Intraspecific Evaluation of Phenotypic Variations of Caryopteris incana (Thunb. ex Houtt.) Miq. in Western Kyushu, Japan
Abstract
1. Introduction
2. Results
2.1. Comparison by Trait Classification
2.1.1. Phenology
2.1.2. Florets and Flower Cluster Traits
2.1.3. Leaf Traits
- Herbaceous form traits
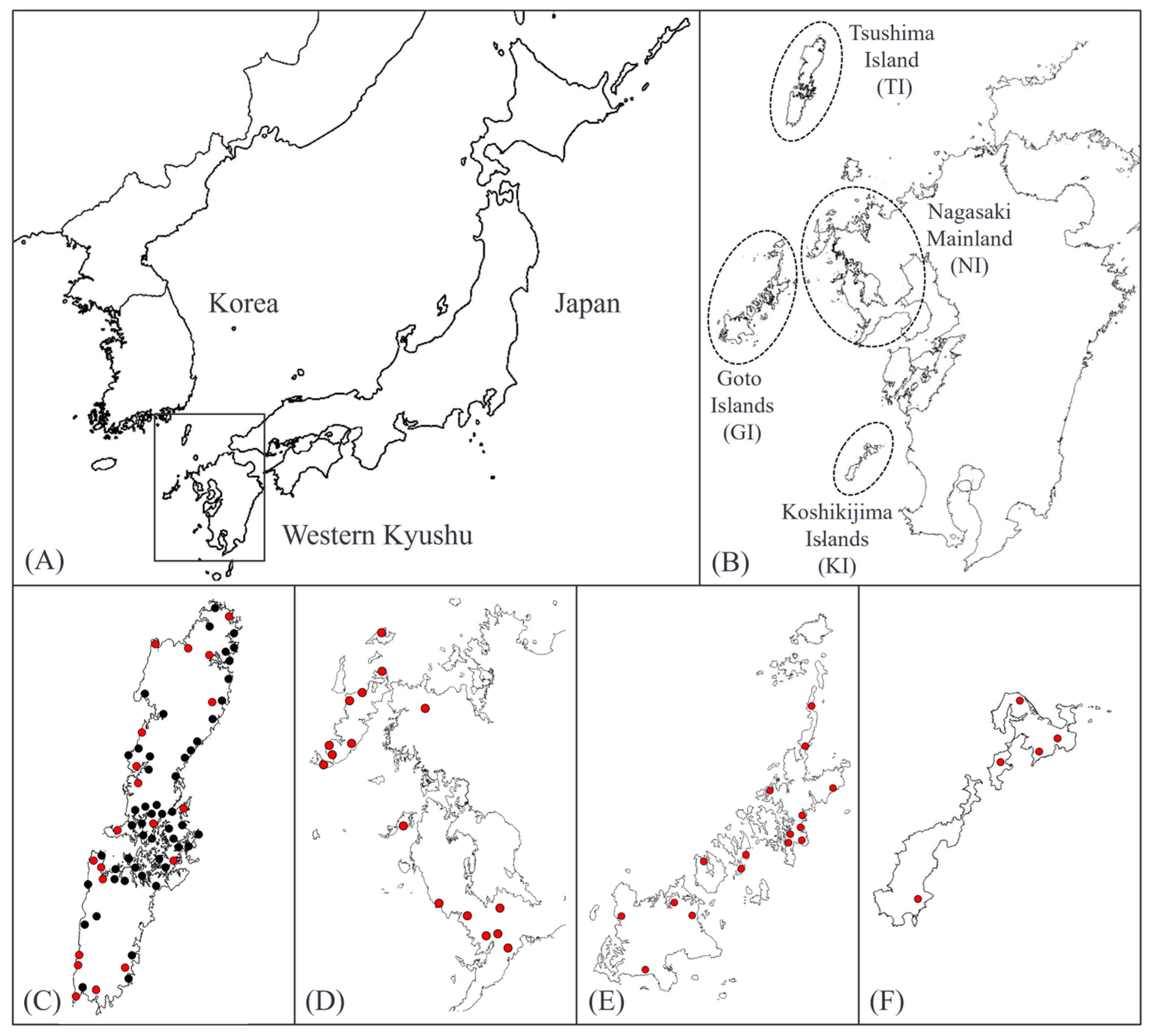
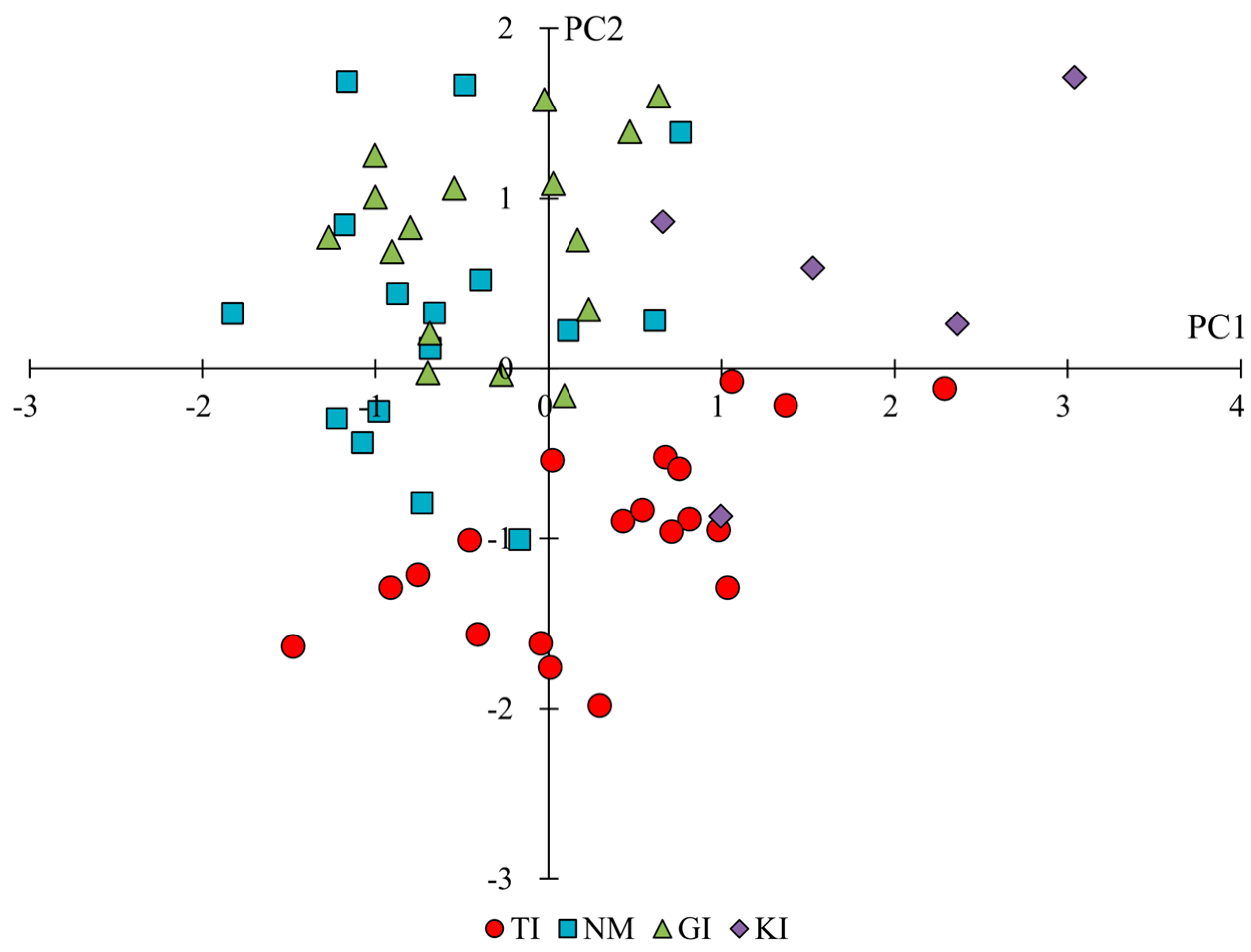
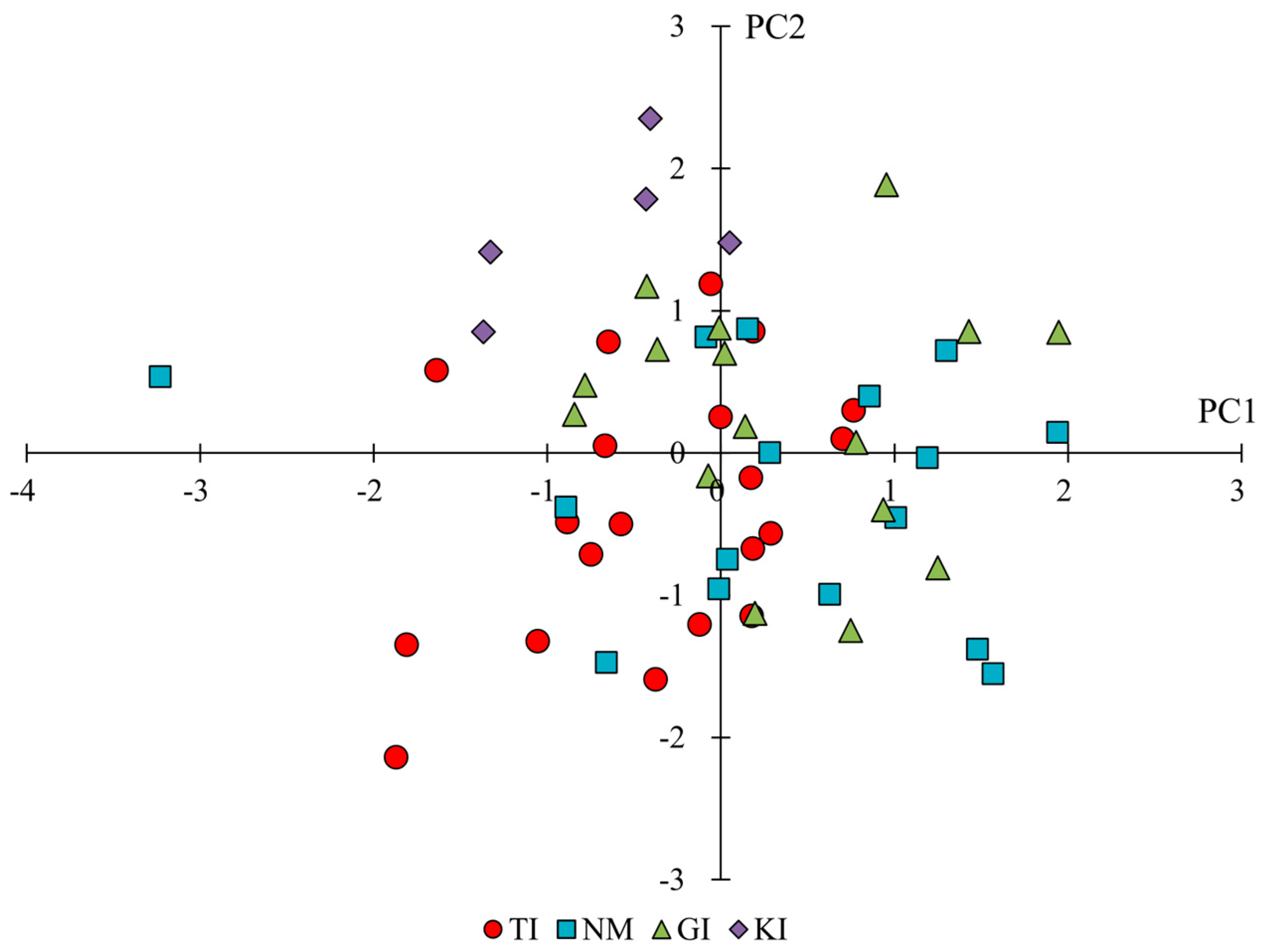
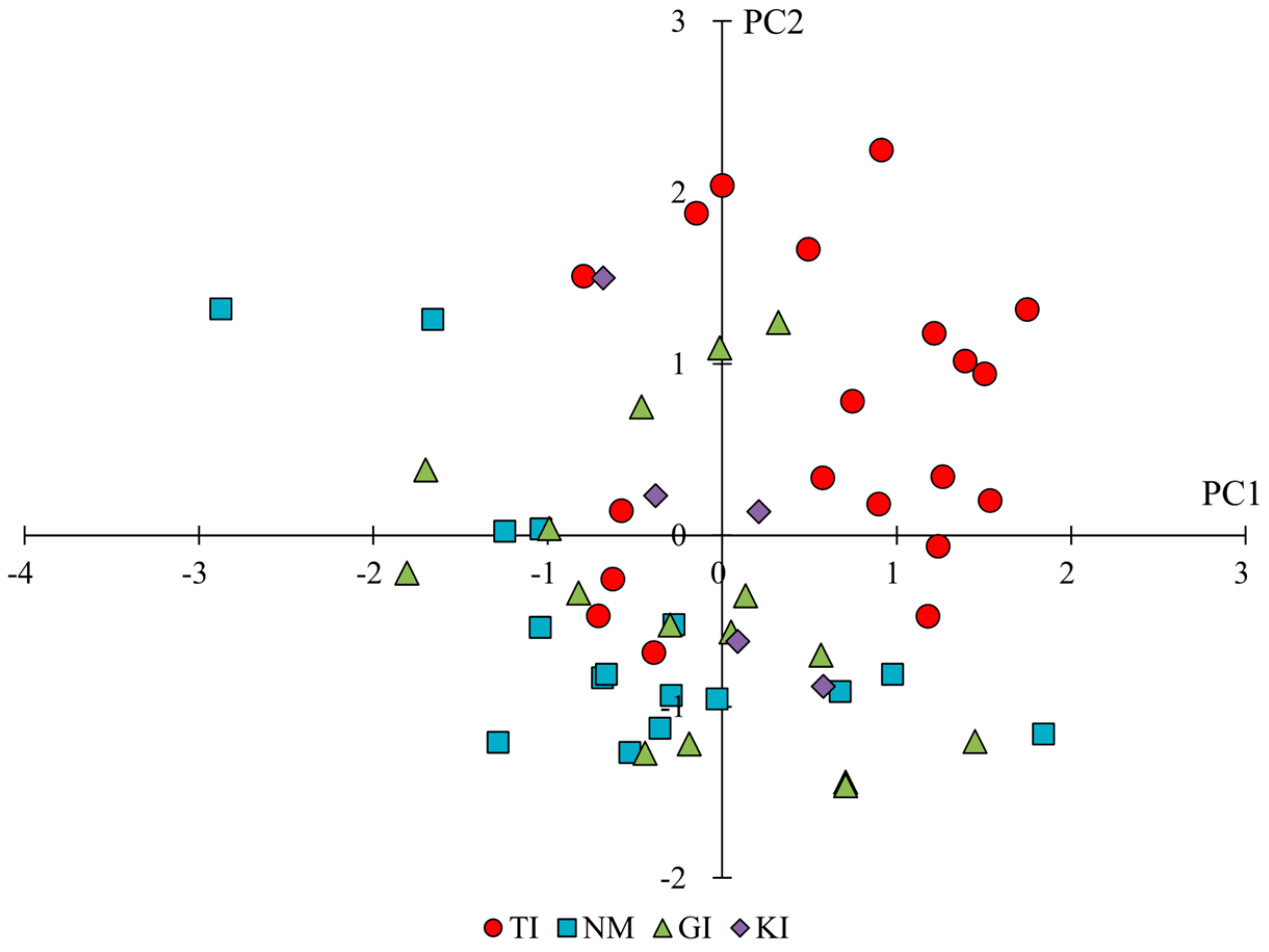
2.2. Phenotypic Variation Across and Within Regions
3. Discussion
3.1. Traits Related to Phenotypic Variation
3.2. Summary of Specific Phenotypes in Each Region
3.3. Factors in the Formation of Phenotypic Variation
3.4. Usefulness as a Plant Resource and Conservation
4. Materials and Methods
4.1. Growth Survey
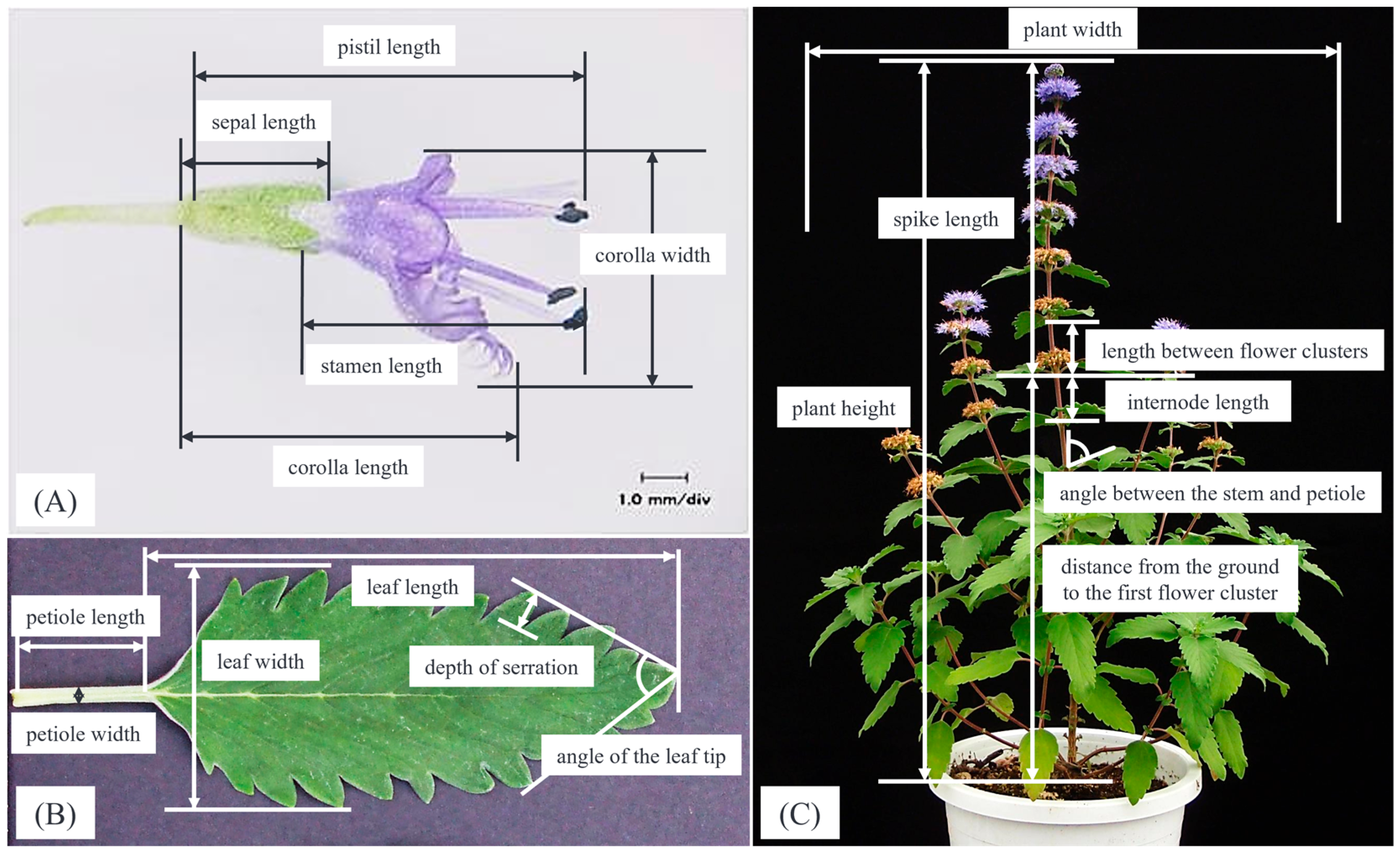
4.2. Measurement Method
4.3. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watanabe, S. The Garden Plant Dictionary, 1st ed.; Syogakukan Inc.: Tokyo, Japan, 1994. [Google Scholar]
- Miller, D. Caryopteris. In RHS Plant Trials and Assessments; Supplementary to RHS Trials and Awards No. 2; Royal Horticultural Society: London, UK, 2007. [Google Scholar]
- Nanba, T. Illustrated Medicinal Plants of Japanese and Chinese in Color; Hoikusha Publishers Co. Ltd.: Osaka, Japan, 1980. [Google Scholar]
- Chu, S.S.; Liu, Q.Z.; Zhou, L.; Du, S.S.; Liu, Z.L. Chemical composition and toxic activity of essential oil of Caryopteris incana against Sitophilus zeamais. Afr. J. Biotechnol. 2011, 10, 8476–8480. [Google Scholar]
- Itow, S. A review of phyto- and vegetation geography in the Japan-Korea strait region. Bull. Fac. Lib. Arts Nagasaki Univ. Nat. Sci. 1997, 38, 25–51. [Google Scholar]
- Itow, S.; Kawasato, H. The Distribution and ecology of Caryopteris incana Maxim. (Verbenaceae) in western Kyushu, Japan. Hikobia 1988, 10, 135–143. [Google Scholar]
- Environment Agency of Japan. Threatened wildlife of Japan. In Red Data Book, 2nd ed.; Japan Wildlife Research Center: Tokyo, Japan, 2000; Volume 8, p. 521. [Google Scholar]
- Ando, M.; Watanabe, H.; Matsubara, K.; Taniguchi, A. Intraspecific phylogenetic relationships of Caryopteris incana in the Tsushima Islands, Japan, using DNA sequence analysis. Am. J. Plant Sci. 2015, 6, 2361–2373. [Google Scholar] [CrossRef]
- Ando, M.; Kuwabara, K.; Matsubara, K.; Watanabe, H. Distribution and phylogeography of Caryopteris incana (Lamiaceae) based on chloroplast DNA sequences in west Kyushu, Japan. Am. J. Plant Sci. 2016, 7, 167–180. [Google Scholar] [CrossRef][Green Version]
- Walker, C. Increases in plant phenotypic diversity in response to aridity and grazing. Nat. Plants 2024, 10, 1277. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.N.; Odell, J.; Cruse-Sanders, J.; Rogers, W.; Anderson, J.T.; Baskauf, C. Phenotypic plasticity and genetic diversity elucidate rarity and vulnerability of an endangered riparian plant. Ecosphere 2022, 13, e3996. [Google Scholar] [CrossRef]
- Tilman, D.; May, R.M.; Lehman, C.L.; Nowak, M.A. Habitat destruction and the extinction debt. Nature 1994, 371, 65–66. [Google Scholar] [CrossRef]
- Helm, A.; Oja, T.; Saar, L.; Takkis, K.; Talve, T.; Partel, T. Human influence lowers plant genetic diversity in communities with extinction debt. J. Ecol. 2009, 97, 1329–1336. [Google Scholar] [CrossRef]

| A | mean value of survey traits for phenology | |||||||||||||||||||||||||||||
| group | A1: number of days to flowering [d] | A2: number of days in the flowering period [d] | ||||||||||||||||||||||||||||
| Tsushima Islands (TI) | 165.12 | a | 77.19 | a | ||||||||||||||||||||||||||
| Nagasaki Mainland (NM) | 166.78 | a | 88.87 | a | ||||||||||||||||||||||||||
| Goto Islands (GI) | 171.73 | b | 83.5 | a | ||||||||||||||||||||||||||
| Koshikijima Islands (KI) | 180.28 | c | 76.23 | a | ||||||||||||||||||||||||||
| All population | 168.62 | 81.83 | ||||||||||||||||||||||||||||
| B | mean value of survey traits for florets and flower cluster | |||||||||||||||||||||||||||||
| group | B1: corolla length [mm] | B2: corolla width [mm] | B3: aspect ratio of corolla (B1/B2) | B4: stamen length [mm] | B5: pistil length [mm] | B6: ratio of stamen/pistil (B4/B5) | ||||||||||||||||||||||||
| TI | 9.51 | b | 7.35 | a | 1.31 | c | 7.54 | a | 10.75 | b | 0.71 | b | ||||||||||||||||||
| NM | 9.01 | a | 7.43 | a | 1.23 | b | 7.55 | a | 10.29 | a | 0.74 | b | ||||||||||||||||||
| GI | 8.99 | a | 7.92 | b | 1.15 | a | 7.75 | a | 10.93 | b | 0.72 | b | ||||||||||||||||||
| KI | 10.26 | c | 8.59 | c | 1.21 | a | b | 7.71 | a | 11.87 | c | 0.65 | a | |||||||||||||||||
| All population | 9.28 | 7.63 | 1.24 | 7.61 | 10.75 | 0.72 | ||||||||||||||||||||||||
| B | mean value of survey traits for florets and flower cluster | |||||||||||||||||||||||||||||
| group | B8: peduncle length [mm] | B9: peduncle width [mm] | B10: number of florets | B11: number of flower clusters on main stem | B12: vertical diameter of flower clusters [mm] | B13: horizontal diameter of flower clusters [mm] | ||||||||||||||||||||||||
| TI | 8.46 | a | b | 1.48 | a | 73.09 | b | 8.52 | a | 44.35 | b | 38.98 | a | |||||||||||||||||
| NM | 8.1 | a | 1.49 | a | 66.73 | a | 8.72 | a | 41.66 | a | 37.58 | a | ||||||||||||||||||
| GI | 8.77 | b | c | 1.44 | a | 61.46 | a | 8.14 | a | 42.48 | a | b | 38.9 | a | ||||||||||||||||
| KI | 9.26 | c | 1.74 | b | 87.17 | c | 9.04 | a | 50.62 | c | 43.6 | b | ||||||||||||||||||
| All population | 8.51 | 1.49 | 69.09 | 8.51 | 43.53 | 38.91 | ||||||||||||||||||||||||
| B | mean value of survey traits for florets and flower cluster | |||||||||||||||||||||||||||||
| group | B15: flower color L-value | B16: flower color a-value | B17: flower color b-value | |||||||||||||||||||||||||||
| TI | 49.29 | a | 10.46 | b | −23.05 | c | ||||||||||||||||||||||||
| NM | 56.72 | c | 7.6 | a | −17.36 | a | ||||||||||||||||||||||||
| GI | 58.2 | c | 7.53 | a | −17.93 | a | b | |||||||||||||||||||||||
| KI | 53.81 | b | 9.8 | b | −19.94 | b | ||||||||||||||||||||||||
| All population | 54.32 | 8.76 | −19.71 | |||||||||||||||||||||||||||
| C | mean value of survey traits for leaf | |||||||||||||||||||||||||||||
| group | C1: leaf length [mm] | C2: leaf width [mm] | C3: aspect ratio of leaf (C1/C2) | C4: petiole length [mm] | C5: petiole width [mm] | C6: number of serrations | ||||||||||||||||||||||||
| TI | 65.85 | b | 33.26 | a | b | 2 | c | 19.11 | a | 2.2 | a | 19.54 | c | |||||||||||||||||
| NM | 61.12 | a | 32.34 | a | 1.93 | b | c | 18.86 | a | 2.2 | a | 16.57 | b | |||||||||||||||||
| GI | 64.39 | b | 34.8 | b | 1.87 | b | 19.79 | a | 2.31 | b | 15.82 | a | b | |||||||||||||||||
| KI | 63.48 | a | b | 41.23 | c | 1.55 | a | 20.06 | a | 2.36 | b | 14.64 | a | |||||||||||||||||
| All population | 63.89 | 34.06 | 1.91 | 19.3 | 2.24 | 17.28 | ||||||||||||||||||||||||
| C | mean value of survey traits for leaf | |||||||||||||||||||||||||||||
| group | C8: angle of the leaf tip [°] | C9: angle between stem and petiole [°] | C10: leaf color L-value | C11: leaf color a-value | C12: leaf color b-value | |||||||||||||||||||||||||
| TI | 63.92 | b | 66.97 | a | 36.4 | a | −7.62 | b | 15.55 | a | ||||||||||||||||||||
| NM | 59.92 | a | 77.95 | b | 38.25 | c | −8.04 | a | 17.09 | b | ||||||||||||||||||||
| GI | 59.06 | a | 77.66 | b | 37.61 | b | c | −8.21 | a | 17.25 | b | |||||||||||||||||||
| KI | 67.19 | c | 74.7 | b | 37.1 | a | b | −7.8 | a | b | 14.83 | a | ||||||||||||||||||
| All population | 61.72 | 73.75 | 37.32 | −7.91 | 16.4 | |||||||||||||||||||||||||
| D | mean value of survey traits for herbaceous form | |||||||||||||||||||||||||||||
| group | D1: length between flower clusters [mm] | D2: distance from ground to the first flower cluster mm] | D3: spike length (D6-D2) | D4: position of first inflorescence node | D5: plant height at flowering [mm] | D6: plant height at fruiting [mm] | ||||||||||||||||||||||||
| TI | 41.32 | c | 258.04 | b | 282.41 | b | 15.66 | a | 441.16 | b | 540.45 | b | ||||||||||||||||||
| NM | 29.77 | a | 229.19 | a | 221.65 | a | 16.32 | b | 364.75 | a | 450.91 | a | ||||||||||||||||||
| GI | 34.37 | b | 229.73 | a | 230.27 | a | 16.47 | b | 369.42 | a | 460 | a | ||||||||||||||||||
| KI | 29.16 | a | 271.65 | b | 251.93 | a | b | 18.67 | c | 390.57 | a | 523.59 | b | |||||||||||||||||
| All population | 35.07 | 242.8 | 247.81 | 16.31 | 394.98 | 490.64 | ||||||||||||||||||||||||
| D | mean value of survey traits for herbaceous form | |||||||||||||||||||||||||||||
| group | D8: plant width [mm] | D9: internode length [mm] | D10: number of flowering side branches | D11: stem diameter [mm] | D12: D6/B11 | D13: D3/D2 | ||||||||||||||||||||||||
| TI | 290.66 | b | 38.71 | c | 5.34 | a | 8.16 | b | 66.03 | c | 1.17 | b | ||||||||||||||||||
| NM | 319.95 | c | 28.98 | a | 9.04 | b | 8.06 | a | b | 54.32 | a | 1.08 | a | b | ||||||||||||||||
| GI | 352.95 | d | 32.52 | b | 8.3 | b | 7.67 | a | 59.69 | a | b | 1.08 | a | b | ||||||||||||||||
| KI | 243.43 | a | 28.85 | a | 5.11 | a | 7.55 | a | 60.75 | b | c | 0.98 | a | |||||||||||||||||
| All population | 312.88 | 33.38 | 7.22 | 7.95 | 60.44 | 1.1 | ||||||||||||||||||||||||
| A | coefficient of variation traits for phenology | |||||||||||||
| group | A1 | A2 | ||||||||||||
| Tsushima Islands (TI) | 0.08 | 0.44 | ||||||||||||
| Nagasaki Mainland (NM) | 0.06 | 0.39 | ||||||||||||
| Goto Islands (GI) | 0.06 | 0.45 | ||||||||||||
| Koshikijima Islands (KI) | 0.07 | 0.41 | ||||||||||||
| All population | 0.07 | 0.43 | ||||||||||||
| B | coefficient of variation for traits for florets and flower cluster | |||||||||||||
| group | ×1 | ×2 | ×3 | ×4 | ×5 | ×6 | ×7 | ×8 | ×9 | ×10 | ×11 | ×12 | ×13 | ×14 |
| TI | 0.09 | 0.14 | 0.15 | 0.1 | 0.15 | 0.12 | 0.11 | 0.25 | 0.12 | 0.34 | 0.26 | 0.14 | 0.14 | 0.08 |
| NM | 0.11 | 0.13 | 0.14 | 0.1 | 0.13 | 0.12 | 0.09 | 0.29 | 0.13 | 0.33 | 0.25 | 0.14 | 0.11 | 0.09 |
| GI | 0.11 | 0.13 | 0.16 | 0.11 | 0.12 | 0.14 | 0.12 | 0.23 | 0.11 | 0.32 | 0.31 | 0.13 | 0.12 | 0.11 |
| KI | 0.09 | 0.1 | 0.14 | 0.09 | 0.1 | 0.11 | 0.08 | 0.16 | 0.12 | 0.32 | 0.28 | 0.15 | 0.11 | 0.09 |
| All population | 0.11 | 0.14 | 0.16 | 0.1 | 0.14 | 0.13 | 0.11 | 0.25 | 0.13 | 0.35 | 0.27 | 0.15 | 0.13 | 0.09 |
| B | coefficient of variation for traits for florets and flower cluster | |||||||||||||
| group | ×39 | ×40 | ×41 | |||||||||||
| TI | 0.09 | 0.19 | 0.12 | |||||||||||
| NM | 0.1 | 0.27 | 0.3 | |||||||||||
| GI | 0.09 | 0.23 | 0.25 | |||||||||||
| KI | 0.08 | 0.15 | 0.16 | |||||||||||
| All population | 0.12 | 0.27 | 0.24 | |||||||||||
| C | coefficient of variation for traits for leaf | |||||||||||||
| group | ×27 | ×28 | ×29 | ×30 | ×31 | ×32 | ×33 | ×34 | ×35 | ×36 | ×37 | ×38 | ||
| TI | 0.11 | 0.13 | 0.12 | 0.23 | 0.12 | 0.14 | 0.3 | 0.13 | 0.14 | 0.07 | 0.16 | 0.21 | ||
| NM | 0.1 | 0.15 | 0.17 | 0.19 | 0.1 | 0.21 | 0.32 | 0.14 | 0.15 | 0.07 | 0.15 | 0.2 | ||
| GI | 0.13 | 0.13 | 0.15 | 0.22 | 0.11 | 0.21 | 0.29 | 0.12 | 0.16 | 0.06 | 0.11 | 0.17 | ||
| KI | 0.11 | 0.11 | 0.11 | 0.19 | 0.12 | 0.15 | 0.36 | 0.11 | 0.15 | 0.06 | 0.11 | 0.17 | ||
| All population | 0.12 | 0.15 | 0.16 | 0.21 | 0.12 | 0.2 | 0.31 | 0.14 | 0.16 | 0.07 | 0.14 | 0.2 | ||
| D | coefficient of variation for traits for herbaceous form | |||||||||||||
| group | ×15 | ×16 | ×17 | ×18 | ×19 | ×20 | ×21 | ×22 | ×23 | ×24 | ×42 | ×43 | ×44 | ×45 |
| TI | 0.24 | 0.25 | 0.29 | 0.14 | 0.21 | 0.19 | 0.15 | 0.38 | 0.27 | 0.47 | 0.17 | 0.23 | 0.41 | 0.2 |
| NM | 0.29 | 0.31 | 0.27 | 0.14 | 0.27 | 0.2 | 0.16 | 0.23 | 0.34 | 0.38 | 0.21 | 0.29 | 0.49 | 0.19 |
| GI | 0.23 | 0.27 | 0.33 | 0.16 | 0.21 | 0.21 | 0.15 | 0.27 | 0.27 | 0.34 | 0.19 | 0.26 | 0.43 | 0.17 |
| KI | 0.18 | 0.19 | 0.34 | 0.11 | 0.17 | 0.18 | 0.18 | 0.31 | 0.16 | 0.48 | 0.17 | 0.22 | 0.46 | 0.14 |
| All population | 0.29 | 0.27 | 0.32 | 0.15 | 0.24 | 0.21 | 0.15 | 0.32 | 0.31 | 0.46 | 0.19 | 0.26 | 0.44 | 0.22 |
| Label | Trait | Characteristic value | ||
|---|---|---|---|---|
| principal component numbers | PC 1 | PC 2 | PC 3 | |
| Eigenvalue | 5.39 | 2.71 | 1.99 | |
| Contribution rate (%) | 31.70 | 15.96 | 11.69 | |
| B1 | corolla length | 0.72 | −0.24 | −0.28 |
| B2 | corolla width | 0.64 | 0.52 | −0.35 |
| B3 | aspect ratio of corolla (B1/B2) | −0.11 | −0.71 | 0.17 |
| B4 | stamen length | 0.39 | 0.34 | −0.35 |
| B5 | pistil length | 0.78 | 0.10 | −0.49 |
| B6 | ratio of stamen/pistil (B4/B5) | −0.61 | 0.15 | 0.31 |
| B7 | sepal length | 0.53 | −0.16 | −0.34 |
| B8 | peduncle length | 0.28 | 0.37 | 0.29 |
| B9 | peduncle width | 0.50 | 0.15 | 0.18 |
| B10 | number of florets | 0.49 | 0.10 | 0.72 |
| B11 | number of flower clusters on the main stem | −0.23 | −0.15 | −0.10 |
| B12 | vertical diameter of flower cluster | 0.84 | 0.30 | 0.40 |
| B13 | horizontal diameter of flower cluster | 0.80 | 0.28 | 0.12 |
| B14 | aspect ratio of flower cluster (B12/B13) | 0.39 | 0.13 | 0.59 |
| B15 | flower color (L-value) | −0.45 | 0.70 | −0.06 |
| B16 | flower color (a-value) | 0.66 | −0.62 | 0.11 |
| B17 | flower color (b-value) | −0.52 | 0.67 | −0.07 |
| Label | Trait | Characteristic value | ||
|---|---|---|---|---|
| principal component numbers | PC 1 | PC 2 | PC 3 | |
| Eigenvalue | 2.93 | 2.60 | 2.18 | |
| Contribution rate (%) | 24.40 | 21.64 | 18.17 | |
| C1 | leaf length | 0.36 | 0.20 | 0.79 |
| C2 | leaf width | −0.14 | 0.92 | 0.06 |
| C3 | aspect ratio of leaf (C1/C2) | 0.36 | −0.80 | 0.40 |
| C4 | petiole length | 0.26 | 0.19 | 0.53 |
| C5 | petiole width | 0.05 | 0.80 | 0.22 |
| C6 | number of serrations | 0.14 | −0.29 | 0.66 |
| C7 | depth of serration | 0.32 | 0.31 | 0.25 |
| C8 | angle of the leaf tip | −0.26 | 0.39 | 0.15 |
| C9 | angle between the stem and petiole | 0.29 | 0.08 | −0.64 |
| C10 | leaf color (L-value) | 0.86 | 0.05 | −0.21 |
| C11 | leaf color (a-value) | −0.84 | −0.21 | 0.22 |
| C12 | leaf color (b-value) | 0.93 | 0.05 | −0.17 |
| Label | Trait | Characteristic value | ||
|---|---|---|---|---|
| principal component numbers | PC 1 | PC 2 | PC 3 | |
| Eigenvalue | 6.14 | 2.82 | 1.36 | |
| Contribution rate (%) | 43.88 | 20.18 | 9.68 | |
| D1 | length between flower clusters | 0.89 | 0.27 | −0.07 |
| D2 | distance from the ground to the first flower cluster | 0.90 | −0.22 | 0.12 |
| D3 | spike length (D6-D2) | 0.37 | 0.81 | 0.37 |
| D4 | position of the first inflorescence node | 0.13 | −0.38 | 0.07 |
| D5 | plant height at flowering | 0.91 | −0.08 | 0.10 |
| D6 | plant height at fruiting | 0.82 | 0.36 | 0.31 |
| D7 | ratio of plant height at flowering/at fruiting (D5/D6) | 0.47 | −0.6 | −0.29 |
| D8 | plant width | 0.25 | −0.38 | 0.75 |
| D9 | internode length | 0.89 | 0.20 | −0.04 |
| D10 | number of flowering side branches | −0.12 | −0.67 | 0.54 |
| D11 | stem diameter | −0.28 | 0.11 | 0.00 |
| D12 | plant height at fruiting/number of flower clusters (D6/B11) | 0.91 | −0.21 | −0.21 |
| D13 | spike length/distance from the ground to the first flower cluster (D3/D2) | −0.51 | 0.77 | 0.21 |
| D14 | spike length/number of flower clusters on the main stem-1 (D3/B11-1) | 0.80 | 0.29 | −0.23 |
| Region | Altitude (m) | Number of Neighbor Populations | Number of Individuals | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ave. | Number of Populations | |||||||||
| <10 | 10–100 | 100< | 0 | 1 | 2≦ | <20 | 20–100 | 100< | ||
| Tsushima Islands | 39.9 ± 9.6 | 5 | 13 | 2 | 1 | 2 | 17 | 7 | 3 | 10 |
| Nagasaki Mainland | 175.3 ± 26.9 | 3 | 1 | 12 | 9 | 5 | 2 | 4 | 6 | 6 |
| Goto Islands | 35.8 ± 7.7 | 5 | 11 | 0 | 9 | 3 | 4 | 6 | 3 | 7 |
| Koshikijima Islands | 123.8 ± 22.0 | 0 | 3 | 2 | 3 | 2 | 0 | 3 | 0 | 2 |
| whole region | 84.1 ± 11.9 | 13 | 28 | 16 | 22 | 12 | 23 | 20 | 12 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, M.; Kuronuma, T.; Watanabe, H. Intraspecific Evaluation of Phenotypic Variations of Caryopteris incana (Thunb. ex Houtt.) Miq. in Western Kyushu, Japan. Plants 2025, 14, 1840. https://doi.org/10.3390/plants14121840
Ando M, Kuronuma T, Watanabe H. Intraspecific Evaluation of Phenotypic Variations of Caryopteris incana (Thunb. ex Houtt.) Miq. in Western Kyushu, Japan. Plants. 2025; 14(12):1840. https://doi.org/10.3390/plants14121840
Chicago/Turabian StyleAndo, Masaya, Takanori Kuronuma, and Hitoshi Watanabe. 2025. "Intraspecific Evaluation of Phenotypic Variations of Caryopteris incana (Thunb. ex Houtt.) Miq. in Western Kyushu, Japan" Plants 14, no. 12: 1840. https://doi.org/10.3390/plants14121840
APA StyleAndo, M., Kuronuma, T., & Watanabe, H. (2025). Intraspecific Evaluation of Phenotypic Variations of Caryopteris incana (Thunb. ex Houtt.) Miq. in Western Kyushu, Japan. Plants, 14(12), 1840. https://doi.org/10.3390/plants14121840






