Characterizing the Flowering Phenology of Rosa rugosa Thunb. as an Ecosystem Service in the Context of Climate Change in Kupinovo (Vojvodina), Serbia
Abstract
1. Introduction
2. Results
2.1. Weather Conditions
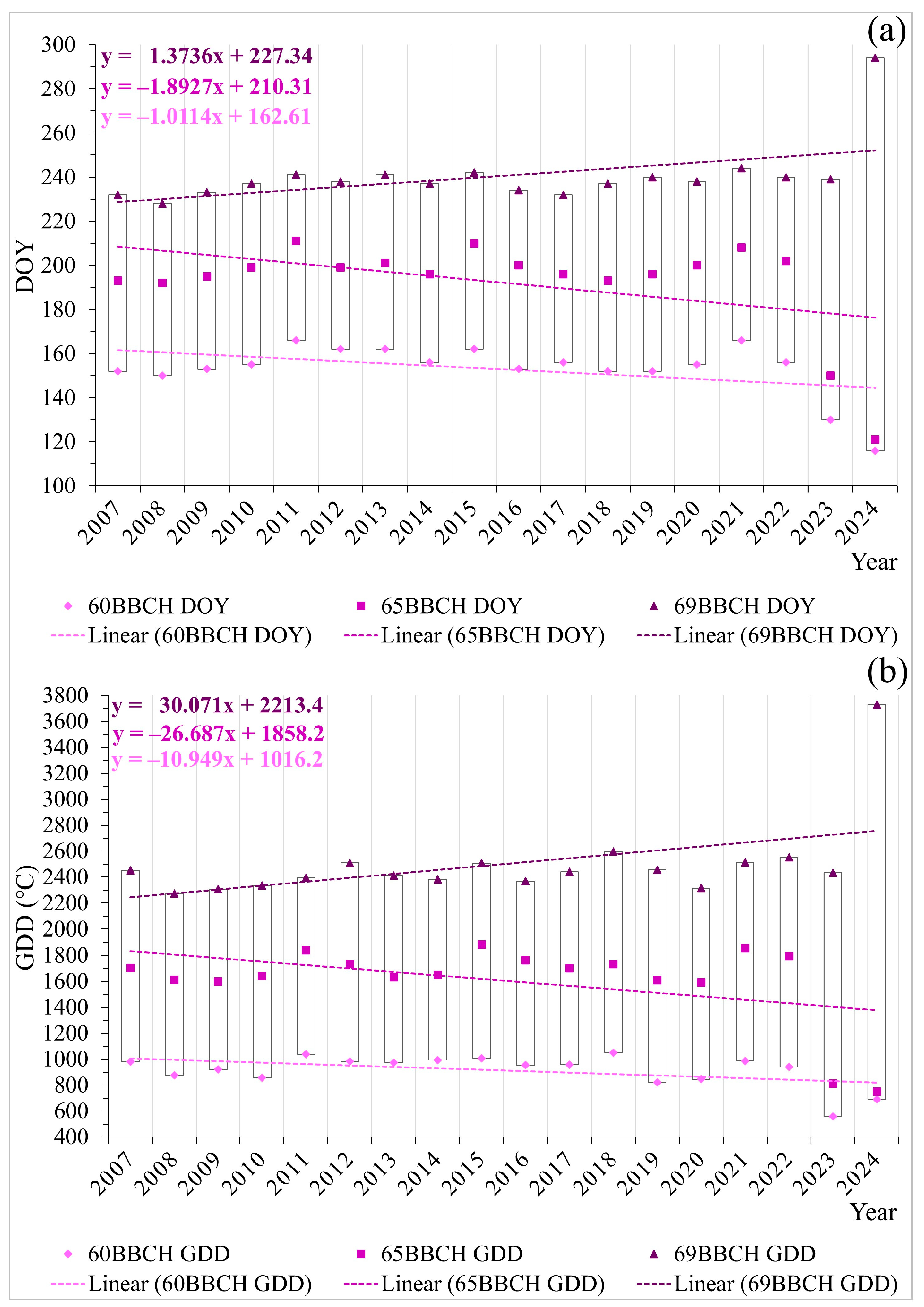
2.2. Phenological Patterns of Primary Flowering
2.3. Evaluation of Secondary Flowering
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Climatic Data
4.3. Phenological Data
4.4. Processing of Data
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Desertification Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Scholte, S.; van Teeffelen, A.; Verburg, P. Integrating socio-cultural perspectives into ecosystem service valuation: A review of concepts and methods. Ecol. Econ. 2016, 114, 67–78. [Google Scholar] [CrossRef]
- Vujičić, D.; Vasiljević, N.; Radić, B.; Tutundžić, A.; Galečić, N.; Skočajić, D.; Ocokoljić, M. Conceptualisation of the Regulatory Framework of Green Infrastructure for Urban Development: Identifying Barriers and Drivers. Land 2024, 13, 692. [Google Scholar] [CrossRef]
- IPBES. Izveštaj o Globalnoj Proceni Satnja Biodiverziteta i Ekosistemskih Usluga. Available online: https://www.undp.org/sites/g/files/zskgke326/files/migration/rs/a84b97e3e718730ea61523ae36a97edcd1067336c966cb398a505abe7f15f909.pdf (accessed on 19 April 2025).
- Eldridge, D.J.; Bowker, M.A.; Maestre, F.T.; Roger, E.; Reynolds, J.F.; Whitford, W.G. Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecol. Lett. 2011, 14, 709–722. [Google Scholar] [CrossRef]
- Moro, M.J.; Pugnaire, F.I.; Haase, P.; Puigdefábregas, J. Mechanisms of interaction between a leguminous shrub understorey in a semi-arid environment. Ecography 1997, 20, 175–184. [Google Scholar] [CrossRef]
- Blaser, W.J.; Shanungu, G.K.; Edwards, P.J.; Olde Venterink, H. Woody encroachment reduces nutrient limitation and promotes soil carbon sequestration. Ecol. Evol. 2014, 4, 1423–1438. [Google Scholar] [CrossRef]
- House, J.I.; Archer, S.R.; Breshears, D.D.; Scholes, R.J. Conundrums in mixed woody–herbaceous plant systems. J. Biogeogr. 2003, 10, 1763–1777. [Google Scholar] [CrossRef]
- Harp, D.; Hammond, G.; Zlesak, D.C.; Church, G.; Chamblee, M.; George, S. Flowering, drought and disease tolerance, and landscape performance of landscape roses grown under low-input conditions in north central Texas. HortTechnology 2019, 29, 234–240. [Google Scholar] [CrossRef]
- Ocokoljić, M.; Petrov, D.J. Decorative Dendrology [Dekorativna dendrologija]; Univerzitet u Beogradu-Šumarski Fakultet: Belgrade, Serbia, 2022; p. 409. (In Serbian) [Google Scholar]
- Krüssmann, G. Rosen Rosen Rosen Unser Wissen über die Rose; Verlag Paul Parey: Berlin/Hamburg, Germany, 1974. [Google Scholar]
- Smulders, M.J.M.; Arens, P.; Bourk, P.M.; Debener, T.; Linde, M.; Riek, J.; Leu, L.; Ruttink, T.; Baudino, S.; Saint-Oyant, L.H.; et al. In the name of the rose: A roadmap for rose research in the genome era. Hortic. Res. 2019, 6, 65. [Google Scholar] [CrossRef]
- Božanić Tanjga, B.; Ljubojević, M.; Đukić, A.; Vukosavljev, M.; Ilić, O.; Narandžić, T. Selection of garden roses to improve the ecosystem services they provide. Horticulturae 2022, 8, 883. [Google Scholar] [CrossRef]
- Market Research Report. Rose Oil Market Size, Share and Trends Analysis Report by Application (Fragrance and Cosmetics, Pharmaceuticals, Food and Beverages), by Product (Organic, Conventional), and Segment Forecasts, 2019–2025; Report ID:GVR-3-68038-655-4; Grand View Research: San Francisco, CA, USA, 2019; Available online: https://www.grandviewresearch.com/industry-analysis/rose-oil-market (accessed on 20 April 2025).
- Popek, R. Wild Roses of Poland—Róze Dziko Rosnące Polski; Wydawnictwo Plantpress: Kraków, Poland, 2002. (In Polish) [Google Scholar]
- Lukasová, V.; Vido, J.; Škvareninová, J.; Bičárová, S.; Hlavatá, H.; Borsányi, P.; Škvarenina, J. Autumn phenological response of European beech to summer drought and heat. Water 2020, 12, 2610. [Google Scholar] [CrossRef]
- Ocokoljić, M.; Petrov, D.J.; Galečić, N.; Skočajić, D.; Košanin, O.; Simović, I. Phenological Flowering Patterns of Woody Plants in the Function of Landscape Design: Case Study Belgrade. Land 2023, 12, 706. [Google Scholar] [CrossRef]
- Ocokoljić, M.; Petrov, D.J.; Galečić, N.; Skočajić, D.; Šišaković, N.; Simović, I. The study of Jasminum nudiflorum Lindl. in urban green infrastructure in conditions of climate change in Belgrade, Serbia. Appl. Ecol. Environ. Res. 2024, 22, 4779–4805. [Google Scholar] [CrossRef]
- Čukanović, J.; Ljubojević, M.; Djordjević, S.; Narandžić, T.; Petrov, D.J.; Ocokoljić, M. The Impact of Climate Variability on the Blooming of Fraxinus ornus ‘Globosa’ as a Component of Novi Sad’s (Serbia) Green Infrastructure. Sustainability 2024, 16, 8404. [Google Scholar] [CrossRef]
- Škvareninová, J.; Lukasová, V.; Borsányi, P.; Kvas, A.; Vido, J.; Štefková, J. The effect of climate change on spring frosts and flowering of Crataegus laevigata—The indicator of the validity of the weather lore about “The Ice Saints”. Ecol. Indic. 2022, 145, 109688. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Buican Stanciu, A.; Ionescu, M. The influence of temperature on phenology of ornamental woody species in urban environment. Horticulture 2020, 44, 61–67. Available online: https://horticulturejournal.usamv.ro/pdf/2020/issue_1/Art8.pdf (accessed on 10 April 2025).
- Inoue, T.; Nagai, S. Influence of temperature change on plant tourism in Japan: A case study of the flowering of Lycoris radiata (red spider lily). Jpn. J. Biometeorol. 2015, 52, 175–184. [Google Scholar]
- Petrov, D.J.; Ocokoljić, M.; Galečić, N.; Skočajić, D.; Simović, I. Adaptability of Prunus cerasifera Ehrh. to Climate Changes in Multifunctional Landscape. Atmosphere 2024, 15, 335. [Google Scholar] [CrossRef]
- Lalić, B.; Ejcinger, J.; Dalamarta, A.; Orlandini, S.; Firanj Sremac, A.; Paher, B. Meteorology and Climatology for Agronomists [Meteorologija i Klimatologija za Agronome]; Univerzitet u Novom Sadu-Poljoprivredni Fakultet: Novi Sad, Serbia, 2021; p. 219. (In Serbian) [Google Scholar]
- RHMZ. Available online: https://www.hidmet.gov.rs/ciril/meteorologija/klimatologija_godisnjaci.php (accessed on 10 April 2025).
- Silva, I.A.; Da Silva, D.M.; De Carvalho, G.H.; Batalha, M.A. Reproductive phenology of Brazilian savannas and riparian forests: Environmental and phylogenetic issues. Ann. For. Sci. 2011, 68, 1207–1215. [Google Scholar] [CrossRef]
- CaraDonna, P.J.; Inouye, D.W. Phenological responses to climate change do not exhibit phylogenetic signal in a subalpine plant community. Ecology 2015, 96, 355–361. [Google Scholar] [CrossRef]
- eFlora. “Flora of China” 2025. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=128746 (accessed on 14 April 2025).
- Hakam, N.; Khanizadeh, S.; DeEll, J.R.; Richer, C. Assessing Chilling Tolerance in Roses using Chlorophyll Fluorescence. HortScience 2000, 35, 184–186. [Google Scholar] [CrossRef]
- Bolmgren, K.; Eriksson, O.; Linder, H.P. Contrasting flowering phenology and species richness in abiotically and biotically pollinated angiosperms. Evolution 2003, 57, 2001–2011. [Google Scholar]
- Butkienė, Z.P. Biological and biochemical characteristics of the rugosa rose. [Биологическая и биохимическая характеристика шиповника морщинистого]. Liet. TSR Moksl. Akad. Darb. Ser. C 1971, 55, 101–108. (In Russian) [Google Scholar]
- Bruun, H.H. Biological Flora of the British Isles. No. 239. Rosa rugosa Thunb. ex Murray. J. Ecol. 2005, 93, 441–470. [Google Scholar]
- Gu, C.; Robertson, K.P. Rosa Linnaeus. Sp. Pl. 1: 491. 1753. Flora China 2003, 9, 339. Available online: http://flora.huh.harvard.edu/china/mss/volume09/Rosaceae.PDF (accessed on 14 April 2025).
- WMO. State of the Global Climate 2021: WMO Provisional Report. World Meteorological Organization (WMO); 47. 2021. Available online: https://library.wmo.int/doc_num.php?explnum_id=10859 (accessed on 14 April 2025).
- Baldzhieva, M.; Popova, M. Some features of phenological development in Rosa rugosa Thunb. Nauchni Trudove, Vissh Selskostopanski Institut “Vasil Kolarov” 1985, 28, 51–57. [Google Scholar]
- Jerzy, M.; Żyła, S.; Czekalski, M. Róże 110 Odmian; Państwowe Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 1992; p. 229. [Google Scholar]
- Wlodarczyk, Z.; Ziernicka-Wojtaszek, A.; Kedizor, R.; Mazur, J. Flowering phenology of shrub roses as a aensitive indicator of meteorological variability in Central Europe. J. Hortic. Res. 2023, 31, 189–204. [Google Scholar] [CrossRef]
- Imperial Household Agency. “Flower Calendar”. Available online: https://www.kunaicho.go.jp/e-event/hanadayori-cal.html (accessed on 14 April 2025).
- Dobson, H.E.M. Pollen and flower fragrances in pollination. Acta Hortic. 1991, 288, 313–320. [Google Scholar] [CrossRef]
- Fukuda, H.; Sakagami, S.F.; Yamauchi, K.; Matsumura, T. Biofaunistic survey of wild bees in Hama-Koshimizu, Eastern Hokkaido. Jpn. J. Ecol. 1973, 23, 160–170. [Google Scholar]
- Kupianskaya, A.N.; Lelej, A.S.; Urbain, B.K. The ants (Hymenoptera, Formicidae) of the Kuril Islands. Far East. Entomol. 2000, 92, 1–21. [Google Scholar]
- SPOS—Saveza Pčelarskih Organizacija Srbije. Available online: https://www.nin.rs/drustvo/vesti/73520/uginuce-pcela-u-srbiji (accessed on 13 April 2025).
- Markov, Z. Fauna Insekata Polinatora u Vojvodini: Diverzitet, Brojnost i Procena Vrednosti Ekosistemske Usluge Polinacije. Doktorska disertacija; Univerzitet u Novom Sadu, Prirodno-matematički fakultet: Novi Sad, Serbia, 2017; p. 240. [Google Scholar]
- Winfree, R.; Bartomeus, I.; Cariveau, D.P. Native pollinators in anthropogenic habitats. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 1–22. [Google Scholar] [CrossRef]
- Hart, R.; Salick, J.; Ranjitkar, S.; Xu, J.C. Herbarium specimens show contrasting phenological responses to Himalayan climate. Proc. Natl. Acad. Sci. USA 2014, 111, 10615–10619. [Google Scholar] [CrossRef]
- Ollerton, J.; Erenler, H.; Edwards, M.; Crockett, R. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 2014, 346, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef]
- Galal, T.M.; Majrashi, A.; Al-Yasi, H.M.; Farahat, E.A.; Eid, E.M.; Ali, E.F. Taif’s Rose (Rosa damascena Mill Var. Trigentipetala) Wastes Are a Potential Candidate for Heavy Metals Remediation from Agricultural Soil. Agriculture 2022, 12, 1319. [Google Scholar]
- Li, X. Study on Physiological and Accumulation Characteristics of Rosa Rugosa-Cv. to Copper, Cadmium and Lead Stress; Jilin Agricultural University: Changchun, China, 2017. [Google Scholar]
- Markićević, M. Protection and improvement of SRP Obedska bara. [Zaštita i unapređenje SRP Obedska bara]. Zb. Rad. Geogr. Fak. 2022, 50, 145–158. Available online: https://scindeks-clanci.ceon.rs/data/pdf/0351-465X/2003/0351-465X0350145M.pdf (accessed on 5 April 2025).
- Pravno Informacioni Sistem. Uredba Vlade o Izmeni Uredbe o Zaštiti Specijalnog Rezervata Prirode “Obedska bara”. 2008. Available online: https://otvorenavlada.rs/uredba-obedska-bara0013-lat-doc/ (accessed on 7 April 2025).
- Biswal, B.K.; Bolan, N.; Zhu, Y.G.; Balasubramanian, R. Nature-based Systems (NbS) for mitigation of stormwater and air pollution in urban areas: A review. Resour. Conserv. Recycl. 2022, 186, 106578. [Google Scholar] [CrossRef]
- JP “Vojvodinašume”. Plan Upravljanja SRP “Obedska bara” 2021–2030. Available online: https://www.vojvodinasume.rs/wp-content/uploads/2022/03/Plan-upravljanja-OB_2021-2030_medium-compressed.pdf (accessed on 14 April 2025).
- Tabaei-aghdaei, S.R.; and Rezaee, M.B. Study of Flower Yield Variation in Rosa damascena L. Genotypes of Kashan. Iran. Rangel. For. Plant Breed. Genet. Res. 2003, 9, 99–110. [Google Scholar]
- Ma, Y.; Lin, X.; Liu, R.F.; Wu, L.L.; Li, J.A. Analysis of Plant Growth and Flower Aromatic Composition in Chinese Rosa rugosa Cultivars Under Cadmium Stress. Horticulturae 2025, 11, 214. [Google Scholar] [CrossRef]
- Maraš, Ž.; Budovlačev Papić, S.; Nikolić Mirković, M.; Đuričić, P.; Pavlović, M.; Filipović, D.; Milutinović, B.; Vidaković, I.; Cvejić, J.; Dimovski, K.; et al. Spatial Plan of the Municipality of Pećinci. [Prostorni Plan Opštine Pećinci]; JUP “Plan”: Ruma, Serbia; JP “Zavod za urbanizam Vojvodine”: Novi Sad, Serbia, 2006; Available online: https://www.ruma.rs/portal2/jupgrade/dokumenta/PROSTORNI%20PLAN%20OPSTINE%20RUMA.pdf (accessed on 5 April 2025).
- Bosnić, D. Stari Gradovi Srbije; Prometej: Novi Sad, Serbia, 2022; p. 25. [Google Scholar]
- Pivac, T. Vinski Turizam Vojvodine; Univerzitet u Novom Sadu—Prirodno-matematički fakultet: Novi Sad, Serbia, 2012; 192p. [Google Scholar]
- Bukurov, B. Geomorfološki prikaz Vojvodine. Zb. Matice Srp. 1953, 4, 100–134. [Google Scholar]
- Bogdanović, Ž. Hidrološki Problem Srema. Ph.D. Thesis, University of Novi Sad, Institute for Geography, Novi Sad, Serbia, 1982. [Google Scholar]
- Ivanišević, P.; Knežević, M. Tipovi Šuma i Šumskog Zemljišta na Području Ravnog Srema. Monografija: “250 Godina Šumarstva Ravnog Srema”; JP ”Vojvodina šume”: Petrovaradin, Serbia, 2008; pp. 87–118. [Google Scholar]
- Koch, E.; Bruns, E.; Chmielewski, F.M.; Defila, C.; Lipa, W.; Menzel, A. Guidelines for Plant Phenological Observations. Available online: https://www.researchgate.net/publication/266211199 (accessed on 1 January 2007).
- Meier, U. (Ed.) BBCH-Monograph. Growth Stages of Plants. Entwicklungsstadien von Pflanzen. Estadios de las Plantas. Stades de Développement des Plantes; Blackwell Wissenschafts-Verlag: Berlin, Germany, 1997; 622p. [Google Scholar]
- Buttler, K.P.; Schmid, W. (Eds.) Anleitung für die Phänologischen Beobachter des Deutschen Wetterdienstes, 3rd ed.; Deutscher Wetterdienst: Offenbach am Main, Germany, 1991. [Google Scholar]
- Stilinović, S. Seed Production of Forest and Ornamental Trees and Shrubs. [Semenarstvo Šumskog i Ukrasnog Drveća i Žbunja]; University of Belgrade—Faculty of Forestry: Belgrade, Serbia, 1985; 399p. (In Serbian) [Google Scholar]
- Hirsch, R.; Slack, J. Non-Parametric Trend Test for Seasonal Data with Serial Dependence. Water Resour. Res. 1984, 20, 727–732. [Google Scholar] [CrossRef]
- Gilbert, R.O. Statistical Methods for Environmental Pollution Monitoring; Van Nostrand Reinhold: New York, NY, USA, 1987. [Google Scholar]
- Horvat, J.; Mijoč, J. Osnove Statistike; Naklada Ljevak d.o.o.: Zagreb, Croatia, 2012; p. 482. [Google Scholar]


| Mean air temperatures (°C) | |||||||||||||
| Months Period | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| 1991/2020 | 1.0 | 3.0 | 7.5 | 12.9 | 17.6 | 21.4 | 23.2 | 23.2 | 18.0 | 12.8 | 7.4 | 2.2 | 12.5 |
| 2007/2023 | 2.0 | 4.2 | 8.1 | 13.3 | 17.8 | 22.1 | 24.2 | 24.0 | 18.8 | 13.2 | 8.3 | 3.4 | 13.3 |
| 2024 | 2.9 | 10.1 | 11.6 | 15.7 | 18.6 | 24.7 | 26.8 | 27.4 | 19.9 | 14.4 | 5.7 | 3.2 | 15.1 |
| Deviation of 2024 from the norm 1991–2020 | 1.8 | 7.1 | 4.2 | 2.8 | 1.0 | 3.3 | 3.6 | 4.2 | 1.9 | 1.6 | −1.7 | 1.1 | 2.6 |
| Deviation of 2024 from the norm 2007–2023 | 0.9 | 5.9 | 3.6 | 2.4 | 0.9 | 2.6 | 2.6 | 3.4 | 1.1 | 1.2 | −2.5 | −0.2 | 1.8 |
| Mean maximum air temperatures (°C) | |||||||||||||
| 1991/2020 | 4.5 | 7.4 | 12.9 | 18.4 | 23.2 | 26.9 | 29.0 | 29.3 | 24.1 | 18.5 | 11.9 | 5.5 | 17.6 |
| 2007/2023 | 5.5 | 8.6 | 13.3 | 18.9 | 23.1 | 27.4 | 29.9 | 30.1 | 24.8 | 18.8 | 12.8 | 6.8 | 18.3 |
| 2024 | 7.1 | 15.5 | 17.2 | 21.6 | 23.7 | 29.9 | 32.2 | 33.6 | 25.9 | 20.3 | 10.4 | 5.9 | 20.3 |
| Deviation of 2024 from the norm 1991–2020 | 2.5 | 8.1 | 4.4 | 3.1 | 0.6 | 3.0 | 3.2 | 4.3 | 1.8 | 1.8 | −1.5 | 0.4 | 2.6 |
| Deviation of 2024 from the norm 2007–2023 | 1.6 | 6.9 | 4.0 | 2.7 | 0.6 | 2.5 | 2.4 | 3.5 | 1.1 | 1.5 | −2.4 | −0.9 | 1.9 |
| Mean minimum air temperatures (°C) | |||||||||||||
| 1991/2020 | −2.3 | −1.0 | 2.6 | 7.1 | 11.8 | 15.4 | 16.8 | 16.9 | 12.6 | 7.9 | 3.6 | −0.9 | 7.5 |
| 2007/2023 | −1.4 | 0.1 | 3.2 | 7.3 | 12.1 | 16.2 | 17.7 | 17.5 | 13.2 | 8.4 | 4.4 | 0.3 | 8.2 |
| 2024 | −0.8 | 5.2 | 6.3 | 9.3 | 13.6 | 18.4 | 20.1 | 19.8 | 14.1 | 9.3 | 1.4 | 0.7 | 9.8 |
| Deviation of 2024 from the norm 1991–2020 | 1.5 | 6.2 | 3.7 | 2.2 | 1.8 | 3.0 | 3.3 | 2.9 | 1.6 | 1.3 | −2.2 | 1.6 | 2.2 |
| Deviation of 2024 from the norm 2007–2023 | 0.6 | 5.1 | 3.1 | 2.1 | 1.5 | 2.2 | 2.4 | 2.3 | 0.9 | 0.9 | −3.0 | 0.4 | 1.5 |
| Sums and mean amounts of relative humidity (%) | |||||||||||||
| Months Period | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ∑ |
| 1991/2020 | 85.0 | 78.0 | 68.8 | 64.9 | 66.2 | 66.4 | 63.1 | 62.5 | 68.8 | 74.6 | 79.9 | 85.3 | 72.0 |
| 2007/2023 | 82.9 | 75.1 | 66.1 | 61.6 | 65.4 | 64.5 | 58.6 | 58.9 | 65.1 | 72.2 | 78.4 | 82.8 | 69.3 |
| 2024 | 77.9 | 63.0 | 62.4 | 56.1 | 65.8 | 60.8 | 54.3 | 47.3 | 61.4 | 68.9 | 78.5 | 86.4 | 65.2 |
| Deviation of 2024 from the norm 1991–2020 | −7.1 | −15.0 | −6.3 | −8.8 | −0.4 | −5.6 | −8.8 | −15.2 | −7.4 | −5.7 | −1.5 | 1.1 | −6.7 |
| Sums and mean amounts of precipitation (mm) | |||||||||||||
| 1991/2020 | 42.4 | 34.0 | 41.7 | 47.4 | 68.1 | 80.1 | 58.2 | 54.0 | 56.0 | 50.7 | 45.5 | 48.3 | 626.4 |
| 2007/2023 | 46.5 | 39.1 | 47.3 | 41.5 | 82.1 | 75.4 | 50.6 | 46.2 | 52.0 | 48.1 | 51.1 | 48.9 | 628.9 |
| 2024 | 37.1 | 4.8 | 27.7 | 23.3 | 99.8 | 92.5 | 69.4 | 6.5 | 86.8 | 44.0 | 45.6 | 63.2 | 600.7 |
| Deviation (%) of 2024 from the norm 1991–2020 | −12.5 | −85.9 | −33.6 | −50.9 | +46.5 | +15.4 | +19.2 | −88.0 | +55.0 | −13.2 | +0.2 | +30.8 | −4.1 |
| Deviation (%) of 2024 from the norm 2007–2023 | −20.2 | −87.7 | −41.5 | −43.8 | +21.5 | +22.7 | +37.2 | −85.9 | +66.9 | −8.5 | −10.8 | +29.2 | −4.5 |
| Number and mean number of days with precipitation ≥0.1 mm | |||||||||||||
| 1991/2020 | 12.4 | 11.0 | 10.5 | 11.6 | 13.0 | 11.6 | 9.6 | 8.0 | 9.3 | 10.0 | 10.2 | 12.7 | 129.9 |
| 2007/2023 | 14.5 | 12.2 | 11.2 | 11.9 | 14.1 | 11.2 | 8.4 | 7.9 | 9.5 | 9.9 | 10.9 | 13.5 | 135.3 |
| 2024 | 15 | 10 | 15 | 7 | 17 | 14 | 5 | 5 | 14 | 7 | 18 | 19 | 135 |
| Deviation of 2024 from the norm 1991–2020 | 2.6 | −1.0 | 4.5 | −4.6 | 4.0 | 2.4 | −4.6 | −3.0 | 4.7 | −3.0 | −2.2 | 5.3 | 5.1 |
| Deviation of 2024 from the norm 2007–2023 | 0.5 | −2.2 | 3.8 | −4.9 | 2.9 | 2.8 | −3.4 | −2.9 | 4.5 | −2.9 | −2.9 | 4.5 | −0.3 |
| Sums and mean duration of sunshine (h) | |||||||||||||
| 1991/2020 | 78.6 | 106.9 | 163.0 | 200.0 | 240.7 | 272.0 | 298.7 | 281.3 | 206.4 | 166.1 | 102.3 | 67.0 | 2183.1 |
| 2007/2023 | 86.7 | 103.9 | 171.1 | 217.2 | 240.2 | 276.7 | 323.0 | 298.1 | 213.1 | 171.2 | 108.7 | 72.7 | 2282.6 |
| 2024 | 101.9 | 133.2 | 166.3 | 253.3 | 244.6 | 281.6 | 341.6 | 318.8 | 196.7 | 200.2 | 117.0 | 79.7 | 2434.9 |
| Deviation of 2024 from the norm 1991–2020 | 23.3 | 26.3 | 3.3 | 53.3 | 3.9 | 9.6 | 42.9 | 37.5 | −9.7 | 34.1 | 14.7 | 12.7 | 251.8 |
| Deviation of 2024 from the norm 2007–2023 | 15.2 | 29.3 | −4.8 | 36.1 | 4.4 | 4.9 | 18.6 | 20.7 | −16.4 | 29.0 | 8.3 | 7.0 | 152.3 |
| Number and mean number of summer days (Tmax ≥ 25 °C) | |||||||||||||
| 1991/2020 | 0.0 | 0.0 | 0.2 | 2.5 | 12.0 | 20.1 | 25.7 | 26.2 | 13.7 | 4.1 | 0.1 | 0.0 | 104.7 |
| 2007/2023 | 0.0 | 0.0 | 0.2 | 2.8 | 11.4 | 21.0 | 27.8 | 27.0 | 15.8 | 4.8 | 0.1 | 0.0 | 110.9 |
| 2024 | 0 | 0 | 2 | 13 | 14 | 25 | 28 | 31 | 17 | 3 | 0 | 0 | 133 |
| Deviation of 2024 from the norm 1991–2020 | 0.0 | 0.0 | 1.8 | 10.5 | 2.0 | 4.9 | 2.3 | 4.8 | 3.3 | −1.1 | −0.1 | 0.0 | 28.3 |
| Deviation of 2024 from the norm 2007–2023 | 0.0 | 0.0 | 1.8 | 10.2 | 2.6 | 4.0 | 0.2 | 4.0 | 1.2 | −1.8 | −0.1 | 0.0 | 22.1 |
| Number and mean number of tropical days (Tmax ≥ 30 °C) | |||||||||||||
| 1991/2020 | 0.0 | 0.0 | 0.0 | 0.1 | 1.8 | 8.5 | 12.7 | 14.2 | 3.2 | 0.1 | 0.0 | 0.0 | 40.7 |
| 2007/2023 | 0.0 | 0.0 | 0.0 | 0.2 | 1.5 | 9.4 | 14.4 | 16.9 | 4.6 | 0.2 | 0.0 | 0.0 | 47.1 |
| 2024 | 0 | 0 | 0 | 1 | 0 | 15 | 22 | 28 | 10 | 0 | 0 | 0 | 76 |
| Deviation of 2024 from the norm 1991–2020 | 0.0 | 0.0 | 0.0 | 0.9 | −1.8 | 6.5 | 9.3 | 13.8 | 6.8 | −0.1 | 0.0 | 0.0 | 35.3 |
| Deviation of 2024 from the norm 2007–2023 | 0.0 | 0.0 | 0.0 | 0.8 | −1.5 | 5.6 | 7.6 | 11.1 | 5.4 | −0.2 | 0.0 | 0.0 | 28.9 |
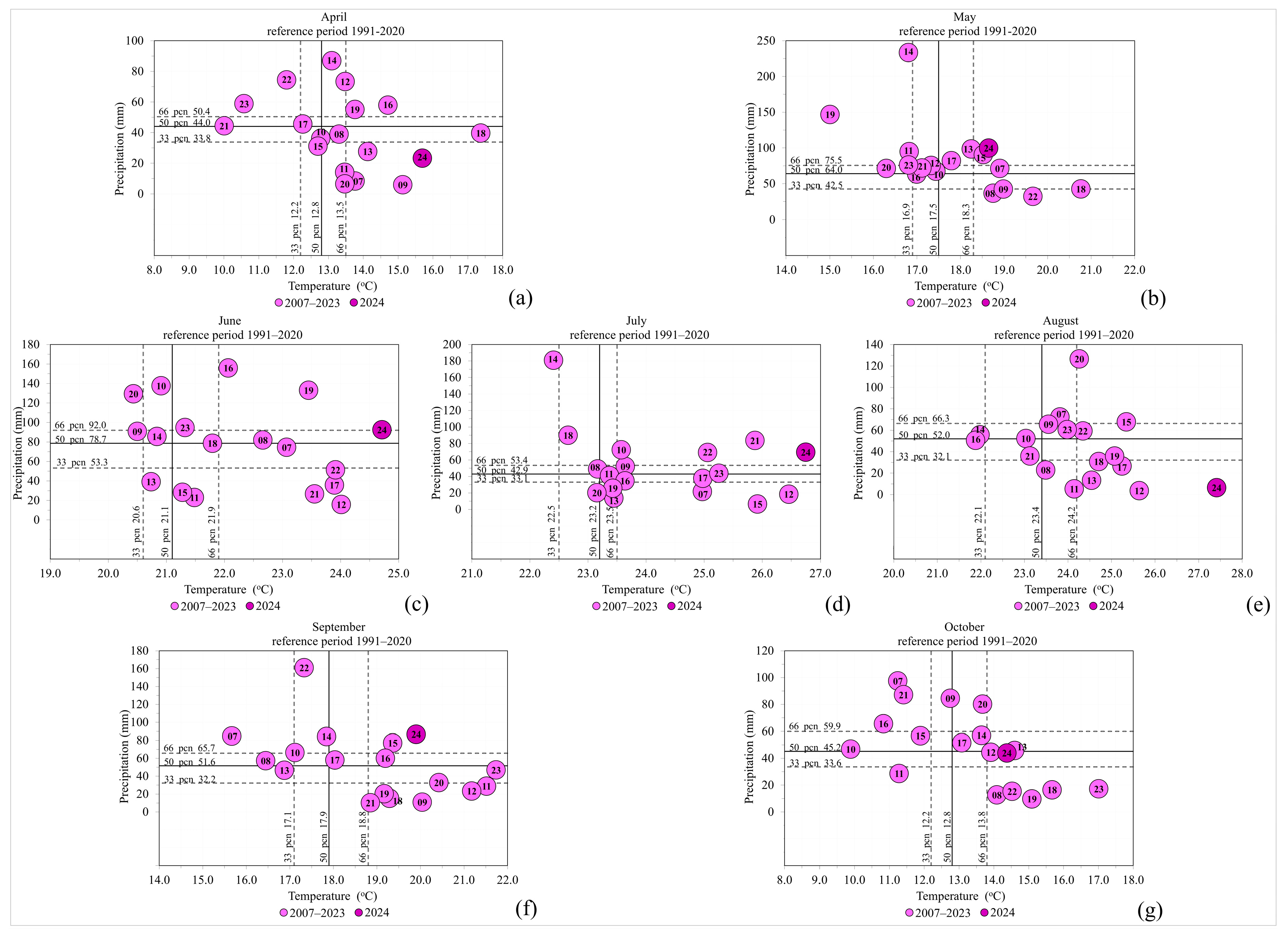
| Tmean (°C) | Perc. Cat. * | Tmean (°C) 1991–2020 | 1991–2020 | 1991–2020 | 1991–2020 | Terciles ** |
|---|---|---|---|---|---|---|
| 1991–2020 | 33.-Perc. | 50.-Perc. | 66.-Perc. | Cat. | ||
| April | ||||||
| 15.7 | VW | 12.9 | 12.2 | 12.8 | 13.5 | 1 |
| May | ||||||
| 18.6 | N | 17.6 | 16.9 | 17.5 | 18.3 | 1 |
| Jun | ||||||
| 24.7 | EW | 21.4 | 20.6 | 21.1 | 21.9 | 1 |
| July | ||||||
| 26.8 | EW | 23.2 | 22.5 | 23.2 | 23.5 | 1 |
| August | ||||||
| 27.4 | EW | 23.2 | 22.1 | 23.4 | 24.2 | 1 |
| September | ||||||
| 19.9 | W | 18.0 | 17.1 | 17.9 | 18.8 | 1 |
| October | ||||||
| 14.4 | W | 12.8 | 12.2 | 12.8 | 13.8 | 1 |
| Sum (mm) | Perc. Cat. * | Sum (mm) 1991–2020 | 1991–2020 | 1991–2020 | 1991–2020 | Terciles ** |
|---|---|---|---|---|---|---|
| 1991–2020 | 33.-Perc. | 50.-Perc. | 66.-Perc. | Cat. | ||
| April | ||||||
| 23.3 | D | 47.4 | 33.8 | 44.0 | 50.4 | −1 |
| May | ||||||
| 99.8 | W | 68.1 | 42.5 | 64.0 | 75.5 | 1 |
| Jun | ||||||
| 92.5 | N | 80.1 | 53.3 | 78.7 | 92.0 | 1 |
| July | ||||||
| 69.4 | W | 58.2 | 33.1 | 42.9 | 53.4 | 1 |
| August | ||||||
| 6.5 | D | 54.0 | 32.1 | 52.0 | 66.3 | −1 |
| September | ||||||
| 86.8 | W | 56.0 | 32.2 | 51.6 | 65.7 | 1 |
| October | ||||||
| 44.0 | N | 50.7 | 33.6 | 45.2 | 59.9 | 0 |
| Parameter\Test | Kendall’s Tau | p-Value * | Sen’s Slope ** | Sen’s Slope Intercept |
|---|---|---|---|---|
| 60BBCH DOY | −0.116 | 0.540 | −0.167 | 490.833 |
| 65BBCH DOY | 0.007 | 1.000 | 0.000 | 197.500 |
| 69BBCH DOY | 0.442 | 0.013 | 0.533 | −837.766 |
| 60BBCH GDD | −0.216 | 0.225 | −5.288 | 11,615.088 |
| 65BBCH GDD | −0.098 | 0.596 | −3.607 | 8928.316 |
| 69BBCH GDD | 0.425 | 0.015 | 14.198 | −26,187.576 |
| Parameter | 60BBCH DOY | 65BBCH DOY | 69BBCH DOY | 60BBCH GDD | 65BBCH GDD | 69BBCH GDD |
|---|---|---|---|---|---|---|
| 60BBCH DOY | 0.056 | 0.154 | 0.005 | 0.002 | 0.823 | |
| 65BBCH DOY | 0.87944 | 0.070 | 0.052 | 0.002 | 0.974 | |
| 69BBCH DOY | 0.35092 | 0.43942 | 0.862 | 0.534 | 0.019 | |
| 60BBCH GDD | 0.63756 | 0.46815 | 0.04456 | 0.087 | 0.335 | |
| 65BBCH GDD | 0.69373 | 0.7043 | 0.15648 | 0.79154 | 0.197 | |
| 69BBCH GDD | 0.057204 | 0.0093217 | 0.5513 | 0.24045 | 0.31889 |
| Parameters | 60BBCH DOY | 65BBCH DOY | 69BBCH DOY | 60BBCH GDD | 65BBCH GDD | 69BBCH GDD |
|---|---|---|---|---|---|---|
| Min | 116 | 121 | 228 | 559.6 | 748.8 | 2273.3 |
| Max | 166 | 211 | 294 | 1049.2 | 1883.7 | 3729.5 |
| Sum | 2754 | 3462 | 4327 | 16,419.4 | 28,884.2 | 44,982.5 |
| Mean | 153 | 192.3333 | 240.3889 | 912.1889 | 1604.678 | 2499.028 |
| Std. error | 2.873135 | 5.17975 | 3.297988 | 29.37805 | 73.89117 | 75.31192 |
| Variance | 148.5882 | 482.942 | 195.781 | 15,535.25 | 98,278.28 | 102,093.9 |
| Stand. dev | 12.18968 | 21.9752 | 13.99218 | 124.6405 | 313.4937 | 319.5214 |
| Median | 155 | 197.5 | 238 | 954.75 | 1674.9 | 2437.3 |
| 25 prcntil | 152 | 193 | 233.75 | 852.975 | 1606.45 | 2360.025 |
| 75 prcntil | 162 | 201.25 | 241 | 986.85 | 1769.55 | 2511.2 |
| Skewness | −2.05238 | −2.6173 | 3.629506 | −1.66862 | −2.27627 | 3.709789 |
| Kurtosis | 4.85839 | 6.9570 | 14.53749 | 2.930922 | 4.612754 | 14.87591 |
| Geom. mean | 152.4873 | 190.8626 | 240.0456 | 902.6261 | 1563.187 | 2483.73 |
| Coeff. var | 7.96719 | 11.42596 | 5.820643 | 13.66389 | 19.53624 | 12.78583 |
| Parameters | № days 60BBCH–65BBCH | Tmean 60BBCH–65BBCH | № Days 65BBCH–69BBCH | Tmean 60BBCH–65BBCH | № Days 60BBCH–69BBCH | Tmean 60BBCH–69BBCH |
|---|---|---|---|---|---|---|
| N | 18 | 18 | 18 | 18 | 18 | 18 |
| Min | 5 | 16.1 | 31 | 22.4 | 76 | 22.1 |
| Max | 48 | 26.3 | 174 | 25.8 | 179 | 25.9 |
| Sum | 708 | 401.8 | 883 | 434.5 | 1591 | 423 |
| Mean | 39.333 | 22.322 | 49.055 | 24.139 | 88.389 | 23.5 |
| Std. error | 2.473 | 0.602 | 7.925 | 0.228112 | 5.6152 | 0.258 |
| Variance | 110.118 | 6.526 | 1130.644 | 0.937 | 567.546 | 1.196 |
| Stand. dev | 10.494 | 2.555 | 33.625 | 0.968 | 23.823 | 1.094 |
| Median | 42 | 22.8 | 39 | 24.1 | 81.5 | 23.3 |
| 25 prcntil | 39.75 | 21.375 | 37 | 23.4 | 79 | 22.6 |
| 75 prcntil | 45 | 23.75 | 42.75 | 25.025 | 85.25 | 24.275 |
| Skewness | −2.616 | −1.078 | 3.472 | −0.111 | 3.646 | 0.652 |
| Kurtosis | 7.057 | 1.802 | 12.574 | −0.695 | 14.010 | −0.346 |
| Geom. mean | 36.253 | 22.170 | 43.899 | 24.120 | 86.388 | 23.476 |
| Coeff. var | 26.679 | 11.445 | 68.545 | 4.009 | 26.953 | 4.655 |
| Parameter\Test | Kendall’s Tau | p-Value * | Sen’s Slope ** | Sen’s Slope Intercept |
|---|---|---|---|---|
| № days 60BBCH–65BBCH | −0.027 | 0.909 | 0.000 | 42.000 |
| Tmean 60BBCH–65BBCH | −0.124 | 0.495 | −0.097 | 216.988 |
| № days 65BBCH–69BBCH | 0.312 | 0.085 | 0.667 | −631.000 |
| Tmean 60BBCH–65BBCH | −0.124 | 0.495 | −0.037 | 99.676 |
| № days 60BBCH–69BBCH | 0.473 | 0.008 | 0.600 | −723.600 |
| Tmean 60BBCH–69BBCH | −0.059 | 0.762 | −0.022 | 67.085 |
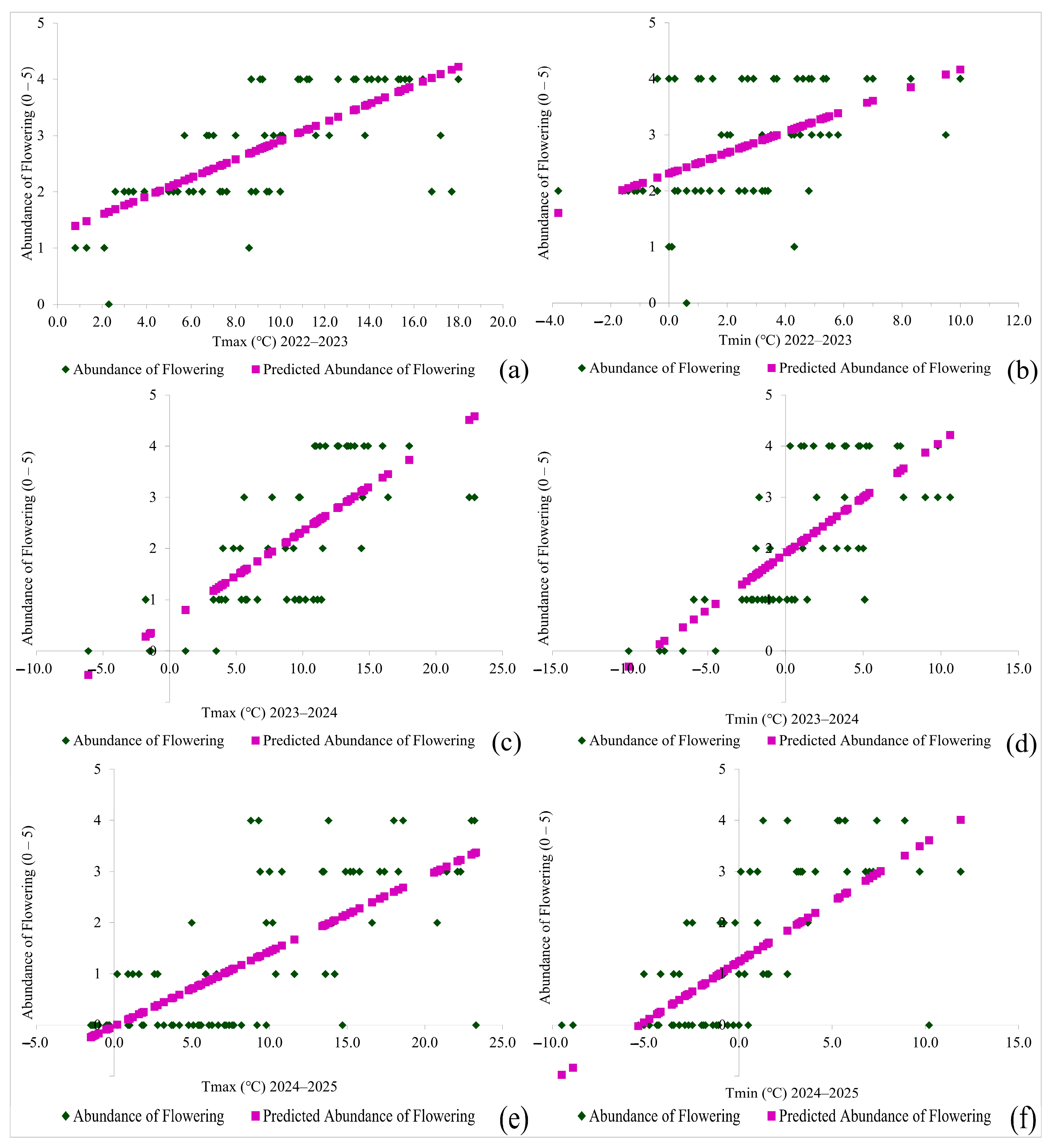
| Parameter | df | SS | MS | F | Significance F |
|---|---|---|---|---|---|
| 2022–2023 | |||||
| Tmax | |||||
| Regression | 1 | 38.1134 | 38.1134 | 73.431136 | 0.0000 |
| Residual | 65 | 33.73734 | 0.519036 | ||
| Total | 66 | 71.85075 | |||
| Tmin | |||||
| Regression | 1 | 16.44495 | 16.44495 | 19.292604 | 0.0000 |
| Residual | 65 | 55.40579 | 0.852397 | ||
| Total | 66 | 71.85075 | |||
| 2023–2024 | |||||
| Tmax | |||||
| Regression | 1 | 55.09212 | 55.09212 | 58.95538 | 0.0000 |
| Residual | 54 | 50.46146 | 0.934471 | ||
| Total | 55 | 105.5536 | |||
| Tmin | |||||
| Regression | 1 | 56.25659 | 56.25659 | 61.62356 | 0.0000 |
| Residual | 54 | 49.29698 | 0.912907 | ||
| Total | 55 | 105.5536 | |||
| 2024–2025 | |||||
| Tmax | |||||
| Regression | 1 | 89.75429 | 89.75429 | 88.8716 | 0.0000 |
| Residual | 80 | 80.79449 | 1.009931 | ||
| Total | 81 | 170.5488 | |||
| Tmin | |||||
| Regression | 1 | 87.56294 | 87.56294 | 84.41241 | 0.0000 |
| Residual | 80 | 82.98584 | 1.037323 | ||
| Total | 81 | 170.5488 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ljubojević, M.; Čukanović, J.; Đorđević, S.; Petrov, D.; Galečić, N.; Skočajić, D.; Ocokoljić, M. Characterizing the Flowering Phenology of Rosa rugosa Thunb. as an Ecosystem Service in the Context of Climate Change in Kupinovo (Vojvodina), Serbia. Plants 2025, 14, 1875. https://doi.org/10.3390/plants14121875
Ljubojević M, Čukanović J, Đorđević S, Petrov D, Galečić N, Skočajić D, Ocokoljić M. Characterizing the Flowering Phenology of Rosa rugosa Thunb. as an Ecosystem Service in the Context of Climate Change in Kupinovo (Vojvodina), Serbia. Plants. 2025; 14(12):1875. https://doi.org/10.3390/plants14121875
Chicago/Turabian StyleLjubojević, Mirjana, Jelena Čukanović, Sara Đorđević, Djurdja Petrov, Nevenka Galečić, Dejan Skočajić, and Mirjana Ocokoljić. 2025. "Characterizing the Flowering Phenology of Rosa rugosa Thunb. as an Ecosystem Service in the Context of Climate Change in Kupinovo (Vojvodina), Serbia" Plants 14, no. 12: 1875. https://doi.org/10.3390/plants14121875
APA StyleLjubojević, M., Čukanović, J., Đorđević, S., Petrov, D., Galečić, N., Skočajić, D., & Ocokoljić, M. (2025). Characterizing the Flowering Phenology of Rosa rugosa Thunb. as an Ecosystem Service in the Context of Climate Change in Kupinovo (Vojvodina), Serbia. Plants, 14(12), 1875. https://doi.org/10.3390/plants14121875










