Plant Growth, Yield, and Quality of Bush Tea (Athrixia phylicoides) as Affected by Deficit Hidrico and Mulching
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Plant Material Preparation
2.3. Experimental Description
2.4. Data Collection
2.4.1. Physiological Parameters
2.4.2. Growth Parameters
2.4.3. Extraction for Proton Nuclear Magnetic Resonance and Mass Spectrometry Studies
2.4.4. Microwave Digestion for Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OEP) Analysis
2.5. Data Analysis
3. Results and Discussion
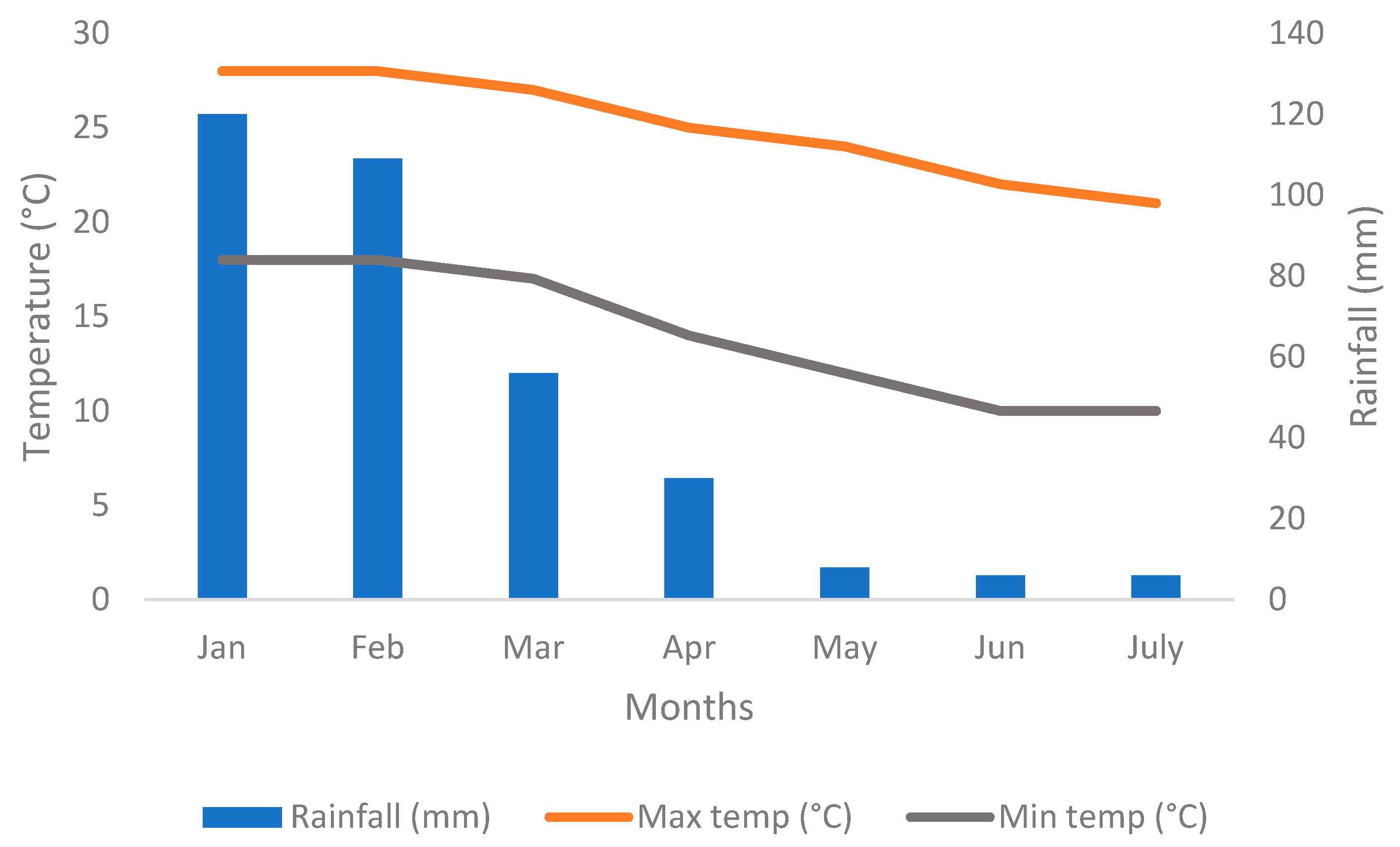
3.1. Influence of Water Regime and Mulching on Proportion of Intercepted Radiation
3.2. Deficit Irrigation on Plant Growth and Yield of Bush Tea
3.3. Influence of Mulching on Plant Growth and Yield of Bush Tea
3.4. Interactive Effect of Water Regimes and Mulching on Plant Growth and Yield of Bush Tea
3.5. Influence of Water Regimes on Quality of Bush Tea
3.6. Influence of Different Types of Mulching on Quality of Bush Tea
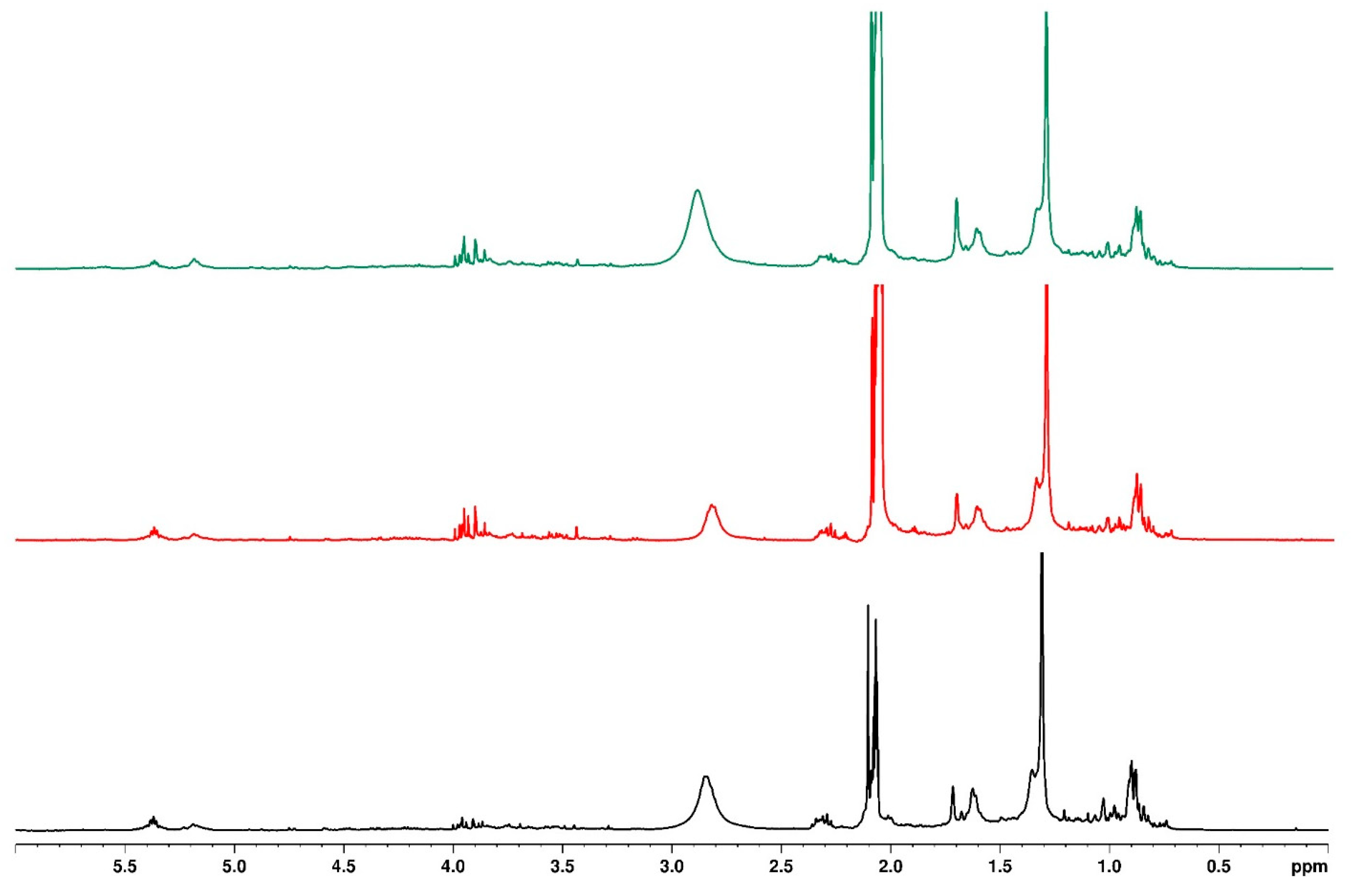
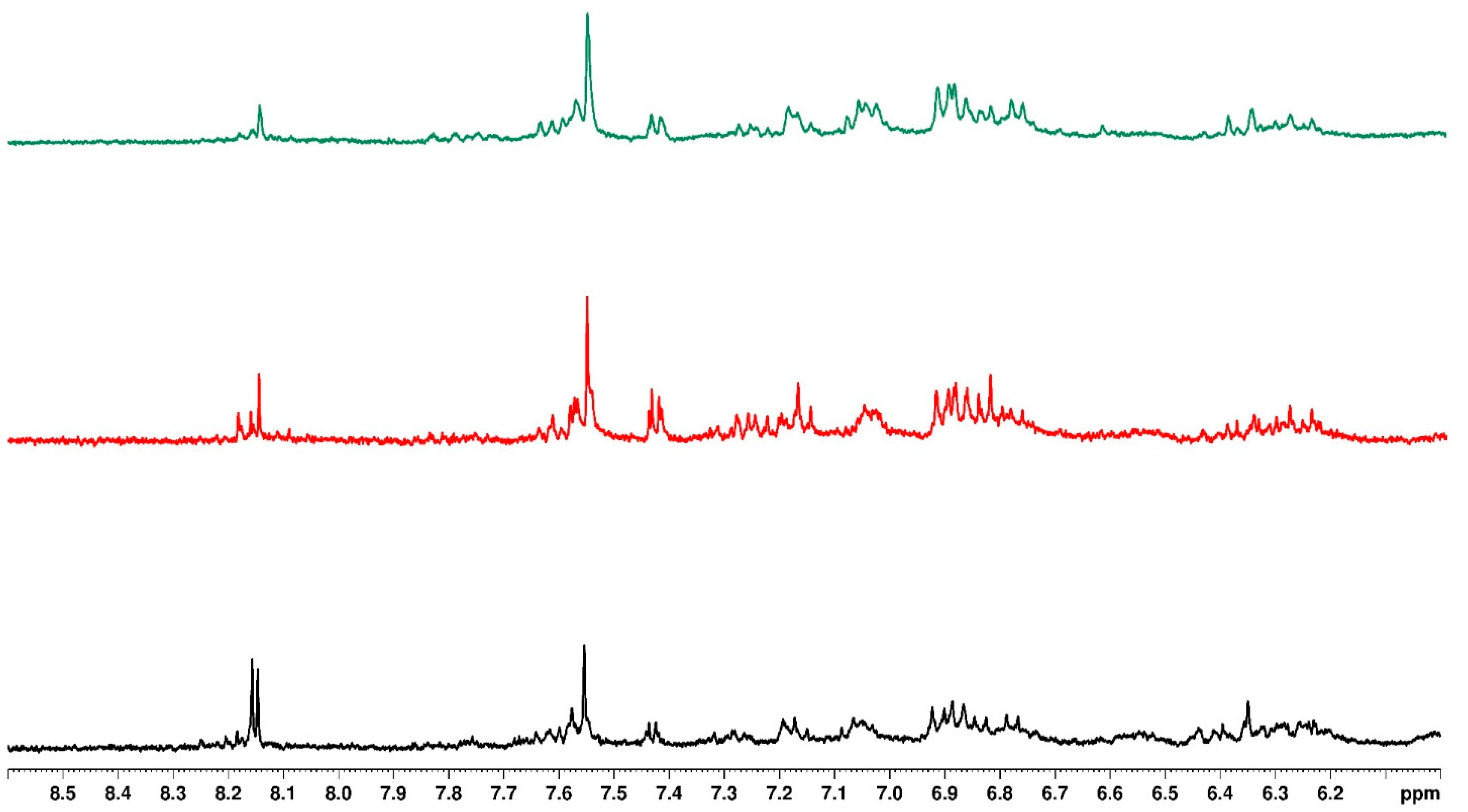
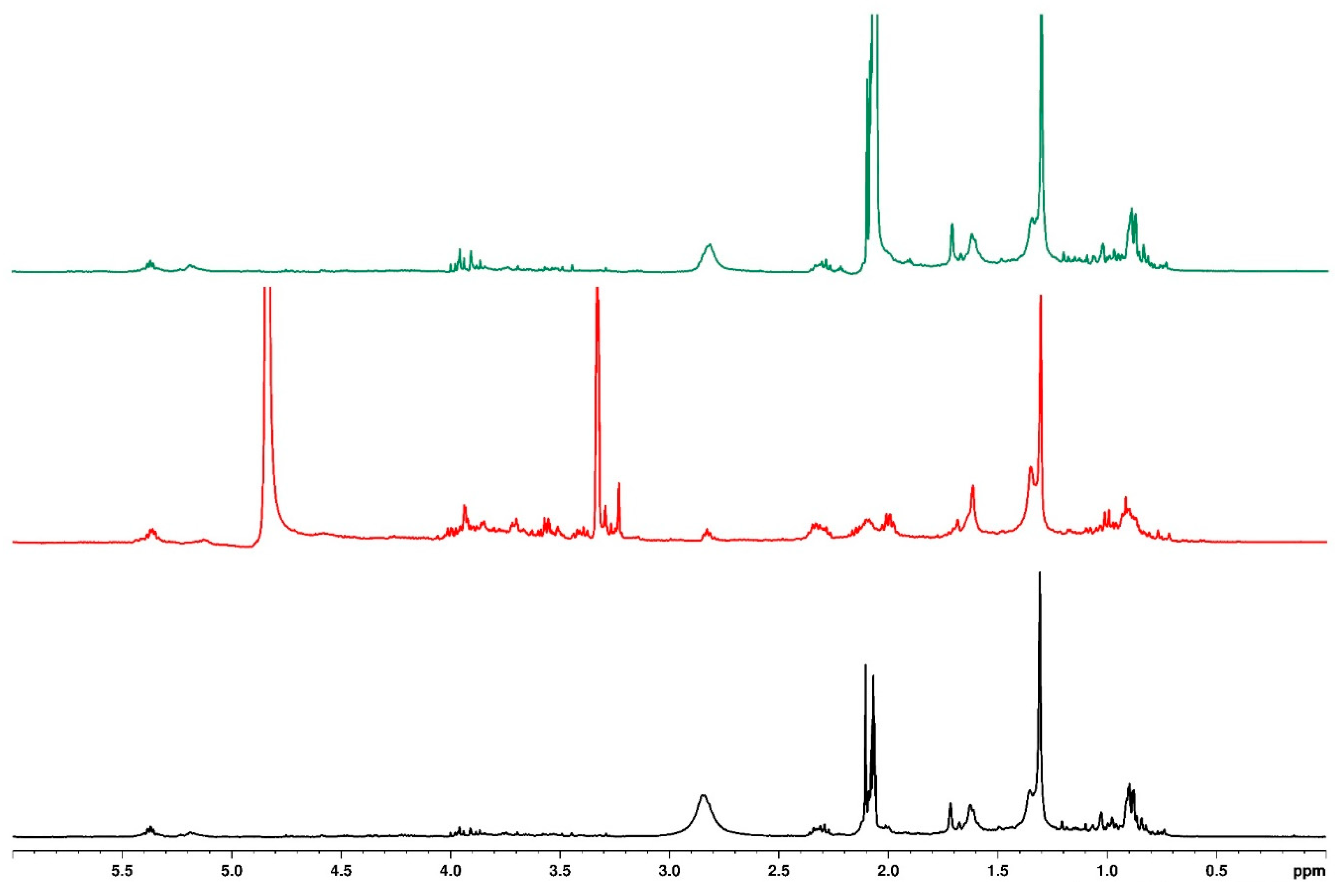
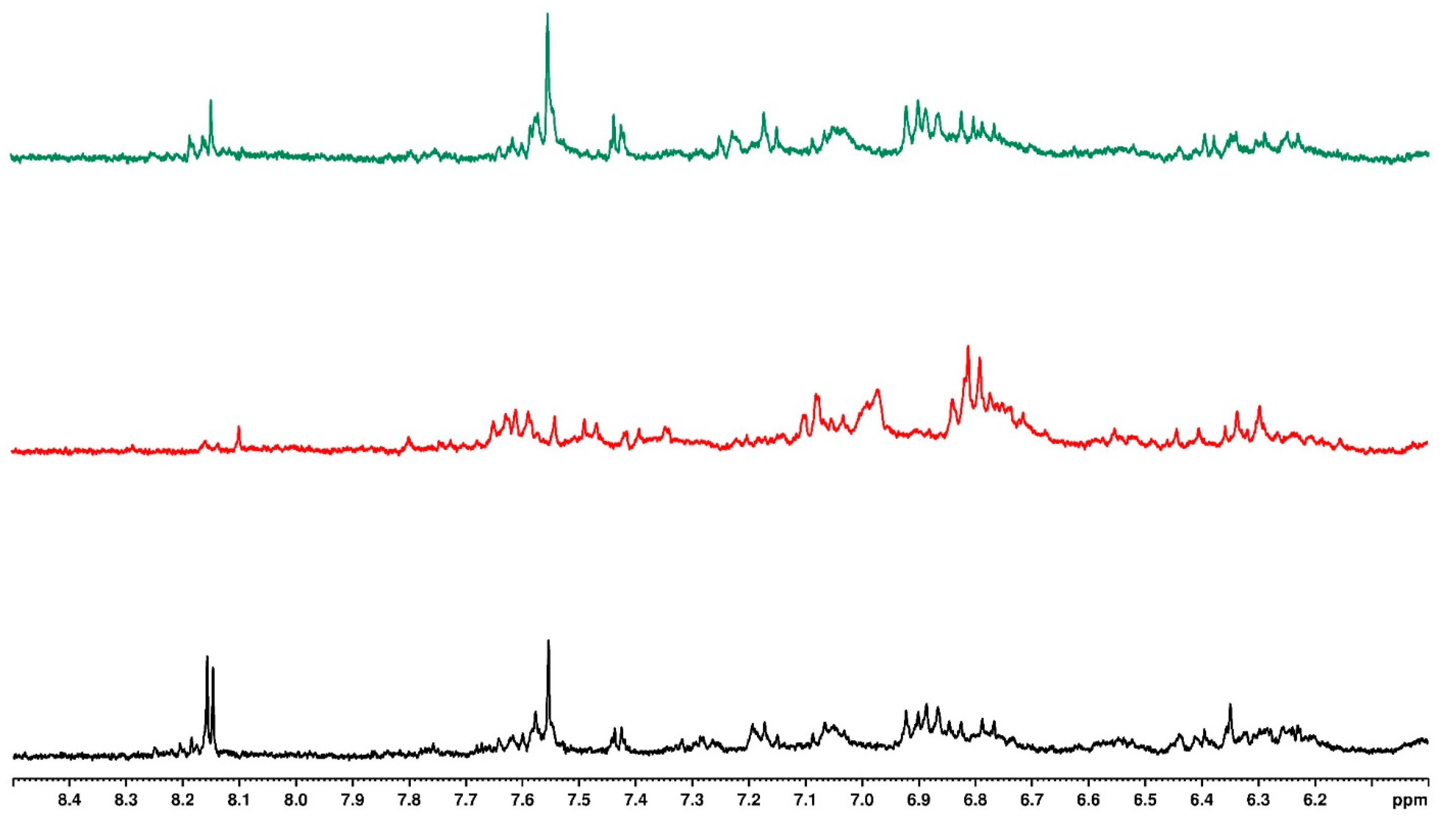
3.7. Mass Spectrometry
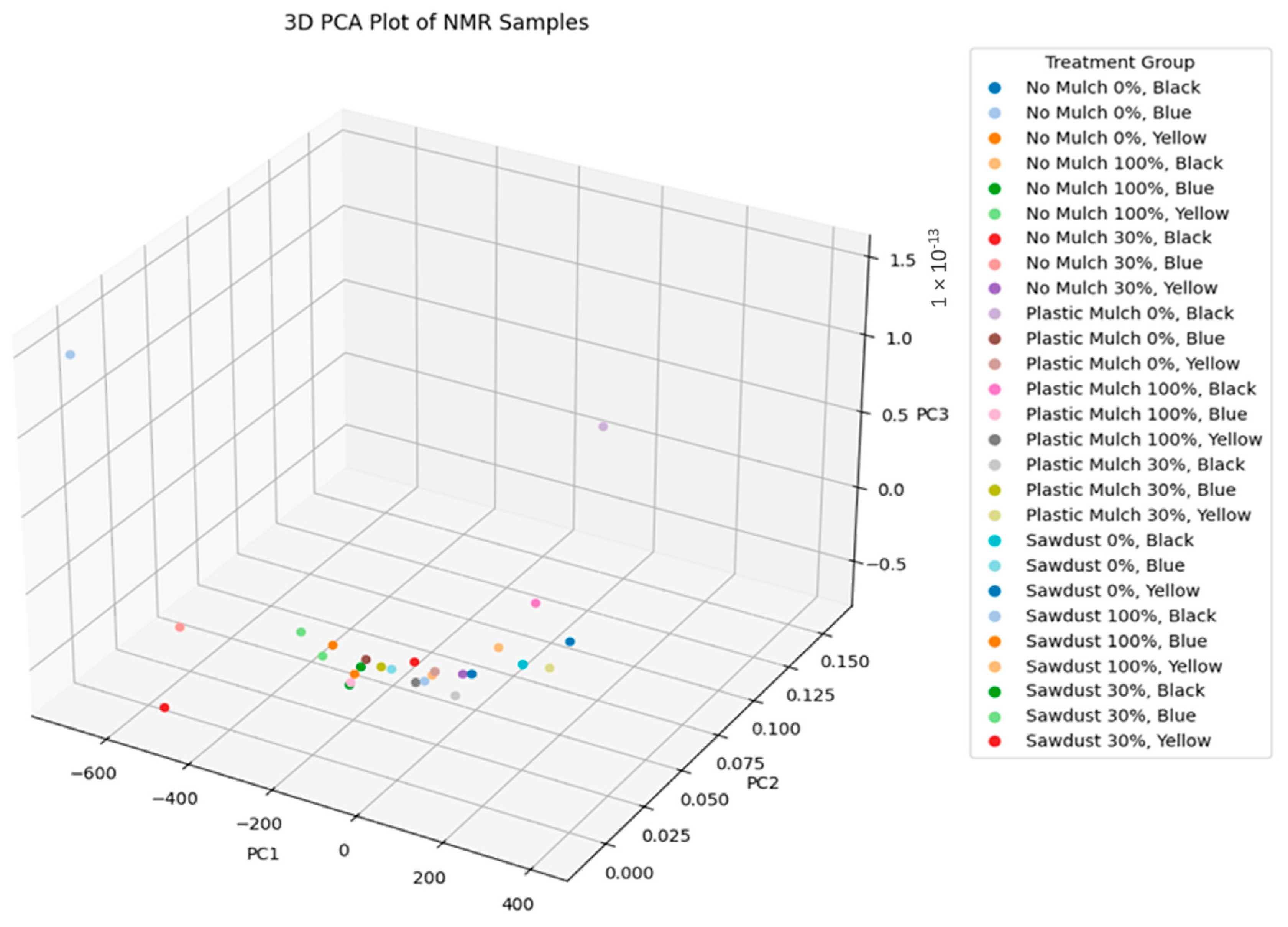
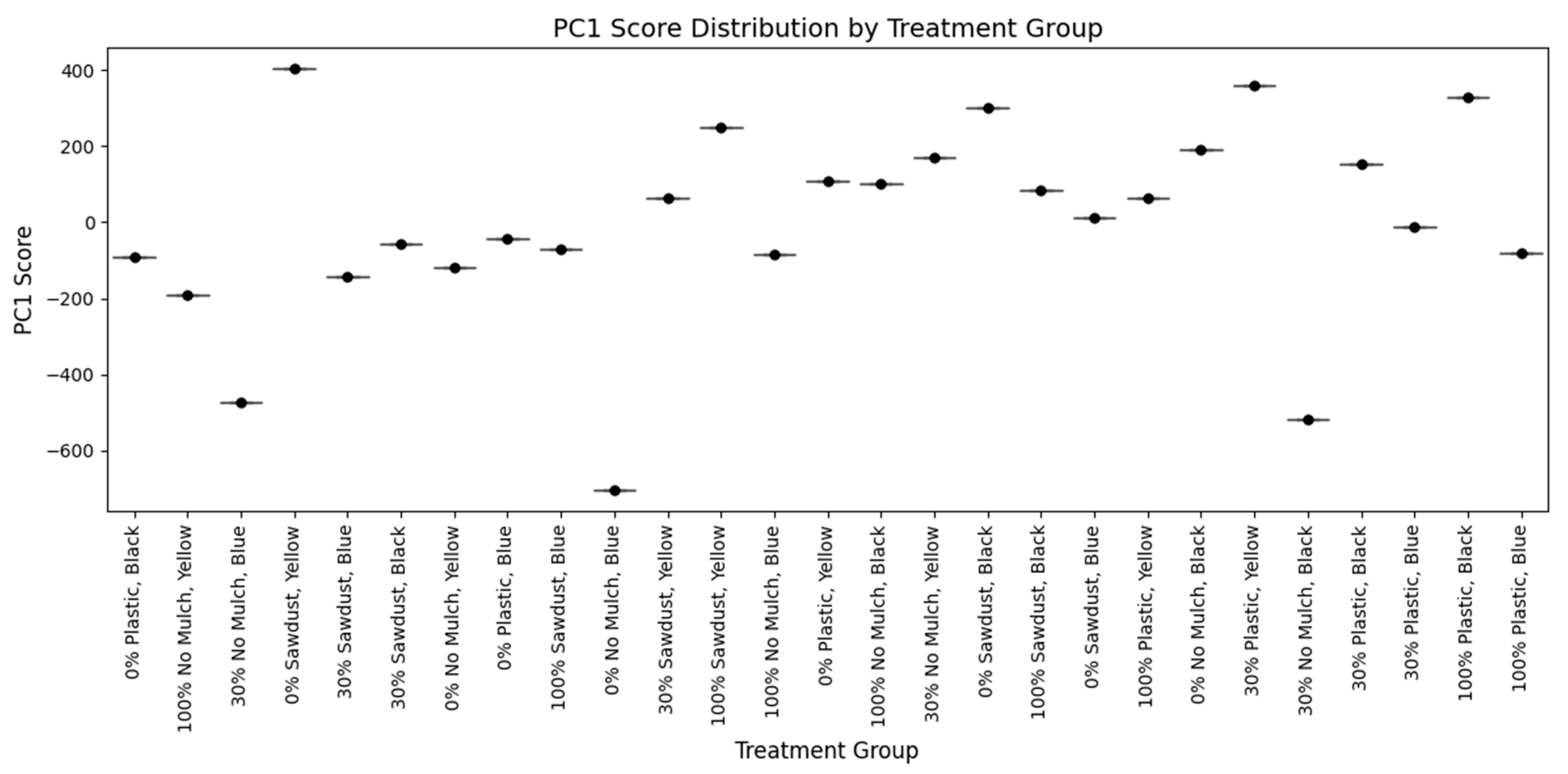
3.8. Principal Component Analysis of Bush Tea Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mudau, F.N.; Araya, H.T.; Du Toit, E.S.; Olivier, J. Bush Tea (Athrixia phylicoides DC.) as an Alternative Herbal and Medicinal Plant in Southern Africa: Opportunity for Commercialization. Med. Aromat. Plant Sci. Biotechnol. 2007, 1, 70–73. [Google Scholar]
- Lerotholi, L.; Chaudhary, S.K.; Combrinck, S.; Viljoen, A. Bush Tea (Athrixia phylicoides): A Review of the Traditional Uses, Bioactivity and Phytochemistry. South Afr. J. Bot. 2017, 110, 4–17. [Google Scholar] [CrossRef]
- Tshikhudo, P.P.; Ntushelo, K.; Kanu, S.A.; Mudau, F.N. Growth Response of Bush Tea (Athrixia phylocoides DC.) to Climatic Conditions in Limpopo Province, South Africa. South Afr. J. Bot. 2019, 121, 500–504. [Google Scholar] [CrossRef]
- Ramphinwa, M.L.; Mchau, G.R.A.; Madala, N.E.; Nengovhela, N.; Ogola, J.B.O.; Mudau, F.N. Response of Plant Growth and Development, and Accumulation of Hydroxyl-Cinnamoyl Acid Derivatives to Selected Shade Nets and Seasonality of Field-Grown Bush Tea (Athrixia phylicoides DC). HortScience 2022, 57, 87–96. [Google Scholar] [CrossRef]
- Rakuambo, Z.J. Indigenous Knowlegde of Bush Tea (Athrixia phylicoides) and Effect of Fertigation Frequency and Growing Medium on Plant Growth. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2007. [Google Scholar]
- McGaw, L.J.; Steenkamp, V.; Eloff, J.N. Evaluation of Athrixia Bush Tea for Cytotoxicity, Antioxidant Activity, Caffeine Content and Presence of Pyrrolizidine Alkaloids. J. Ethnopharmacol. 2007, 110, 16–22. [Google Scholar] [CrossRef]
- Mudau, F.N.; Mariga, I.K. Bush Tea as a Herbal Beverage and Medicinal Plant in South Africa. In Tea in Health and Disease Prevention, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 183–189. [Google Scholar]
- Tshivhandekano, I.; Nixwell Mudau, F.; Ngezimana, W. Effect of cultural practices and environmental conditions on yield and quality of herbal plants: Prospects Leading to Research on Influence of Nitrogen Fertilization, Planting Density and Eco-Physiological Parameters on Yield and Quality of Field-Grown Bush Tea (Athrixia phylicoides DC.). J. Med. Plants Res. 2013, 7, 2489–2493. [Google Scholar] [CrossRef]
- Azhar, N.; Hussain, B.; Yasin Ashraf, M.; Abbasi, K.Y. Water Stress Mediated Changes in Growth, Physiology and Secondary Metabolites of Desi Ajwain (Trachyspermum ammi L.). Pak. J. Bot. 2011, 43, 15–19. [Google Scholar]
- Lungu, M. Effect of Ridging and Mulching on Soil Moisture, Rainwater Use Efficiency and Maize yield in Lundazi, Zambia. Master’s Thesis, University of Zambia, Lundazi, Zambia, 2019. [Google Scholar]
- Mudau, F.N.; Du Toit, E.S. Nitrogen, Phosphorus, and Potassium Nutrition Increases Growth and Total Polyphenol Concentrations of Bush Tea in a Shaded Nursery Environment. HortTechnology 2007, 17, 107–110. [Google Scholar] [CrossRef]
- Maudu, M.; Mudau, F.N.; Mariga, I.K. The Effect of Pruning on Growth and Chemical Composition of Cultivated Bush Tea (Athrixia phylicoides D.C). J. Med. Plants Res. 2010, 4, 2353–2358. [Google Scholar]
- Mohale, K.C.; Hintsa, A.T.; Emanue, M.A.; Mudau, F.N. Metabolic Profiling of Cultivated Bush Tea (Athrixia phylicoides DC.) in Response to Different Pruning Types. HortScience 2018, 53, 993–998. [Google Scholar] [CrossRef]
- Maudu, M.E.; Mudau, F.N.; Mariga, I.K. Quality Profiles of Cultivated and Wild Bush Tea (Athrixia phylicoides) Harvested at Various Phenological Stages. Int. J. Agric. Biol. 2012, 14, 552–556. [Google Scholar]
- Ponmurugan, P.; Manjukarunambika, K.; Manjukarunambika, B.M. Impact of Various Foliar Diseases on the Biochemical, Volatile and Quality Constituents of Green and Black Teas. Australas. Plant Pathol. 2016, 45, 175–185. [Google Scholar] [CrossRef]
- Liu, E.K.; Mei, X.R.; Yan, C.R.; Gong, D.Z.; Zhang, Y.Q. Effects of Water Stress on Photosynthetic Characteristics, Dry Matter Translocation and WUE in Two Winter Wheat Genotypes. Agric. Water Manag. 2016, 167, 75–85. [Google Scholar] [CrossRef]
- Xue, J.; Zhou, S.; Wang, W.; Huo, L.; Zhang, L.; Fang, X.; Yang, Z. Water Availability Effects on Plant Growth, Seed Yield, Seed Quality in Cassia obtusifolia L., a Medicinal Plant. Agric. Water Manag. 2018, 195, 104–113. [Google Scholar] [CrossRef]
- Chivenge, P.; Mabhaudhi, T.; Modi, A.T.; Mafongoya, P. The Potential Role of Neglected and Underutilised Crop Species as Future Crops under Water Scarce Conditions in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2015, 12, 5685–5711. [Google Scholar] [CrossRef]
- Rostamza, M.; Chaichi, M.R.; Jahansouz, M.R.; Alimadadi, A. Forage Quality, Water Use and Nitrogen Utilization Efficiencies of Pearl Millet (Pennisetum americanum L.) Grown under Different Soil Moisture and Nitrogen Levels. Agric. Water Manag. 2011, 98, 1607–1614. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.d.R.; Sosa Alderete, L.G.; Palermo, T.B.; Banchio, E. Plant Growth Promoting Rhizobacteria Improve the Antioxidant Status in Mentha piperita Grown under Drought Stress Leading to an Enhancement of Plant Growth and Total Phenolic Content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Albergaria, E.T.; Oliveira, A.F.M.; Albuquerque, U.P. The Effect of Water Deficit Stress on the Composition of Phenolic Compounds in Medicinal Plants. South Afr. J. Bot. 2022, 131, 12–17. [Google Scholar] [CrossRef]
- Rumani, M.; Mabhaudhi, T.; Ramphinwa, M.L.; Ramabulana, A.T.; Madala, N.E.; Magwaza, L.S.; Mudau, F.N. Response to Various Water Regimes of the Physiological Aspects, Nutritional Water Productivity, and Phytochemical Composition of Bush Tea (Athrixia phylicoides DC.) Grown under a Protected Environment. Horticulturae 2024, 10, 590. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, H.; Wan, X.; Li, Y. The Effects of Different Types of Mulch on Soil Properties and Tea Production and Quality. J. Sci. Food Agric. 2020, 100, 5292–5300. [Google Scholar] [CrossRef]
- Bhardwaj, R.L. Effect of Mulching on Crop Production under Rainfed Condition—A Review. Agric. Rev. 2013, 34, 188. [Google Scholar] [CrossRef]
- Karsen, P.A.; Lötze, E.; Valentine, A.J.; Hoffman, E.W. Propagation and Cultivation Practices of Honeybush (Cyclopia spp.) for the Sustainable Production of an Export Quality Indigenous South African Tea. Crop Sci. 2022, 62, 1702–1733. [Google Scholar] [CrossRef]
- Ni, X.; Song, W.; Zhang, H.; Yang, X.; Wang, L. Effects of Mulching on Soil Properties and Growth of Tea Olive (Osmanthus fragrans). PLoS ONE 2016, 11, e0158228. [Google Scholar] [CrossRef]
- Selolo, K.R.; Mzezewa, J.; Odhiambo, J.J. Short-Term Effects of Tillage and Leaf Mulch on Soil Properties and Sunflower Yield under Semi-Arid Conditions. Plant Soil Env. 2023, 69, 55–61. [Google Scholar] [CrossRef]
- Mudau, F.N.; Du Toit, E.S. Effects of Nitrogen, Phosphorus, and Potassium Nutrition on Total Polyphenol Content of Bush Tea (Athrixia phylicoides L.) Leaves in Shaded Nursery Environment. HortScience 2007, 42, 334–338. [Google Scholar] [CrossRef]
- Makita, C.; Chimuka, L.; Steenkamp, P.; Cukrowska, E.; Madala, E. Comparative Analyses of Flavonoid Content in Moringa Oleifera and Moringa Ovalifolia with the Aid of UHPLC-QTOF-MS Fingerprinting. South Afr. J. Bot. 2016, 105, 116–122. [Google Scholar] [CrossRef]
- Batool, A.; Rashid, A.; Aziz, I.; Ahmed, M. Analysis of Water Stress Characteristics in Phaseolus vulgaris. Pak. J. Bot. 2023, 55, 2029–2035. [Google Scholar] [CrossRef]
- Greaves, G.E.; Wang, Y.M. The Effect of Water Stress on Radiation Interception, Radiation Use Efficiency and Water Use Efficiency of Maize in a Tropical Climate. Turk. J. Field Crops 2017, 22, 114–125. [Google Scholar] [CrossRef]
- Abiodum, F.O.; Isola, J.O.; Smart, M.O. Influence of Sawdust Mulch on Soil Properties, Growth and Yield Performance of Okra Abelmoschus Esculentus (L.) Moench in an Alfisol. FUTY J. Environ. 2022, 16, 12–22. [Google Scholar]
- Ramadhani, A.M.; Nassary, E.K.; Rwehumbiza, F.B.; Massawe, B.H.J.; Nchimbi-Msolla, S. Impact of Mulching Treatments on Growth, Yields, and Economics of Common Bean (Phaseolus vulgaris L.) in Eastern Tanzania. Front. Sustain. Food Syst. 2024, 8. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Riaz, M.; Arif, M.S. Environmental Stress and Secondary Metabolites in Plants: An Overview. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 153–167. [Google Scholar]
- Mahlare, M.S.; Lewu, M.N.; Lewu, F.B.; Bester, C. Cyclopia subternata growth, yield, proline and relative water content in response to water deficit stress. Water SA 2023, 49, 64–72. [Google Scholar] [CrossRef]
- Seifu, W.; Yemane, T.; Bedada, S.; Alemu, T. Evaluation of Different Mulching Practices on Garlic (Allium sativum L.) Growth Parameters under Irrigated Condition in Fiche, North Shoa Ethiopia. J. Biol. Agric. Healthc. 2017, 7, 25–31. [Google Scholar]
- Hajiboland, R. Environmental and Nutritional Requirements for Tea Cultivation. Folia Hortic. 2017, 29, 199–220. [Google Scholar] [CrossRef]
- Mahadeen, A.Y. Effect of Polyethylene Black Plastic Mulch on Growth and Yield of Two Summer Vegetable Crops under Rain-Fed Conditions under Semi-Arid Region Conditions. Am. J. Agric. Biol. Sci. 2014, 9, 202–207. [Google Scholar] [CrossRef]
- Mohale, K.C.; Bodede, O.; Araya, H.T.; Mudau, F.N. Metabolomic Analysis for Compositional Differences of Bush Tea (Athrixia Phylicoides DC.) Subjected to Seasonal Dynamics. Agronomy 2020, 10, 892. [Google Scholar] [CrossRef]
- Hua, S.; Dal-Bianco, M.; Chen, Z.H. Editorial: Crop Yield and Quality Response to the Interaction Between Environment and Genetic Factors. Front. Genet. 2022, 13, 823279. [Google Scholar] [CrossRef]
- Jojic, A.; Liga, G.; Utu, D.; Ruse, G.; Suciu, L.; Motoc, A.; Soica, S.; Tchiakpe-Antal, D. Beyond Essentials Oils: Diterpenes, Lignas, and Biflavonoids from Juniperus communis L. as a Source of Multi-Target Lead Compounds. Plants 2024, 13, 3233. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Alavi, M.S.; Ghane Nikookar Toos, M.; Jamialahmadi, T.; Sahebkar, A. 6-Gingerol, an Ingredient of Zingiber officinale, Abrogates Lipopolysaccharide-Induced Cardiomyocyte Injury by Reducing Oxidative Stress and Inflammation. J. Agric. Food Res. 2024, 15, 101034. [Google Scholar] [CrossRef]
- Vieux, F.; Maillot, M.; Drewnowski, A. Dietary Flavonoid Intakes in France Are Linked to Brewed Tea Consumption and to Socioeconomic Status: Analyses of the Third French Individual and National Food Consumption (INCA3) Survey for Children and Adults. Nutrients 2024, 16, 1118. [Google Scholar] [CrossRef]
- Yue, C.; Wang, Z.; Peng, H.; Jiang, L.; Yang, P.; Li, W. Analysis of Taste Quality Differences Between High and Low Grades of Ninghong Tea: From the Perspective of Sensory, Metabolite, and Taste Activity Values. Foods 2024, 13, 3957. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.; Zabolian, A.; Saleki, H.; Farhani, M.; Hamzehlous, S.; Far, F.; Shrifzadeh, S.; Samrghandan, S.; Khan, H. Caffeic Acid and Its Derivatives Ad Potential Modulators of Oncogeric Molecular Pathways: New Hope in the Fight against Cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-K.; Yu, J.; Le, D.; Han, S.; Yu, S.; Lee, M. Anti-Inflammatory Activity of Caffeic Acid Derivatives from Ilex Rotunda. Int. Immunopharmacol. 2023, 115, 109610. [Google Scholar] [CrossRef]
| Chlorophyll Content Index (CCI) (µmol m−2) | Dry Weight (g) | Fresh Weight (g) | Intercepted Radiation (%) | No. of Branches | Plant Height (cm) | |
|---|---|---|---|---|---|---|
| Mulching | ||||||
| Black Plastic | 3.04 a | 229.34 a | 343.22 a | 65.49 a | 23.44 b | 65.49 a |
| No Mulch | 3.05 a | 229.72 a | 343.37 a | 64.02 a | 30.22 ab | 64.02 a |
| Sawdust | 2.82 a | 189.49 a | 342.13 a | 62.73 a | 33.67 a | 62.73 a |
| Water Regime | ||||||
| 0% | 2.80 a | 167.50 b | 259.33 b | 60.18 b | 17.00 b | 70.67 b |
| 30% | 3.30 a | 283.73 a | 429.17 b | 67.95 a | 45.44 a | 71.67 b |
| 100% | 2.81 a | 198.32 a | 340.22 b | 64.12 a | 25.89 a | 70.67 a |
| p-value | ns | ns | ns | ns | * | ns |
| Mulching | ns | * | ns | ns | * | ns |
| Water Regime | * | * | ** | *** | ** | ns |
| Mulching × Water Regime | ns | * | ** | ns | ** | ns |
| L.S. D | 1.769 | 133.0 | 163.4 | 5.68 | 13.6 | 26.3 |
| CV (%) | 34.7 | 35.9 | 27.8 | 27.4 | 27.3 | 20.8 |
| Sample | Mass to Charge (m/z) | Retention Time (min) | Fragmentation Ion | Molecular Formula | Compound Name | Water Treatment | ||
|---|---|---|---|---|---|---|---|---|
| 0% | 30% | 100% | ||||||
| A | 353.1 | 7.75 | 354, 191, 179 | C16H18O9 | 4-caffeoylquinic acid | ✓ | ||
| 493.1 | 9.93 | 331, 210 | C23H25O12+ | malvidin-3-O-monoglucoside (oenin) | ||||
| AA | 297.1 | 21.12 | 163 | C20H30O2 | communic acid | ✓ | ||
| 433.1 | 22.29 | 403, 225 | C25H22O7 | 5′-hydroxycudraflavone A | ||||
| B | 463.1 | 9.73 | 301 | C21H20O12 | quercetin-3′-O-glucoside | ✓ | ||
| 493.1 | 9.93 | 331, 210 | C23H25O12+ | malvidin-3-O-monoglucoside (oenin) | ||||
| C | 353.1 | 7.50 | 135, 224 | C16H18O9 | 4-caffeoylquinic acid | ✓ | ||
| D | 493.1 | 9.95 | 331 | C23H25O12+ | malvidin-3-O-monoglucoside (oenin) | ✓ | ||
| E | 293 | 29.88 | 276 | C17H26O4 | 6-gingerol | ✓ | ||
| F | 293 | 29.88 | 276 | C17H26O4 | 6-gingerol | ✓ | ||
| G | 353.1 | 7.50 | 354, 191, 179 | C16H18O9 | 4-caffeoylquinic acid | ✓ | ||
| 419.1 | 18.18 | 389, 214 | C20H20O10 | 5,4′,5′-trihydroxy-3,6,7,8,2′-pentamethoxyflavone | ||||
| 433.1 | 22.29 | 403, 225 | C25H22O7 | 5′-hydroxycudraflavone A | ||||
| H | 433.1 | 22.29 | 403, 225 | C25H22O7 | 5′-hydroxycudraflavone A | ✓ | ||
| I | 297 | 21.11 | 163, 117 | C16H22O10 | gardoside | ✓ | ||
| 293 | 29.88 | 276 | C17H26O4 | 6-gingerol | ||||
| J | 293 | 29.88 | 276 | C17H26O4 | 6-gingerol | ✓ | ||
| K | 297 | 21.11 | 163, 117 | C16H22O10 | gardoside (glucoside) | ✓ | ||
| 433.1 | 22.29 | 403, 225 | C25H22O7 | 5′-hydroxycudraflavone A | ||||
| L | 433.1 | 22.29 | 403, 225 | C25H22O7 | 5′-hydroxycudraflavone A | ✓ | ||
| M | 419.1 | 18.18 | 389, 212 | C20H20O10 | 5,4′,5′-trihydroxy-3,6,7,8,2′-pentamethoxyflavone | ✓ | ||
| N | 433.1 | 22.30 | 403, 214 | C25H22O7 | 5′-hydroxycudraflavone A | ✓ | ||
| O | 293 | 29.88 | 276 | C17H26O4 | 6-gingerol | ✓ | ||
| P | 293 | 29.88 | 276 | C17H26O4 | 6-gingerol | ✓ | ||
| Q | 353.1 | 7.76 | 191, 230 | C16H18O9 | 4-caffeoylquinic acid | ✓ | ||
| 419.1 | 18.18 | 389, 212 | C20H20O10 | 5,4′,5′-trihydroxy-3,6,7,8,2′-pentamethoxyflavone | ||||
| R | 419.1 | 18.18 | 389, 212 | C20H20O10 | 5,4′,5′-trihydroxy-3,6,7,8,2′-pentamethoxyflavone | ✓ | ||
| 433.1 | 22.30 | 403, 225 | C25H22O7 | 5′-hydroxycudraflavone A | ||||
| S | 297 | 21.11 | 163, 117 | C16H22O10 | gardoside | ✓ | ||
| 433.1 | 22.30 | 403, 225 | C25H22O7 | 5′-hydroxycudraflavone A | ||||
| T | 293 | 29.88 | 276 | C17H26O4 | 6-gingerol | ✓ | ||
| U | 419.1 | 18.18 | 389, 212 | C20H20O10 | 5,4′,5′-trihydroxy-3,6,7,8,2′-pentamethoxyflavone | ✓ | ||
| 433.1 | 22.30 | 403, 225 | C25H22O7 | 5′-hydroxycudraflavone A | ||||
| V | 293 | 29.88 | 276 | C17H26O4 | 6-gingerol | ✓ | ||
| W | 293 | 29.88 | 276 | C17H26O4 | 6-gingerol | ✓ | ||
| X | 293 | 29.88 | 276 | C17H26O4 | 6-gingerol | ✓ | ||
| Y | 293 | 29.88 | 276 | C17H26O4 | 6-gingerol | ✓ | ||
| Z | Weak peaks on chromatogram | ✓ | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndou, V.T.; Mabhaudhi, T.; Goge, M.; Papo, T.; Shozi, M.; Ramphinwa, M.L.; Mudau, F.N. Plant Growth, Yield, and Quality of Bush Tea (Athrixia phylicoides) as Affected by Deficit Hidrico and Mulching. Plants 2025, 14, 1743. https://doi.org/10.3390/plants14121743
Ndou VT, Mabhaudhi T, Goge M, Papo T, Shozi M, Ramphinwa ML, Mudau FN. Plant Growth, Yield, and Quality of Bush Tea (Athrixia phylicoides) as Affected by Deficit Hidrico and Mulching. Plants. 2025; 14(12):1743. https://doi.org/10.3390/plants14121743
Chicago/Turabian StyleNdou, Vhuhwavho Tshilidzi, Tafadzwanashe Mabhaudhi, Mangaliso Goge, Tshephiso Papo, Mzamo Shozi, Maanea Lonia Ramphinwa, and Fhatuwani Nixwell Mudau. 2025. "Plant Growth, Yield, and Quality of Bush Tea (Athrixia phylicoides) as Affected by Deficit Hidrico and Mulching" Plants 14, no. 12: 1743. https://doi.org/10.3390/plants14121743
APA StyleNdou, V. T., Mabhaudhi, T., Goge, M., Papo, T., Shozi, M., Ramphinwa, M. L., & Mudau, F. N. (2025). Plant Growth, Yield, and Quality of Bush Tea (Athrixia phylicoides) as Affected by Deficit Hidrico and Mulching. Plants, 14(12), 1743. https://doi.org/10.3390/plants14121743








