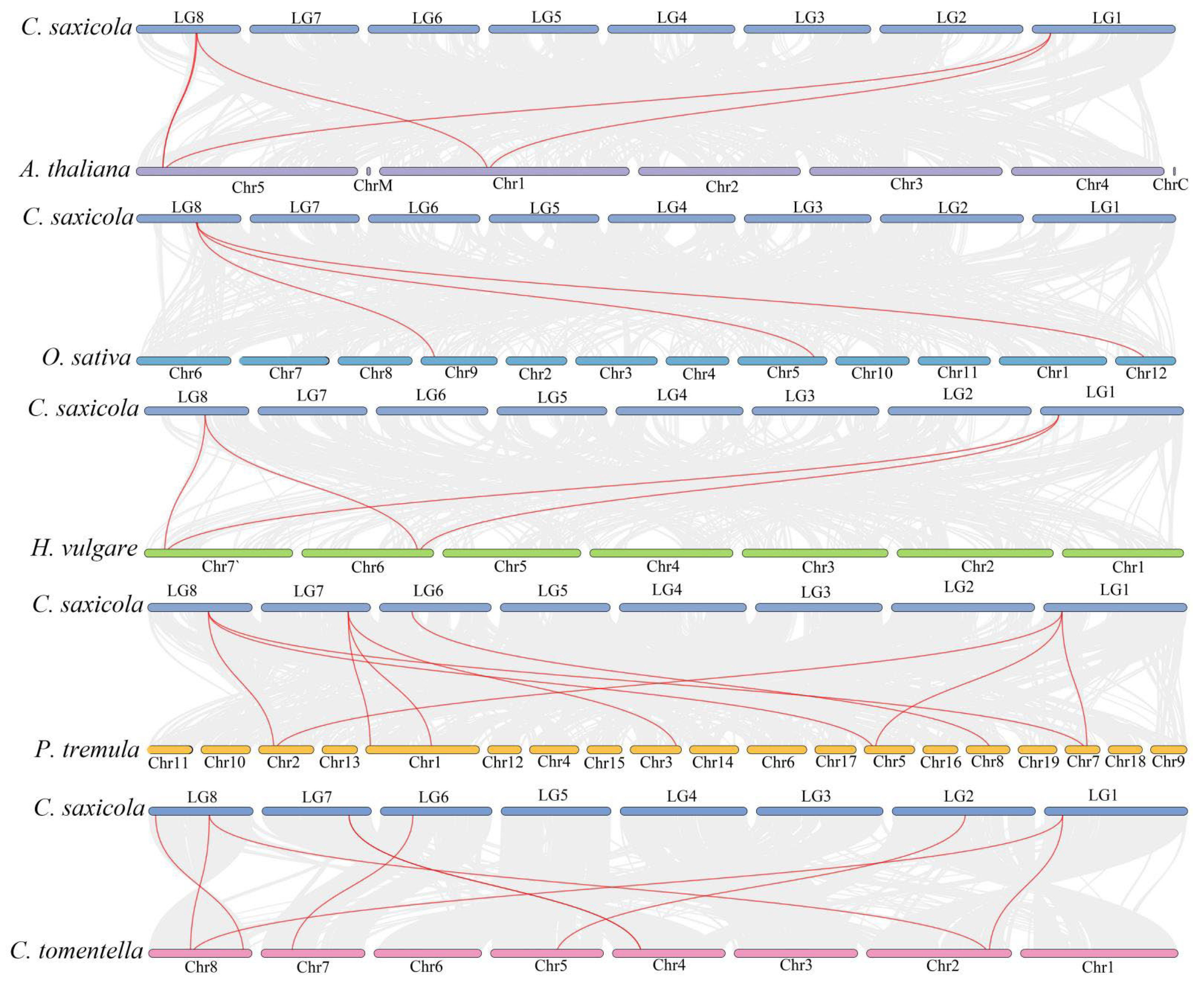
2.4. Chromosomal Location and Collinearity Analysis of CsANNs
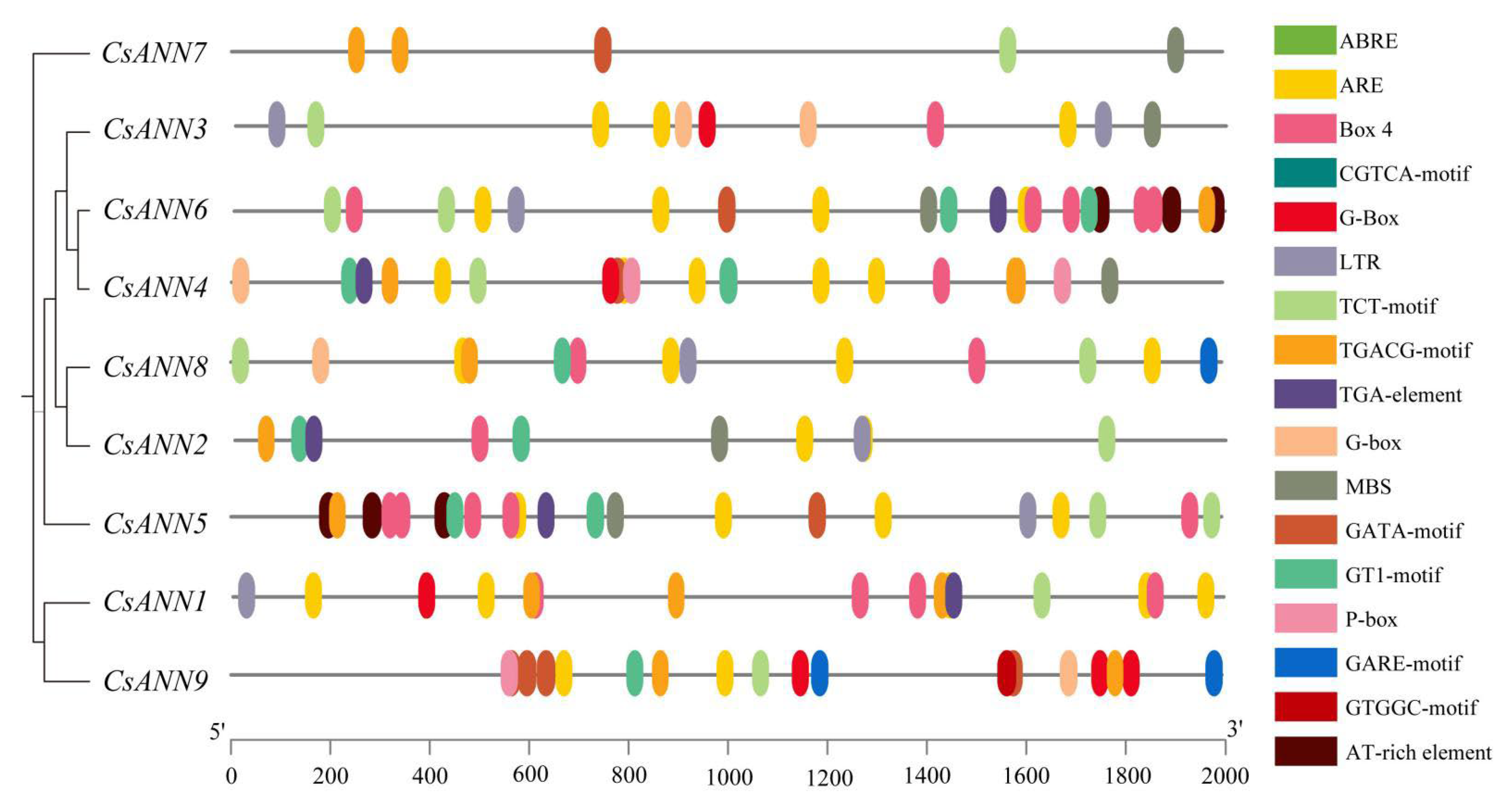
2.5. Analysis of Cis-Acting Elements in CsANN Promoters
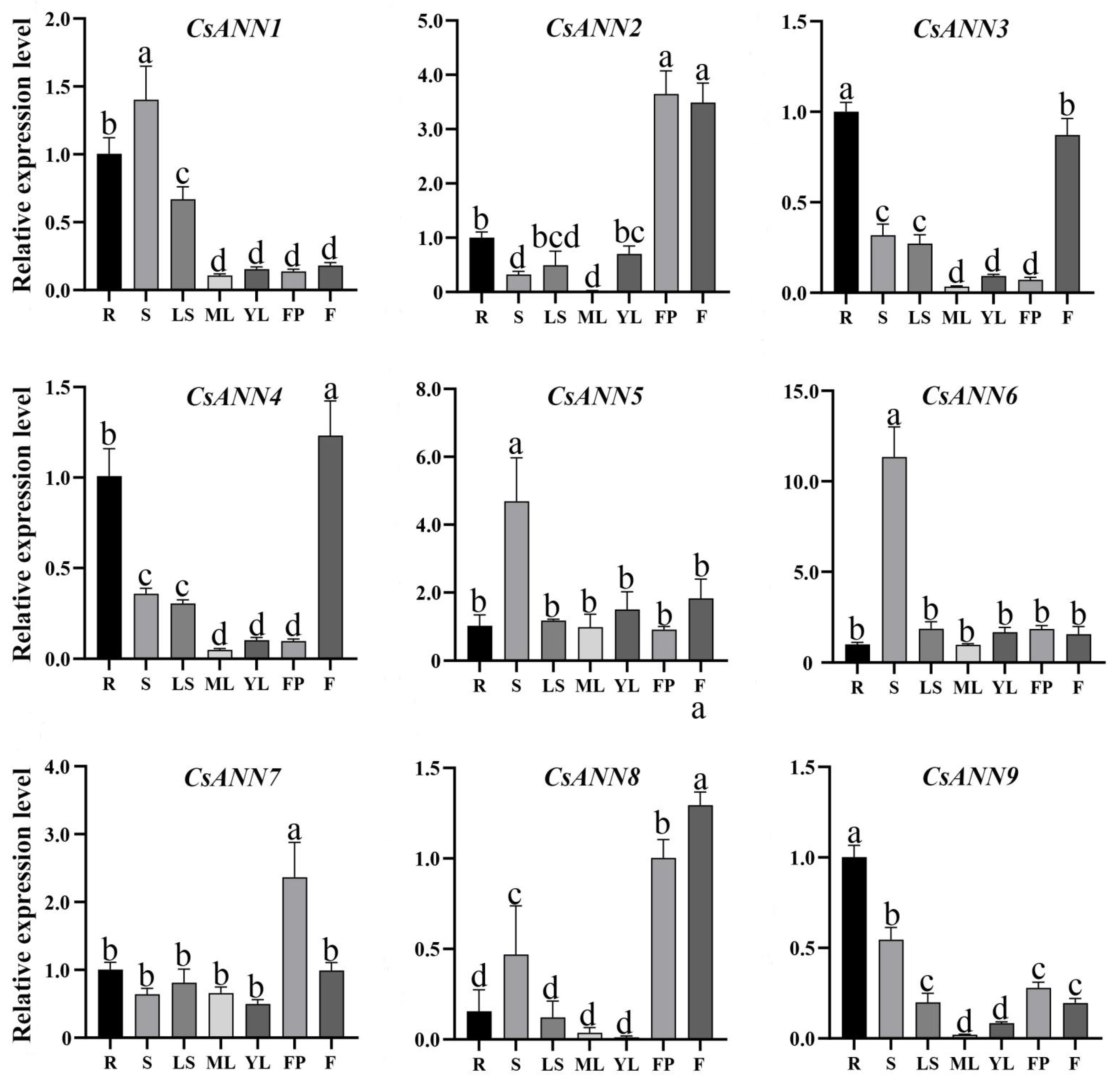
2.6. Tissue-Specific Expression Profile of CsANNs
2.7. The Effects of Exogenous CaCl2 Treatments on C. saxicola Seedlings
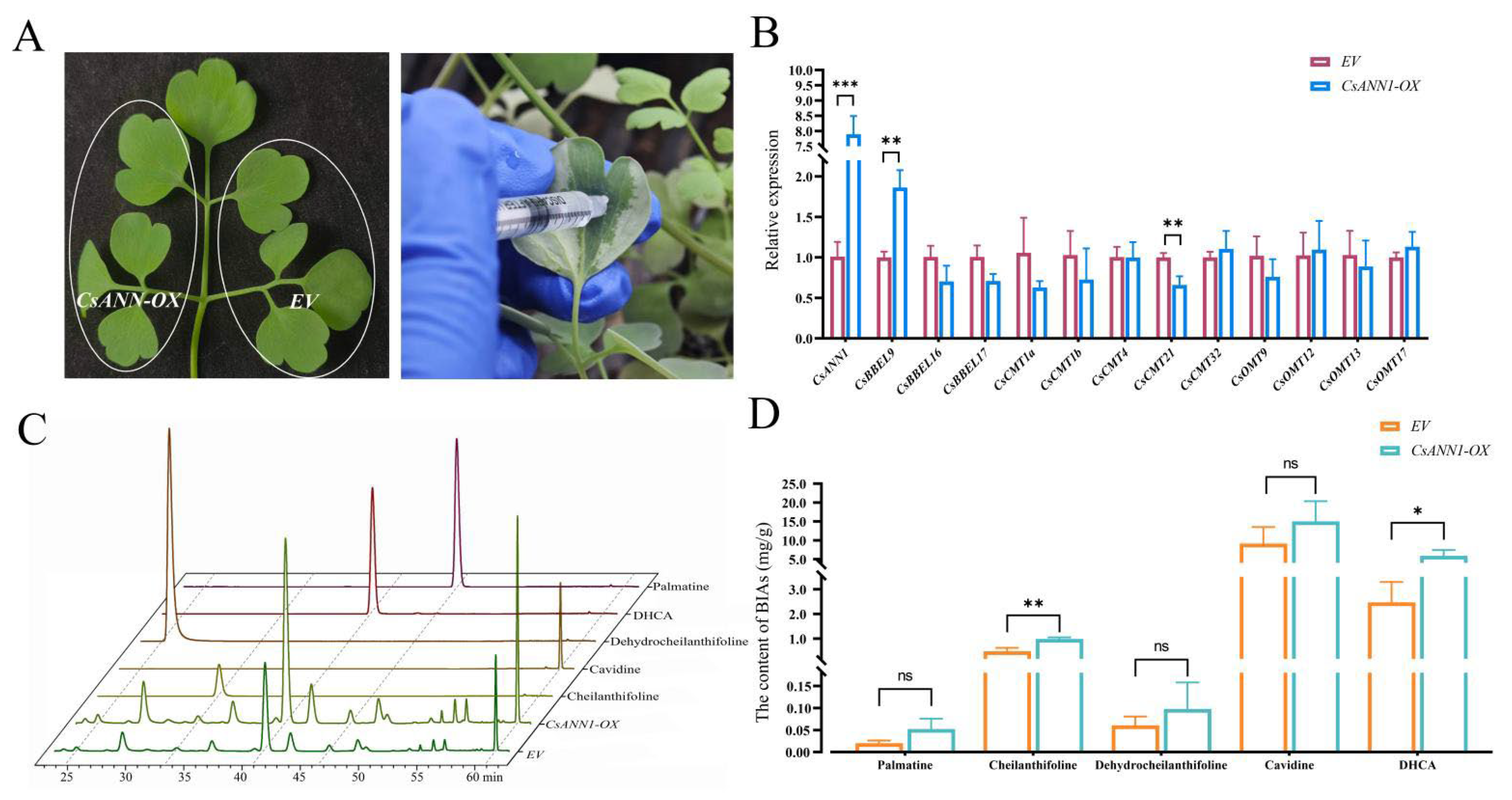
2.8. CsANNs Correlated with DHCA Biosynthesis
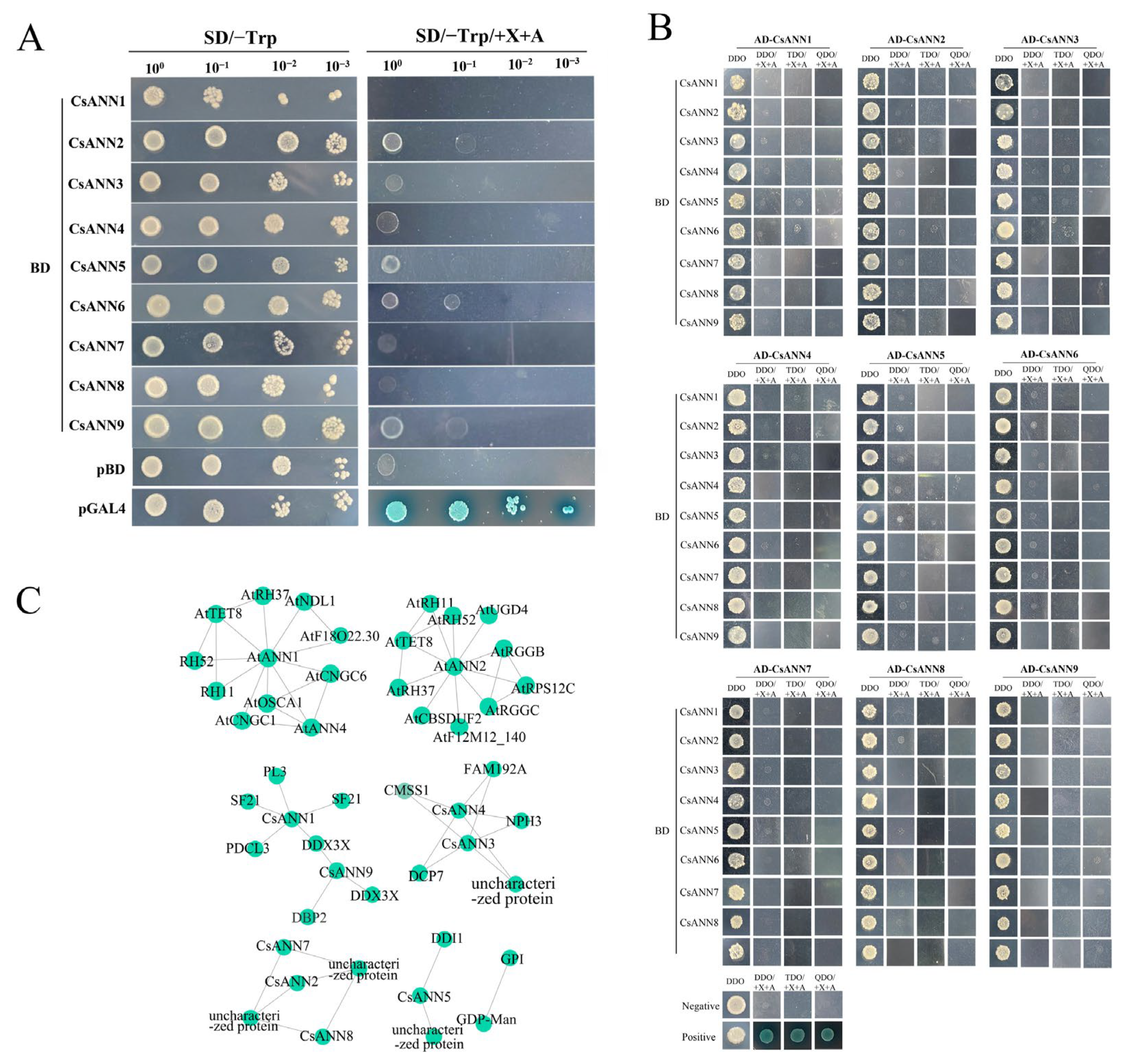
2.9. Yeast Two Hybrid Assays and Protein Interaction Networks of the CsANNs
3. Discussion
3.1. The Structure of CsANNs Were Conserved
3.2. Involvement of CsANNs in the Biosynthesis of DHCA
3.3. The Role of CsANNs in the Adaptability of C. saxicola to a Karst Environment
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. RNA Sequencing and Transcriptomic Data Analysis
4.3. Genome-Wide Identification of the CsANN Genes
4.4. Phylogenetic Relationship, Conserved Motif, and Gene Structure Analysis
4.5. Chromosomal Location, Collinearity, and Gene Duplication Analysis
4.6. Cis-Acting Elements, and Protein–Protein Interaction Analysis
4.7. Measurement of the BIAs, Calcium, Proline, and Soluble Sugar Contents
4.8. CsANN Cloning
4.9. qRT-PCR Analysis
4.10. Yeast Two-Hybrid Assay
4.11. Transient Overexpression of CsANN1 and CsANN9 in C. saxicola Leaves
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, G.; Lin, Z.; Bo, C.; Jia, Y.; Jiang, B.; Qi, Y.; Jun, L. The traditional uses, phytochemistry, pharmacokinetics, pharmacology, toxicity, and applications of Corydalis saxicola Bunting: A review. Front. Pharmacol. 2022, 13, 822792. [Google Scholar]
- Xu, L.; Chao, T.; Hua, Z.; Jin, W.; Fang, W.; Yi, Y.M.; Xi, L.; Hong, Z.; Chun, Y.; Bang, C.; et al. Investigation of the hepato-protective effect of Corydalis saxicola Bunting on carbon tetrachloride-induced liver fibrosis in rats by 1H-NMR-based metabonomics and network pharmacology approaches. J. Pharm. Biomed. Anal. 2018, 159, 252–261. [Google Scholar]
- Fan, Z.; Yang, X.; Zhen, L.; Xiao, W.; Dan, H.; Sui, Z.; Min, L.; De, Y.; Dong, W.; Yi, W. Anti-hepatitis B virus effects of dehydrocheilanthifoline from Corydalis saxicola. Am. J. Chin. Med. 2013, 41, 119–130. [Google Scholar]
- Wang, T.; Sun, N.L.; Zhang, W.D.; Li, H.L.; Lu, G.C.; Yuan, B.J.; Jiang, H.; She, J.H.; Zhang, C. Protective effects of dehydrocavidine on carbon tetrachloride-induced acute hepatotoxicity in rats. J. Ethnopharmacol. 2008, 117, 300–308. [Google Scholar] [CrossRef]
- Li, C.; Liu, H.; Qin, M.; Tan, Y.J.; Ou, X.L.; Chen, X.Y.; Wei, Y.; Zhang, Z.J.; Lei, M. RNa editing events and expression profiles of mitochondrial protein-coding genes in the endemic and endangered medicinal plant, Corydalis saxicola. Front. Plant Sci. 2024, 15, 1332460. [Google Scholar] [CrossRef]
- Liu, C.X.; Duan, Y.; Xie, H.T.; Tian, J. Challenges in Research and Development of Traditional Chinese Medicines; Institute of Pharmaceutical Research: Nanjing, China, 2007; pp. 7102–7108. [Google Scholar]
- Hui, L.; Wei, Z.; Chuan, Z.; Ting, H.; Run, L.; Jiang, H.; Hai, C. Comparative analysis of the chemical profile of wild and cultivated populations of Corydalis saxicola by high-performance liquid chromatography. Phytochem. Anal. 2007, 18, 393–400. [Google Scholar]
- Xiao, W.; Yan, W.; Yu, B.; Yan, R.; Xiao, X.; Fu, Z.; Hai, D. A critical review on plant annexin: Structure, function, and mechanism. Plant Physiol. Biochem. 2022, 190, 81–89. [Google Scholar]
- Volker, G.; Carl, C.; Stephen, M. Annexins: Linking Ca; signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar]
- Mortimer, J.C.; Laohavisit, A.; Macpherson, N.; Webb, A.; Brownlee, C.; Battey, N.H.; Davies, J.M. Annexins: Multifunctional components of growth and adaptation. J. Exp. Bot. 2008, 59, 533–544. [Google Scholar] [CrossRef]
- Xiao, W.; Ali, M.; Hui, W.; Xiao, W.; Jia, Z.; Wei, S.; Dawei, L.; Qiang, Z. Overexpression of PtAnnexin1 from Populus trichocarpa enhances salt and drought tolerance in transgenic poplars. Tree Genet. Genomes 2020, 16, 9137–9146. [Google Scholar]
- Gerke, V.; Moss, S.E. Annexins: From structure to function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.; Ali, M.; Guo, L.; Yi, L.; Shi, L.; Chun, Y.; Yan, C.; Fei, Z.; Jian, Z. Genome-wide characterization and abiotic stresses expression analysis of annexin family genes in poplar. Int. J. Mol. Sci. 2022, 23, 515. [Google Scholar]
- Hofmann, A.; Proust, J.; Dorowski, A.; Schantz, R.; Huber, R. Annexin 24 from Capsicum annuum x-ray structure and biochemical characterization. J. Biol. Chem. 2000, 275, 8072–8082. [Google Scholar] [CrossRef] [PubMed]
- Dorota, P.; Greg, C.; Grazyna, G.; Janusz, D.; Krzysztof, F.; Araceli, C.; Bartlomiej, F.; Stanley, R.; Jacek, H. The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol. 2009, 150, 1394–1410. [Google Scholar]
- Hee, Y.; Pat, H.; Venkatesan, S. Analysis of the female gametophyte transcriptome of Arabidopsis by comparative expression profiling. Plant Physiol. 2005, 139, 1853–1869. [Google Scholar]
- Jami, K.; Clark, B.; Ayele, T.; Ashe, P.; Kirti, B. Genome-wide comparative analysis of annexin superfamily in plants. PLoS ONE 2012, 7, e47801. [Google Scholar] [CrossRef]
- Feng, Y.M.; Wei, X.K.; Liao, W.X.; Huang, L.H.; Zhang, H.; Liang, S.C.; Peng, H. Molecular analysis of the annexin gene family in soybean. Biol. Plant 2013, 57, 655–662. [Google Scholar] [CrossRef]
- Jami, S.K.; Clark, G.B.; Ayele, B.T.; Roux, S.J.; Kirti, P.B. Identification and characterization of annexin gene family in rice. Plant Cell Rep. 2012, 31, 813–825. [Google Scholar] [CrossRef]
- Feng, S.; Jia, Y.; Liang, X.; Xiao, S.; Ji, W.; Yan, W.; Yi, M.; Yue, Z.; Li, L. Characterization of annexin gene family and func-tional analysis of RsANN1a involved in heat tolerance in Radish (Raphanus sativus L.). Physiol. Mol. Biol. Plants 2021, 27, 2027–2041. [Google Scholar]
- Mei, Z.; Xiong, Y.; Qian, Z.; Ming, Z.; En, Z.; Yi, T.; Xue, Z.; Ji, S.; Yan, W. Induction of annexin by heavy metals and jasmonic acid in Zea mays. Funct. Integr. Genomics 2013, 13, 241–251. [Google Scholar]
- Clark, G.B.; Sessions, A.; Eastburn, D.J.; Roux, S.J. Differential expression of members of the annexin multigene family in Arabidopsis. Plant Physiol. 2001, 126, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Bassani, M.; Neumann, P.M.; Gepstein, S. Differential expression profiles of growth-related genes in the elongation zone of maize primary roots. Plant Mol. Biol. 2004, 56, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.; Cantero, A.; Butterfield, T.; Dauwalder, M.; Roux, J. Secretion as a key component of gravitropic growth: Implications for annexin involvement in differential growth. Gravitational Space Res. 2025, 18, 113–114. [Google Scholar]
- Clark, G.B.; Lee, D.W.; Dauwalder, M.; Roux, S.J. Immunolocalization and histochemical evidence for the association of two different Arabidopsis annexins with secretion during early seedling growth and development. Planta 2005, 220, 621–631. [Google Scholar] [CrossRef]
- Blackbourn, H.D.; Walker, J.H.; Battey, N.H. Calcium-dependent phospholipid-binding proteins in plants their characterization and potential for regulating cell-growth. Planta 1991, 184, 67–73. [Google Scholar] [CrossRef]
- Cantero, A.; Barthakur, S.; Bushart, T.; Chou, S.; Morgan, R.; Fernandez, M.; Clark, G.; Roux, S. Expression profiling of the Arabidopsis annexin gene family during germination, de-etiolation and abiotic stress. Plant Physiol. Biochem. 2006, 44, 13–24. [Google Scholar] [CrossRef]
- Blackbourn, H.D.; Barker, P.J.; Huskisson, N.S.; Battey, N.H. Properties and partial protein-sequence of plant annexins. Plant Physiol. 1992, 99, 864–871. [Google Scholar] [CrossRef]
- Barnes, A.; Bale, J.; Constantinidou, C.; Ashton, P.; Jones, A.; Pritchard, J. Determining protein identity from sieve element sap in Ricinus communis L. by quadrupole time of flight (Q-TOF) mass spectrometry. J. Exp. Bot. 2004, 55, 1473–1481. [Google Scholar] [CrossRef]
- Giavalisco, P.; Kapitza, K.; Kolasa, A.; Buhtz, A.; Kehr, J. Towards the proteome of Brassica napus phloem sap. Proteomics 2006, 6, 896–909. [Google Scholar] [CrossRef]
- Tang, J.; Tang, X.; Qin, Y.; He, Q.; Yi, Y.; Ji, Z. Karst Rocky Desertification progress: Soil calcium as a possible driving force. Sci. Total. Environ. 2019, 649, 1250–1259. [Google Scholar] [CrossRef]
- Zhi, L.; Li, Z.; Zhong, Y.; Zhi, R.; Feng, H.; Xin, T.; Zhi, X.; Fang, D.; Zuo, Y.; Guang, L.; et al. Genomic mechanisms of physiological and morphological adaptations of Limestone langurs to Karst Habitats. Mol. Biol. Evol. 2020, 37, 952–968. [Google Scholar]
- Cheng, C.; Hao, C.; Yi, Z.; Hannah, T.; Margaret, F.; Ye, H.; Rui, X. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Magali, L.; Patrice, D.; Gert, T.; Kathleen, M.; Yves, M.; Peer, V.; Pierre, R.; Stephane, R. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar]
- Xu, Z.; Li, Z.; Ren, F.; Gao, R.; Wang, Z.; Zhang, J.; Zhao, T.; Ma, X.; Pu, X.; Xin, T.; et al. The genome of Corydalis reveals the evolution of benzylisoquinoline alkaloid biosynthesis in Ranunculales. Plant J. 2022, 111, 217–230. [Google Scholar] [CrossRef]
- Ferré, S.; Casadó, V.; Devi, L.A.; Filizola, M.; Jockers, R.; Lohse, M.J.; Milligan, G.; Pin, P.; Guitart, X. G protein–coupled re-ceptor oligomerization revisited: Functional and pharmacological perspectives. Pharmacol. Rev. 2014, 66, 413–434. [Google Scholar] [CrossRef]
- Xin, H.; Li, L.; Sai, X.; Min, Y.; Pan, X.; Wei, L.; Yu, K.; Lu, H.; Mei, W.; Lun, Q.; et al. Comprehensive analyses of the annexin (ANN) gene family in Brassica rapa, Brassica oleracea and Brassica napus reveals their roles in stress response. Sci. Rep. 2020, 10, 4295. [Google Scholar]
- Xu, L.; Tang, Y.; Gao, S.; Hong, L.; Wang, W.W.; Fang, Z.F.; Li, X.Y.; Ma, J.X.; Quan, W.; Li, X.; et al. Comprehensive analyses of the annexin gene family in wheat. BMC Genom. 2016, 17, 415–423. [Google Scholar] [CrossRef]
- Mortimer, J.C.; Coxon, K.M.; Laohavisit, A.; Davies, J.M. Heme-independent soluble and membrane-associated peroxidase activity of a Zea mays annexin preparation. Plant Signal. Behav. 2009, 4, 428–430. [Google Scholar] [CrossRef]
- Gorecka, K.M.; Konopka-Postupolska, D.; Hennig, J.; Buchet, R.; Pikula, S. Peroxidase activity of annexin 1 from Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2005, 336, 868–875. [Google Scholar] [CrossRef]
- Raina, I.; Javeria, E.; Sheng, G.; Teng, L.; Muhammad, I.; Zhi, Y.; Taotao, W. Overexpression of annexin gene AnnSp2, enhances drought and salt tolerance through modulation of ABA synthesis and scavenging ROS in tomato. Sci. Rep. 2017, 7, 12087. [Google Scholar]
- Hoshino, D.; Hayashi, A.; Temi, Y.; Kan, N.; Tsuchiya, T. Biochemical and immunohistochemical characterization of Mimosa annexin. Planta 2004, 219, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.J.; Yusof, A.M.; Winter, A.; Osman, A.; Reeve, A.K.; Hofmann, A. The crystal structure of calcium-bound annexin Gh1 from Gossypium hirsutum and its implications for membrane binding mechanisms of plant annexins. J. Biol. Chem. 2008, 283, 18314–18322. [Google Scholar] [CrossRef] [PubMed]
- Jeffares, D.C.; Penkett, J.; Bähler, J. Rapidly regulated genes are intron poor. Trends Genet. 2008, 24, 375–378. [Google Scholar] [CrossRef] [PubMed]
- William, R.S.; Walter, G. The evolution of spliceosomal introns: Patterns, puzzles and progress. Nat. Rev. Genet. 2006, 7, 211–221. [Google Scholar] [CrossRef]
- Michaela, T.; Hendrik, R.; Miroslav, O.; Nicola, M.; Miroslava, H.; Petr, D.; Jozef, Š.; Olga, Š. Advanced microscopy reveals complex developmental and subcellular localization patterns of annexin 1 in Arabidopsis. Front. Plant Sci. 2020, 11, 1153. [Google Scholar]
- Joanna, P.; Aneta, K.; Ruud, D.; Marcin, G.; Michael, K.; Jean, L.; Slawomir, P.; René, B. A putative consensus sequence for the nucleotide-binding site of annexin A6. Biochemistry 2003, 42, 9137–9146. [Google Scholar]
- Zhou, Y.; Fan, W.; Zhang, H. Marsdenia tenacissima genome reveals calcium adaptation and tenacissoside biosynthesis. Plant J. 2022, 113, 1146–1159. [Google Scholar] [CrossRef]
- Volk, G.M.; Lynch, V.J.; Kostman, T.A.; Goss, L.J.; Franceschi, V.R. The role of druse and raphide calcium oxalate crystals in tissue calcium regulation in Pistia stratiotes leaves. Plant Biol. 2002, 4, 34–45. [Google Scholar] [CrossRef]
- Wei, X.; Deng, X.; Xiang, W. Calcium content and high calcium adaptation of plants in karst areas of southwestern hunan, China. Biogeosciences 2018, 15, 2991–3002. [Google Scholar] [CrossRef]
- Musetti, R.; Favali, M. Cytochemical localization of calcium and x-ray microanalysis of Catharanthus roseus L. infected with phytoplasmas. Micron 2003, 34, 387–393. [Google Scholar] [CrossRef]
- Hanger, B.C. The movement of calcium in plants. Commun. Soil. Sci. Plant Anal. 1979, 10, 171–193. [Google Scholar] [CrossRef]
- Poovaiah, B.W.; Reddy, A.S.N.; Leopold, A.C. Calcium messenger system in plants. Crit. Rev. Plant Sci. 1987, 6, 47–103. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Frangne, N.; Gomes, E.; Martinoia, E.; Palmgren, M.G. The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 2000, 124, 1814–1827. [Google Scholar] [CrossRef]
- Cheng, H.; Pittman, J.K.; Barkla, B.J.; Shigaki, T.; Hirschi, K.D. The Arabidopsis CAX1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell. 2003, 15, 347–364. [Google Scholar] [CrossRef]
- Huang, H.; Yang, Y.; Li, J. Effects of Rocky Desertification Stress on Oat (Avena sativa L.) Seed Germination and Seedling Growth in the Karst Areas of Southwest China. Plants 2024, 13, 3260. [Google Scholar] [CrossRef]
- Zheng, J.; Wen, D.; Tang, C.; Lai, S.; Yan, Y.; Du, C.; Zhang, Z. The transcriptional regulation of Arabidopsis ECT8 by ABA-responsive element binding transcription factors in response to ABA and abiotic stresses. Physiol. Mol. Biol. Plants 2025, 31, 343–355. [Google Scholar] [CrossRef]
- Wang, X.; Wei, H.; Wang, K.; Tang, X.; Li, S.; Zhang, N.; Si, H. MYB transcription factors: Acting as molecular switches to regulate different signaling pathways to modulate plant responses to drought stress. Ind. Crops Prod. 2025, 226, 120676. [Google Scholar] [CrossRef]
- Erina, T.; Daichi, K.; Yusuke, U.; Ken, I. Nrf2 induction potency of plant-derived compounds determined using an antioxidant response element luciferase reporter and conventional NAD(P)H-quinone acceptor oxidoreductase 1 activity assay. BMC Res. Notes 2024, 17, 373. [Google Scholar]
- Liu, L.; Yahaya, S.; Li, J.; Wu, F. Enigmatic role of auxin response factors in plant growth and stress tolerance. Front. Plant Sci. 2024, 15, 1398818. [Google Scholar] [CrossRef]
- Yuan, Y.; Stéphanie, S.; Aurore, C. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 2007, 19, 2470–2483. [Google Scholar] [CrossRef]
- Kazuko, Y.S.; Kazuo, S. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant. Sci. 2005, 10, 88–94. [Google Scholar]
- Sesia, M.; Sabatti, C.; Candès, E.J. Gene hunting with hidden markov model knockoffs. Biometrika 2019, 106, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2018, 7, gix120. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Lorna, R.; Neil, R.; Gustavo, S.; Alexandre, A.; David, H.; Gregory, D.; Granger, S.; Robert, F. Genome properties in 2019: A new companion database to InterPro for the inference of complete functional attributes. Nucleic Acids Res. 2019, 47, D564–D572. [Google Scholar]
- Séverine, D.; Chiara, G.; Frédérique, L.; Heinz, S.; Vassilios, I.; Christine, D. Expasy, the swiss bioinformatics resource portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar]
- Letunic, I.; Goodstadt, L.; Dickens, N.J.; Doerks, T.; Schultz, J.; Mott, R.; Ciccarelli, F.; Copley, R.R.; Ponting, C.P.; Bork, P. Recent improvements to the SMART domain-based sequence annotation resource. Nucleic Acids Res. 2002, 30, 242–244. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.; Obayashi, T.; Fujita, N.; Harada, H.; Adams, C.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, 585–587. [Google Scholar] [CrossRef]
- Kuo, C.; Hong, S. Cell-PLoc: A package of web servers for predicting subcellular localization of proteins in various organisms. Nat. Protoc. 2008, 3, 153–162. [Google Scholar]
- Georg, N.; Sebastian, M.; Birgit, E.; Andreas, H.; Frank, E. Prediction of peroxisomal targeting signal 1 containing proteins from amino acid sequence. J. Mol. Biol. 2003, 3, 581–592. [Google Scholar]
- Kuo, C.; Hong, S. Euk-mPLoc: A fusion classifier for large-scale eukaryotic protein subcellular location prediction by incorporating multiple sites. J. Proteome Res. 2007, 6, 1728–1734. [Google Scholar]
- Andrew, W.; Jim, P.; David, M.; Geoffrey, B. Jalview: Visualization and analysis of molecular sequences, alignments, and structures. BMC Bioinform. 2005, 6, 28. [Google Scholar]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Nastou, K.; Koutrouli, M.; Kirsch, R.; Mehryary, F.; Hachilif, R.; Hu, D.; Peluso, M.E.; Huang, Q.; Fang, T.; et al. The STRING database in 2025: Protein networks with directionality of regulation. Nucleic Acids Res. 2025, 53, D730–D737. [Google Scholar] [CrossRef]
- David, O.; John, M.; Jorge, B.; Alexander, P.; Barry, D. Cytoscape automation: Empowering Workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar]
- Wen, L.; Fa, X.; Shi, C.; Zhen, Z.; Yan, Z.; Yu, J.; Mei, L.; Yan, Z.; Yong, L.; Yi, Y.; et al. A comparative study on Ca content and distribution in two Gesneriaceae species reveals distinctive mechanisms to cope with high rhizospheric soluble calcium. Front. Plant Sci. 2014, 20, 647. [Google Scholar]
- Yi, W.; Hui, L.; Shan, M.; Hong, M.; Long, T. Physiological and differential protein expression analyses of the calcium stress response in the Drynaria roosii rhizome. Heliyon 2024, 10, e38260. [Google Scholar]
- Wen, M.; Jing, R.; Quan, D.; Na, T.; Ting, L.; Qing, R. Morphological and physiological adaptation characteristics of lithophytic bryophytes to karst high calcium environment. BMC Plant Biol. 2023, 23, 160. [Google Scholar]
- Zhu, Y.; Niu, S.; Lin, J.; Yang, H.; Zhou, X.; Wang, S.; Liu, X.; Yang, Q.; Zhang, C.; Zhuang, Y.; et al. Genome-wide identification and expression analysis of TCP transcription factors responding to multiple stresses in Arachis hypogaea L. Int. J. Mol. Sci. 2025, 26, 1069. [Google Scholar] [CrossRef]
- Jia, W.; Ya, W.; Xu, L.; Hui, P.; Dian, J.; Jia, W.; Can, J.; Kumar, S.S.; Jian, S.; Xin, L.; et al. An integrated multi-omics approach reveals polymethoxylated flavonoid biosynthesis in Citrus reticulata cv. Chachiensis. Nat. Commun. 2024, 15, 3991. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, J.; Wen, Z.; Qin, M.; Lu, Y.; Huang, L.; Ou, X.; Kang, L.; Li, C.; Lei, M.; et al. Genome-Wide Characterization of the ANN Gene Family in Corydalis saxicola Bunting and the Role of CsANN1 in Dehydrocavidine Biosynthesis. Plants 2025, 14, 1974. https://doi.org/10.3390/plants14131974
Liu H, Wang J, Wen Z, Qin M, Lu Y, Huang L, Ou X, Kang L, Li C, Lei M, et al. Genome-Wide Characterization of the ANN Gene Family in Corydalis saxicola Bunting and the Role of CsANN1 in Dehydrocavidine Biosynthesis. Plants. 2025; 14(13):1974. https://doi.org/10.3390/plants14131974
Chicago/Turabian StyleLiu, Han, Jing Wang, Zhaodi Wen, Mei Qin, Ying Lu, Lirong Huang, Xialian Ou, Liang Kang, Cui Li, Ming Lei, and et al. 2025. "Genome-Wide Characterization of the ANN Gene Family in Corydalis saxicola Bunting and the Role of CsANN1 in Dehydrocavidine Biosynthesis" Plants 14, no. 13: 1974. https://doi.org/10.3390/plants14131974
APA StyleLiu, H., Wang, J., Wen, Z., Qin, M., Lu, Y., Huang, L., Ou, X., Kang, L., Li, C., Lei, M., & Zhang, Z. (2025). Genome-Wide Characterization of the ANN Gene Family in Corydalis saxicola Bunting and the Role of CsANN1 in Dehydrocavidine Biosynthesis. Plants, 14(13), 1974. https://doi.org/10.3390/plants14131974





