Physiological Responses of Asparagus Plants to Soil Disinfection Strategies Targeting Asparagus Decline Syndrome
Abstract
1. Introduction
2. Results
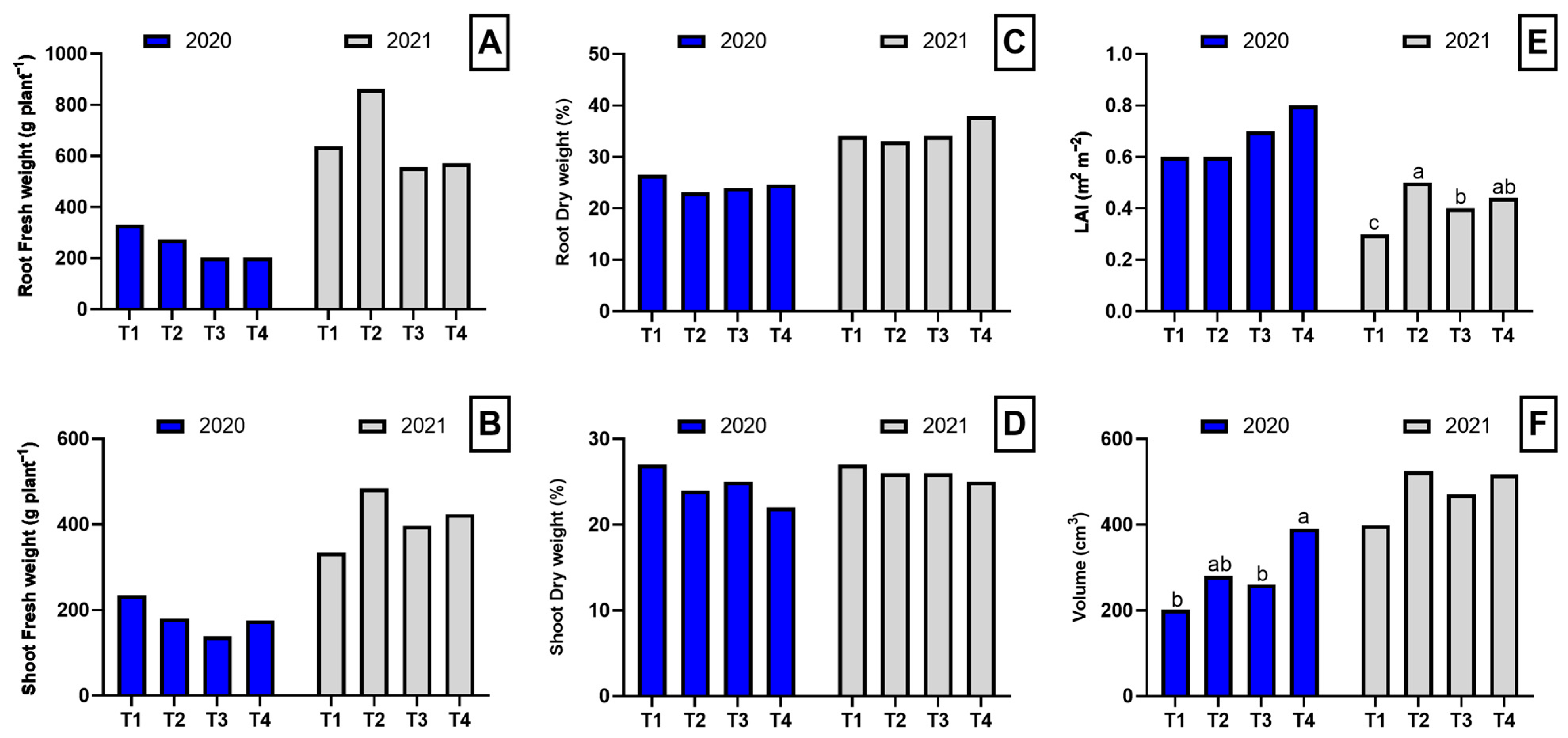
2.1. Biomass Production
2.2. Photosynthetic Pigments
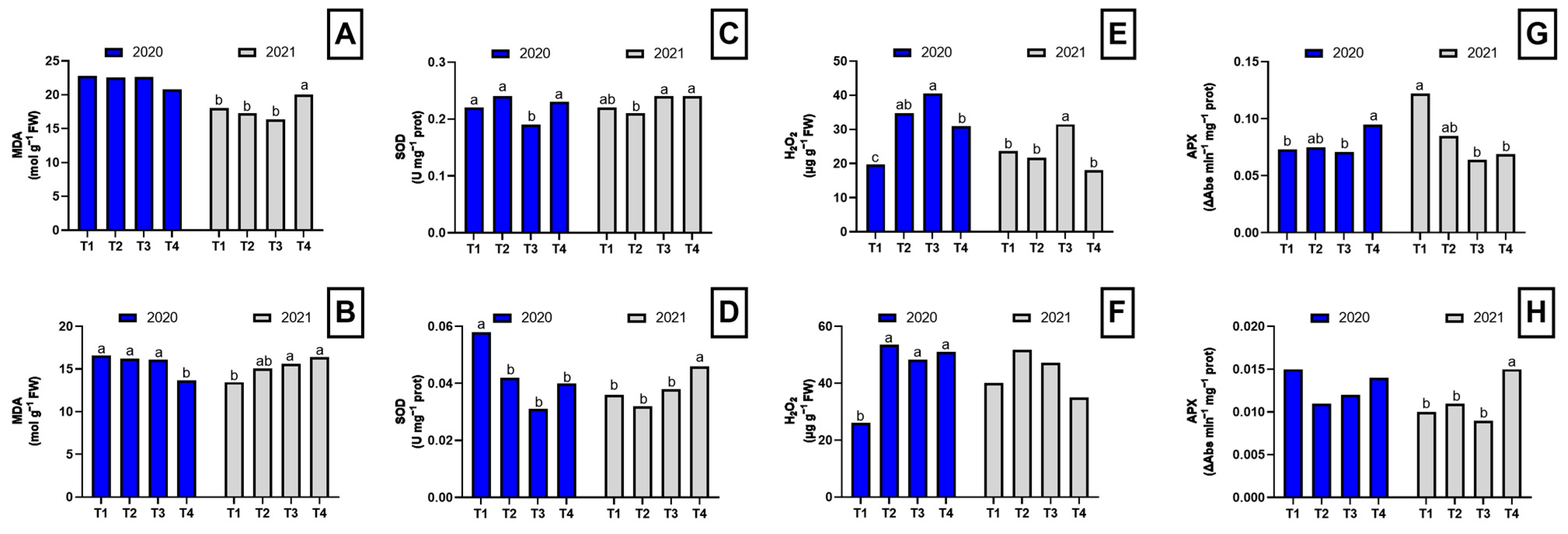
2.3. Oxidative Metabolism
2.4. Phytohormone Profile
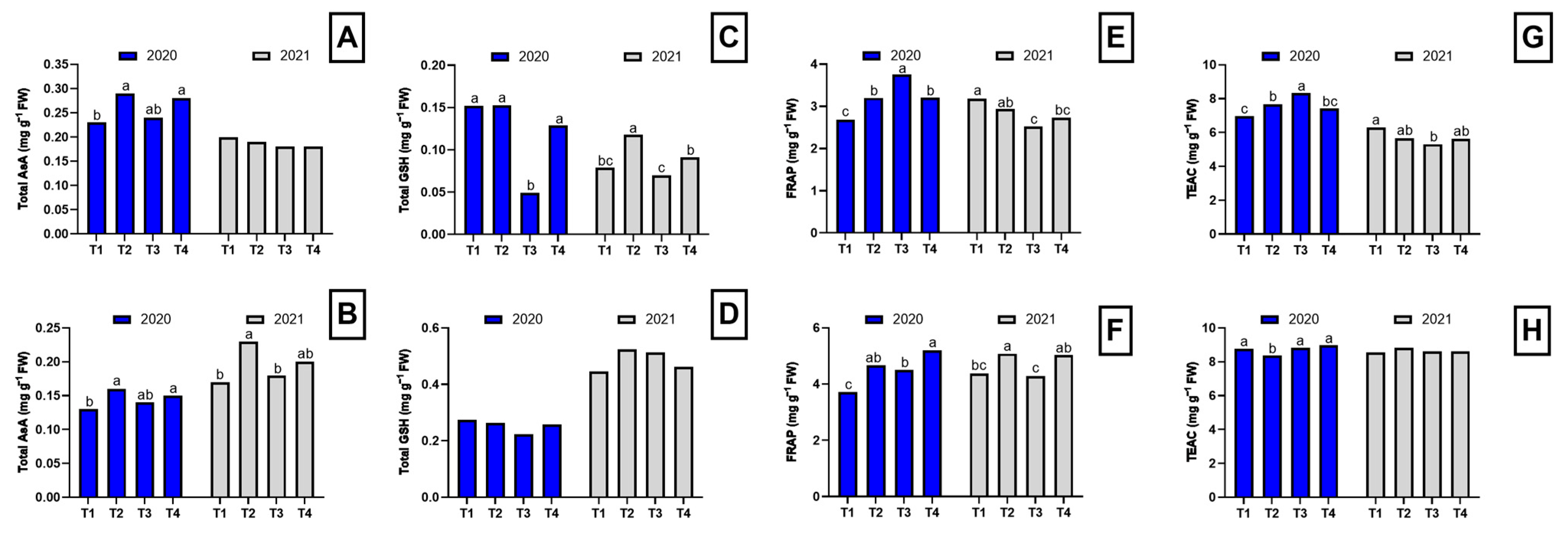
2.5. Phenolic Compounds Profile
2.6. Mineral Nutrient Profile
3. Discussion
4. Materials and Methods
4.1. Plan Material and Experimental Setup
4.2. Sampling and Biomass Parameters
4.3. Photosynthetic Pigments Concentration
4.4. Evaluation of Oxidative Metabolism and Antioxidant Tests
4.5. Phytohormone Analysis
4.6. Phenolic Compound Profiling
4.7. Determination of Mineral Elements
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elmer, W.H. Epidemiology and Management of the Diseases Causal to Asparagus Decline. Plant Dis. 1996, 80, 117. [Google Scholar] [CrossRef]
- Knaflewski, M. Genealogy of Asparagus Cultivars. Acta Hortic. 1996, 415, 87–92. [Google Scholar] [CrossRef]
- Elmer, W. Fusarium Diseases of Asparagus. In Paul E. Nelson Memorial Symposium; Summerell, B.A., Leslie, J.F., Backhouse, D., Eds.; American Phytopathological Society Press: St. Paul, MN, USA, 2001; pp. 248–262. [Google Scholar]
- Baayen, R.P.; O’Donnell, K.; Bonants, P.J.M.; Cigelnik, E.; Kroon, L.P.N.M.; Roebroeck, E.J.A.; Waalwijk, C. Gene Genealogies and AFLP Analyses in the Fusarium Oxysporum Complex Identify Monophyletic and Nonmonophyletic Formae Speciales Causing Wilt and Rot Disease. Phytopathology 2000, 90, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Benjumea, A.; Basallote-Ureba, M.J.; Melero-Vara, J.M. Eficacia de Enmiendas Orgánicas, Temperatura de Suelo y Cultivares En El Control de La Podredumbre de Raíces y Cuello de Espárrago. In Proceedings of the Resúmenes del XV Congreso de la Sociedad Española de Fitopatología, Vitoria-Gasteiz, Vitoria-Gasteiz, Spain, 27 September 2010; p. 383. [Google Scholar]
- Blok, W.J.; Bollen, G.J. The Role of Autotoxins from Root Residues of the Previous Crop in the Replant Disease of Asparagus. Neth. J. Plant Pathol. 1993, 99, 29–40. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and Quantification of Phenolic Compounds from Pomegranate (Punica granatum L.) Peel, Mesocarp, Aril and Differently Produced Juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Schofield, P.E. Asparagus Decline and Replant Problem in New Zealand. N. Z. J. Crop Hortic. Sci. 1991, 19, 213–220. [Google Scholar] [CrossRef][Green Version]
- Kato-Noguchi, H.; Nakamura, K.; Okuda, N. Involvement of an Autotoxic Compound in Asparagus Decline. J. Plant Physiol. 2018, 224–225, 49–55. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Nakamura, K.; Ohno, O.; Suenaga, K.; Okuda, N. Asparagus Decline: Autotoxicity and Autotoxic Compounds in Asparagus Rhizomes. J. Plant Physiol. 2017, 213, 23–29. [Google Scholar] [CrossRef]
- Elmer, W. Asparagus Decline and Replant Problem: A Look Back and a Look Forward at Strategies for Mitigating Losses. Acta Hortic. 2018, 1223, 195–204. [Google Scholar] [CrossRef]
- Corpas-Hervias, C.; Melero-Vara, J.M.; Molinero-Ruiz, M.L.; Zurera-Muñoz, C.; Basallote-Ureba, M.J. Characterization of Isolates of Fusarium spp. Obtained from Asparagus in Spain. Plant Dis. 2006, 90, 1441–1451. [Google Scholar] [CrossRef]
- Ito, T.; Ochiai, T.; Fukuda, T.; Ashizawa, H.; Kanno, A.; Kameya, T.; Sonoda, T. Potential of Interspecific Hybrids in the Genus Asparagus. Acta Hortic. 2008, 776, 279–284. [Google Scholar] [CrossRef]
- Kathe, L.; Krämer, R.; Budahn, H.; Pillen, K.; Rabenstein, F.; Nothnagel, T. Development of a Bioassay to Assess Resistance to Fusarium Oxysporum (Schlecht.) in Asparagus (Asparagus officinalis L.). J. Phytopathol. 2019, 167, 558–566. [Google Scholar] [CrossRef]
- Fravel, D.; Olivain, C.; Alabouvette, C. Fusarium Oxysporum and Its Biocontrol. New Phytol. 2003, 157, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Antignani, V.; Pane, C.; Scala, F. Suppression of Soilborne Fungal Diseases with Organic Amendments. J. Plant Pathol. 2007, 89, 311–324. [Google Scholar]
- dos Santos, C.A.; de Souza Abboud, A.C.; Carmo, M.G.F.d. Biofumigation with Species of the Brassicaceae Family: A Review. Ciência Rural. 2021, 51, e20200440. [Google Scholar] [CrossRef]
- Zhao, R.; Suo, X.; Meng, X.; Wang, Y.; Dai, P.; Hu, T.; Cao, K.; Wang, S.; Li, B. Global Analysis of MicroRNA-like RNAs Reveals Differential Regulation of Pathogenicity and Development in Fusarium Oxysporum HS2 Causing Apple Replant Disease. J. Fungi 2024, 10, 883. [Google Scholar] [CrossRef]
- Wilson, D.R.; Sinton, S.M.; Butler, R.C.; Drost, D.T.; Paschold, P.J.; van Kruistum, G.; Poll, J.T.K.; Garcin, C.; Pertierra, R.; Vidal, I.; et al. Carbohydrates and Yield Physiology of Asparagus—A Global Overview. Acta Hortic. 2008, 776, 413–428. [Google Scholar] [CrossRef]
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Winkelmann, T. Biofumigation for Fighting Replant Disease- A Review. Agronomy 2020, 10, 425. [Google Scholar] [CrossRef]
- Del Carmen Martínez-Ballesta, M.; Moreno, D.; Carvajal, M. The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef]
- Nguyen, V.P.T.; Stewart, J.D.; Allais, F.; Ioannou, I. Optimization of the Recovery of Secondary Metabolites from Defatted Brassica Carinata Meal and Its Effects on the Extractability and Functional Properties of Proteins. Foods 2022, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, G.H.; Ay, E.B.; Şengür, Ş. Effects of Different Fertilizer Types on Pigment Content and Some Stress Molecules in Perennial Ryegrass (Lolium perenne L.). Legume Res.—An. Int. J. 2024, 48, 224. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.-H.; Ahmad, H.; Li, F.B. Mechanisms Regulating the Dynamics of Photosynthesis Under Abiotic Stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef]
- Boutahiri, S.; Benrkia, R.; Tembeni, B.; Idowu, O.E.; Olatunji, O.J. Effect of Biostimulants on the Chemical Profile of Food Crops under Normal and Abiotic Stress Conditions. Curr. Plant Biol. 2024, 40, 100410. [Google Scholar] [CrossRef]
- Gavelienė, V.; Mockevičiūtė, R.; Jankovska-Bortkevič, E.; Šveikauskas, V.; Zareyan, M.; Žalnierius, T.; Jankauskienė, J.; Jurkonienė, S. Synergistic Effects of Microbial Biostimulants and Calcium in Alleviating Drought Stress in Oilseed Rape. Microorganisms 2025, 13, 530. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Dąbrowski, P.; Cetner, M.D.; Samborska, I.A.; Łukasik, I.; Brestic, M.; Zivcak, M. A Comparison between Different Chlorophyll Content Meters under Nutrients Deficiency Conditions. J. Plant Nutr. 2016, 40, 1024–1034. [Google Scholar] [CrossRef]
- Bashri, G.; Prasad, S.M. Exogenous IAA Differentially Affects Growth, Oxidative Stress and Antioxidants System in Cd Stressed Trigonella Foenum-Graecum L. Seedlings: Toxicity Alleviation by up-Regulation of Ascorbate-Glutathione Cycle. Ecotoxicol. Environ. Saf. 2016, 132, 329–338. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The Multiple Roles of Ascorbate in the Abiotic Stress Response of Plants: Antioxidant, Cofactor, and Regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant Nutrition: An Effective Way to Alleviate Abiotic Stress in Agricultural Crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef]
- Hatamleh, A.A.; Danish, M.; Al-Dosary, M.A.; El-Zaidy, M.; Ali, S. Physiological and Oxidative Stress Responses of Solanum Lycopersicum (L.) (Tomato) When Exposed to Different Chemical Pesticides. RSC Adv. 2022, 12, 7237–7252. [Google Scholar] [CrossRef]
- Cuypers, A.; Vangronsveld, J.; Clijsters, H. The Redox Status of Plant Cells (AsA and GSH) Is Sensitive to Zinc Imposed Oxidative Stress in Roots and Primary Leaves of Phaseolus Vulgaris. Plant Physiol. Biochem. 2001, 39, 657–664. [Google Scholar] [CrossRef]
- Blázquez, M.A.; Nelson, D.C.; Weijers, D. Evolution of Plant Hormone Response Pathways. Annu. Rev. Plant Biol. 2020, 71, 327–353. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Saeed, M.S.; Fan, X.; Salam, A.; Munir, R.; Yasin, M.U.; Khan, A.R.; Muhammad, S.; Ali, B.; Ali, I.; et al. The Multifaceted Role of Jasmonic Acid in Plant Stress Mitigation: An Overview. Plants 2023, 12, 3982. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Zhang, Q.; Cui, M.; Zhao, M.; Li, N.; Wang, S.; Wu, R.; Zhang, L.; Cao, Y.; et al. The Interaction of ABA and ROS in Plant Growth and Stress Resistances. Front. Plant Sci. 2022, 13, 1050132. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on Their Biosynthesis, Genetics, and Ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Chowdhary, V.; Alooparampil, S.; Pandya, R.V.; Tank, J.G. Physiological Function of Phenolic Compounds in Plant Defense System. In Phenolic Compounds: Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; IntechOpen: London, UK, 2022; ISBN 978-1-83969-347-2. [Google Scholar]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Bailey, K.L.; Lazarovits, G. Suppressing Soil-Borne Diseases with Residue Management and Organic Amendments. Soil. Tillage Res. 2003, 72, 169–180. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.; Cardinali, A. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Friedman, M. Overview of Antibacterial, Antitoxin, Antiviral, and Antifungal Activities of Tea Flavonoids and Teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.B.; Turatti, I.C.C.; Gouveia, D.R.; Ernst, M.; Teixeira, S.P.; Lopes, N.P. Mass Spectrometry of Flavonoid Vicenin-2, Based Sunlight Barriers in Lychnophora Species. Sci. Rep. 2014, 4, 4309. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Recent Insights into the Biological Functions of Apigenin. EXCLI J. 2020, 19, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2012. [Google Scholar]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. The Effects of Biochar, Compost and Their Mixture and Nitrogen Fertilizer on Yield and Nitrogen Use Efficiency of Barley Grown on a Nitisol in the Highlands of Ethiopia. Sci. Total Environ. 2016, 569–570, 869–879. [Google Scholar] [CrossRef]
- Matthiessen, J.N.; Kirkegaard, J.A. Biofumigation and Enhanced Biodegradation: Opportunity and Challenge in Soilborne Pest and Disease Management. CRC Crit. Rev. Plant Sci. 2006, 25, 235–265. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Sarwar, M. Biofumigation Potential of Brassicas. Plant Soil. 1998, 201, 71–89. [Google Scholar] [CrossRef]
- Lazzeri, L.; Leoni, O.; Manici, L.M. Biocidal Plant Dried Pellets for Biofumigation. Ind. Crops Prod. 2004, 20, 59–65. [Google Scholar] [CrossRef]
- Shennan, C.; Muramoto, J.; Lamers, J.; Mazzola, M.; Rosskopf, E.N.; Kokalis-Burelle, N.; Momma, N.; Butler, D.M.; Kobara, Y. Anaerobic Soil Disinfestation for Soil Borne Disease Control in Strawberry and Vegetable Systems: Current Knowledge and Future Directions. Acta Hortic. 2014, 1044, 165–175. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Fu, J.; Huang, B. Involvement of Antioxidants and Lipid Peroxidation in the Adaptation of Two Cool-Season Grasses to Localized Drought Stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Yu, Q.; Osborne, L.; Rengel, Z. Micronutrient Deficiency Changes Activities of Superoxide Dismutase and Ascorbate Peroxidase in Tobacco Plants. J. Plant Nutr. 1998, 21, 1427–1437. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized Assay for Hydrogen Peroxide Determination in Plant Tissue Using Potassium Iodide. Am. J. Anal. Chem. 2014, 5, 730–736. [Google Scholar] [CrossRef]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and Ascorbic Acid in Spinach (Spinacia Oleracea) Chloroplasts. The Effect of Hydrogen Peroxide and of Paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Simultaneous Measurement of Foliar Glutathione, γ-Glutamylcysteine, and Amino Acids by High-Performance Liquid Chromatography: Comparison with Two Other Assay Methods for Glutathione. Anal. Biochem. 1998, 264, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Aeasure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant Activity and Phenolic Compounds of 112 Traditional Chinese Medicinal Plants Associated with Anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Albacete, A.; Ghanem, M.E.; Martinez-Andujar, C.; Acosta, M.; Sanchez-Bravo, J.; Martinez, V.; Lutts, S.; Dodd, I.C.; Perez-Alfocea, F. Hormonal Changes in Relation to Biomass Partitioning and Shoot Growth Impairment in Salinized Tomato (Solanum lycopersicum L.) Plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B. A Comprehensive System of Leaf Analyses and Its Use for Diagnosing Crop Nutrient Status. Commun. Soil. Sci. Plant Anal. 1982, 13, 1035–1059. [Google Scholar] [CrossRef]
- Krom, M.D. Spectrophotometric Determination of Ammonia: A Study of a Modified Berthelot Reaction Using Salicylate and Dichloroisocyanurate. Analyst 1980, 105, 305–316. [Google Scholar] [CrossRef]
| Total Chls mg g−1 FW | Chl a/b | Carotenoids mg g−1 FW | ||
|---|---|---|---|---|
| 2020 | T1 | 0.34 b | 2.13 b | 0.108 b |
| T2 | 0.38 a | 2.86 b | 0.123 a | |
| T3 | 0.32 b | 1.64 b | 0.095 c | |
| T4 | 0.32 b | 5.49 a | 0.115 ab | |
| p value | * | *** | *** | |
| 2021 | T1 | 0.20 c | 1.50 b | 0.059 c |
| T2 | 0.33 a | 2.15 a | 0.104 a | |
| T3 | 0.25 b | 2.13 a | 0.081 b | |
| T4 | 0.28 b | 2.15 a | 0.093 b | |
| p value | *** | *** | *** |
| Indole Acetic Acid | Trans-Zeatin | Zeatin Riboside | Isopentenyl Adenine | GA1 | GA3 | GA4 | ACC | ABA | Jasmonic Acid | Salicylic Acid | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root | 2020 | T1 | 4.43 | 65.16 | ND | 28.73 | ND | ND | 1.37 | 189.48 | 2.20 | 6.45 ab | 350.57 |
| T2 | 5.09 | 70.20 | ND | 18.33 | ND | ND | 0.82 | 260.95 | 2.08 | 5.03 b | 375.38 | ||
| T3 | 4.78 | 83.68 | ND | 25.48 | ND | ND | 1.78 | 175.00 | 2.32 | 8.42 a | 462.76 | ||
| T4 | 4.97 | 61.08 | ND | 22.91 | ND | ND | 0.46 | 280.85 | 2.73 | 6.66 ab | 358.60 | ||
| p value | NS | NS | - | NS | - | - | NS | NS | NS | * | NS | ||
| 2021 | T1 | 4.39 | 77.76 | ND | 21.19 | ND | ND | 0.51 | 237.18 | 1.11 b | 6.47 | 287.86 | |
| T2 | 4.68 | 89.02 | ND | 17.27 | ND | ND | 0.44 | 210.20 | 0.69 c | 5.91 | 287.98 | ||
| T3 | 5.53 | 83.69 | ND | 23.95 | ND | ND | 0.61 | 209.74 | 1.14 b | 6.09 | 271.5 | ||
| T4 | 4.86 | 91.97 | ND | 18.85 | ND | ND | 0.41 | 273.38 | 1.43 a | 5.48 | 279.76 | ||
| p value | NS | NS | - | NS | - | - | NS | NS | *** | NS | NS | ||
| Shoot | 2020 | T1 | 5.67 a | 10.22 | ND | 3.81 | ND | ND | 0.02 b | 28.00 | 40.87 | 31.45 | 102.63 |
| T2 | 4.04 c | 13.09 | ND | 2.80 | ND | ND | 0.04 b | 22.87 | 46.91 | 31.02 | 109.49 | ||
| T3 | 4.50 bc | 11.10 | ND | 4.33 | ND | ND | 0.07 a | 29.93 | 49.95 | 34.15 | 123.64 | ||
| T4 | 5.25 ab | 12.31 | ND | 2.54 | ND | ND | 0.04 b | 29.99 | 53.66 | 32.08 | 128.13 | ||
| p value | ** | NS | - | NS | - | - | ** | NS | NS | NS | NS | ||
| 2021 | T1 | 8.22 | 9.85 | ND | 3.90 | ND | ND | 0.41 | 20.06 | 38.07 | 39.96 a | 114.57 b | |
| T2 | 7.78 | 12.83 | ND | 4.09 | ND | ND | 0.29 | 24.28 | 40.92 | 28.67 b | 125.73 a | ||
| T3 | 9.71 | 9.69 | ND | 3.97 | ND | ND | 0.55 | 21.47 | 32.91 | 34.47 ab | 115.02 b | ||
| T4 | 9.83 | 11.48 | ND | 3.53 | ND | ND | 0.42 | 19.89 | 37.37 | 39.01 a | 128.20 a | ||
| p value | NS | NS | - | NS | - | - | NS | NS | NS | * | * |
| 4-CQA | Caffeic Acid Hexose | Caffeic Acid | Feruloil-QA | p-Coumaric Acid Derivative | Ferulic Acid Derivative | Apigenin Diglucoside (Vicenin2) | Q-Rutin-Hexose | Q-Rutin | Kaempferol Rutinoside | Quercetin Rutinoside Derivative | Isorhamnetin Rutinoside | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root | 2020 | T1 | ND | 0.74 | 3.40 ab | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| T2 | ND | 0.57 | 3.00 c | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| T3 | ND | 0.67 | 3.17 bc | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| T4 | ND | 0.57 | 3.58 a | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| p value | - | NS | ** | - | - | - | - | - | - | - | - | - | ||
| 2021 | T1 | ND | 0.88 a | 3.52 | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| T2 | ND | 0.69 ab | 3.38 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| T3 | ND | 0.51 ab | 3.55 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| T4 | ND | 0.39 b | 3.96 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ||
| p value | - | * | NS | - | - | - | - | - | - | - | - | - | ||
| Shoot | 2020 | T1 | 0 | ND | ND | 0.13 bc | 0.43 b | 0.12 b | 1.93 | 0.27 b | 9.70 b | 0.56 a | 0.20 a | 0.16 b |
| T2 | 0.05 | ND | ND | 0.08 c | 0.41 b | 0.10 b | 1.56 | 0.56 a | 8.95 c | 0.44 b | 0 b | 0.10 b | ||
| T3 | 0 | ND | ND | 0.22 a | 0.47 b | 0.12 b | 1.62 | 0.16 b | 9.48 bc | 0.53 ab | 0 b | 0.36 a | ||
| T4 | 0 | ND | ND | 0.16 ab | 0.78 a | 0.25 a | 1.90 | 0.55 a | 10.60 a | 0.63 a | 0.10 ab | 0.43 a | ||
| p value | * | - | - | ** | *** | ** | NS | *** | *** | * | * | *** | ||
| 2021 | T1 | 0.19 | ND | ND | ND | 0.13 | 0.46 ab | 1.04 a | ND | 5.51 a | 0.29 | ND | ND | |
| T2 | 0.20 | ND | ND | ND | 0.09 | 0.31 b | 0.78 b | ND | 4.68 b | 0.24 | ND | ND | ||
| T3 | 0.21 | ND | ND | ND | 0.16 | 0.61 a | 0.85 b | ND | 5.23 a | 0.24 | ND | ND | ||
| T4 | 0.20 | ND | ND | ND | 0.09 | 0.33 b | 0.71 b | ND | 4.75 b | 0.26 | ND | ND | ||
| p value | NS | - | - | - | NS | * | * | - | ** | NS | - | - |
| N | P | K | S | Ca | Mg | Fe | Mn | Zn | Cu | B | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root | 2020 | T1 | 99.12 a | 1.30 a | 17.37 b | 3.14 | 4.12 b | 1.85 c | 1016.76 | 38.81 | 23.39 | 8.19 | 3.86 |
| T2 | 97.10 a | 1.27 ab | 20.90 a | 3.39 | 6.10 a | 2.38 ab | 1611.04 | 44.57 | 23.84 | 8.35 | 4.19 | ||
| T3 | 73.81 b | 1.11 bc | 21.52 a | 3.37 | 5.49 ab | 1.70 c | 1495.32 | 43.64 | 21.54 | 8.01 | 3.49 | ||
| T4 | 67.17 b | 1.05 c | 21.05 a | 3.23 | 6.38 a | 2.48 a | 1486.81 | 45.91 | 22.99 | 7.43 | 4.28 | ||
| p value | *** | ** | * | NS | * | * | NS | NS | NS | NS | NS | ||
| 2021 | T1 | 107.70 bc | 1.33 | 20.08 | 4.23 | 7.01 b | 1.80 b | 219.61 b | 56.75 b | 27.71 | 8.16 | 27.25 ab | |
| T2 | 116.35 ab | 1.27 | 19.16 | 3.68 | 7.64 a | 2.00 ab | 277.20 a | 53.68 b | 24.30 | 7.96 | 27.14 ab | ||
| T3 | 128.14 a | 1.30 | 20.53 | 4.02 | 7.28 b | 2.21 a | 195.70 b | 55.84 b | 23.02 | 7.68 | 24.10 b | ||
| T4 | 92.89 c | 1.34 | 20.41 | 4.34 | 8.69 a | 2.25 a | 305.6 a | 73.92 a | 23.18 | 8.37 | 32.52 a | ||
| p value | ** | NS | NS | NS | * | * | *** | *** | NS | NS | * | ||
| Shoot | 2020 | T1 | 97.81 a | 1.15 a | 12.26 a | 2.97 | 5.29 | 1.69 | 2771.59 | 68.19 | 27.91 a | 8.83 | 4.97 |
| T2 | 95.64 a | 1.14 a | 12.36 a | 2.97 | 4.79 | 1.66 | 2545.04 | 64.22 | 26.32 a | 8.13 | 4.53 | ||
| T3 | 75.20 b | 1.08 ab | 12.45 a | 3.02 | 4.80 | 1.56 | 2482.70 | 62.51 | 25.67 ab | 8.74 | 4.62 | ||
| T4 | 65.97 b | 0.94 b | 10.38 b | 2.74 | 4.44 | 1.51 | 2481.12 | 63.21 | 22.36 b | 7.98 | 3.77 | ||
| p value | ** | * | *** | NS | NS | NS | NS | NS | * | NS | NS | ||
| 2021 | T1 | 109.26 a | 1.80 a | 14.87 | 4.13 a | 5.37 a | 0.79 b | 192.59 | 58.81 a | 31.79 a | 8.25 a | 36.33 a | |
| T2 | 115.89 a | 1.86 a | 14.70 | 3.40 b | 3.50 b | 1.04 a | 151.57 | 36.16 c | 29.37 a | 7.36 b | 19.67 c | ||
| T3 | 127.51 a | 1.76 ab | 14.15 | 4.18 a | 4.92 a | 1.04 a | 182.00 | 61.76 a | 25.25 b | 7.96 ab | 30.52 ab | ||
| T4 | 88.72 b | 1.49 b | 14.17 | 3.24 b | 4.69 a | 1.05 a | 160.73 | 48.31 b | 21.83 c | 7.23 b | 25.51 bc | ||
| p value | ** | * | NS | ** | *** | ** | NS | *** | *** | * | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Moreno, F.J.; Navarro-León, E.; de Cara, M.; Soriano, T.; Ruiz, J.M. Physiological Responses of Asparagus Plants to Soil Disinfection Strategies Targeting Asparagus Decline Syndrome. Plants 2025, 14, 1992. https://doi.org/10.3390/plants14131992
López-Moreno FJ, Navarro-León E, de Cara M, Soriano T, Ruiz JM. Physiological Responses of Asparagus Plants to Soil Disinfection Strategies Targeting Asparagus Decline Syndrome. Plants. 2025; 14(13):1992. https://doi.org/10.3390/plants14131992
Chicago/Turabian StyleLópez-Moreno, Francisco Javier, Eloy Navarro-León, Miguel de Cara, Teresa Soriano, and Juan Manuel Ruiz. 2025. "Physiological Responses of Asparagus Plants to Soil Disinfection Strategies Targeting Asparagus Decline Syndrome" Plants 14, no. 13: 1992. https://doi.org/10.3390/plants14131992
APA StyleLópez-Moreno, F. J., Navarro-León, E., de Cara, M., Soriano, T., & Ruiz, J. M. (2025). Physiological Responses of Asparagus Plants to Soil Disinfection Strategies Targeting Asparagus Decline Syndrome. Plants, 14(13), 1992. https://doi.org/10.3390/plants14131992









