Physiological, Chemical and Metabolite Profiling of Pectobacterium carotovorum-Inoculated Tomato Plants Grown in Nutrient-Amended Soils
Abstract
1. Introduction
2. Results and Discussion
2.1. Growth Measurements
2.2. Gas Exchange Responses
2.3. Nutrient Assimilation Analysis
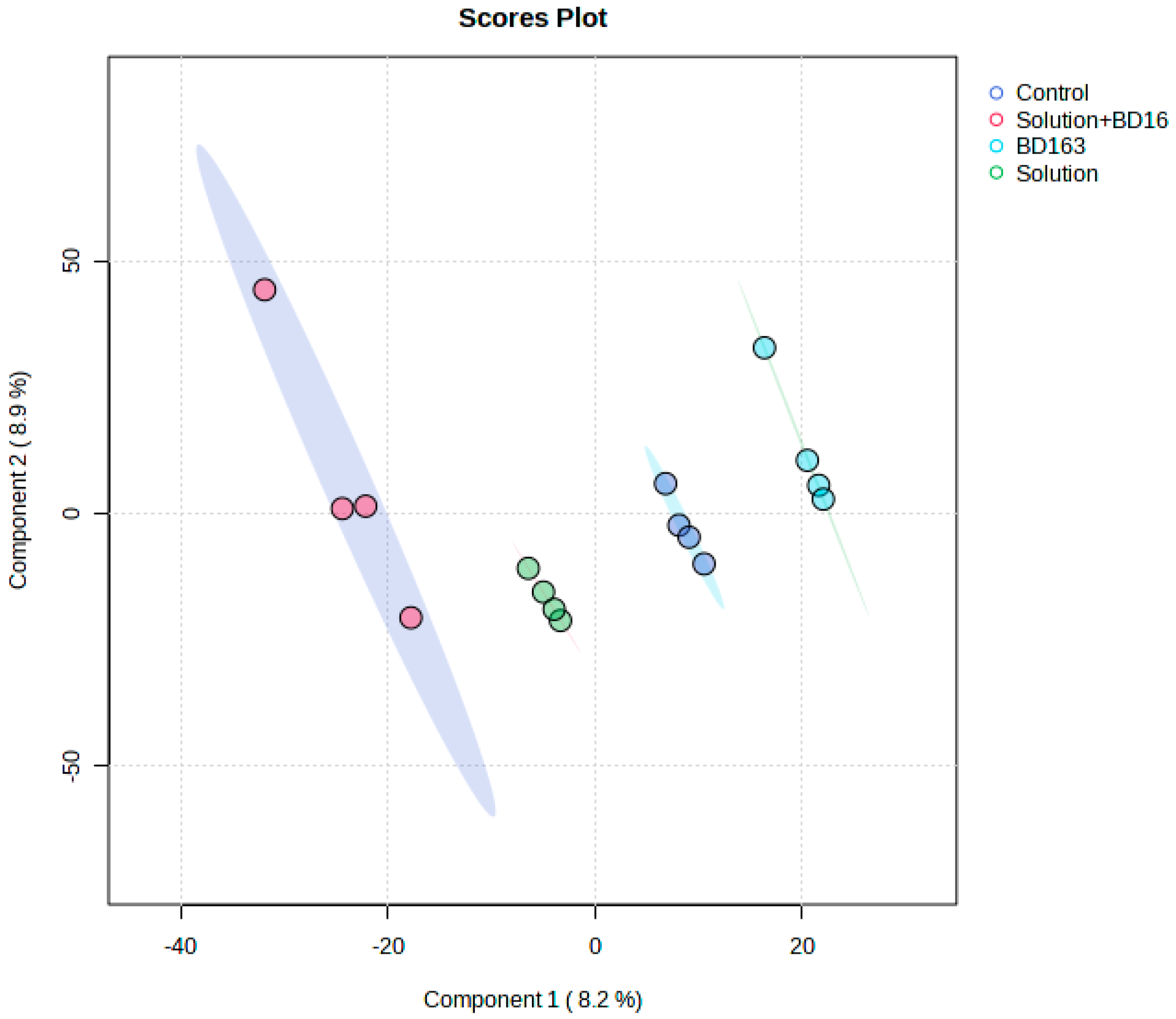
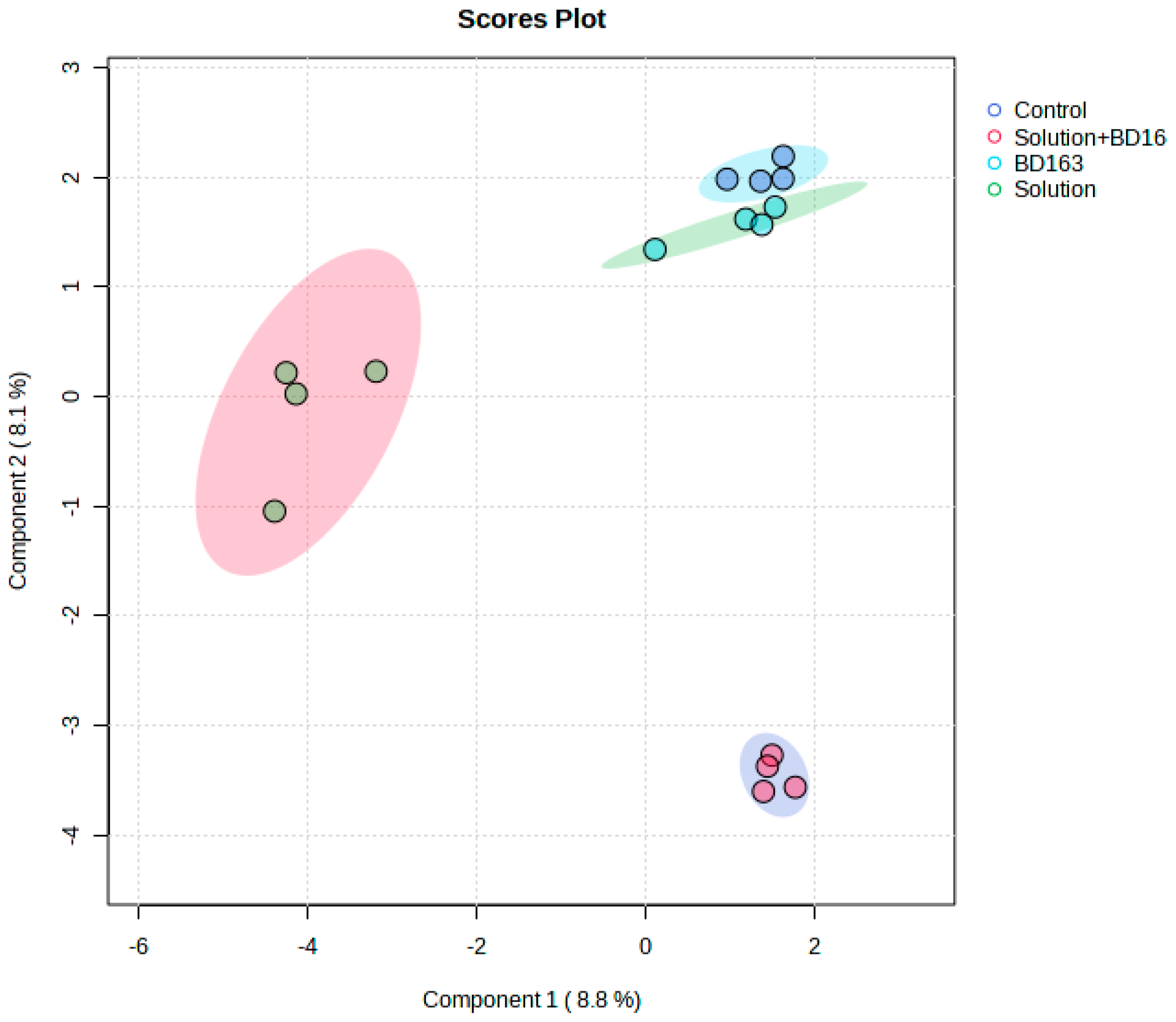
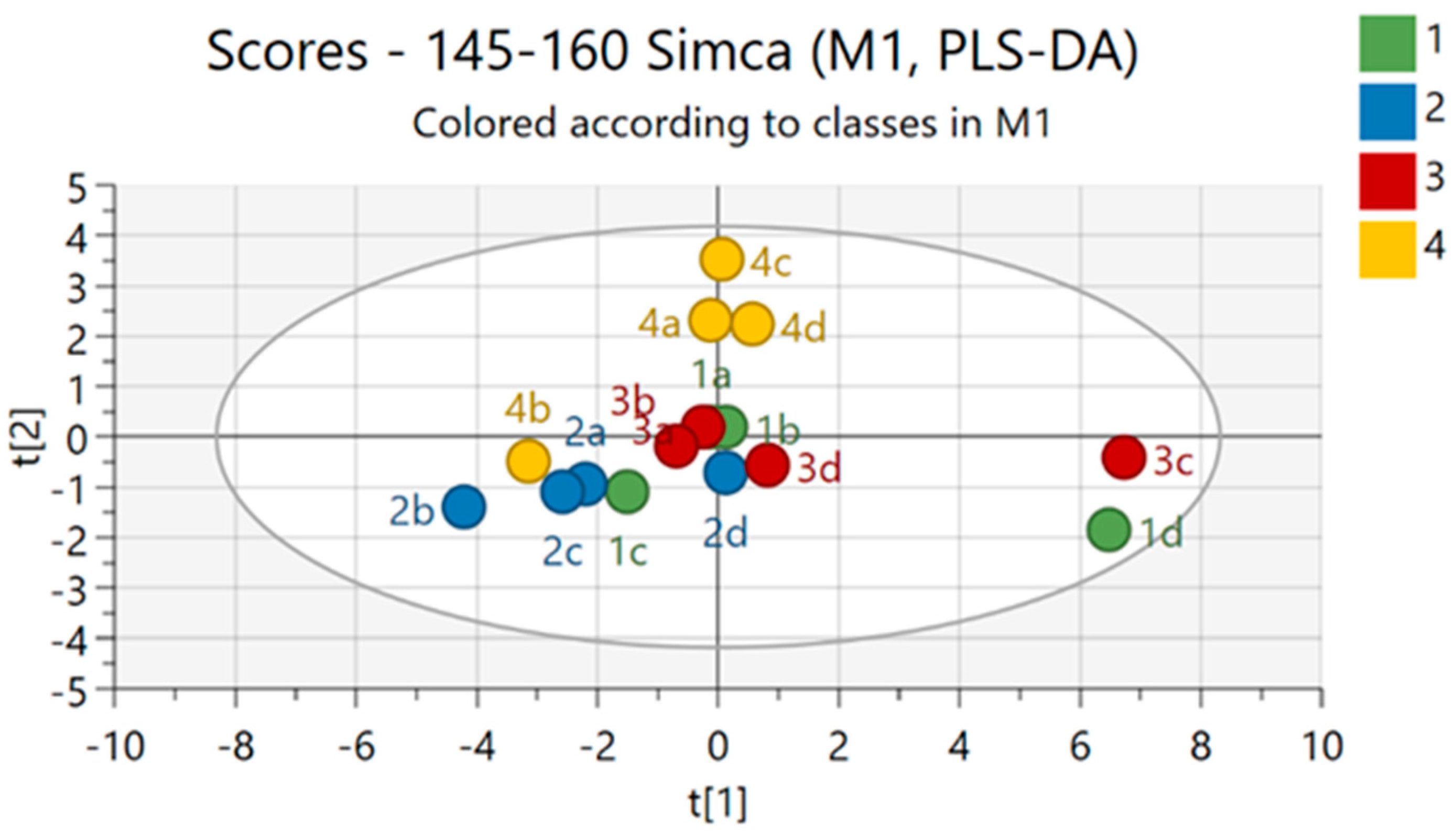
2.4. Liquid Chromatography–Mass Spectrometry and Nuclear Magnetic Resonance Spectroscopy
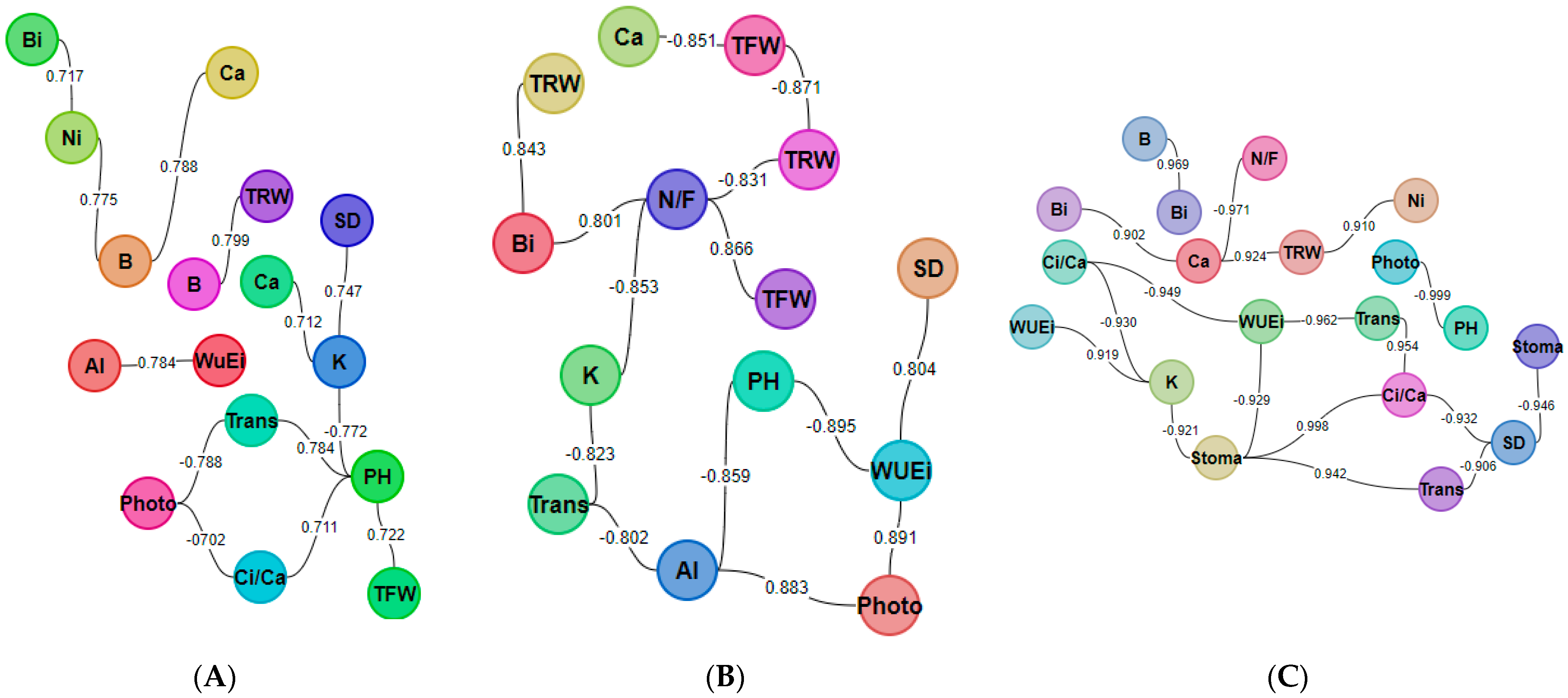
2.5. Correlation Analysis of Measured Variables
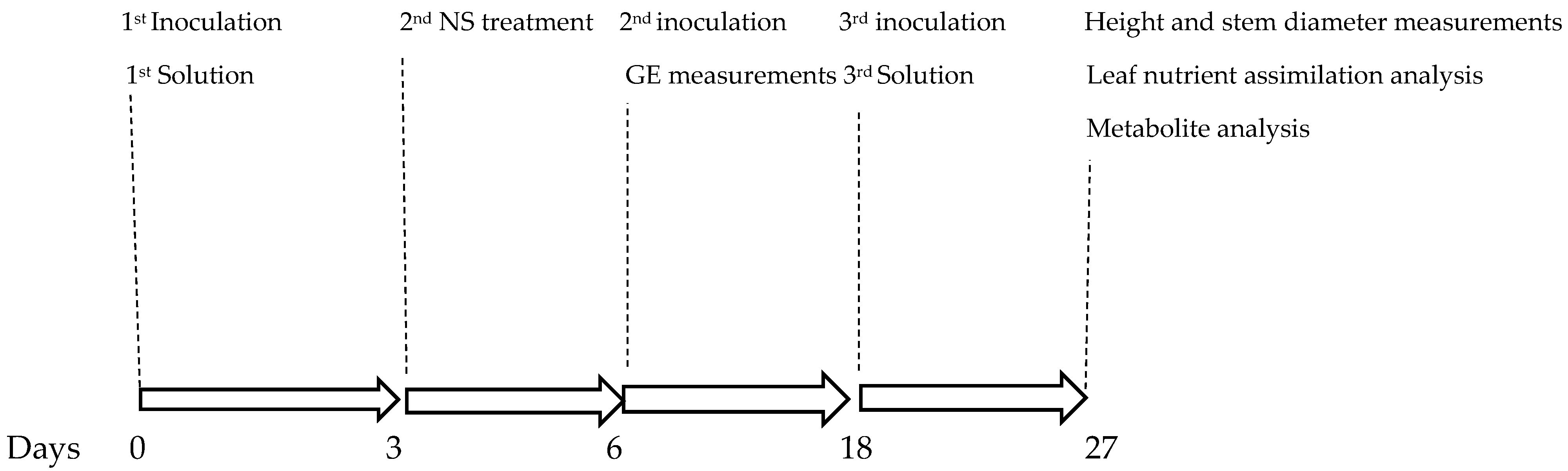
3. Materials and Methods
3.1. Plant Material
3.2. Experimental Treatments
3.3. Bacterial Inoculation and Nutrient Mixture Application
3.4. Gas Exchange Measurements and Nutrient Assimilation Determination
Data Analysis
3.5. Liquid Chromatography
3.6. Secondary Metabolites Identification and Quantification
3.7. Nuclear Magnetic Resonance Spectroscopy
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gatahi, D.M. Challenges and opportunities in tomato production chain and sustainable standards. Int. J. Hortic. Sci. Technol. 2020, 7, 235–262. [Google Scholar]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional composition and bioactive compounds in tomatoes and their impact on human health and disease: A review. Foods 2020, 10, 45. [Google Scholar] [CrossRef]
- Xing, M.; Li, W.; Yu, H.; Wang, Y.; Wu, F.; Wu, M.; Xu, J. Physiological and transcriptome analyses reveal copper toxicity responses in tomato plants. Environ. Exp. Bot. 2024, 224, 105819. [Google Scholar] [CrossRef]
- Ahmed, F.A.; Arif, M.; Alvarez, A.M. Antibacterial effect of potassium tetraborate tetrahydrate against soft rot disease agent Pectobacterium carotovorum in tomato. Front. Microbiol. 2017, 8, 1728. [Google Scholar] [CrossRef] [PubMed]
- Gardan, L.; Gouy, C.; Christen, R.; Samson, R. Elevation of three subspecies of Pectobacterium carotovorum to species level: Pectobacterium atrosepticum sp. nov., Pectobacterium betavasculorum sp. nov. and Pectobacterium wasabiae sp. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 381–391. [Google Scholar] [CrossRef]
- Munyaneza, J.E.; Crosslin, J.M.; Buchman, J.L.; Sengoda, V.G. Susceptibility of different potato plant growth stages to purple top disease. Am. J. Potato Res. 2010, 87, 60–66. [Google Scholar] [CrossRef]
- Rodriguez-Algaba, J.; Sørensen, C.K.; Labouriau, R.; Justesen, A.F.; Hovmøller, M.S. Susceptibility of winter wheat and triticale to yellow rust influenced by complex interactions between vernalisation, temperature, plant growth stage and pathogen race. Agronomy 2019, 10, 13. [Google Scholar] [CrossRef]
- Vergnes, D.M.; Renard, M.; Duveiller, E.; Maraite, H. Effect of growth stage on host sensitivity to helminthosporol toxin and susceptibility to Cochliobolus sativus causing spot blotch on wheat. Physiol. Mol. Plant Pathol. 2006, 68, 14–21. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Bacteria and fungi controlling plant growth by manipulating auxin: Balance between development and defense. J. Plant Physiol. 2015, 172, 4–12. [Google Scholar] [CrossRef]
- Meline, V.; Hendrich, C.G.; Truchon, A.N.; Caldwell, D.; Hiles, R.; Leuschen-Kohl, R.; Tran, T.; Mitra, R.M.; Allen, C.; Iyer-Pascuzzi, A.S. Tomato deploys defence and growth simultaneously to resist bacterial wilt disease. Plant Cell Environ. 2023, 46, 3040–3058. [Google Scholar] [CrossRef]
- Agbavor, C.; Mirza, B.S.; Wait, A. The effects of phyllosphere bacteria on plant physiology and growth of soybean infected with Pseudomonas syringae. Plants 2022, 11, 2634. [Google Scholar] [CrossRef] [PubMed]
- Piccinni, G.; Rush, C.M. Determination of optimum irrigation regime and water use efficiency of sugar beet grown in pathogen-infested soil. Plant Dis. 2000, 84, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, M.K.; John Foulkes, M.; Paveley, N.D. Foliar pathogenesis and plant water relations: A review. J. Exp. Bot. 2012, 63, 4321–4331. [Google Scholar] [CrossRef]
- Su, C.; Evans, L.J.; Bates, T.E.; Spiers, G.A. Extractable soil boron and alfalfa uptake: Calcium carbonate effects on acid soil. Soil Sci. Soc. Am. J. 1994, 58, 1445–1450. [Google Scholar] [CrossRef]
- Smith, T.E.; Grattan, S.R.; Grieve, C.M.; Poss, J.A.; Suarez, D.L. Salinity’s influence on boron toxicity in broccoli: II. Impacts on boron uptake, uptake mechanisms and tissue ion relations. Agric. Water Manag. 2010, 97, 783–791. [Google Scholar]
- Ma, Y.; Rajkumar, M.; Freitas, H. Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J. Hazard. Mater. 2009, 166, 1154–1161. [Google Scholar] [CrossRef]
- Abou-Shanab, R.; Angle, J.S.; Chaney, R.L. Bacterial inoculants affecting nickel uptake by Alyssum murale from low, moderate and high Ni soils. Soil Biol. Biochem. 2006, 38, 2882–2889. [Google Scholar] [CrossRef]
- Boyd, R.S. Ecology of metal hyperaccumulation. New Phytol. 2004, 1, 563–567. [Google Scholar] [CrossRef]
- Ni, L.; Punja, Z.K. Effects of a foliar fertilizer containing boron on the development of Sclerotinia stem rot (Sclerotinia sclerotiorum) on canola (Brassica napus L.) leaves. J. Phytopathol. 2020, 168, 47–55. [Google Scholar]
- López-Gresa, M.P.; Torres, C.; Campos, L.; Lisón, P.; Rodrigo, I.; Bellés, J.M.; Conejero, V. Identification of defence metabolites in tomato plants infected by the bacterial pathogen Pseudomonas syringae. Environ. Exp. Bot. 2011, 74, 216–228. [Google Scholar] [CrossRef]
- Lowe-Power, T.M.; Hendrich, C.G.; von Roepenack-Lahaye, E.; Li, B.; Wu, D.; Mitra, R.; Dalsing, B.L.; Ricca, P.; Naidoo, J.; Cook, D.; et al. Metabolomics of tomato xylem sap during bacterial wilt reveals Ralstonia solanacearum produces abundant putrescine, a metabolite that accelerates wilt disease. Environ. Microbiol. 2018, 20, 1330–1349. [Google Scholar] [CrossRef]
- Galeano Garcia, P.; Neves dos Santos, F.; Zanotta, S.; Eberlin, M.N.; Carazzone, C. Metabolomics of Solanum lycopersicum infected with Phytophthora infestans leads to early detection of late blight in asymptomatic plants. Molecules 2018, 23, 3330. [Google Scholar] [CrossRef]
- López-Gresa, M.P.; Maltese, F.; Bellés, J.M.; Conejero, V.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Metabolic response of tomato leaves upon different plant–pathogen interactions. Phytochem. Anal. 2010, 21, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.J.; Awika, H.O.; Jayaprakasha, G.K.; Avila, C.A.; Crosby, K.M.; Patil, B.S. Tomato metabolic changes in response to tomato-potato psyllid (Bactericera cockerelli) and its vectored pathogen Candidatus Liberibacter Solanacearum. Plants 2020, 9, 1154. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, E.; Weinert, C.H.; Egert, B.; Trierweiler, B.; Schmidt-Heydt, M.; Horneburg, B.; Graeff-Hönninger, S.; Kulling, S.E.; Geisen, R. Chlorogenic acid, a metabolite identified by untargeted metabolome analysis in resistant tomatoes, inhibits the colonization by Alternaria alternata by inhibiting alternariol biosynthesis. Eur. J. Plant Pathol. 2014, 139, 735–747. [Google Scholar] [CrossRef]
- Sade, D.; Shriki, O.; Cuadros-Inostroza, A.; Tohge, T.; Semel, Y.; Haviv, Y.; Willmitzer, L.; Fernie, A.R.; Czosnek, H. Comparative metabolomics and transcriptomics of plant response to tomato yellow leaf curl virus infection in resistant and susceptible tomato cultivars. Metabolomics 2015, 11, 81–97. [Google Scholar] [CrossRef]
- Singh, D.P.; Maurya, S.; Yerasu, S.R.; Bisen, M.S.; Farag, M.A.; Prabha, R.; Shukla, R.; Chaturvedi, K.K.; Farooqi, M.S.; Srivastava, S.; et al. Metabolomics of early blight (Alternaria solani) susceptible tomato (Solanum lycopersicum) unfolds key biomarker metabolites and involved metabolic pathways. Sci. Rep. 2023, 13, 21023. [Google Scholar] [CrossRef]
- Ward, J.L.; Forcat, S.; Beckmann, M.; Bennett, M.; Miller, S.J.; Baker, J.M.; Hawkins, N.D.; Vermeer, C.P.; Lu, C.; Lin, W.; et al. The metabolic transition during disease following infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato. Plant J. 2010, 63, 443–457. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Tugizimana, F.; Mhlongo, M.I. Comparative metabolite profiling of wheat cultivars (Triticum aestivum) reveals signatory markers for resistance and susceptibility to stripe rust and aluminium (Al3+) toxicity. Metabolites 2022, 12, 98. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A.; Mhlongo, M.I. Metabolite profiling of susceptible and resistant wheat (Triticum aestivum) cultivars responding to Puccinia striiformis f. sp. tritici infection. BMC Plant Biol. 2023, 23, 293. [Google Scholar] [CrossRef]
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64. [Google Scholar]
- Sung, J.; Lee, Y.; Lee, S.; Lim, J.; Lee, D. Mineral-and tissue-specific metabolic changes in tomato (Lycopersicon esculentum L.) plants grown under NPK-starved conditions. Korean J. Soil Sci. Fert. 2016, 49, 689–698. [Google Scholar]
- Iglesias, M.J.; García-López, J.; Collados-Luján, J.F.; López-Ortiz, F.; Díaz, M.; Toresano, F.; Camacho, F. Differential response to environmental and nutritional factors of high-quality tomato varieties. Food Chem. 2015, 176, 278–287. [Google Scholar] [CrossRef]
- Zeiss, D.R.; Mhlongo, M.I.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A. Comparative metabolic phenotyping of tomato (Solanum lycopersicum) for the identification of metabolic signatures in cultivars differing in resistance to Ralstonia solanacearum. Int. J. Mol. Sci. 2018, 19, 2558. [Google Scholar] [CrossRef]
- Camañes, G.; Scalschi, L.; Vicedo, B.; González-Bosch, C.; García-Agustín, P. An untargeted global metabolomic analysis reveals the biochemical changes underlying basal resistance and priming in Solanum lycopersicum and identifies 1-methyltryptophan as a metabolite involved in plant responses to Botrytis cinerea and Pseudomonas syringae. Plant J. 2015, 84, 125–139. [Google Scholar] [PubMed]
- Yogendra, K.N.; Pushpa, D.; Mosa, K.A.; Kushalappa, A.C.; Murphy, A.; Mosquera, T. Quantitative resistance in potato leaves to late blight associated with induced hydroxycinnamic acid amides. Funct. Integr. Genom. 2014, 14, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Ikeda, S.; Tsuda, S.; Someya, N.; Asano, K.; Kikuchi, J.; Chikayama, E.; Ono, H.; Sekiyama, Y. A survey of metabolic changes in potato leaves by NMR-based metabolic profiling in relation to resistance to late blight disease under field conditions. Magn. Reson. Chem. 2017, 55, 120–127. [Google Scholar] [CrossRef]
- Wang, L.; Shen, X.; Chen, X.; Ouyang, Q.; Tan, X.; Tao, N. Exogenous application of melatonin to green horn pepper fruit reduces chilling injury during postharvest cold storage by regulating enzymatic activities in the antioxidant system. Plants 2022, 11, 2367. [Google Scholar] [CrossRef]
- Wu, S.; Li, R.; Bu, C.; Zhu, C.; Miao, C.; Zhang, Y.; Cui, J.; Jiang, Y.; Ding, X. Photoperiodic effect on growth, photosynthesis, mineral elements, and metabolome of tomato seedlings in a plant factory. Plants 2024, 13, 3119. [Google Scholar] [CrossRef]
- Zhou, L.; Cai, Y.; Yang, L.; Zou, Z.; Zhu, J.; Zhang, Y. Comparative metabolomics analysis of stigmas and petals in Chinese saffron (Crocus sativus) by widely targeted metabolomics. Plants 2022, 11, 2427. [Google Scholar] [CrossRef]
- El-Nagar, A.; Elzaawely, A.A.; Xuan, T.D.; Gaber, M.; El-Wakeil, N.; El-Sayed, Y.; Nehela, Y. Metal complexation of bis-chalcone derivatives enhances their efficacy against Fusarium wilt disease, caused by Fusarium equiseti, via induction of antioxidant defense machinery. Plants 2022, 11, 2418. [Google Scholar] [CrossRef] [PubMed]
- Hacisalihoglu, G.; Ji, P.; Longo, L.M.; Olson, S.; Momol, T.M. Bacterial wilt induced changes in nutrient distribution and biomass and the effect of acibenzolar-S-methyl on bacterial wilt in tomato. Crop Prot. 2007, 26, 978–982. [Google Scholar] [CrossRef]
- Majeed, H.H.; Bayati, A.S.A. Role of foliar application of organic liquid fertilizers fortified with phosphorus and calcium in tomato yield and fruit quality. Int. J. Aquat. Sci. 2023, 14, 96–105. [Google Scholar]
- Sajid, M.; Ullah, I.; Rab, A.; Shah, S.T.; Basit, A.; Bibi, F.; Ahmad, M. 2. Foliar application of calcium improves growth, yield and quality of tomato cultivars. Pure Appl. Biol. 2020, 9, 10–19. [Google Scholar] [CrossRef]
- Hernández-Pérez, O.I.; Valdez-Aguilar, L.A.; Alia-Tejacal, I.; Cartmill, A.D.; Cartmill, D.L. Tomato fruit yield, quality, and nutrient status in response to potassium: Calcium balance and electrical conductivity in the nutrient solution. J. Soil Sci. Plant Nutr. 2020, 20, 484–492. [Google Scholar] [CrossRef]
- Leigh, R.A.; Wyn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Institute of Plant Nutrition University of Hohenheim: Stuttgart, Germany, 1995. [Google Scholar]
- Takahashi, K.; Kinoshita, T. The regulation of plant cell expansion: Auxin-induced turgor-driven cell elongation. In Molecular Cell Biology of the Growth and Differentiation of Plant Cells; CRC Press: Boca Raton, FL, USA, 2016; pp. 156–173. [Google Scholar]
- Osakabe, Y.; Arinaga, N.; Umezawa, T.; Katsura, S.; Nagamachi, K.; Tanaka, H.; Ohiraki, H.; Yamada, K.; Seo, S.-U.; Abo, M.; et al. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 2013, 25, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, R.P.; Nagpal, P.; Reed, J.W. A mutation in the Arabidopsis KT2/KUP2 potassium transporter gene affects shoot cell expansion. Plant Cell 2002, 14, 119–131. [Google Scholar] [CrossRef]
- Liu, C.; Lakshman, D.K.; Tavantzis, S.M. Quinic acid induces hypovirulence and expression of a hypovirulence-associated double-stranded RNA in Rhizoctonia solani. Curr. Genet. 2003, 43, 103–111. [Google Scholar] [CrossRef]
- Imazaki, I. Effects of alkaline calcareous and magnesium soil amendments on the incidence of bacterial wilt of tomato. PhytoFront. 2022, 2, 380–390. [Google Scholar] [CrossRef]
- Sediqui, N.; Amin, M.W.; Dawlatzai, N.; Gulab, G.; Poyesh, D.S.; Terada, N.; Sanada, A.; Kamata, A.; Koshio, K. Elucidation of shoot and root growth, physiological responses, and quality traits of tomato (Solanum lycopersicon L.) exposed to elevated calcium carbonate concentrations. Horticulturae 2024, 10, 573. [Google Scholar] [CrossRef]
- Zushi, K.; Matsuzoe, N. Effect of soil water deficit on vitamin C, sugar, organic acid, amino acid and carotene contents of large-fruited tomatoes. J. Jpn. Soc. Hortic. Sci. 1998, 67, 927–933. [Google Scholar] [CrossRef]
- Li, Y.; Niu, W.; Cao, X.; Wang, J.; Zhang, M.; Duan, X.; Zhang, Z. Effect of soil aeration on root morphology and photosynthetic characteristics of potted tomato plants (Solanum lycopersicum) at different NaCl salinity levels. BMC Plant Biol. 2019, 19, 331. [Google Scholar] [CrossRef]
- Christou, A.; Georgiadou, E.C.; Zissimos, A.M.; Christoforou, I.C.; Christofi, C.; Neocleous, D.; Dalias, P.; Ioannou, A.; Fotopoulos, V. Uptake of hexavalent chromium by tomato (Solanum lycopersicum L.) plants and mediated effects on their physiology and productivity, along with fruit quality and safety. Environ. Exp. Bot. 2021, 189, 104564. [Google Scholar] [CrossRef]
- Varbanets, L.D.; Vasil’ev, V.N.; Brovarskaya, O.S. Characterization of lipopolysaccharides from Ralstonia solanacearum. Microbiology 2003, 72, 12–17. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, Y.; Yi, G.; Li, M.; Liao, L.; Yang, C.; Cho, C.; Zhang, B.; Zhu, J.; Zou, K.; et al. Quinic acid: A potential antibiofilm agent against clinical resistant Pseudomonas aeruginosa. Chin. Med. 2021, 16, 72. [Google Scholar] [CrossRef] [PubMed]
- Nnabuo-Eguzozie, E.C.; Kibechu, R.W.; Eguzozie, K.U.; Ntushelo, K.; Mamba, B.B.; Nyoni, H.; Nkambule, T.T.; Msagati, T.A. Impact of biotic stress on greenhouse-cultivated tomato (Solanum lycopersicum) using UPLC-ESI-qToF-MS-based integrated metabolomics and microwave-assisted digested ICP-OES nutrient assimilation analysis. Agric. Res. 2023, 12, 214–225. [Google Scholar] [CrossRef]
- Ramabulana, A.T.; Steenkamp, P.A.; Madala, N.E.; Dubery, I.A. Application of plant growth regulators modulates the profile of chlorogenic acids in cultured Bidens pilosa cells. Plants 2021, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Clarke, D.B. Glucosinolates, structures and analysis in food. Anal. Methods 2010, 2, 310–325. [Google Scholar] [CrossRef]


| Treatments | Plant Height | Stem Diameter | Number of Fruits | Total Fruit Weight | Total Root Weight |
|---|---|---|---|---|---|
| cm | mm | Grams | Grams | ||
| Solution + BD163 | 41.25 ± 1.03 ab | 9.04 ± 0.25 a | 1.25 ± 0.25 a | 25.75 ± 8.54 a | 69.00 ± 17.78 a |
| Solution | 44.00 ± 3.29 a | 9.55 ± 0.62 a | 1.50 ± 0.29 a | 98.5 ± 44.81 a | 34.00 ± 14.70 a |
| BD163 | 35.25 ± 3.86 b | 9.71 ± 0.39 a | 1.25 ± 0.25 a | 25.25 ± 12.46 a | 48.75 ± 19.59 a |
| Control (No Solution + No BD163) | 41.75 ± 0.63 ab | 9.22 ± 0.49 a | 1.75 ± 0.25 a | 96.75 ± 37.13 a | 20.00 ± 3.14 a |
| p Value | 0.16 ns | 0.73 ns | 0.50 ns | 0.18 ns | 0.18 ns |
| Treatments | Photosynthesis Rate | Stomatal Conductance | Intercellular CO2 Concentration | Transpiration Rate | Ci/Ca * | Water Use Efficiency |
|---|---|---|---|---|---|---|
| µmol(CO2) m−2 s−1 | mol(H2O) m−2 s−1 | mol m−2 s−1 | mol (H2O) ms−2 s−1 | µmol (CO2) m−2 s−1 | µmol (CO2) m−1 H2O | |
| Solution + BD163 | 10.54 ± 1.39 a | 0.09 ± 0.01 a | 216.72 ± 26.19 a | 2.98 ± 0.19 a | 0.55 ± 0.06 a | 114.73 ± 15.08 b |
| Solution | 9.73 ± 1.32 a | 0.08 ± 0.02 a | 198.18 ± 22.99 a | 2.65 ± 0.44 a | 0.46 ± 0.05 a | 128.35 ± 15.08 b |
| BD163 | 12.39 ± 1.00 a | 0.07 ± 0.01 a | 214.05 ± 14.55 a | 2.09 ± 0.16 a | 0.41 ± 0.06 a | 208.79 ± 29.76 a |
| Control (No Solution + No BD163) | 10.51 ± 1.56 a | 0.09 ± 0.01 a | 213.14 ± 10.18 a | 2.75 ± 0.22 a | 0.50 ± 0.02 a | 116.11 ± 4.90 b |
| p Value | 0.56 ns | 0.47 ns | 0.91 ns | 0.19 ns | 0.50 ns | 0.01 s |
| Treatments | Aluminum | Boron | Bismuth | Calcium | Potassium | Nickel |
|---|---|---|---|---|---|---|
| Solution + BD163 | 0.06 ± 0.02 a | 0.19 ± 0.01 a | 0.47 ± 0.01 b | 40.31 ± 4.56 a | 39.76 ± 6.14 a | 0.02 ± 0.00 a |
| Solution | 0.06 ± 0.01 a | 0.17 ± 0.01 ab | 0.40 ± 0.01 b | 33.58 ± 2.78 a | 41.48 ± 1.95 a | 0.01 ± 0.00 b |
| BD163 | 0.09 ± 0.01 a | 0.14 ± 0.01 bc | 0.35 ± 0.02 ab | 38.93 ± 6.54 a | 53.38 ± 7.63 a | 0.01 ± 0.00 b |
| Control (No Solution + No BD163) | 0.08 ± 0.02 a | 0.10 ± 0.13 c | 0.13 ± 0.16 a | 25.05 ± 11.57 a | 32.45 ± 11.98 a | 0.01 ± 0.00 b |
| p Value | 0.58 ns | 0.05 s | 0.06 s | 0.45 ns | 0.34 ns | 0.04 s |
| PH | SD | N/f | TFW | TRW | Photo | Stoma | Inter | Trans | Ci/Ca | WUE | Al | B | Bi | Ca | K | Ni | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH | 1 | −0.464 | 0.611 | 0.722 | −0.302 | −0.999 | 0.669 | −0.573 | 0.784 | 0.711 | −0.895 | −0.859 | 0.209 | −0.017 | −0.437 | −0.772 | 0.027 |
| SD | 1 | −0.32 | 0.027 | −0.256 | 0.464 | −0.946 | −0.453 | −0.906 | −0.932 | 0.804 | 0.508 | −0.181 | 0.016 | 0.127 | 0.747 | −0.623 | |
| N/f | 1 | 0.866 | −0.831 | −0.571 | 0.592 | −0.228 | 0.418 | 0.605 | −0.626 | −0.137 | −0.638 | −0.801 | −0.971 | −0.853 | −0.528 | ||
| TFW | 1 | −0.871 | −0.693 | 0.298 | −0.683 | 0.255 | 0.335 | −0.51 | −0.292 | −0.431 | −0.567 | −0.851 | −0.635 | −0.667 | |||
| TRW | 1 | 0.259 | −0.045 | 0.441 | 0.128 | −0.062 | 0.139 | −0.215 | 0.799 | 0.843 | 0.924 | 0.424 | 0.91 | ||||
| Photo | 1 | −0.659 | 0.578 | −0.788 | −0.702 | 0.891 | 0.883 | −0.257 | −0.033 | 0.392 | 0.749 | −0.063 | |||||
| Stoma | 1 | 0.22 | 0.942 | 0.998 | −0.929 | −0.567 | 0.019 | −0.212 | −0.405 | −0.921 | 0.372 | ||||||
| Inter | 1 | 0.035 | 0.164 | 0.148 | 0.448 | −0.153 | −0.11 | 0.22 | 0.035 | 0.463 | |||||||
| Trans | 1 | 0.954 | −0.962 | −0.802 | 0.327 | 0.09 | −0.193 | −0.823 | 0.521 | ||||||||
| Ci/Ca | 1 | −0.949 | −0.605 | 0.038 | −0.197 | −0.413 | −0.93 | 0.357 | |||||||||
| WUE | 1 | 0.784 | −0.153 | 0.096 | 0.423 | 0.919 | −0.27 | ||||||||||
| Al | 1 | −0.675 | −0.482 | −0.08 | 0.489 | −0.468 | |||||||||||
| B | 1 | 0.969 | 0.788 | 0.245 | 0.775 | ||||||||||||
| Bi | 1 | 0.902 | 0.477 | 0.717 | |||||||||||||
| Ca | 1 | 0.712 | 0.696 | ||||||||||||||
| K | 1 | 0.01 | |||||||||||||||
| Ni | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maluleke, S.; Ogugua, U.V.; Mdluli, N.; Madala, N.E.; Ntushelo, K. Physiological, Chemical and Metabolite Profiling of Pectobacterium carotovorum-Inoculated Tomato Plants Grown in Nutrient-Amended Soils. Plants 2025, 14, 1876. https://doi.org/10.3390/plants14121876
Maluleke S, Ogugua UV, Mdluli N, Madala NE, Ntushelo K. Physiological, Chemical and Metabolite Profiling of Pectobacterium carotovorum-Inoculated Tomato Plants Grown in Nutrient-Amended Soils. Plants. 2025; 14(12):1876. https://doi.org/10.3390/plants14121876
Chicago/Turabian StyleMaluleke, Sandra, Udoka Vitus Ogugua, Njabulo Mdluli, Ntakadzeni Edwin Madala, and Khayalethu Ntushelo. 2025. "Physiological, Chemical and Metabolite Profiling of Pectobacterium carotovorum-Inoculated Tomato Plants Grown in Nutrient-Amended Soils" Plants 14, no. 12: 1876. https://doi.org/10.3390/plants14121876
APA StyleMaluleke, S., Ogugua, U. V., Mdluli, N., Madala, N. E., & Ntushelo, K. (2025). Physiological, Chemical and Metabolite Profiling of Pectobacterium carotovorum-Inoculated Tomato Plants Grown in Nutrient-Amended Soils. Plants, 14(12), 1876. https://doi.org/10.3390/plants14121876







