Evaluating Spherical Trees in the Urban Environment in Budapest (Hungary)
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Evaluated Taxa with a Spherical Crown
2.3. Data Origin and Processing
2.4. The Tree Inspection and Assessment Methods
2.5. Statistical Analyses
3. Results
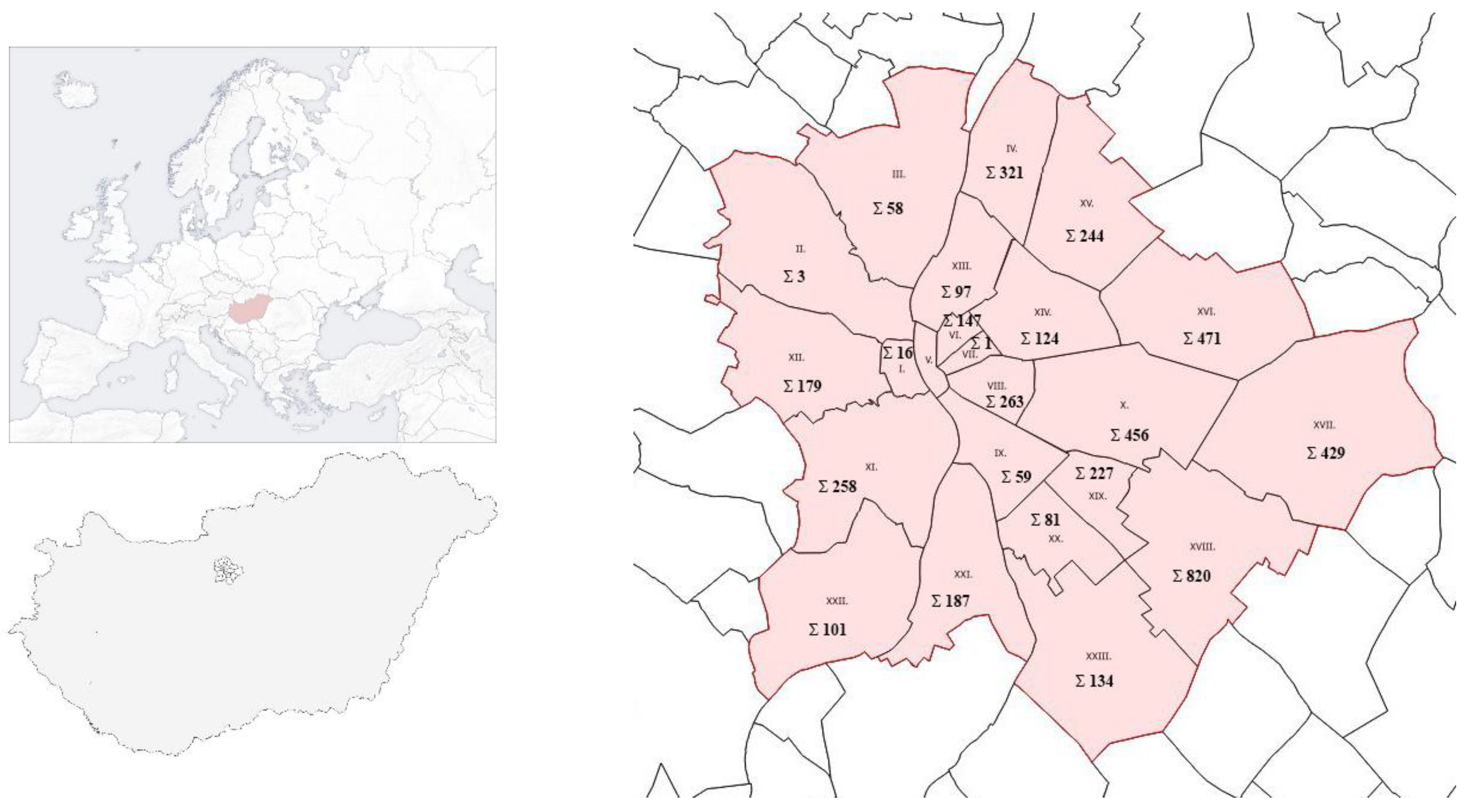
3.1. Taxon Abundance, District Distribution, and Site Character
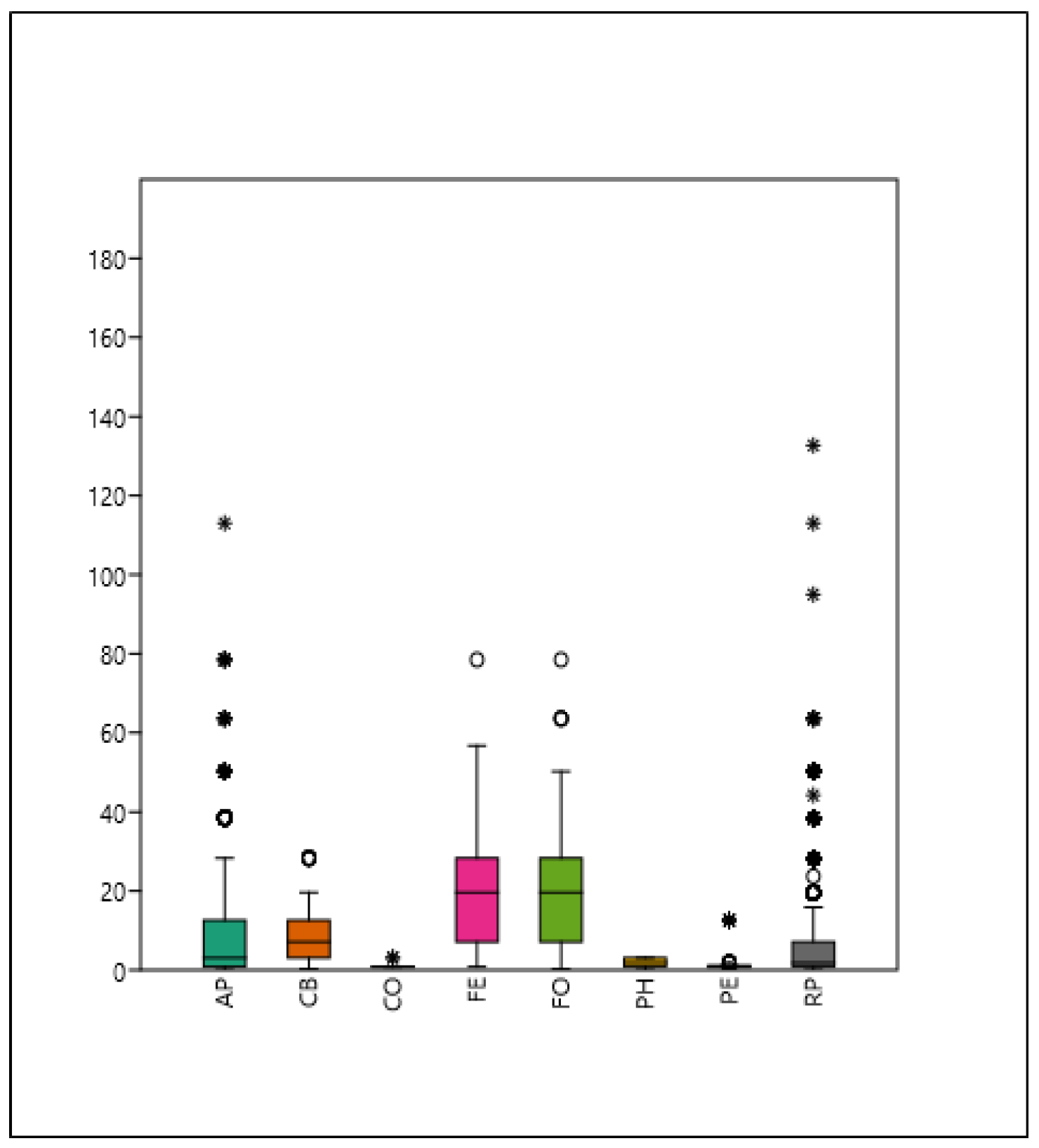
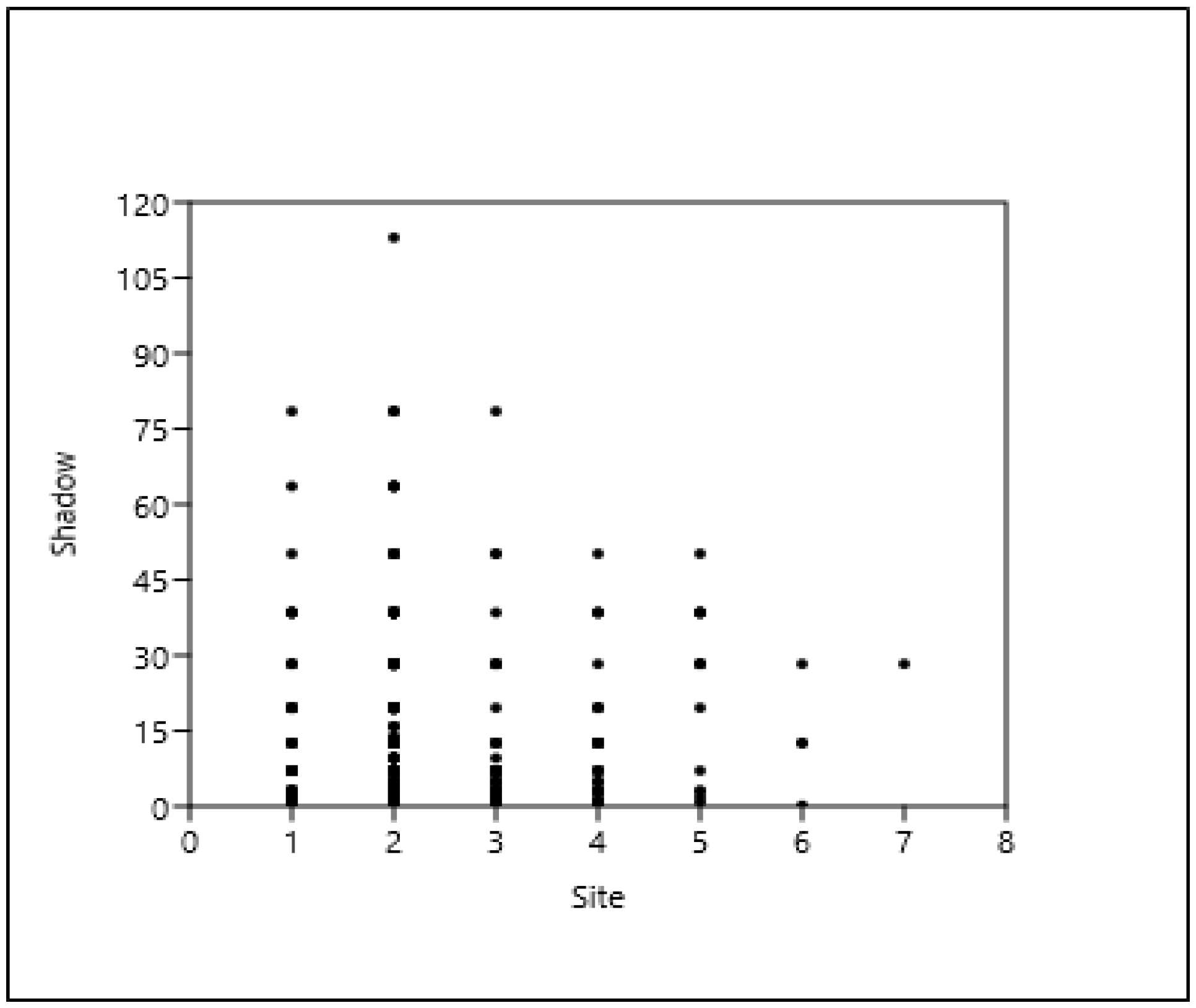
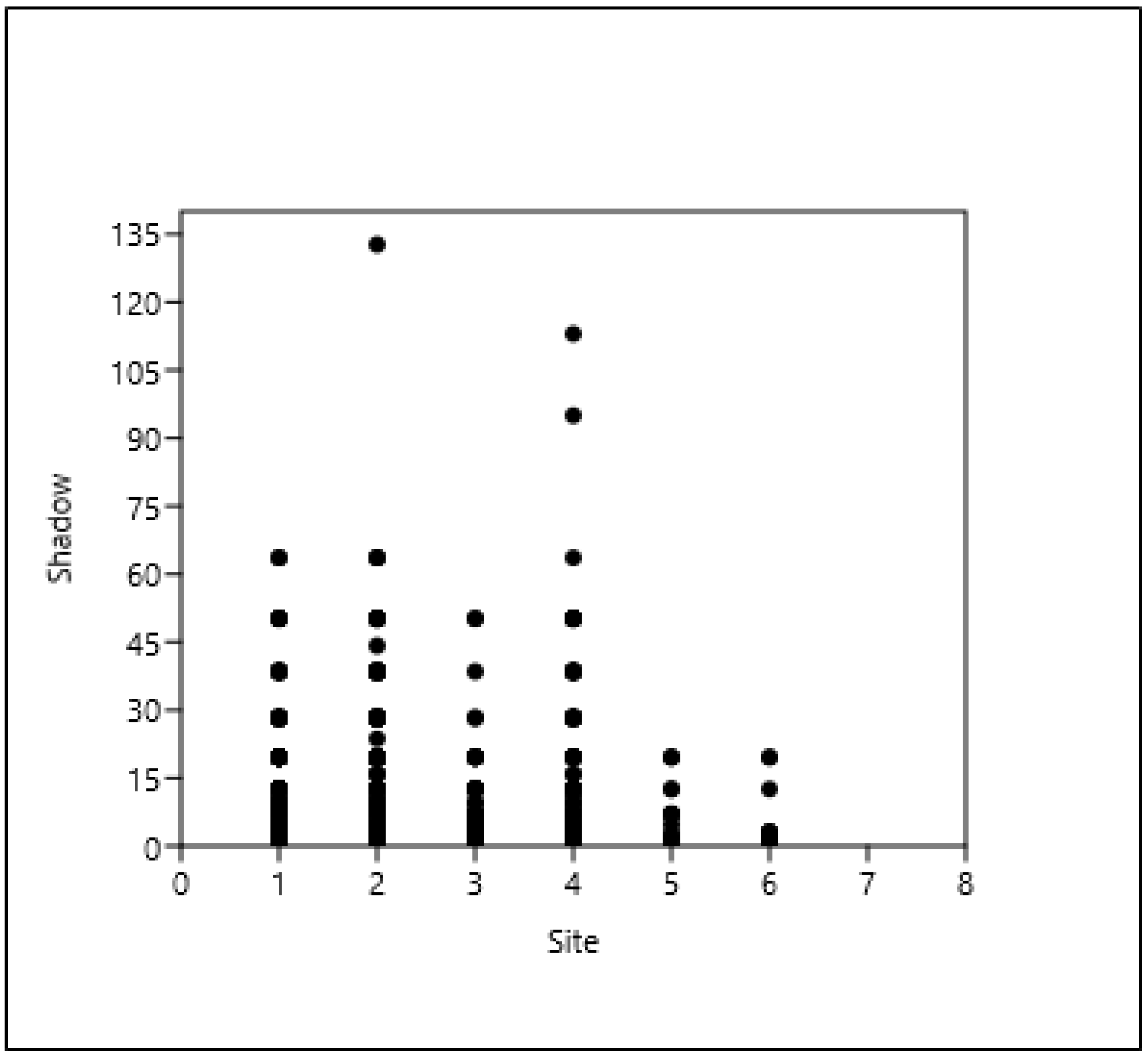
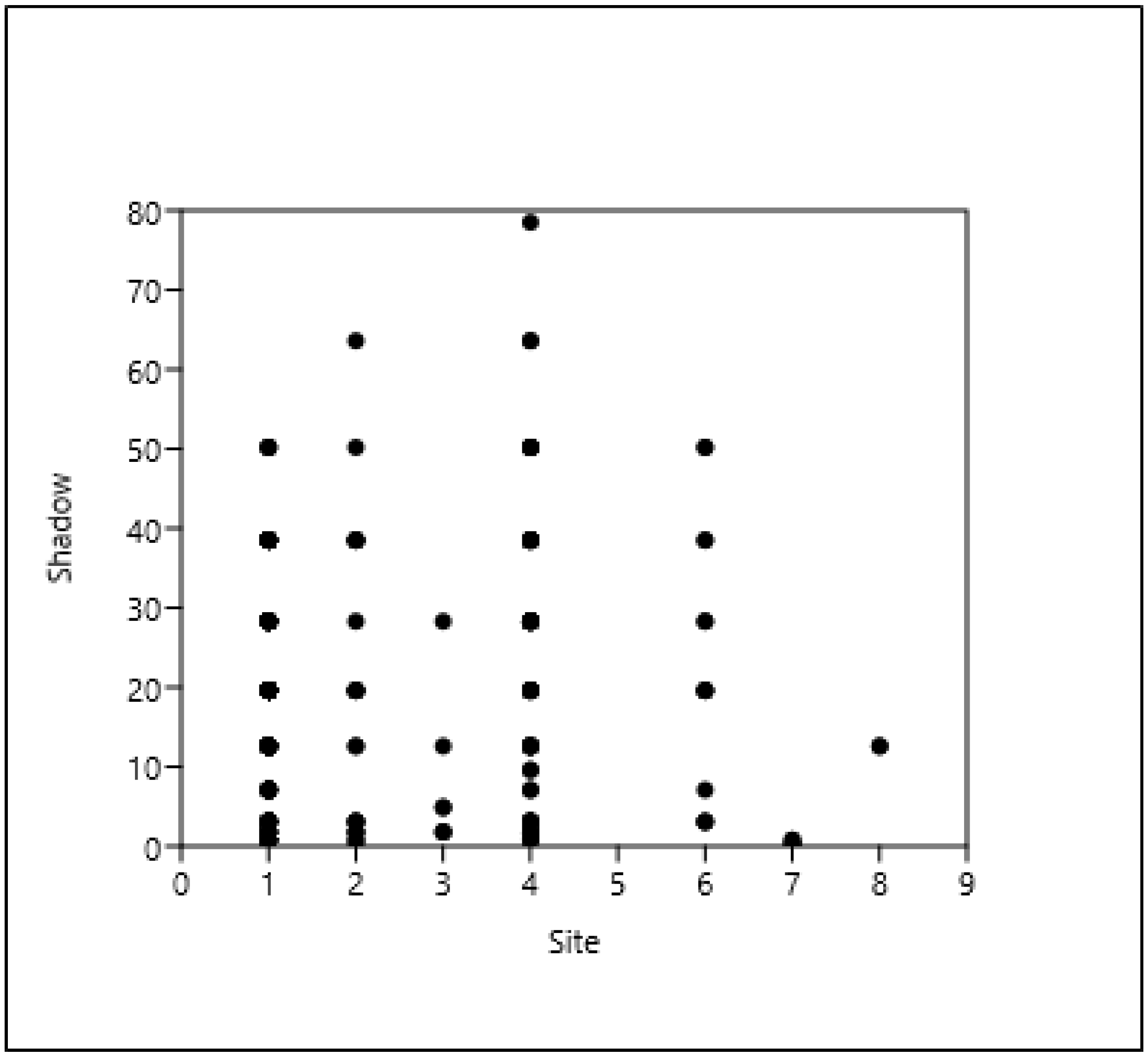
3.2. The Context (Relationship) of the Shade Projection Areas
3.3. The Relationship of the Trunk Diameter
3.4. The Relationship of the Trunk Diameter and Shade Projection Area
3.5. Correlations Between Trees and Different Tree Locations
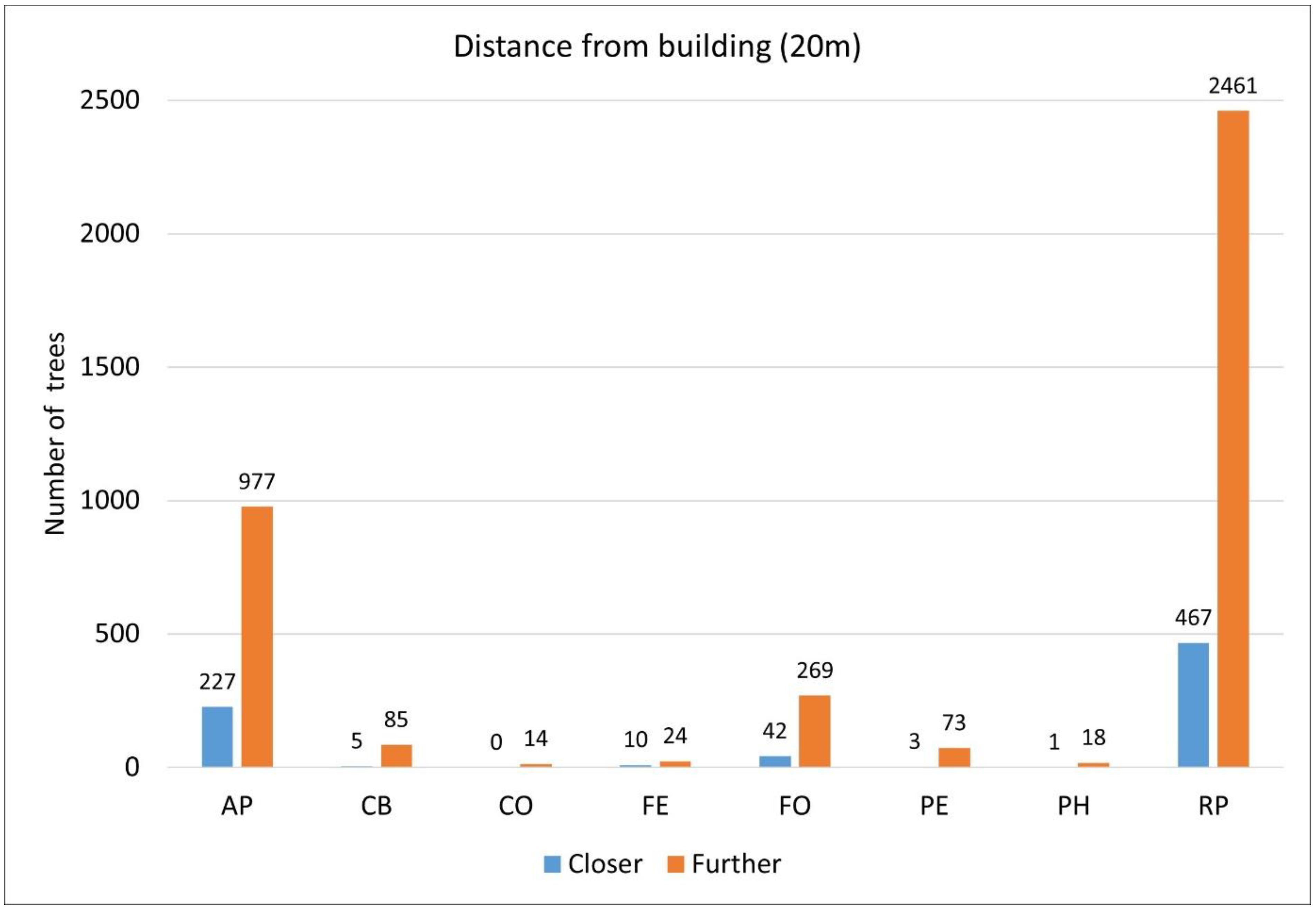
3.6. The Relationship Between the Distance of a Tree from a Building and the Shade Projection Area
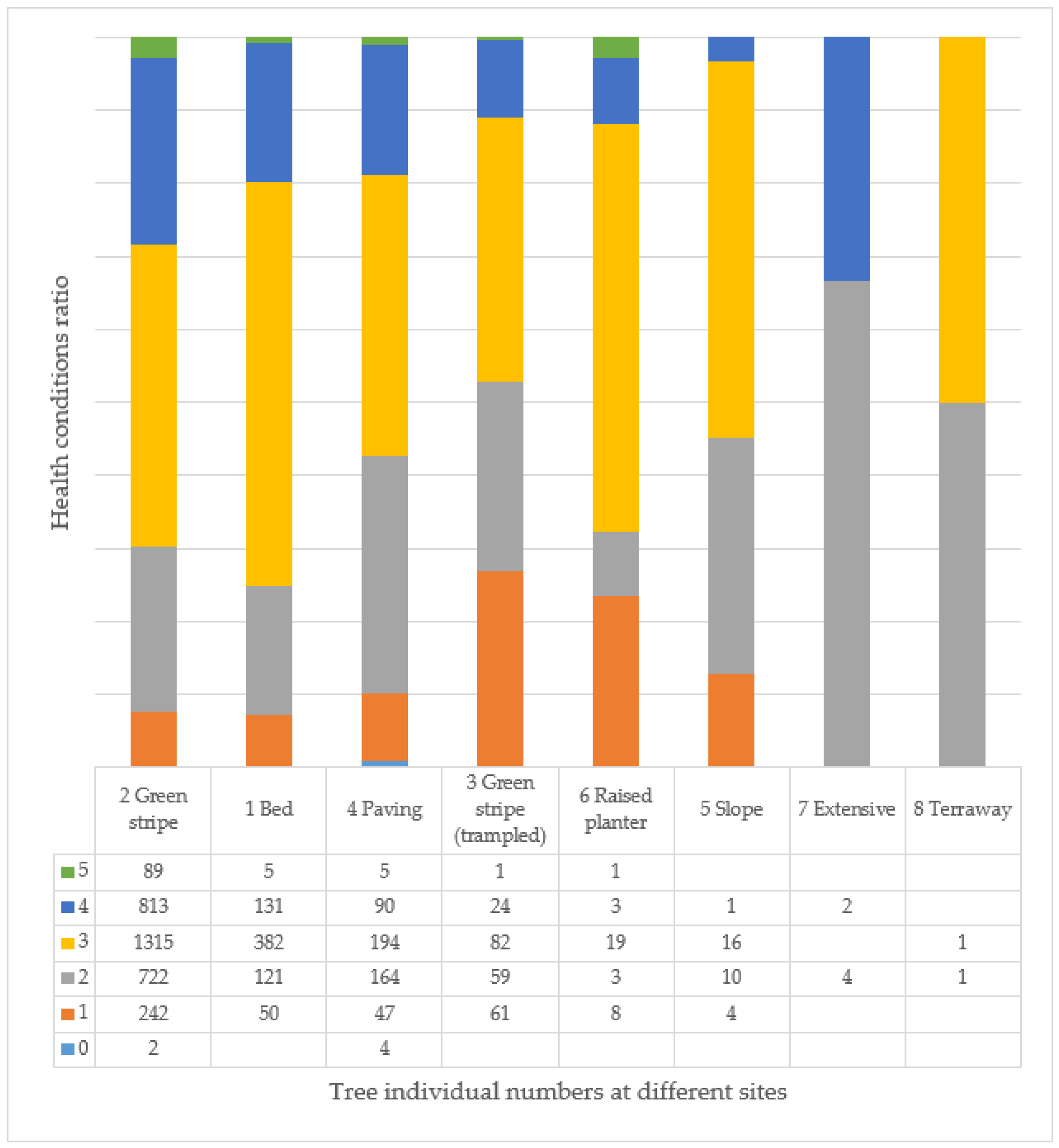
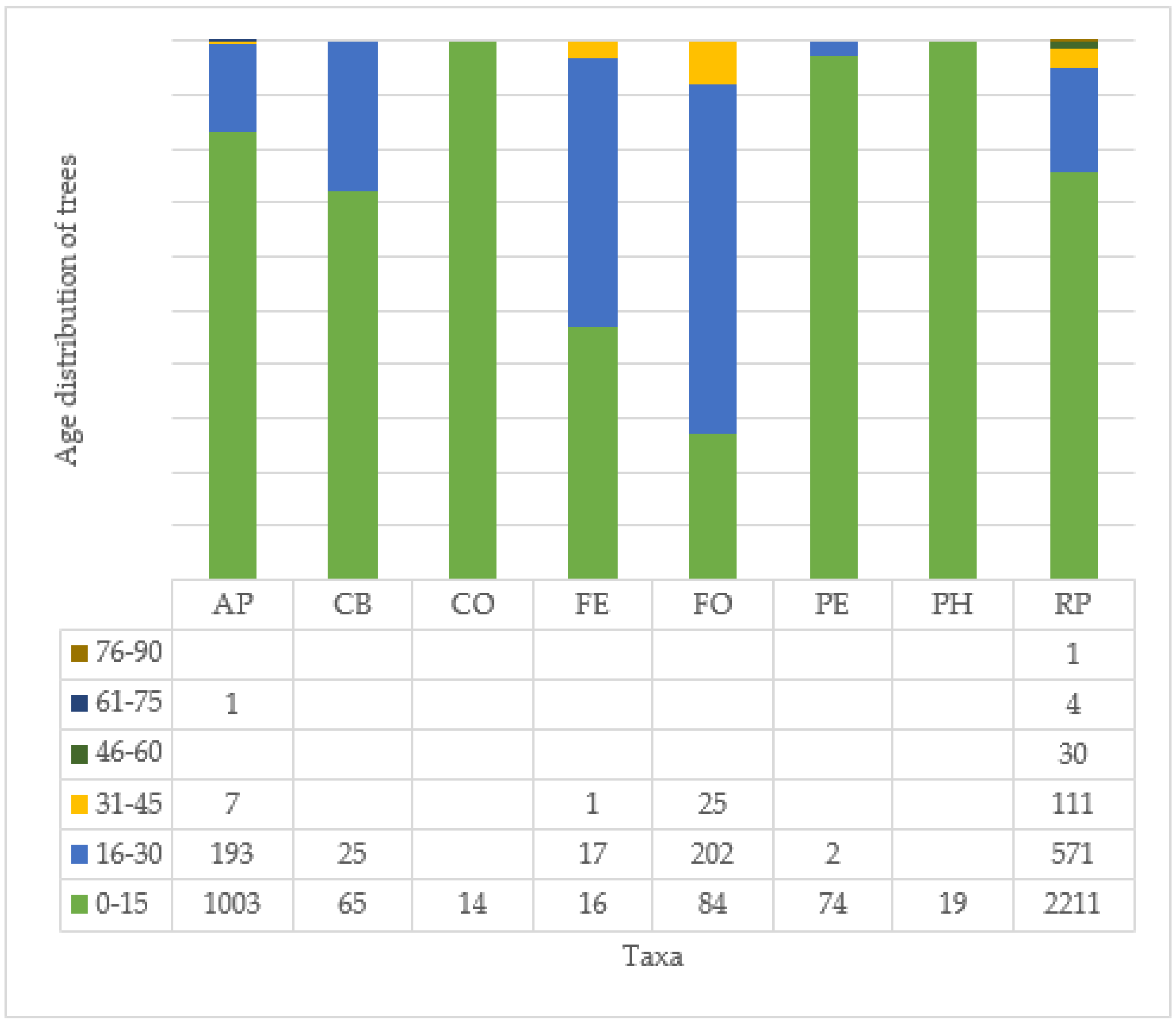
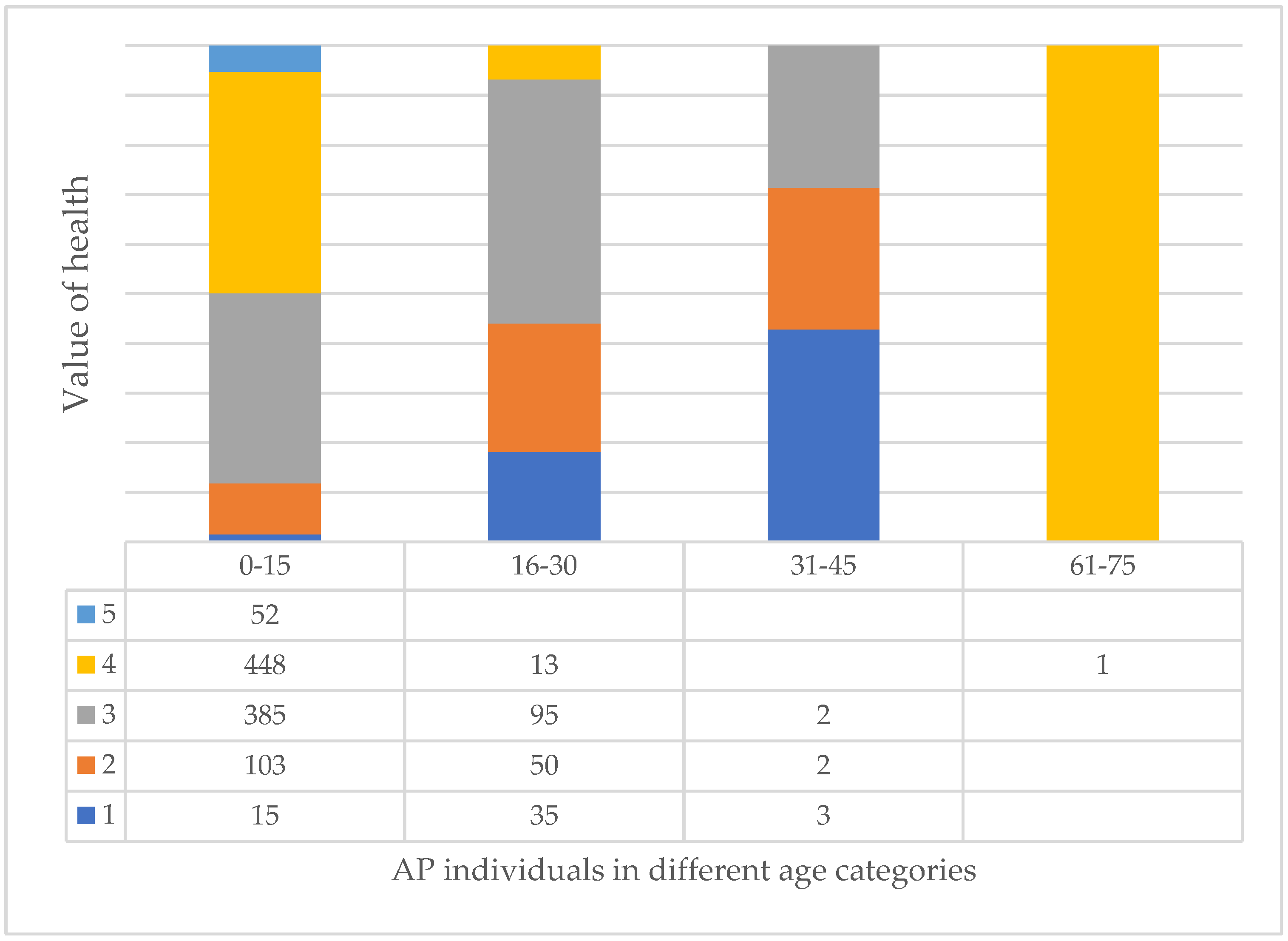
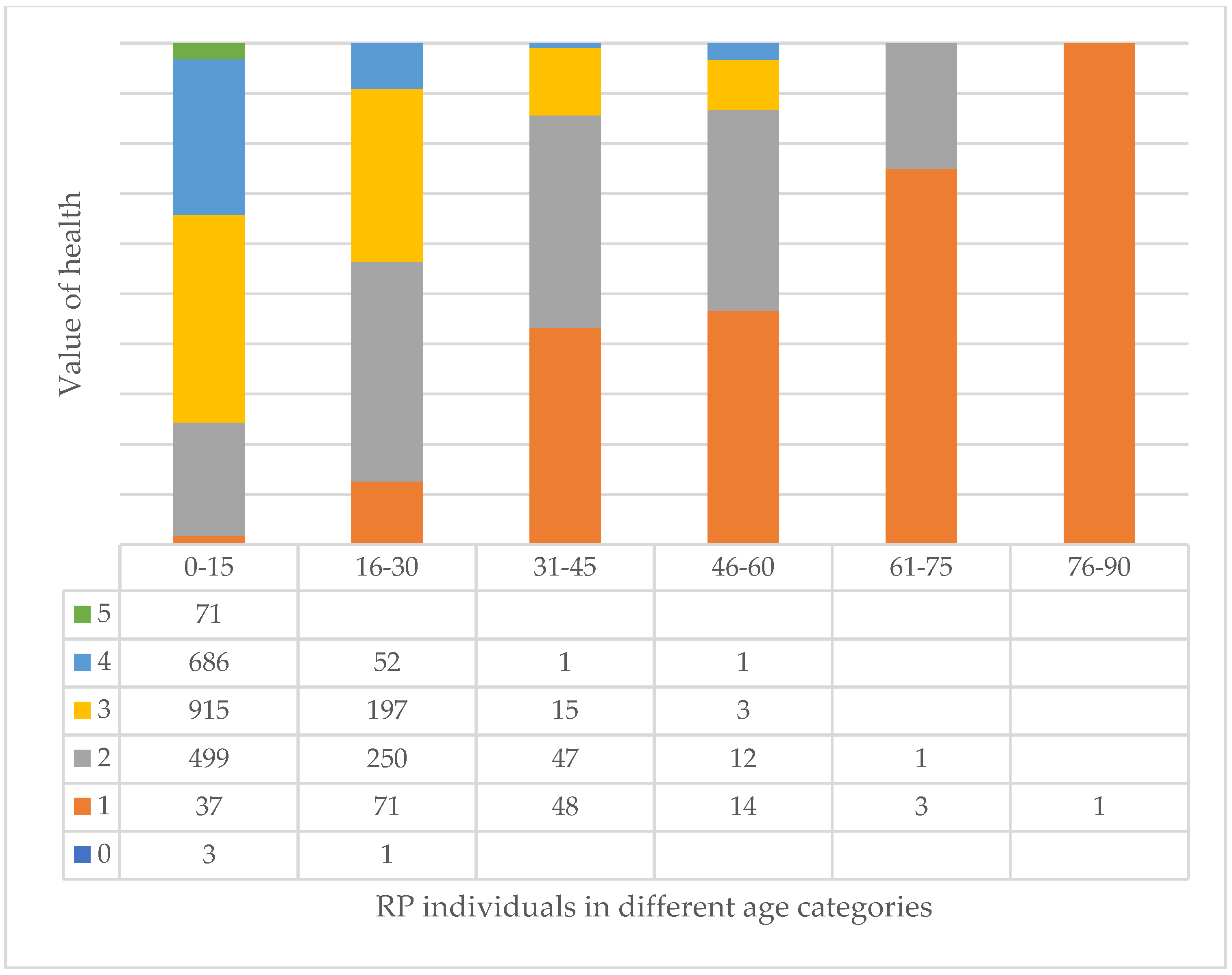
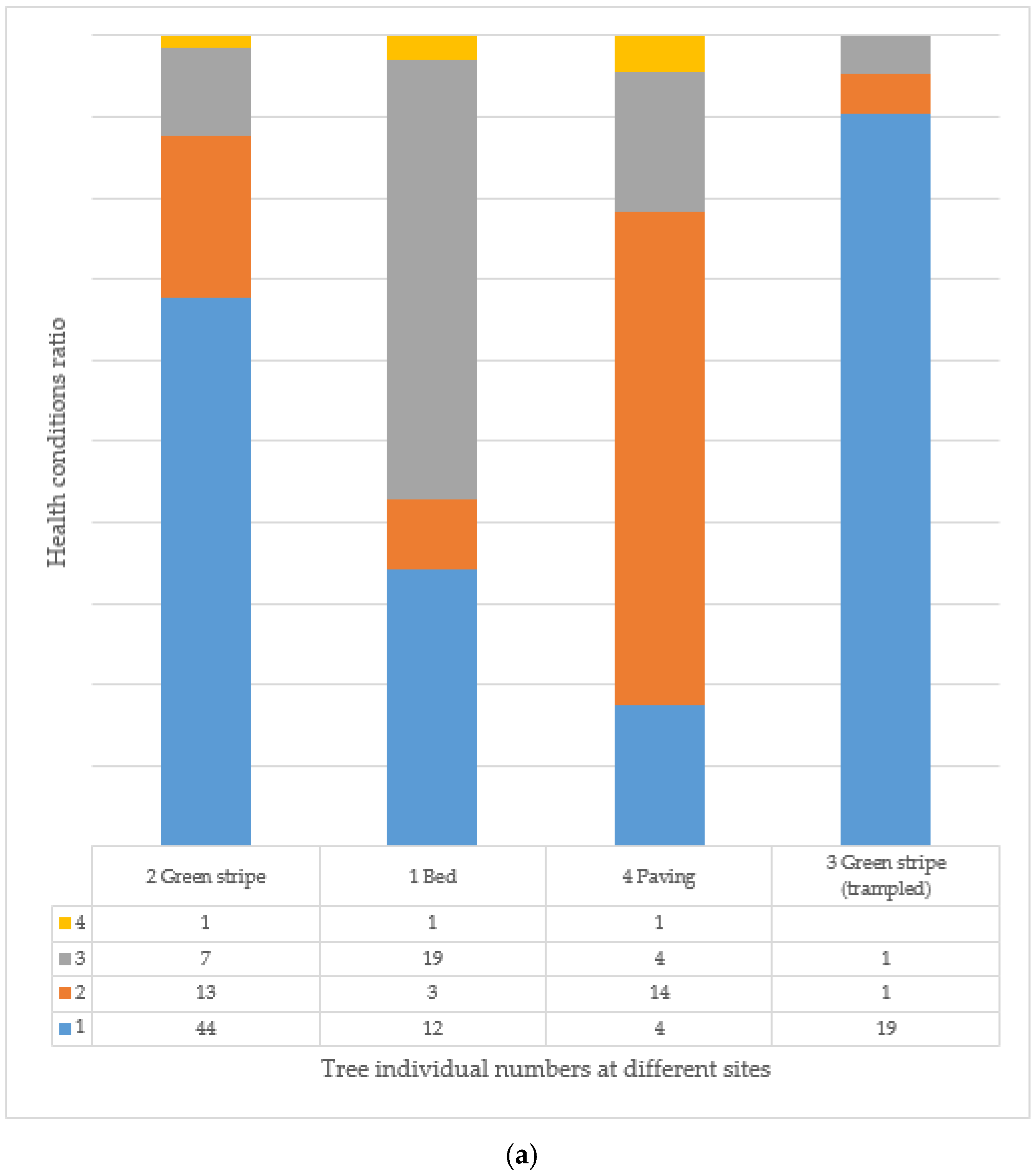
3.7. Age and Health of Trees and Their Correlation at Different Tree Sites
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dye, C. Health and Urban Living. Science 2008, 319, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Volder, A.; Anderson, L.J.; Smart, D.R.; Bloom, A.J.; Lakso, A.N.; Eissenstat, D.M. Estimating Nitrogen Uptake of Individual Roots in Container- and Field-Grown Plants Using a 15N-Depletion Approach. Funct. Plant Biol. 2009, 36, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Radó, D. Fák a Betonrengetegben; Mezőgazdasági Kiadó: Budapest, Hungary, 1981; ISBN 978-963-231-058-9. [Google Scholar]
- Nowak, D.J.; Aevermann, T. Tree Compensation Rates: Compensating for the Loss of Future Tree Values. Urban For. Urban Green. 2019, 41, 93–103. [Google Scholar] [CrossRef]
- Lin, J. Developing a Composite Indicator to Prioritize Tree Planting and Protection Locations. Sci. Total Environ. 2020, 717, 137269. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, E.; Moser-Reischl, A.; Rahman, M.; Pauleit, S.; Pretzsch, H.; Rötzer, T. Crown Shapes of Urban Trees-Their Dependences on Tree Species, Tree Age and Local Environment, and Effects on Ecosystem Services. Forests 2022, 13, 748. [Google Scholar] [CrossRef]
- Tóth, I. Lomblevelű Díszfák, Díszcserjék Kézikönyve, 2012th ed; Tarkavirág Kereskedelmi és Szolgáltató Kft.: Budapest, Hungary, 2012; ISBN 978-963-08-4345-4. [Google Scholar]
- Schmidt, G.; Tóth, I.; Danyi, G. Kertészeti Dendrológia; Mezogazda: Budapest, Hungary, 2006; ISBN 978-963-286-318-4. [Google Scholar]
- Némethy, Z. Érdemes-e Gömbkoronájú Fákat Ültetni? Milyen Speciális Gondozást Igényelnek? Available online: https://agroforum.hu/szaktanacsadas-kerdesek/erdemes-e-gombkoronaju-fakat-ultetni-milyen-specialis-gondozast-igenyelnek/ (accessed on 8 December 2024).
- Szabó, K. Klímafák És Városfásítás, 2023th ed; Starkiss Kft.: Budapest, Hungary, 2023; ISBN 978-615-01-7157-9. [Google Scholar]
- Sitzia, T.; Cierjacks, A.; de Rigo, D.; Caudullo, G. Robinia Pseudoacacia in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016; ISBN 978-92-79-36740-3. [Google Scholar]
- Mally, R.; Ward, S.F.; Trombik, J.; Buszko, J.; Medzihorský, V.; Liebhold, A.M. Non-Native Plant Drives the Spatial Dynamics of Its Herbivores: The Case of Black Locust (Robinia pseudoacacia) in Europe. NeoBiota 2021, 69, 155–175. [Google Scholar] [CrossRef]
- Martin, G.D. Addressing Geographical Bias: A Review of Robinia Pseudoacacia (Black Locust) in the Southern Hemisphere. S. Afr. J. Bot. 2019, 125, 481–492. [Google Scholar] [CrossRef]
- GBIF. Available online: https://www.gbif.org/ (accessed on 8 December 2024).
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black Locust (Robinia pseudoacacia) Beloved and Despised: A Story of an Invasive Tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef] [PubMed]
- DAISIE-Inventory of Alien Invasive Species in Europe. Available online: https://www.gbif.org/dataset/39f36f10-559b-427f-8c86-2d28afff68ca (accessed on 8 December 2024).
- Rédei, K.; Osváth-Bujtás, Z.; Veperdi, I. Black Locust (Robinia pseudoacacia L.) Improvement in Hungary: A Review. Acta Silv. Lignaria Hung. 2008, 4, 127–132. [Google Scholar] [CrossRef]
- Keserű, Z.; Borovics, A.; Ábri, T.; Rédei, K.; Lee, I.H.; Lim, H. Growing of Black Locust (Robinia pseudoacacia L.) Candidate Cultivars on Arid Sandy Site. Acta Silv. Lignaria Hung. 2021, 17, 51–61. [Google Scholar] [CrossRef]
- Bartha, D. Fekete Lista. Magyarország Inváziós Fa- És Cserjefajai./Black List. Invasive Tree and Shrub Species of Hungary. * Szürke Lista. Magyarország Potenciálisan Inváziós Fa- És Cserjefajai./Grey List. Potentially Invasive Tree and Shrub Species of Hungary; Soproni Egyetem Kiadó/University of Sopron Press: Sopron, Hungary, 2020; ISBN 978-963-334-357-9. [Google Scholar]
- Halupa, L.; Rédei, K. Establishment of Forests Primarily for Energetic Purpose. Erdészeti Kut. 1991, 82–83, 267–286. [Google Scholar]
- 346/2008 (XII.30.) Korm. Rendelet A Fás Szárú Növények Védelme. Available online: https://njt.hu/jogszabaly/2008-346-20-22 (accessed on 8 December 2024).
- 282/2024. (IX.30.) Korm. Rendelet A Települési Zöldinfrastruktúráról, a Zöldfelületi Tanúsítványról És a Zöld Védjegyről. Available online: https://net.jogtar.hu/jogszabaly?docid=a2400282.kor (accessed on 8 December 2024).
- Tóth, I.; Retkes, J. Lombos Fák, Cserjék; Nyugat-Dunántúli Díszfaiskolások Egyesülete: Szombathely, Hungary, 2015; ISBN 978963123238. [Google Scholar]
- Kaszab, L. Faápolók Dendrológiája I.: Lombhullatók; Szent István Egyetemen: Gödöllő, Hungary, 2019. [Google Scholar]
- Li, G.; Xu, G.; Guo, K.; Du, S. Mapping the Global Potential Geographical Distribution of Black Locust (Robinia Pseudoacacia L.) Using Herbarium Data and a Maximum Entropy Model. Forests 2014, 5, 2773–2792. [Google Scholar] [CrossRef]
- Tóth, B. Kertművészeti Fejlesztési Lehetőségek a Budai Arborétumban, Magyar Agrár- és Élettudományi Egyetem, 2022.
- Krussmann, G. Manual of Cultivated Broad-Leaved Trees and Shrubs; Timber Press: Portland, OR, USA, 1985; ISBN 978-0-88192-005-5. [Google Scholar]
- Gencsi, L.; Vancsura, R. Dendrológia; Mezőgazdasági Kiadó: Budapest, Hungary, 1997. [Google Scholar]
- Szabó, K.; Gergely, A.; Tóth, B.; Szilágyi, K. Assessing the Spontaneous Spread of Climate-Adapted Woody Plants in an Extensively Maintained Collection Garden. Plants 2023, 12, 1989. [Google Scholar] [CrossRef] [PubMed]
- Csiszár, Á. Inváziós Növényfajok Magyarországon; Nyugat-magyarországi Egyetem K: Sopron, Hungary, 2012; ISBN 978-963-334-050-9. [Google Scholar]
- Ifju, Z. Dísznövény Fajtái És Felszaporítás Alatt Levő Fajta Jelöltek. 2019. Available online: https://ifjufaiskola.hu/data/uploads/szakmai-anyagok/fajtaim-2020-01-17-01-vegleges-jo-2.-var.pdf (accessed on 8 December 2024).
- Platanus ×hispanica “Alphen’s Globe. Available online: https://www.vdberk.hu/fak/platanus-hispanica-alphen-s-globe/ (accessed on 8 December 2024).
- Szaller, V. “TREE ASSESSOR” Favizsgáló A Fák Értéke, Fanyilvántartás; FAKOPP Enterprise Bt.: Bécsi út, Hungary, 2021. [Google Scholar]
- Szaller, V. Útmutató a Fák Nyilvántartásához És Egyedi Értékük Kiszámításához; Magyar Faápolók Egyesületének: Budapest, Hungary, 2013. [Google Scholar]
- Lukács, Z.; Szaller, V.; Divós, F.; Kelemen, G. Útmutató a Vizuális És Műszeres Favizsgálatok Elvégzéséhez És Dokumentálásához [Guidelines for Performing and Documenting Visual and Instrumental Tree Evaulation]; Magyar Faápolók Egyesülete: Budapest, Hungary, 2017. [Google Scholar]
- Maco, S.; McPherson, E.G. Assessing Canopy Cover Over Streets and Sideswalks in Street Tree Populations. J. Arboric. 2002, 28, 270–276. [Google Scholar] [CrossRef]
- Nowak, D.J. Estimating Leaf Area and Leaf Biomass of Open-Grown Deciduous Urban Trees. For. Sci. 1996, 42, 504–507. [Google Scholar] [CrossRef]
- Timilsina, N.; Staudhammer, C.L.; Escobedo, F.J.; Lawrence, A. Tree Biomass, Wood Waste Yield, and Carbon Storage Changes in an Urban Forest. Landsc. Urban Plan. 2014, 127, 18–27. [Google Scholar] [CrossRef]
- Lawrence, E. The Illustrated Book of Trees and Shrubs; Gallery Books: New York, NY, USA, 1985; ISBN 978-0-8317-8820-9. [Google Scholar]
- Hammer, Ø.; Harper, D.T. Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Kirisits, T.; Matlakova, M.; Mottinger-Kroupa, S.; Halmschlager, E.; Lakatos, F. Chalara Fraxinea Associated with Dieback of Narrow-leafed Ash (Fraxinus angustifolia). Plant Pathol. 2010, 59, 411. [Google Scholar] [CrossRef]
- Sikorski, P.; Gawryszewska, B.; Sikorska, D.; Chormański, J.; Schwerk, A.; Jojczyk, A.; Ciężkowski, W.; Archiciński, P.; Łepkowski, M.; Dymitryszyn, I.; et al. The Value of Doing Nothing—How Informal Green Spaces Can Provide Comparable Ecosystem Services to Cultivated Urban Parks. Ecosyst. Serv. 2021, 50, 101339. [Google Scholar] [CrossRef]
- Schwanda, K.; Kirisits, T. Pathogenicity of Hymenoscyphus Fraxineus towards Leaves of Three European Ash Species: Fraxinus Excelsior, F. Angustifolia and F. Ornus. Plant Pathol. 2016, 65, 1071–1083. [Google Scholar] [CrossRef]
- Dujesiefken, D.; Fay, N.; de Groot, J.W.; de Berker, N.; Witkos-Gnach, K. Trees—A Lifespan Approach: Contributions to Arboriculture from European Practitioners; Fundacja EkoRozwoju: Wrocław, Poland, 2016; ISBN 978-83-63573-14-0. [Google Scholar]
- Olchowik, J.; Suchocka, M.; Malewski, T.; Baczewska-Dąbrowska, A.; Studnicki, M.; Hilszczańska, D. The Ectomycorrhizal Community of Crimean Linden Trees in Warsaw, Poland. Forests 2020, 11, 926. [Google Scholar] [CrossRef]
- Czaja, M.; Kołton, A.; Muras, P. The Complex Issue of Urban Trees—Stress Factor Accumulation and Ecological Service Possibilities. Forests 2020, 11, 932. [Google Scholar] [CrossRef]
- Suchocka, M.; Heciak, J.; Błaszczyk, M.; Adamczyk, J.; Gaworski, M.; Gawłowska, A.; Mojski, J.; Kalaji, H.M.; Kais, K.; Kosno-Jończy, J.; et al. Comparison of Ecosystem Services and Replacement Value Calculations Performed for Urban Trees. Ecosyst. Serv. 2023, 63, 101553. [Google Scholar] [CrossRef]
- Sanaphre-Villanueva, L.; Dupuy, J.; Andrade, J.; Reyes-García, C.; Paz, H.; Jackson, P. Functional Diversity of Small and Large Trees along Secondary Succession in a Tropical Dry Forest. Forests 2016, 7, 163. [Google Scholar] [CrossRef]
- Song, P.; Kim, G.; Mayer, A.; He, R.; Tian, G. Assessing the Ecosystem Services of Various Types of Urban Green Spaces Based on I-Tree Eco. Sustainability 2020, 12, 1630. [Google Scholar] [CrossRef]
- Popkin, G. How Much Can Forests Fight Climate Change? Nature 2019, 565, 280–282. [Google Scholar] [CrossRef]
- Suryaningrum, F.; Jarvis, R.M.; Buckley, H.L.; Hall, D.; Case, B.S. Large-Scale Tree Planting Initiatives as an Opportunity to Derive Carbon and Biodiversity Co-Benefits: A Case Study from Aotearoa New Zealand. New For. 2022, 53, 589–602. [Google Scholar] [CrossRef]
- Mestre, L.; Jansson, N.; Ranius, T. Saproxylic Biodiversity and Decomposition Rate Decrease with Small-Scale Isolation of Tree Hollows. Biol. Conserv. 2018, 227, 226–232. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Likens, G.E. The Science and Application of Ecological Monitoring. Biol. Conserv. 2010, 143, 1317–1328. [Google Scholar] [CrossRef]
- Luyssaert, S.; Marie, G.; Valade, A.; Chen, Y.-Y.; Njakou Djomo, S.; Ryder, J.; Otto, J.; Naudts, K.; Lansø, A.S.; Ghattas, J.; et al. Trade-Offs in Using European Forests to Meet Climate Objectives. Nature 2018, 562, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Goodburn, J.M.; Lorimer, C.G. Cavity Trees and Coarse Woody Debris in Old-Growth and Managed Northern Hardwood Forests in Wisconsin and Michigan. Can. J. For. Res. 1998, 28, 427–438. [Google Scholar] [CrossRef]
- Ding, W.; Wang, R.; Yuan, Y.; Liang, X.; Liu, J. Effects of Nitrogen Deposition on Growth and Relationship of Robinia Pseudoacacia and Quercus Acutissima Seedlings. Dendrobiology 2012, 67, 3–13. [Google Scholar]
- Luo, Y.; Guo, W.; Yuan, Y.; Liu, J.; Du, N.; Wang, R. Increased Nitrogen Deposition Alleviated the Competitive Effects of the Introduced Invasive Plant Robinia Pseudoacacia on the Native Tree Quercus Acutissima. Plant Soil 2014, 385, 63–75. [Google Scholar] [CrossRef]
- Horváthová, E.; Badura, T.; Duchková, H. The Value of the Shading Function of Urban Trees: A Replacement Cost Approach. Urban For. Urban Green. 2021, 62, 127166. [Google Scholar] [CrossRef]
- Tóth, B.; Szabó, K. A Budai Arborétum Hárs Taxonjainak Fenológiai Vizsgálata. In A Lippay János-Ormos Imre-Vas Károly (LOV) Tudományos Ülésszak Tanulmányai, 2022th ed; MATE Budai Campus: Budapest, Hungary, 2022. [Google Scholar]
- Korcz, N.; Janeczko, E.; Bielinis, E.; Urban, D.; Koba, J.; Szabat, P.; Małecki, M. Influence of Informal Education in the Forest Stand Redevelopment Area on the Psychological Restoration of Working Adults. Forests 2021, 12, 993. [Google Scholar] [CrossRef]
- Bernaciak, A.; Wojcieszak, M. The Valuation of Trees in the Urbanized Areas with the Compensation/Replacement Method and Benefits Analysis (the Case of the City of Gniezno). Ekon. Sr. 2014, 4, 187–197. [Google Scholar]
- Arbab, N.; Grabosky, J.; Leopold, R. Economic Assessment of Urban Ash Tree Management Options in New Jersey. Sustainability 2022, 14, 2172. [Google Scholar] [CrossRef]

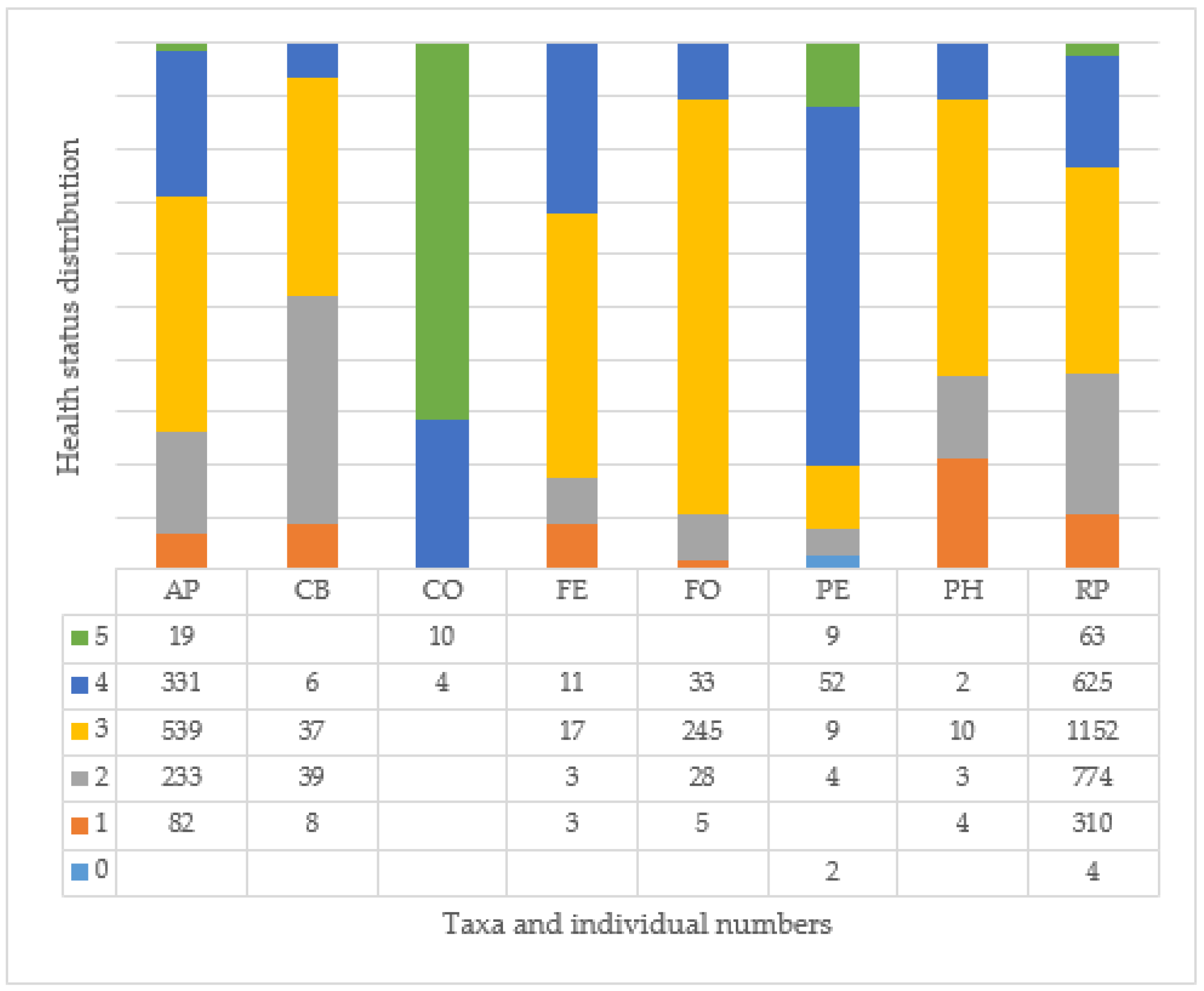
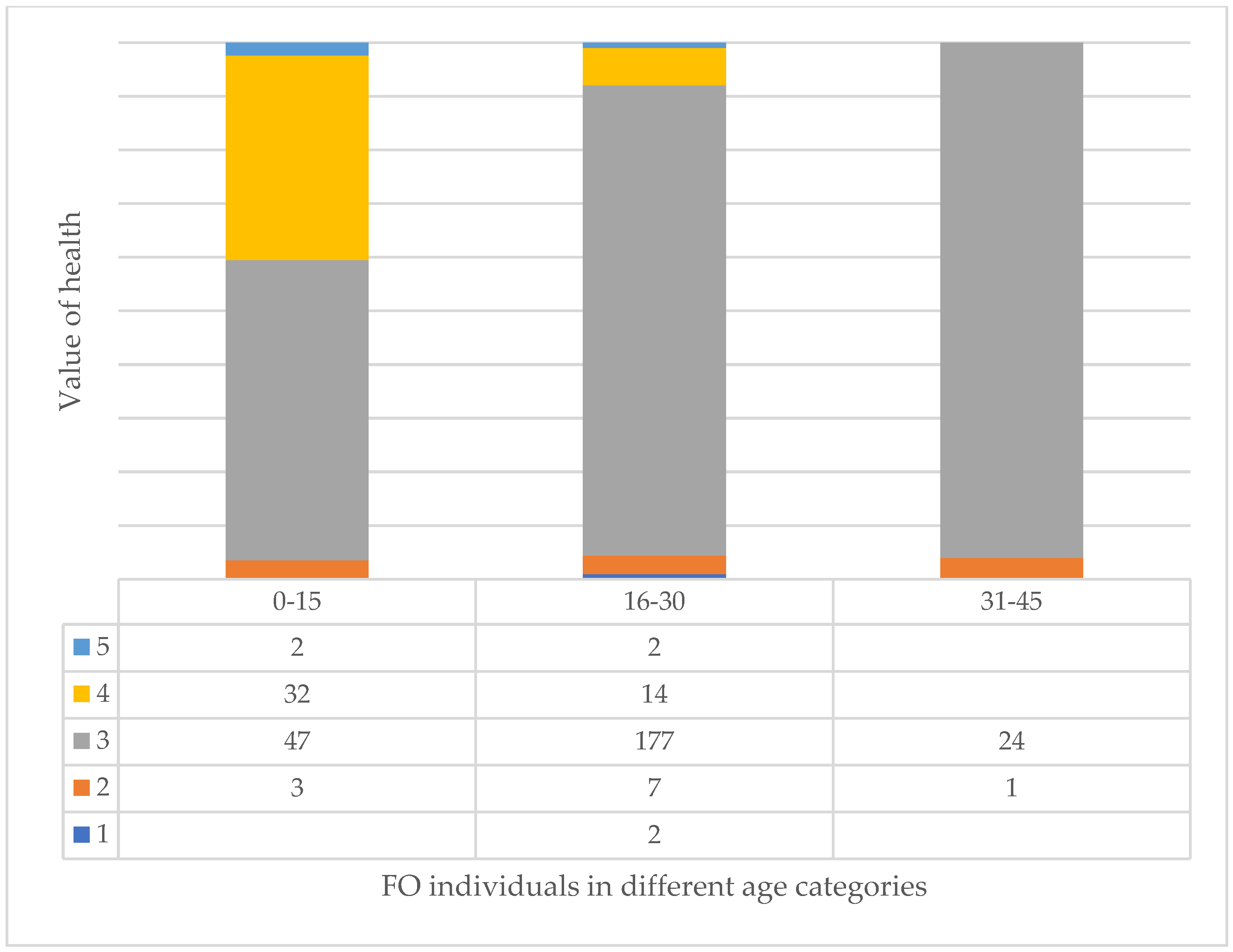


| Root Condition | Trunk Condition | ||
|---|---|---|---|
| Evaluation | Grade | Evaluation | Grade |
| intact root necks, optimal growing conditions | 5 | intact | 5 |
| intact root necks, acceptable growing conditions | 4 | superficial injuries | 4 |
| less damage to root crowns, acceptable growing conditions | 3 | some superficial wounds and aging | 3 |
| heavy visible yellowing, unfavorable growing conditions | 2 | large surface wounds, serious decay | 2 |
| severe damage, very poor site | 1 | progressive decay, necrosis | 1 |
| dead roots, empty tree site | 0 | empty tree site | 0 |
| Crown Condition | Health and Viability of the Tree | ||
| Evaluation | Grade | Evaluation | Grade |
| crown shape intact, foliage loss (1–10%) | 5 | excellent condition | 5 |
| foliage loss (11–25%) | 4 | with intervention near maximum age | 4 |
| significant defoliation (26–50%) | 3 | to be replaced before maximum age | 3 |
| severe crown damage ((over 50%) | 2 | to be replaced within 10 years | 2 |
| dead crown, total defoliation | 1 | to be replaced urgently | 1 |
| empty tree site | 0 | empty tree site | 0 |
| Districts | Robinia pseudoacacia ‘Umraculifera’ | Acer platanoides ‘Globosum’ | Fraxinus ornus ‘Mecsek’ | Fraxinus excelsior ‘Nana’ | Celtis occidentalis ‘Globosa’ | Prunus × eminens ‘Umbraculifera’ | Platanus × hispanica ‘Alphen’s Globe’ | Catalpa bignonioides ‘Nana’ |
|---|---|---|---|---|---|---|---|---|
| RP | AP | FO | FE | CO | PE | PH | CB | |
| I. | 2 | 14 | ||||||
| II. | 1 | 1 | 1 | |||||
| III. | 24 | 12 | 4 | 10 | 8 | |||
| IV. | 318 | 2 | 1 | |||||
| V. | ||||||||
| VI. | 145 | 2 | ||||||
| VII. | 1 | |||||||
| VIII: | 238 | 11 | 10 | 4 | ||||
| IX. | 50 | 9 | ||||||
| X. | 305 | 109 | 9 | 3 | 30 | |||
| XI. | 43 | 21 | 169 | 1 | 16 | 8 | ||
| XII. | 105 | 10 | 64 | |||||
| XIII. | 64 | 12 | 14 | 7 | ||||
| XIV. | 84 | 7 | 7 | 2 | 17 | 7 | ||
| XV. | 144 | 100 | 3 | |||||
| XVI. | 74 | 392 | 1 | 1 | ||||
| XVII. | 190 | 198 | 12 | 19 | 10 | |||
| XVIII. | 699 | 106 | 5 | 6 | 4 | |||
| XIX. | 189 | 27 | 11 | |||||
| XX. | 62 | 19 | ||||||
| XXI. | 137 | 47 | 3 | |||||
| XXII. | 5 | 58 | 3 | 35 | ||||
| XXIII. | 51 | 62 | 7 | 14 | ||||
| Total | 2928 | 1204 | 311 | 34 | 14 | 76 | 19 | 90 |
| Acer platanoides | AP | 0.74 |
| Catalpa bignonioides | CB | 0.87 |
| Celtis occidentalis | CO | −0.47 |
| Fraxinus excelsior | FE | 0.88 |
| Fraxinus ornus | FO | 0.78 |
| Platanus × hispanica | PH | −0.11 |
| Prunus × eminens | PE | 0.76 |
| Robinia pseudoacacia | RP | 0.68 |
| NO | Types | Descriptions | Number of Individuals |
|---|---|---|---|
| 1 | Bed | the soil surface is also covered with some type of shrub, perennial, or annual plant | 689 |
| 2 | Green stripe | in the tree line, a continuous strip covered with earth and/or turf of a given width | 3183 |
| 3 | Green stripe (rampled) | the soil is compacted, which is usually caused by vehicles parked in the wooded area | 227 |
| 4 | Paving | the tree site is surrounded by some kind of closed covering, for example, concrete, asphalt, paving stones | 504 |
| 5 | Slope | tree site in a sloping area, e.g., it is on the bank of a ditch, on the side of an overpass, on a hillside | 31 |
| 6 | Raised planter | the wood site has a raised, concrete, brick, or stone border and the ground surface in the wood site is higher than the surface or pavement surrounding the wood site | 34 |
| 7 | Extensive | the environment of the tree is not well kept, usually outside, neglected areas on the side of the road | 6 |
| 8 | Terraway | the floor of the enclosure is covered by a water-permeable covering called Terraway | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, K.; Tőke, E.; Gergely, A. Evaluating Spherical Trees in the Urban Environment in Budapest (Hungary). Plants 2025, 14, 228. https://doi.org/10.3390/plants14020228
Szabó K, Tőke E, Gergely A. Evaluating Spherical Trees in the Urban Environment in Budapest (Hungary). Plants. 2025; 14(2):228. https://doi.org/10.3390/plants14020228
Chicago/Turabian StyleSzabó, Krisztina, Eszter Tőke, and Attila Gergely. 2025. "Evaluating Spherical Trees in the Urban Environment in Budapest (Hungary)" Plants 14, no. 2: 228. https://doi.org/10.3390/plants14020228
APA StyleSzabó, K., Tőke, E., & Gergely, A. (2025). Evaluating Spherical Trees in the Urban Environment in Budapest (Hungary). Plants, 14(2), 228. https://doi.org/10.3390/plants14020228