Metabolomic Insights into Sexual Multi-Morphism of Sinomenine Accumulation in Sinomenium acutum
Abstract
1. Introduction
2. Results
2.1. Morphological Characteristics of S. acutum Plants
2.2. Overview of S. acutum Metabolome
2.2.1. Differential Accumulated Metabolites (DAMs) in S. acutum
2.2.2. Pathway Enrichment of DAMs
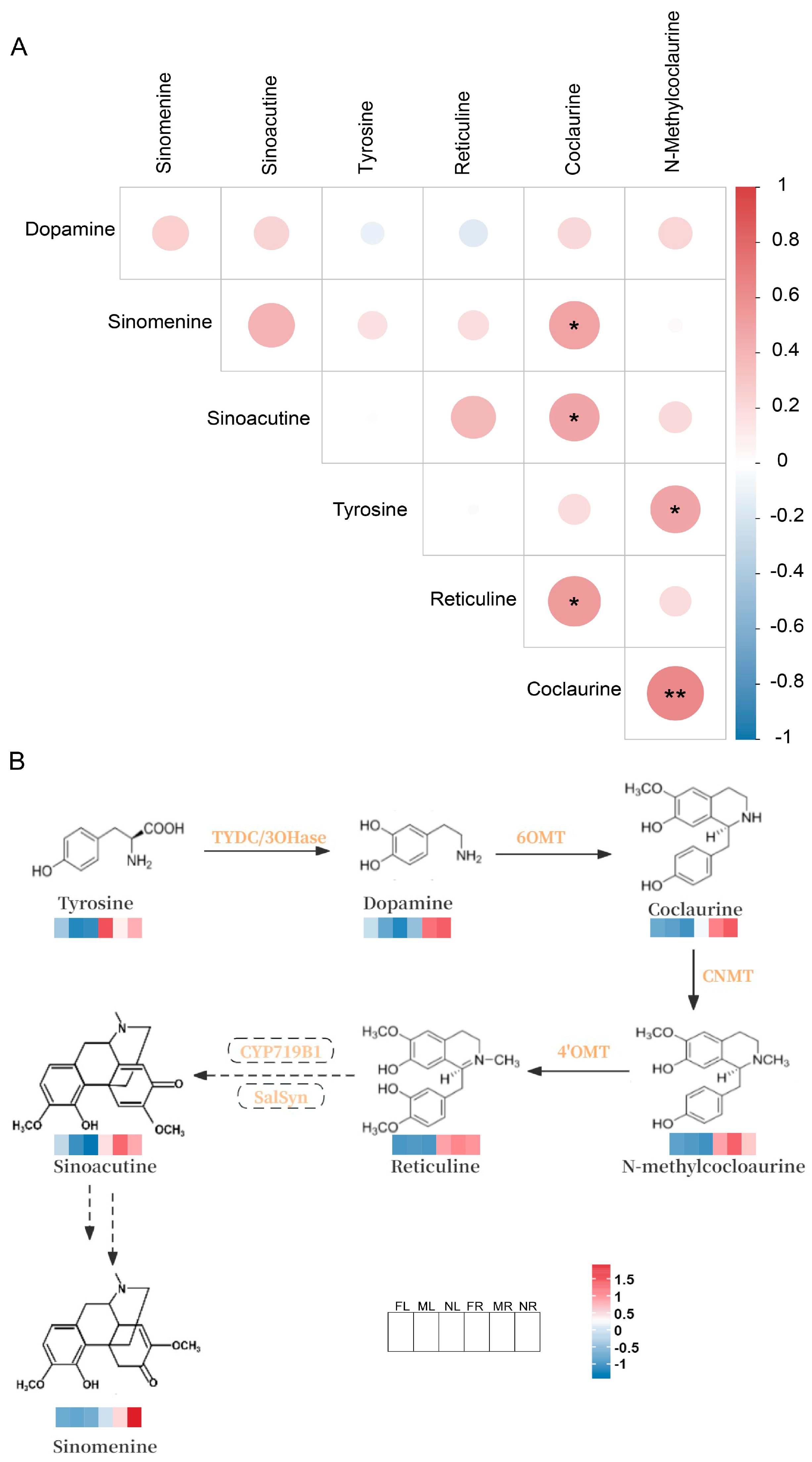
2.2.3. Prediction of Sinomenine Biosynthesis Pathway Based on Metabolome Data
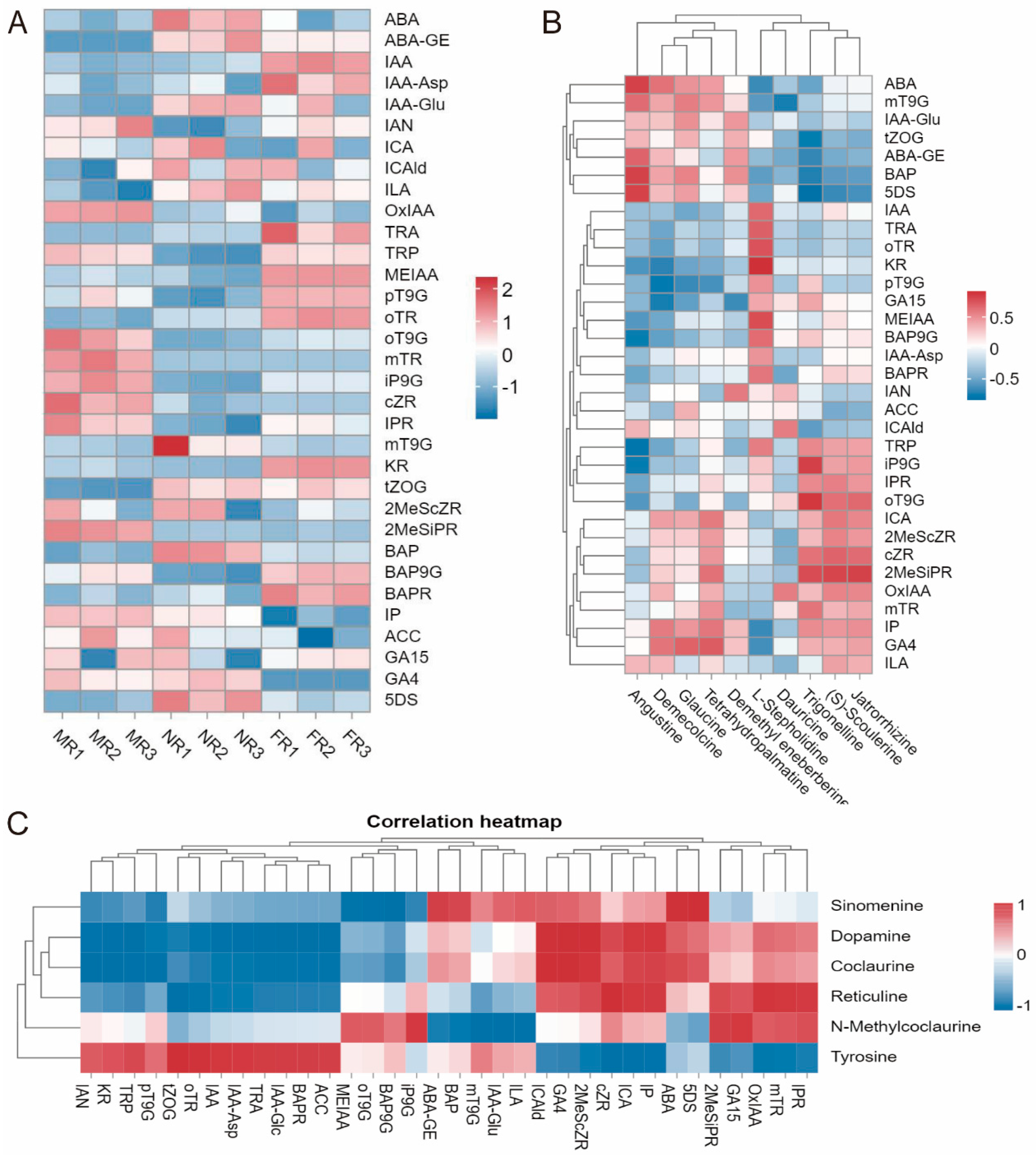
2.2.4. Correlation Analysis of IQA Content and Phytohormone Levels
2.2.5. Exogenous ABA Treatment Improves Sinomenine Accumulation in S. acutum
3. Discussion
3.1. Metabolomics Analysis of Male and Female S. acutum Plants
3.2. Analysis of Phytohormonal Differences Between the Roots of Male and Female S. acutum Plants
4. Materials and Methods
4.1. Plant Materials
4.2. Metabolome and LC-MS Analysis
4.3. Phytohormone Level Measurement
4.4. The Impact of Exogenous Hormone Treatment on the Alkaloids in S. acutum
4.5. Detecting the Expression Levels of Genes Associated with Sinomenine
4.6. Data Processing and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China: Volume One; China Medical Science and Technology Press: Beijing, China, 2020; Volume 204, Available online: https://ydz.chp.org.cn/#/item?bookId=1&entryId=300 (accessed on 30 December 2020).
- Chen, X.; Wang, Y.L. The research status and prospect of caulis sinomenii. Jiangxi J. Tradit. Chin. Med. 2011, 42, 69–72. Available online: https://kns.cnki.net/kcms2/article/abstract?v=tQ02qvqxHS-ekIsuo4TS2_z-8Y38EYDGuGrJUAoulKKysIyLj4CCv_QzMOWwXBtGshHI3Zy0s8j9P6khwjFvGCf3BJT0haf4DlrIImb7CO-q7pC7984dbFVSz7fpmmk1hSCo0SQSWuJBbAwSFqyahWLK9yqNELWzxqbe8qZi0pmEwaAJDVohz5M7hB2oh9jy&uniplatform=NZKPT&language=CHS (accessed on 1 June 2011).
- Ding, C.; Li, Y.; Sun, Y.; Wu, Y.; Wang, F.; Liu, C.; Zhang, H.; Jiang, Y.; Zhang, D.; Song, X. Sinomenium acutum: A Comprehensive Review of its Botany, Phytochemistry, Pharmacology and Clinical Application. Am. J. Chin. Med. 2022, 50, 1219–1253. [Google Scholar] [CrossRef]
- Huang, Y.F.; He, F.; Wang, C.J.; Xie, Y.; Zhang, Y.Y.; Sang, Z.; Qiu, P.; Luo, P.; Xiao, S.Y.; Li, J.; et al. Discovery of chemical markers for improving the quality and safety control of Sinomenium acutum stem by the simultaneous determination of multiple alkaloids using UHPLC-QQQ-MS/MS. Sci. Rep. 2020, 10, 14182. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.P.L.; Coghi, P.; Law, B.Y.K.; Liu, L.; Wong, V.K.W. The present and future synthetic strategies of structural modifications of sinomenine. Org. Chem. Front. 2020, 7, 4089–4107. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, W.; Gao, T.; Li, T.; Yin, Z.; Guo, H.; Wang, L.; Han, Y.; Jiang, J.D. Analgesic Mechanism of Sinomenine against Chronic Pain. Pain Res. Manag. 2020, 2020, 1876862. [Google Scholar] [CrossRef]
- Lagerström, M.C. Sinomenine is a promising analgesic and antihyperalgesic for pain and hypersensitivity in rheumatoid arthritis. Scand. J. Pain 2015, 7, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, S.; Wang, X.; Wu, H.; Tahara, K.; Tanaka, S.; Sugiyama, K.; Yamada, H.; Sawada, T.; Hirano, T. Effects of sinomenine on the proliferation, cytokine production, and regulatory T-cell frequency in peripheral blood mononuclear cells of rheumatoid arthritis patients. Drug Dev. Res. 2021, 82, 251–258. [Google Scholar] [CrossRef]
- Yan, J.; Yang, J.; Shen, H.; Gao, R.; Lv, S. Sinomenine regulates circTRPM7-related pathway to inhibit gastric cancer cell growth and metastasis. Chem. Biol. Drug Des. 2023, 102, 870–881. [Google Scholar] [CrossRef]
- Liu, W.B.; Yu, X.F.; Zhou, L.; Li, J.G.; Li, M.; Li, W.; Gao, F. Sinomenine Inhibits Non-Small Cell Lung Cancer via Downregulation of Hexokinases II-Mediated Aerobic Glycolysis. Onco Targets Ther. 2020, 13, 3209–3221. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J. Sinomenine alleviates glomerular endothelial permeability by activating the C/EBP-α/claudin-5 signaling pathway. Hum. Cell 2022, 35, 1453–1463. [Google Scholar] [CrossRef]
- Yuan, M.; Zhao, B.; Jia, H.; Zhang, C.; Zuo, X. Sinomenine ameliorates cardiac hypertrophy by activating Nrf2/ARE signaling pathway. Bioengineered 2021, 12, 12778–12788. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Jin, X.; Wang, F.; Jiang, J.; Cheng, L.; Hu, S.; Zhang, G.; Xu, H. Morphinan and isoquinoline alkaloids from the tuberous roots of Stephania cepharantha. Nat. Prod. Res. 2025, 39, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Hoffarth, E.; Eugenio, L.; Savtchouk, J.; Chen, X.; Morris, J.S.; Facchini, P.J.; Ng, K.K. Structural and Functional Studies of Pavine N-Methyltransferase from Thalictrum flavum Reveal Novel Insights into Substrate Recognition and Catalytic Mechanism. J. Biol. Chem. 2016, 291, 23403–23415. [Google Scholar] [CrossRef]
- Misiurek, J.; Plech, T.; Kaproń, B.; Makuch-Kocka, A.; Szultka-Młyńska, M.; Buszewski, B.; Petruczynik, A. Determination of Some Isoquinoline Alkaloids in Extracts Obtained from Selected Plants of the Ranunculaceae, Papaveraceae and Fumarioideae Families by Liquid Chromatography and In Vitro and In Vivo Investigations of Their Cytotoxic Activity. Molecules 2023, 28, 3503. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Wang, L.J.; Pang, H.Q.; Guo, Y.; Xiao, P.T.; Chu, C.; Guo, L.; Liu, E.H. Rapid profiling of alkaloid analogues in Sinomenii Caulis by an integrated characterization strategy and quantitative analysis. J. Pharm. Biomed. Anal. 2019, 174, 376–385. [Google Scholar] [CrossRef]
- Sato, F. Improved Production of Plant Isoquinoline Alkaloids by Metabolic Engineering. Adv. Bot. Res. 2013, 68, 163–181. [Google Scholar] [CrossRef]
- Lin, Z.; Hu, Z.W.; Qu, X.D.; Lin, S.J. Advances and challenges in microbial production of benzylisoquinoline alkaloids. Synth. Biol. 2021, 2, 716–733. [Google Scholar]
- Fossati, E.; Narcross, L.; Ekins, A.; Falgueyret, J.P.; Martin, V.J. Synthesis of Morphinan Alkaloids in Saccharomyces cerevisiae. PLoS ONE 2015, 10, e0124459. [Google Scholar] [CrossRef]
- Fiehn, O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comp. Funct. Genom. 2001, 2, 55–168. [Google Scholar] [CrossRef]
- He, F.; Wu, Z.; Zhao, Z.; Chen, G.; Wang, X.; Cui, X.; Zhu, T.; Chen, L.; Yang, P.; Bi, L.; et al. Drought stress drives sex-specific differences in plant resistance against herbivores between male and female poplars through changes in transcriptional and metabolic profiles. Sci. Total Environ. 2022, 845, 157171. [Google Scholar] [CrossRef]
- Jiao, P.; Li, C.Y.; Zhai, W.H.; Dai, J.J.; Zhao, Y.L.; Xu, Z.G. Integrative Metabolome and Transcriptome Analysis of Flavonoid Biosynthesis Genes in Broussonetia papyrifera Leaves From the Perspective of Sex Differentiation. Front. Plant Sci. 2022, 13, 900030. [Google Scholar] [CrossRef]
- Yu, C.; Huang, J.; Wu, Q.; Zhang, C.; Li, X.L.; Xu, X.; Feng, S.; Zhan, X.; Chen, Z.; Wang, H.; et al. Role of female-predominant MYB39-bHLH13 complex in sexually dimorphic accumulation of taxol in Taxus media. Hortic. Res. 2022, 9, 3083–3095. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Zhu, G.; Zhou, G.; Song, X.; Hussein Ibrahim, M.E.; Ibrahim Salih, E.G.; Hussain, S.; Younas, M.U. Pivotal Role of Phytohormones and Their Responsive Genes in Plant Growth and Their Signaling and Transduction Pathway under Salt Stress in Cotton. Int. J. Mol. Sci. 2022, 23, 7339. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Shahsavar, A. The Effect of Foliar Application of Melatonin on Changes in Secondary Metabolite Contents in Two Citrus Species Under Drought Stress Conditions. Front. Plant Sci. 2021, 12, 692735. [Google Scholar] [CrossRef]
- Kianersi, F.; Pour-Aboughadareh, A.; Majdi, M.; Poczai, P. Effect of Methyl Jasmonate on Thymol, Carvacrol, Phytochemical Accumulation, and Expression of Key Genes Involved in Thymol/Carvacrol Biosynthetic Pathway in Some Iranian Thyme Species. Int. J. Mol. Sci. 2021, 22, 11124. [Google Scholar] [CrossRef] [PubMed]
- Sulochana, S.B.; Arumugam, M. Influence of abscisic acid on growth, biomass and lipid yield of Scenedesmus quadricauda under nitrogen starved condition. Bioresour. Technol. 2016, 213, 198–203. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Yu, F. Regulation of Abscisic Acid on the Biosynthesis of Monoterpenoid Indole Alkaloids in Catharanthus roseus. Mol. Breed. 2019, 17, 3371–3377. [Google Scholar]
- Chang, B.; Liu, J.; Zhong, P.; Guo, X. Effects of Exogenous Ethylene on Physiology and Alkaloid Accumulations in Catharanthus roseus. Bull. Bot. Res. 2018, 38, 284–291. Available online: https://kns.cnki.net/kcms2/article/abstract?v=Nyg97wmOeE4l5yFWh172CMV7ZH1PkImd7ClxV_tPnLYK3dyq7o8CngQp6wtnngKoHRnVW-7DooQHmuLyefJ7U7ll1tRJgIFOecVWtGqPk_cWbj-X5q3b6yIHk8XAfr6fr1ECKv_ss-f-5ehgzwI6UFTZuYJOwlmAMd07OhgEMTH8M05tbJCtf5qP-AIxXIr8kaedBwNE9xg=&uniplatform=NZKPT&language=CHS%25W (accessed on 15 October 2018).
- Hoekstra, S.S.; Harkes, P.A.; Verpoorte, R.; Libbenga, K.R. Effect of auxinon cytodifferentiation and production of quinoline alkaloids in compact globular structures of Cinchona ledgeriana. Plant. Cell Rep. 1990, 8, 571–574. [Google Scholar] [CrossRef]
- Fujii, N.; Inui, T.; Iwasa, K.; Morishige, T.; Sato, F. Knockdown of berberine bridge enzyme by RNAi accumulates (S)-reticuline and activates a silent pathway in cultured California poppy cells. Transgenic Res. 2007, 16, 363–375. [Google Scholar] [CrossRef]
- He, S.M.; Song, W.L.; Cong, K.; Wang, X.; Dong, Y.; Cai, J.; Zhang, J.J.; Zhang, G.H.; Yang, J.L.; Yang, S.C.; et al. Identification of candidate genes involved in isoquinoline alkaloids biosynthesis in Dactylicapnos scandens by transcriptome analysis. Sci. Rep. 2017, 7, 9119. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, E.B.; Yi, O.Y.; Wu, H.; Deng, Y.M.; Huang, G.M.; Liu, W.L.; Yan, J.Y.; Cai, X. Sex-related differences in safety profiles, pharmacokinetics and tissue distribution of sinomenine hydrochloride in rats. Arch. Toxicol. 2022, 96, 3245–3255. [Google Scholar] [CrossRef] [PubMed]
- Okayama, M.; Matsumoto, T.; Kitagawa, T.; Nakamura, S.; Ohta, T.; Yoshida, T.; Watanabe, T. Cytotoxic activities of alkaloid constituents from the climbing stems and rhizomes of Sinomenium acutum against cancer stem cells. J. Nat. Med. 2024, 78, 226–235. [Google Scholar] [CrossRef]
- Shen, D.D.; Hua, Y.P.; Huang, J.Y.; Yu, S.T.; Wu, T.B.; Zhang, Y.; Chen, H.L.; Yue, C.P. Multiomic Analysis Reveals Core Regulatory Mechanisms underlying Steroidal Glycoalkaloid Metabolism in Potato Tubers. J. Agric. Food Chem. 2022, 70, 415–426. [Google Scholar] [CrossRef]
- Luo, S.; Wang, K.; Li, Z.; Li, H.; Shao, J.; Zhu, X. Salicylic Acid Enhances Cadmium Tolerance and Reduces Its Shoot Accumulation in Fagopyrum tataricum Seedlings by Promoting Root Cadmium Retention and Mitigating Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 14746. [Google Scholar] [CrossRef]
- Luhach, K.; Kulkarni, G.T.; Singh, V.P.; Sharma, B. Vinpocetine ameliorates developmental hyperserotonemia induced behavioral and biochemical changes: Role of neuronal function, inflammation, and oxidative stress. Acta Neurobiol. Exp. 2022, 82, 35–51. [Google Scholar] [CrossRef]
- Du, Q.; Meng, X.; Wang, S.A. Comprehensive Review on the Chemical Properties, Plant Sources, Pharmacological Activities, Pharmacokinetic and Toxicological Characteristics of Tetrahydropalmatine. Front. Pharmacol. 2022, 13, 890078. [Google Scholar] [CrossRef]
- Zhong, L.; Qin, Y.; Liu, M.; Sun, J.; Tang, H.; Zeng, Y.; Zhang, J.; Wang, W.; Liang, G.; Zhao, X. Magnoflorine improves cognitive deficits and pathology of Alzheimer’s disease via inhibiting of JNK signaling pathway. Phytomedicine 2023, 112, 154714. [Google Scholar] [CrossRef] [PubMed]
- Potočnjak, I.; Šimić, L.; Vukelić, I.; Batičić, L.; Domitrović, R. Oleanolic acid induces HCT116 colon cancer cell death through the p38/FOXO3a/Sirt6 pathway. Chem. Biol. Interact. 2022, 363, 110010. [Google Scholar] [CrossRef]
- Hu, J.; Chen, R.; An, J.; Wang, Y.; Liang, M.; Huang, K. Corrigendum: Dauricine attenuates vascular endothelial inflammation through inhibiting NF-κB pathway. Front. Pharmacol. 2023, 14, 1236892. [Google Scholar] [CrossRef]
- Kimura, M.; Kimura, I.; Takahashi, K. The neuromuscular blocking actions of coclaurine derivatives and of paeoniflorin derivatives. Planta Med. 1982, 45, 136. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Kong, L.; Li, Q.; Wang, Y.; Wang, Y.; An, Z.; Ma, Y.; Tian, L.; Duan, B.; Sun, W.; et al. Structural diversity, evolutionary origin, and metabolic engineering of plant specialized benzylisoquinoline alkaloids. Nat. Prod. Rep. 2024, 41, 1787–1810. [Google Scholar] [CrossRef]
- Verhage, A.; van Wees, S.C.; Pieterse, C.M. Plant immunity: It’s the hormones talking, but what do they say? Plant Physiol. 2010, 154, 536–540. [Google Scholar] [CrossRef]
- Huang, S.; Zheng, W.; Wang, Y.; Yan, H.; Zhou, C.; Ma, T. Dynamic Changes of Endogenous Hormones in Different Seasons of Idesia polycarpa Maxim. Life 2023, 13, 788. [Google Scholar] [CrossRef]
- Xiong, Y.T.; Xu, M.; Gu, W. Comparison of Endogenous Hormones of Leaves between Female and Male Plants of Schisandra sphenanthera Rehd, et Wils. during Flowering Stage. Plant Physiol. J. 2012, 48, 156–160. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Luo, X.F.; Wang, X.; Ma, J.; Jiang, Y.G.; Zhao, J.G.; Ao, Y. The role of phytohormones and their related miRNAs in sex differentiation of Xanthoceras sorbifolium Bunge. Sci. Hortic. 2023, 307, 111498. [Google Scholar] [CrossRef]
- Villalobos-González, L.; PeñA-Neira, A.; Ibáñez, F.; Pastenes, C. Long-term effects of abscisic acid (ABA) on the grape berry phenylpropanoid pathway: Gene expression and metabolite content. Plant Physiol. Biochem. 2016, 105, 213–223. [Google Scholar] [CrossRef]
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the Basal Role of ABA—Roles Outside of Stress. Trends Plant Sci. 2019, 24, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, B.; Yu, H.; Guo, H.Y.; Lin, T.; Kou, L.Q.; Wang, A.Q.; Shao, N.; Ma, H.Y.; Xiong, G.S.; et al. Transcriptional regulation of strigolactone signalling in Arabidopsis. Nature 2020, 583, 277–281. [Google Scholar] [CrossRef]
- Wani, K.I.; Zehra, A.; Choudhary, S.; Naeem, M.; Khan, M.A.; Khan, R.; Aftab, T. Exogenous Strigolactone (GR24) Positively Regulates Growth, Photosynthesis, and Improves Glandular Trichome Attributes for Enhanced Artemisinin Production in Artemisia annua. J. Plant Growth Regul. 2023, 42, 4606–4615. [Google Scholar] [CrossRef]
- Jie, H.; Zhao, L.; Ma, Y.; Rasheed, A.; Jie, Y. Integrated Transcriptome and Metabolome Analysis Reveal That Exogenous Gibberellin Application Regulates Lignin Synthesis in Ramie. Agronomy 2023, 13, 1450. [Google Scholar] [CrossRef]
- Liu, Z.; Shen, S.; Wang, Y.; Sun, S.; Yu, T.; Fu, Y.; Zhou, R.; Li, C.; Cao, R.; Zhang, Y.; et al. The genome of Stephania japonica provides insights into the biosynthesis of cepharanthine. Cell Rep. 2024, 43, 113832. [Google Scholar] [CrossRef]
- Facchini, P.J.; Yu, M.; Penzesyost, C. Decreased cell wall digestibility in canola transformed with chimeric tyrosine decarboxylase genes from opium poppy. Plant Physiol. 1999, 120, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Thompson, M.L.; Shepherd, S.A.; Dunstan, M.S.; Herbert, A.J.; Smith, D.R.M.; Cronin, V.A.; Menon, B.R.K.; Levy, C.; Micklefield, J. Structure and Biocatalytic Scope of Coclaurine N-Methyltransferase. Angew. Chem. 2018, 57, 10600–10604. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Y.; Liu, Y.; Ma, M.N.; Li, X.P.; Zhang, D.N.; Ding, K.; Li, C.; Zou, Y.G.; Ma, F.W. Overexpression of tyrosine decarboxylase (MdTYDC) enhances drought tolerance in Malus domestica. Sci. Hortic. 2021, 289, 110425. [Google Scholar] [CrossRef]
- Meelaph, T.; Kobtrakul, K.; Chansilpa, N.N.; Han, Y.; Rani, D.; De-Eknamkul, W.; Vimolmangkang, S. Coregulation of Biosynthetic Genes and Transcription Factors for Aporphine-Type Alkaloid Production in Wounded Lotus Provides Insight into the Biosynthetic Pathway of Nuciferine. ACS Omega 2018, 3, 8794–8802. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.X.; Zhong, J.; Shu, D.; Luo, D.; Li, Z.M.; Zhou, J.Y.; Yang, J.; Tan, H.; Ma, X.R. Exogenous 1′,4′-trans-Diol-ABA Induces Stress Tolerance by Affecting the Level of Gene Expression in Tobacco (Nicotiana tabacum L.). Int. J. Mol. Sci. 2021, 22, 2555. [Google Scholar] [CrossRef]
- Juvany, M.; Munné-Bosch, S. Sex-related differences in stress tolerance in dioecious plants: A critical appraisal in a physiological context. J. Exp. Bot. 2015, 66, 6083–6092. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Zhang, W.E.; Li, C.; Wang, R.; Pan, X.; Peng, J. Combined transcriptional and metabolomic analysis of flavonoids in the regulation of female flower bud differentiation in Juglans sigillata Dode. BMC Plant Biol. 2025, 25, 168. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Zhao, H.; Korpelainen, H.; Li, C. Sex-related adaptive responses to interaction of drought and salinity in Populus yunnanensis. Plant Cell Environ. 2010, 33, 1767–1778. [Google Scholar] [CrossRef]
- Cao, P.Y.; Chen, W.J.; Zong, Y.; Shi, K.; Li, J.M.; He, Z.M.; DU, R. Extraction Process of Total Alkaloids from Sinomenii caulis and Its Anti Gout Effect. J. Jilin Agric. Univ. 2024, 46, 280–289. [Google Scholar]
- Zeng, X.Y.; Qiao, Q.W.; Li, Y.Y.; Zhou, X.X.; Yang, H.; Xiong, X.Y. Analysis of sinomenine synthesis pathway based on transcriptome sequencing in Sinomenii Caulis. Chin. Herb. Med. 2019, 50, 5537–5544. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]




| S. acutum Plants | Pedicel Length (cm) | Carpels (Number) | Stamens (Number) | Sepals (Number) | Total Plants Observed (Number) |
|---|---|---|---|---|---|
| Female | 8–20 | 3 | 9 (degenerated) | - | 183 |
| Male | 13–20 | - | 8–12 | 6 | 424 |
| Undifferentiated | - | - | - | - | 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Xu, W.; Fan, Y.; Ma, X.; Deng, Q.; Li, M.; Sun, W. Metabolomic Insights into Sexual Multi-Morphism of Sinomenine Accumulation in Sinomenium acutum. Plants 2025, 14, 1885. https://doi.org/10.3390/plants14121885
Luo Y, Xu W, Fan Y, Ma X, Deng Q, Li M, Sun W. Metabolomic Insights into Sexual Multi-Morphism of Sinomenine Accumulation in Sinomenium acutum. Plants. 2025; 14(12):1885. https://doi.org/10.3390/plants14121885
Chicago/Turabian StyleLuo, Yanxian, Wen Xu, Yanling Fan, Xinyu Ma, Qian Deng, Meng Li, and Wei Sun. 2025. "Metabolomic Insights into Sexual Multi-Morphism of Sinomenine Accumulation in Sinomenium acutum" Plants 14, no. 12: 1885. https://doi.org/10.3390/plants14121885
APA StyleLuo, Y., Xu, W., Fan, Y., Ma, X., Deng, Q., Li, M., & Sun, W. (2025). Metabolomic Insights into Sexual Multi-Morphism of Sinomenine Accumulation in Sinomenium acutum. Plants, 14(12), 1885. https://doi.org/10.3390/plants14121885






