Transcriptome and Metabolome Reveal Ferulic Acid as a Critical Phenylpropanoid for Drought Resistance in Dendrobium sinense
Abstract
1. Introduction
2. Results
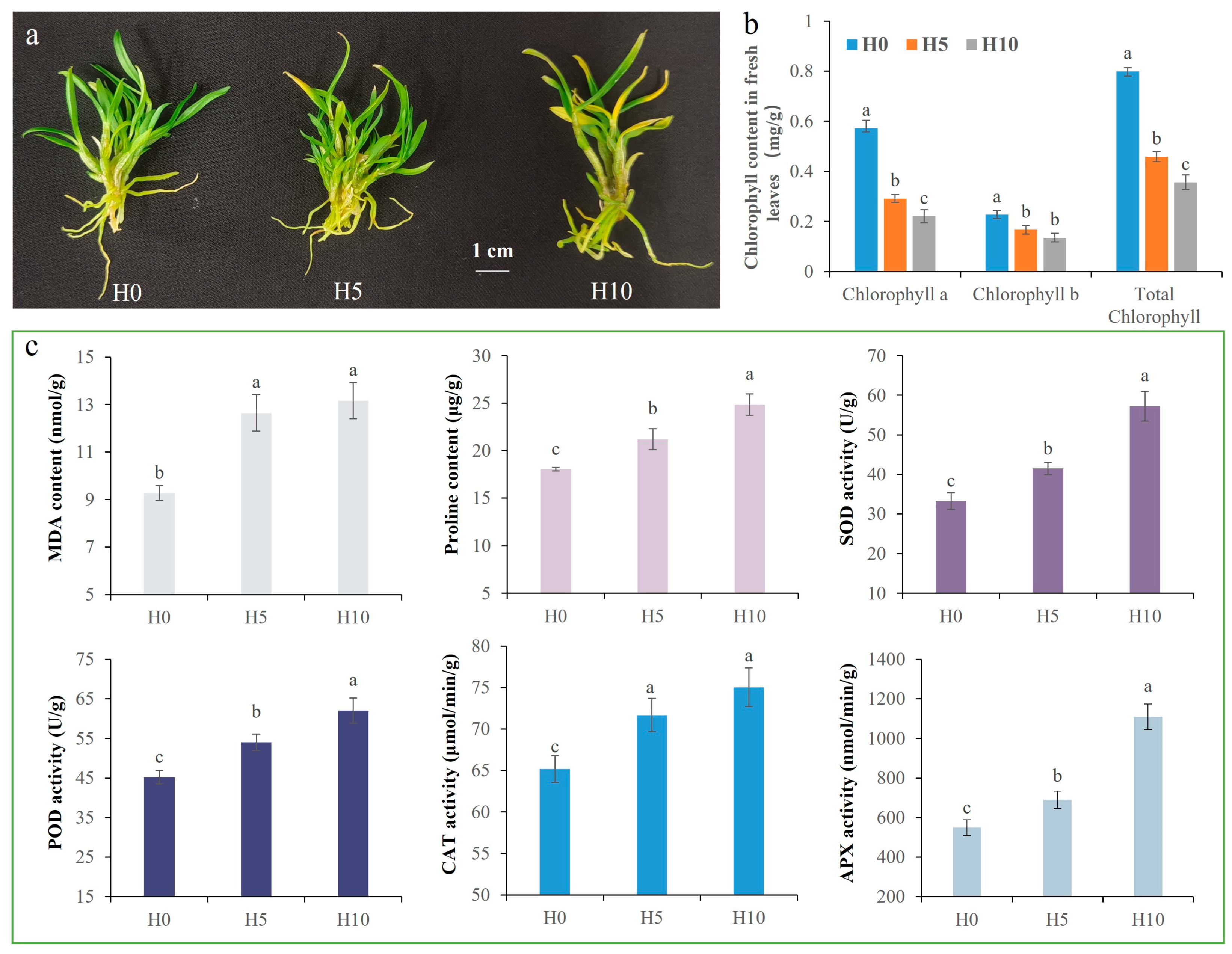
2.1. Physiological and Biochemical Indicators Related to Drought Stress
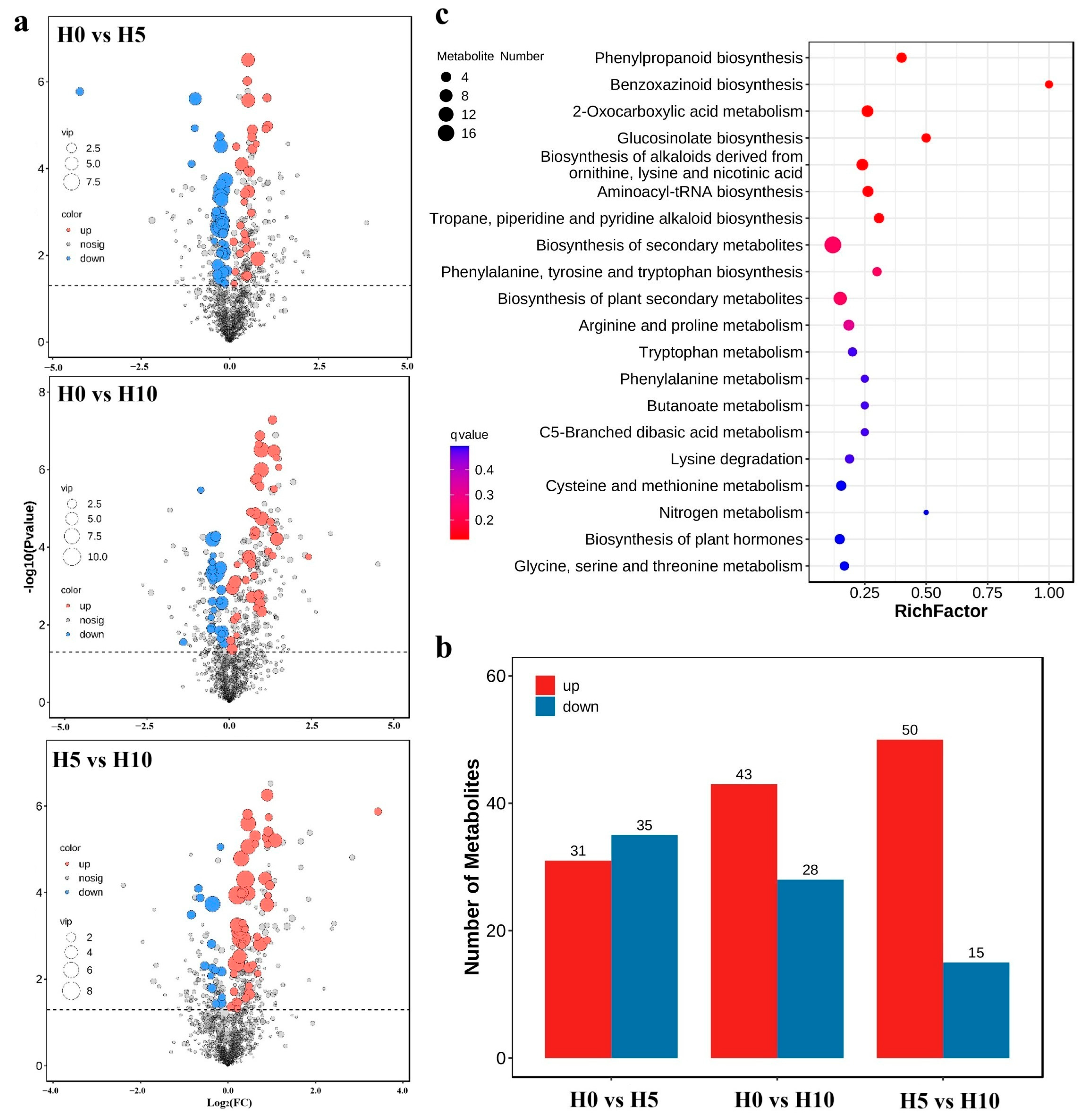
2.2. Identification of Metabolites Responsive to Drought Stress
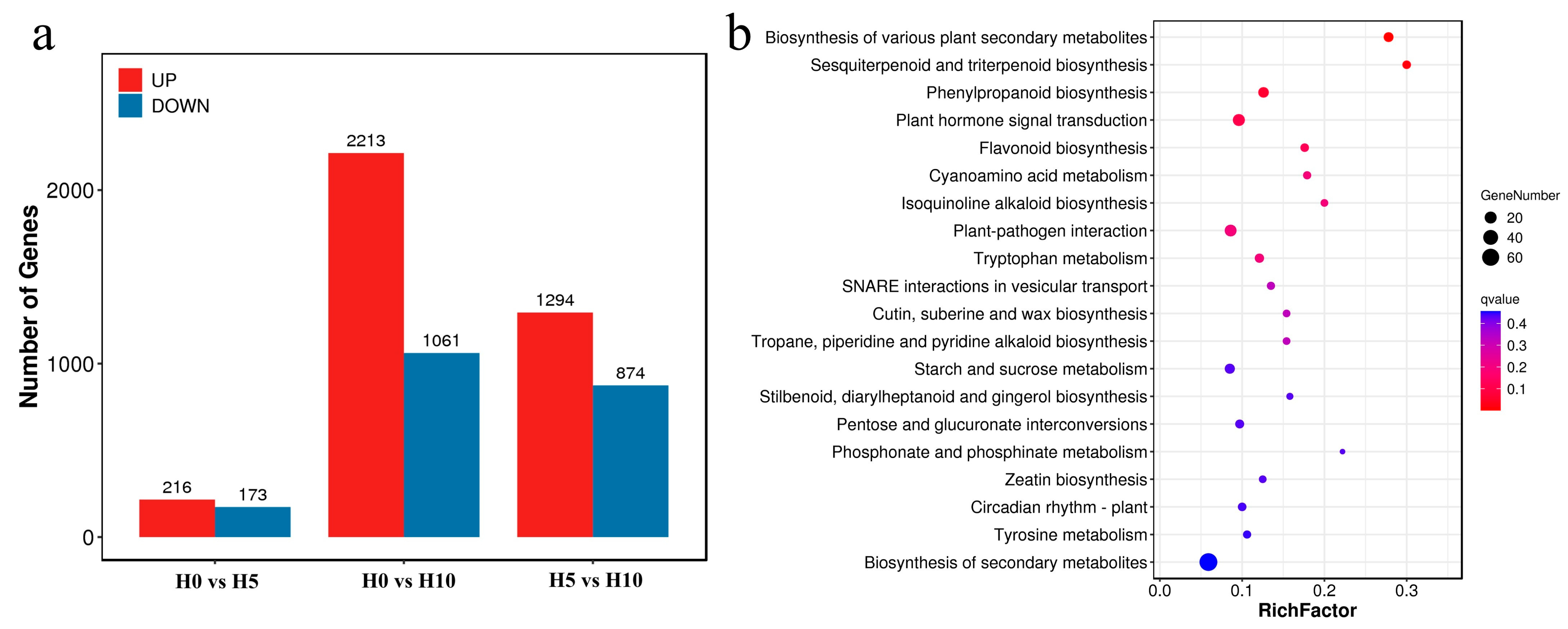
2.3. Transcriptome Analysis Results
2.4. The Response of Phenylpropanoid Biosynthesis to Drought Stress
2.5. Alleviating Effect of Ferulic Acid on Drought Stress of D. sinense
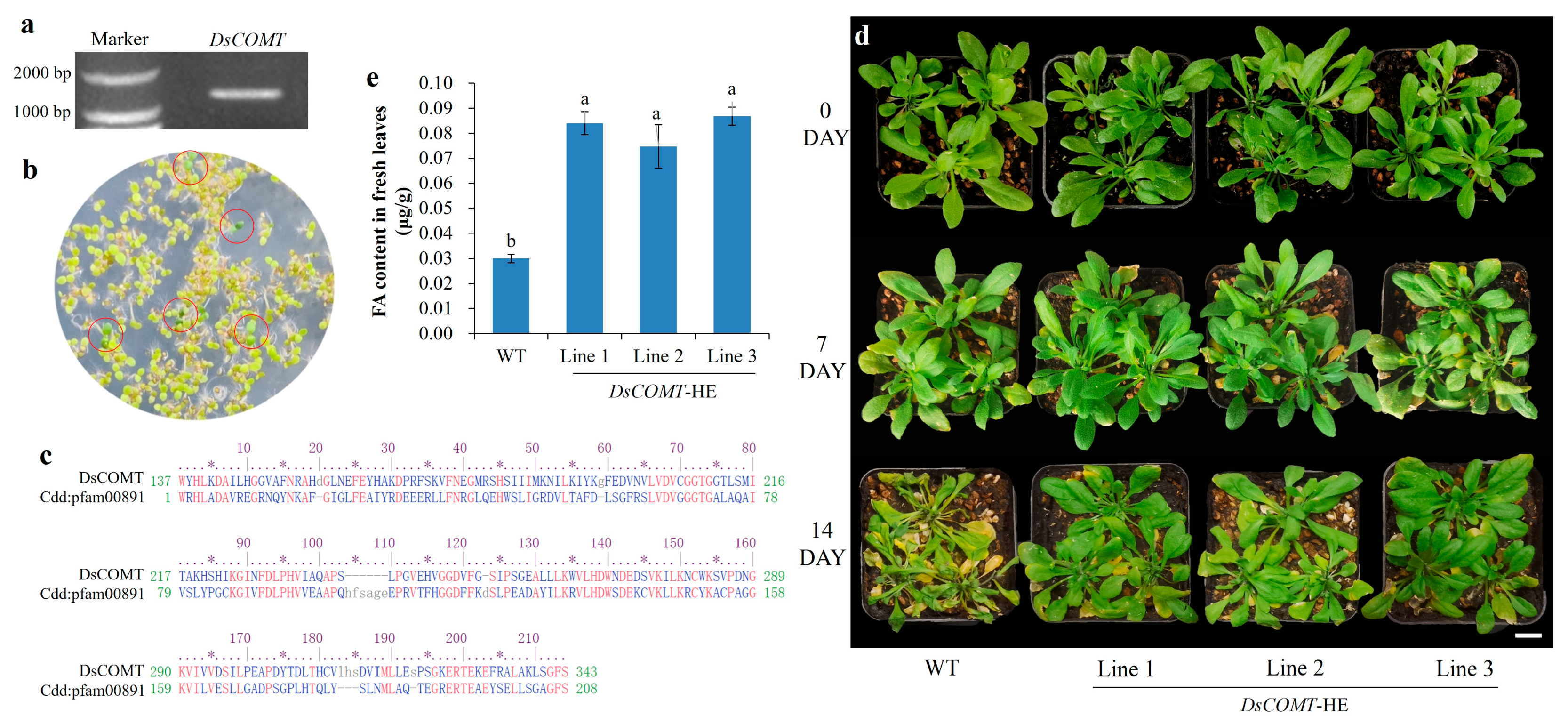
2.6. Heterologous Expression of DsCOMT in A. thaliana
3. Discussion
4. Material and Methods
4.1. Experimental Materials and Drought Treatment
4.2. Physiological and Biochemical Index Determination
4.3. Metabolome Analysis
4.4. Metabolites Identification and Quantification
4.5. RNA Extraction, Library Construction, and Sequencing
4.6. Gene Expression Profiles
4.7. Genetic Transformation and Drought Treatment in A. thaliana
4.8. HPLC Analysis of Ferulic Acid Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; You, H.; Ye, L.; Ou, Q.; Zhao, Y.; Wang, J.; Niu, J. Unveiling the Catalytic Roles of DsBBS1 and DsBBS2 in the Bibenzyl Biosynthesis of Dendrobium sinense. Molecules 2024, 29, 3682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Huang, W.; Song, X.; Niu, J. Transcriptomics and Metabolomics Reveal Purine and Phenylpropanoid Metabolism Response to Drought Stress in Dendrobium sinense, an Endemic Orchid Species in Hainan Island. Front. Genet. 2021, 12, 692702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Ou, Q.; You, H.; Wang, J.; Niu, J. Alternative First Exons Drive Enzymatic Activity Variation in Chalcone Synthase 3 of Dendrobium sinense. Forests 2023, 14, 1702. [Google Scholar] [CrossRef]
- Chen, X.-J.; Mei, W.-L.; Cai, C.-H.; Guo, Z.-K.; Song, X.-Q.; Dai, H.-F. Four New Bibenzyl Derivatives from Dendrobium sinense. Phytochem. Lett. 2014, 9, 107–112. [Google Scholar] [CrossRef]
- Chen, X.-J.; Mei, W.-L.; Zuo, W.-J.; Zeng, Y.-B.; Guo, Z.-K.; Song, X.-Q.; Dai, H.-F. A New Antibacterial Phenanthrenequinone from Dendrobium sinense. J. Asian Nat. Prod. Res. 2013, 15, 67–70. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, D.; Jiang, W.; Chen, G.; Li, W.; Li, N. Dihydrophenanthrenes from Medicinal Plants of Orchidaceae: A Review. Chin. Herb. Med. 2021, 13, 480–493. [Google Scholar] [CrossRef]
- Ketsa, S.; Warrington, I.J. The Dendrobium Orchid: Botany, Horticulture, and Utilization. Crop Sci. 2023, 63, 1829–1888. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Sardans, J.; Alrefaei, A.F.; Klem, K.; Fuchslueger, L.; Ramírez-Rojas, I.; Donald, J.; Leroy, C.; Langenhove, L.V.; Verbruggen, E.; et al. Tree Species and Epiphyte Taxa Determine the “Metabolomic Niche” of Canopy Suspended Soils in a Species-Rich Lowland Tropical Rainforest. Metabolites 2021, 11, 718. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Song, X.; Meng, Q.; Zhu, J.; Zhao, Y.; Yu, W. Influence of Host Tree Species on Isolation and Communities of Mycorrhizal and Endophytic Fungi from Roots of a Tropical Epiphytic Orchid, Dendrobium sinense (Orchidaceae). Mycorrhiza 2017, 27, 709–718. [Google Scholar] [CrossRef]
- Vanderschuren, H.; Nyaboga, E.; Poon, J.S.; Baerenfaller, K.; Grossmann, J.; Hirsch-Hoffmann, M.; Kirchgessner, N.; Nanni, P.; Gruissem, W. Large-Scale Proteomics of the Cassava Storage Root and Identification of a Target Gene to Reduce Postharvest Deterioration. Plant Cell 2014, 26, 1913–1924. [Google Scholar] [CrossRef]
- Dang, X.; Zhang, B.; Li, C.; Nagawa, S. FvNST1b NAC Protein Induces Secondary Cell Wall Formation in Strawberry. Int. J. Mol. Sci. 2022, 23, 13212. [Google Scholar] [CrossRef] [PubMed]
- Pratyusha, D.S.; Sarada, D.V. MYB Transcription Factors-Master Regulators of Phenylpropanoid Biosynthesis and Diverse Developmental and Stress Responses. Plant Cell Rep. 2022, 41, 2245–2260. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Wu, X.; Wu, J.; Luo, Y.; Bragg, J.; Anderson, O.; Vogel, J.; Gu, Y.Q. Phylogenetic, Molecular, and Biochemical Characterization of Caffeic Acid o-Methyltransferase Gene Family in Brachypodium distachyon. Int. J. Plant Genom. 2013, 2013, 423189. [Google Scholar] [CrossRef]
- Liu, L.-L.; Deng, Y.-Q.; Dong, X.-X.; Wang, C.-F.; Yuan, F.; Han, G.-L.; Wang, B.-S. ALDH2C4 Regulates Cuticle Thickness and Reduces Water Loss to Promote Drought Tolerance. Plant Sci. 2022, 323, 111405. [Google Scholar] [CrossRef]
- Chao, N.; Huang, S.; Kang, X.; Yidilisi, K.; Dai, M.; Liu, L. Systematic Functional Characterization of Cinnamyl Alcohol Dehydrogenase Family Members Revealed Their Functional Divergence in Lignin Biosynthesis and Stress Responses in Mulberry. Plant Physiol. Bioch. 2022, 186, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, W.; Zhang, H.; Zhan, Y.; Zeng, F. Fraxinus Mandshurica 4-Coumarate-CoA Ligase 2 Enhances Drought and Osmotic Stress Tolerance of Tobacco by Increasing Coniferyl Alcohol Content. Plant Physiol. Bioch. 2020, 155, 697–708. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Lee, K.; Back, K. Caffeic Acid O-Methyltransferase Is Involved in the Synthesis of Melatonin by Methylating N-Acetylserotonin in A Rabidopsis. J. Pineal Res. 2014, 57, 219–227. [Google Scholar] [CrossRef]
- Ge, L.; Yang, X.; Liu, Y.; Tang, H.; Wang, Q.; Chu, S.; Hu, J.; Zhang, N.; Shi, Q. Improvement of Seed Germination under Salt Stress via Overexpressing Caffeic Acid O-Methyltransferase 1 (SlCOMT1) in Solanum lycopersicum L. Int. J. Mol. Sci. 2023, 24, 734. [Google Scholar] [CrossRef]
- Gonçalves, A.Z.; Mercier, H. Transcriptomic and Biochemical Analysis Reveal Integrative Pathways between Carbon and Nitrogen Metabolism in Guzmania monostachia (Bromeliaceae) under Drought. Front. Plant Sci. 2021, 12, 715289. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Pittermann, J.; Brodersen, C.; Watkins, J.E., Jr. The Physiological Resilience of Fern Sporophytes and Gametophytes: Advances in Water Relations Offer New Insights into an Old Lineage. Front. Plant Sci. 2013, 4, 285. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Pérez, C.I.; Delgado-Sánchez, P.; Torres-Castillo, J.A.; Flores-Rivas, J.D.; Mendieta-Leiva, G.; De La Rosa-Manzano, E. Epiphytic Orchids Stanhopea tigrina and Prosthechea cochleata Are Differentially Affected by Drought in a Subtropical Cloud Forest. Photosynthetica 2019, 57, 1053–1065. [Google Scholar] [CrossRef]
- Gao, L.-Z.; Liu, Y.-L.; Zhang, D.; Li, W.; Gao, J.; Liu, Y.; Li, K.; Shi, C.; Zhao, Y.; Zhao, Y.-J.; et al. Evolution of Oryza Chloroplast Genomes Promoted Adaptation to Diverse Ecological Habitats. Commun. Biol. 2019, 2, 278. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yang, S.-J.; Zhang, J.-L.; Bartlett, M.; Zhang, S.-B. Correlated Evolution in Traits Influencing Leaf Water Balance in Dendrobium (Orchidaceae). Plant Ecol. 2014, 215, 1255–1267. [Google Scholar] [CrossRef]
- Ma, X.; Xia, H.; Liu, Y.; Wei, H.; Zheng, X.; Song, C.; Chen, L.; Liu, H.; Luo, L. Transcriptomic and Metabolomic Studies Disclose Key Metabolism Pathways Contributing to Well-Maintained Photosynthesis under the Drought and the Consequent Drought-Tolerance in Rice. Front. Plant Sci. 2016, 7, 1886. [Google Scholar] [CrossRef]
- Hura, T.; Grzesiak, S.; Hura, K.; Thiemt, E.; Tokarz, K.; Wędzony, M. Physiological and Biochemical Tools Useful in Drought-Tolerance Detection in Genotypes of Winter Triticale: Accumulation of Ferulic Acid Correlates with Drought Tolerance. Ann. Bot. 2007, 100, 767–775. [Google Scholar] [CrossRef]
- Liang, Y.; Han, C.; Yun, L.; Yang, Y.; Cao, Y. Transcriptomic and Metabolomic Analysis of the Mechanism of Temperature-Regulated Anthocyanin Biosynthesis in Purple Asparagus Spears. Sci. Hortic. 2022, 295, 110858. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Xu, N.; Sun, Y.; Li, Q.; Yue, L.; Zhou, Y.; He, M. Chrysanthemum × Grandiflora Leaf and Root Transcript Profiling in Response to Salinity Stress. BMC Plant Biol. 2022, 22, 240. [Google Scholar] [CrossRef]
- Okano, K.; Sato, Y.; Inoue, S.; Kawakami, S.; Kitani, S.; Honda, K. Enhancement of S-Adenosylmethionine-Dependent Methylation by Integrating Methanol Metabolism with 5-Methyl-Tetrahydrofolate Formation in Escherichia Coli. Catalysts 2020, 10, 1001. [Google Scholar] [CrossRef]
- Castro-Moretti, F.R.; Gentzel, I.N.; Mackey, D.; Alonso, A.P. Metabolomics as an Emerging Tool for the Study of Plant–Pathogen Interactions. Metabolites 2020, 10, 52. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Li, H.; Du, H.; Huang, H.; Li, Y.; Hu, Y.; Liu, H.; Liu, Y.; Yu, G.; et al. Identification of Transcription Factors ZmMYB111 and ZmMYB148 Involved in Phenylpropanoid Metabolism. Front. Plant Sci. 2016, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.; Alegre, L.; Van Breusegem, F.; Munné-Bosch, S. How Relevant Are Flavonoids as Antioxidants in Plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in Modulation of Cell Survival Signalling Pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Cui, H.; Li, M.; Yang, G.; Wang, Z.; Zhang, K. Carex rigescens Caffeic Acid O-Methyltransferase Gene CrCOMT Confer Melatonin-Mediated Drought Tolerance in Transgenic Tobacco. Front. Plant Sci. 2022, 13, 971431. [Google Scholar] [CrossRef]
- Fan, H.; Li, T.; Li, Z.; Lin, Y.; Cai, Y.; Jin, Q. Msap Analysis of Epigenetic Changes in Dendrobium huoshanense under Peg Simulated Drought Stress. J. Nucl. Agr. Sci. 2011, 25, 0363. [Google Scholar]
- Saccenti, E.; Hoefsloot, H.C.; Smilde, A.K.; Westerhuis, J.A.; Hendriks, M.M. Reflections on Univariate and Multivariate Analysis of Metabolomics Data. Metabolomics 2014, 10, 361–374. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate Transcript Quantification from RNA-Seq Data with or without a Reference Genome. BMC Bioinformatics 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Zhang, X.; Henriques, R.; Lin, S.-S.; Niu, Q.-W.; Chua, N.-H. Agrobacterium-Mediated Transformation of Arabidopsis thaliana Using the Floral Dip Method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, H.; Yi, A.; Ou, Q.; Wang, J.; Niu, J. Transcriptome and Metabolome Reveal Ferulic Acid as a Critical Phenylpropanoid for Drought Resistance in Dendrobium sinense. Plants 2025, 14, 1841. https://doi.org/10.3390/plants14121841
You H, Yi A, Ou Q, Wang J, Niu J. Transcriptome and Metabolome Reveal Ferulic Acid as a Critical Phenylpropanoid for Drought Resistance in Dendrobium sinense. Plants. 2025; 14(12):1841. https://doi.org/10.3390/plants14121841
Chicago/Turabian StyleYou, Huiyan, Ao Yi, Qiongjian Ou, Jia Wang, and Jun Niu. 2025. "Transcriptome and Metabolome Reveal Ferulic Acid as a Critical Phenylpropanoid for Drought Resistance in Dendrobium sinense" Plants 14, no. 12: 1841. https://doi.org/10.3390/plants14121841
APA StyleYou, H., Yi, A., Ou, Q., Wang, J., & Niu, J. (2025). Transcriptome and Metabolome Reveal Ferulic Acid as a Critical Phenylpropanoid for Drought Resistance in Dendrobium sinense. Plants, 14(12), 1841. https://doi.org/10.3390/plants14121841







