Modulations of Photosynthetic Membrane Lipids and Fatty Acids in Response to High Light in Brown Algae (Undaria pinnatifida)
Abstract
1. Introduction
2. Results
2.1. Lipid Content and Lipid Class Profiles
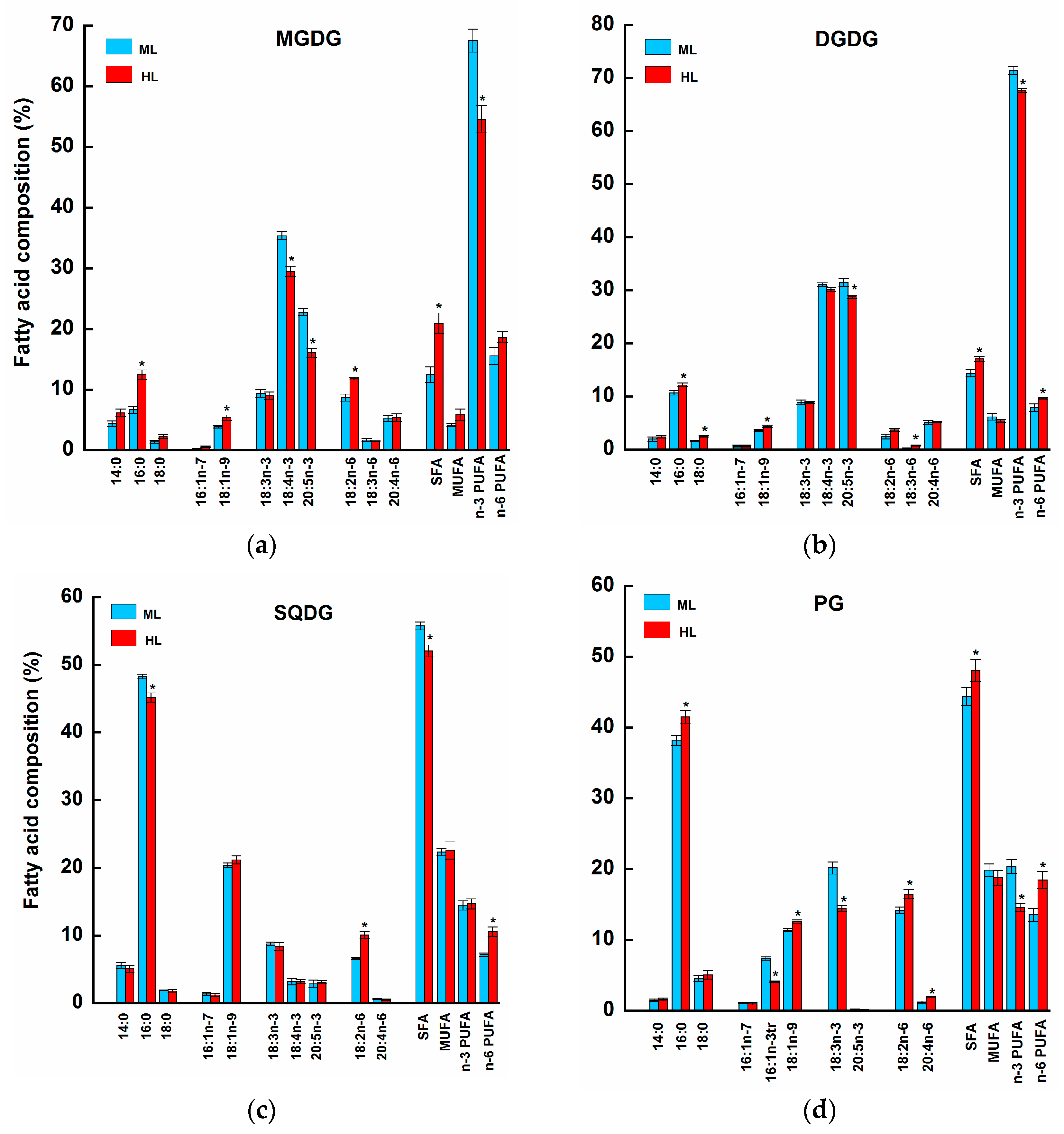
2.2. Fatty Acid Profiles
2.3. Chloroplast Ultrastructure
2.4. Photosynthetic Performance and Lipid Peroxidation
3. Discussion
4. Materials and Methods
4.1. Experimental Protocol
4.2. Lipid Analysis
4.3. Chloroplast Ultrastructure Studies
4.4. Lipid Peroxidation Analysis
4.5. Photosynthetic Performance and Pigment Analyses
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Chl | Chlorophyll |
| DGDG | Digalactosyldiacylglycerol |
| Ek | Minimum irradiance to saturate photosynthesis |
| Fv/Fm | Maximum photochemical efficiency of photosystem II |
| HL | High light |
| LPX | Lipid peroxidation |
| MDA | Malondialdehyde |
| MGDG | Monogalactosyldiacylglycerol |
| ML | Moderate light |
| MUFA | Monounsaturated fatty acids |
| PAR | Photosynthetically active radiation |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| PI | Phosphatidylinositol |
| Pmax | Maximum rate of net photosynthesis |
| PSII | Photosystem II |
| PUFA | Polyunsaturated fatty acids |
| PG | Phosphatidylglycerol |
| PC | Phosphatidylcholine |
| SFA | Saturated fatty acids |
| SQDG | Sulfoquinovosyldiacylglycerol |
| TAG | Triacylglycerols |
References
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The role of light in regulation plant growth, development and sugar metabolism: A review. Front. Plant Sci. 2024, 15, 1507628. [Google Scholar]
- Koch, K.; Thiel, M.; Hagen, W.; Graeve, M.; Gomez, I.; Jofre, D.; Hofmann, L.C.; Tala, F.; Bischof, K. Short- and long-term acclimation patterns of the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae) along a depth gradient. J. Phycol. 2016, 52, 260–273. [Google Scholar] [CrossRef]
- Morales, A.; Kaiser, E. Photosynthetic acclimation to fluctuating irradiance in plants. Front. Plant Sci. 2020, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Ostermeier, M.; Garibay-Hernández, A.; Holzer, V.J.C.; Schroda, M.; Nickelsen, J. Structure, biogenesis, and evolution of thylakoid membranes. Plant Cell. 2024, 36, 4014–4035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lepetit, B.; Goss, R.; Jakob, T.; Wilhelm, C. Molecular dynamics of the diatom thylakoid membrane under different light conditions. Photosynth. Res. 2012, 111, 245–257. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Suguru, K.; Atsuki, O.; Nodoka, T.; Tomomi, I.; Haruka, H.; Noriko, M.; Yasuo, I. Quality control of PSII: Behavior of PSII in the highly crowded grana thylakoids under excessive light. Plant Cell Physiol. 2014, 55, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka-Nishimura, M. Close relationships between the PSII repair cycle and thylakoid membrane dynamics. Plant Cell Physiol. 2016, 57, 1115–1122. [Google Scholar] [CrossRef]
- Hernandez, M.L.; Cejudo, F.J. Chloroplast lipids metabolism and function. A redox Perspective. Front. Plant Sci. 2021, 12, 2021. [Google Scholar] [CrossRef]
- Garab, G.; Yaguzhinsky, L.S.; Dlouhý, O.; Nesterov, S.V.; Špunda, V.; Gasanoff, E.S. Structural and functional roles of non-bilayer lipid phases of chloroplast thylakoid membranes and mitochondrial inner membranes. Progr. Lipid Res. 2022, 86, 101163. [Google Scholar] [CrossRef]
- Billah, M.; Aktar, S.; Sikder, R.K.; Ahammed, G.J.; Hu, W.; Li, F.; Yang, Z. Exploring regulatory roles of plant thylakoid-bound proteins involved in abiotic stress responses. J. Plant Growth Regul. 2024, 43, 1570–1591. [Google Scholar] [CrossRef]
- Kobayashi, K. Role of membrane glycerolipids in photosynthesis, thylakoid biogenesis and chloroplast development. J. Plant Res. 2016, 129, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Klyachko-Gurvich, G.L.; Pronina, N.A.; Ladygin, V.G.; Tsoglin, L.N.; Semenenko, V.E. Uncoupled functioning of separate photosystems: 1. Characteristics of fatty acid desaturation and its role. Russ. J. Plant Physiol. 2000, 47, 603–612. [Google Scholar]
- Koch, K.; Hagen, W.; Graeve, M.; Bischof, K. Fatty acid compositions associated with high-light tolerance in the intertidal rhodophytes Mastocarpus stellatus and Chondrus crispus. Helgol. Mar. Res. 2017, 71, 15. [Google Scholar] [CrossRef][Green Version]
- van Rooijen, R.; Harbinson, J.; Aarts, M.G.M. Photosynthetic response to increased irradiance correlates to variation in transcriptional response of lipid-remodeling and heat-shock genes. Plant Direct 2018, 2, e00069. [Google Scholar] [CrossRef]
- Yu, L.; Fan, J.; Fan, J.; Zhou, C.; Xu, C. Chloroplast lipid biosynthesis is fine-turned to thylakoid membrane remodeling during light acclimation. Plant Physiol. 2021, 185, 94–107. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Z.; Hu, Q.; Sommerfeld, M.; Lu, Y.; Han, D. Cellular capacities for high-light acclimation and changing lipid profiles across life cycle stages of the green alga Haematococcus pluvialis. PLoS ONE 2014, 9, e106679. [Google Scholar] [CrossRef][Green Version]
- Dong, H.P.; Dong, Y.L.; Cui, L.; Balamurugan, S.; Gao, J.; Lu, S.H.; Jiang, T. High light stress triggers distinct proteomic responses in the marine diatom Thalassiosira pseudonana. BMC Genom. 2016, 17, 994. [Google Scholar] [CrossRef]
- Widzgowski, J.; Vogel, A.; Altrogge, L.; Pfaff, J.; Schoof, H.; Usadel, B.; Nedbal, L.; Schurr, U.; Pfaff, C. High light induces species specific changes in the membrane lipid composition of Chlorella. Biochem. J. 2020, 477, 2543–2559. [Google Scholar] [CrossRef]
- Ferrer-Ledo, N.; van Oossanen, S.; Wijffels, R.H.; Evers, W.A.; Südfeld, C.; Janssen, M. Effect of incident light and light gradients on eicosapentaenoic acid distribution between lipid classes in Nannochloropsis oceanica. J. Appl. Phycol. 2025, 37, 163–179. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Yakovleva, I.M. Effect of solar irradiance on lipids of the green alga Ulva fenestrata Postels et Ruprecht. Bot. Mar. 2004, 47, 395–401. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Yakovleva, I.M. Lipid composition of the red alga Tichocarpus crinitus exposed to different levels of photon irradiance. Phytochemistry 2005, 66, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Giossi, C.E.; Cruz, S.; Rey, F.; Marques, R.; Melo, T.; Domingues, M.D.R.; Cartaxana, P. Light induced changes in pigment and lipid profiles of Bryopsidales algae. Front. Mar. Sci. 2021, 8, 745083. [Google Scholar] [CrossRef]
- Gu, K.; Liu, Y.; Jiang, T.; Cai, C.; Zhao, H.; Liu, X.; He, P. Molecular response of Ulva prolifera to short-term high light stress revealed by a multi-omics approach. Biology 2022, 11, 1563. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Yakovleva, I.M. Low light acclimation strategy of the brown macroalga Undaria pinnatifida: Significance of lipid and fatty acid remodeling for photosynthetic competence. J. Phycol. 2021, 57, 1792–1804. [Google Scholar] [CrossRef] [PubMed]
- Epstain, G.; Smale, D.A. Undaria pinnatifida: A case study to highlight challenges in marine invasion ecology and management. Ecol. Evol. 2017, 7, 8624–8642. [Google Scholar] [CrossRef]
- Skriptsova, A.V. Biology and ecology of Undaria pinnatifida (Phaeophyta) in Peter the Great Bay of the Sea of Japan. In The Current State of Aquatic Biological Resources, Proceedings of the Conference to the 70th Anniversary of the S.M. Konovalov; TINRO-Center: Vladivostok, Russia, 2008; pp. 254–258. [Google Scholar]
- South, P.M.; Floerl, O.; Forrest, B.M.; Thomsen, M.S. A review of three decades of research on the invasive kelp Undaria pinnatifida in Australasia: An assessment of its success, impacts and status as one of the world’s worst invaders. Mar. Environ. Res. 2017, 131, 243–257. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef]
- Khotimchenko, S.V. Distribution of glyceroglycolipids in marine algae and grasses. Chem. Nat. Compd. 2002, 38, 223–229. [Google Scholar] [CrossRef]
- Wada, H.; Murata, N. Lipids in thylakoid membranes and photosynthetic cells. In Lipids in Photosynthesis: Essential and Regulatory Function; Wada, H., Murata, N., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 1–9. [Google Scholar]
- Fujii, S.; Wada, H.; Kobayashi, K. Role of galactolipids in plastid differentiation before and after light exposure. Plants 2019, 8, 357. [Google Scholar] [CrossRef]
- Rocha, J.; Nitenberg, M.; Girard-Egrot, A.; Jouhet, J.; Maréchal, E.; Block, M.A.; Breton, C. Do galactolipid synthases play a key role in the biogenesis of chloroplast membranes of higher plants? Front. Plant Sci. 2018, 9, 126. [Google Scholar] [CrossRef]
- Haferkamp, S.; Haase, W.; Pascal, A.A.; van Amerongen, H.; Kirchhoff, H. Efficient light harvesting by photosystem II requires an optimized protein packing density in grana thylakoids. J. Biol. Chem. 2010, 285, 17020–17028. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, C.; Fan, J.; Shanklin, J.; Xu, C. Mechanisms and functions of membrane lipid remodeling in plants. Plant J. 2021, 107, 37–53. [Google Scholar] [CrossRef]
- Jones, M.R. Lipids in photosynthetic reaction centres: Structural roles and functional holes. Prog. Lipid Res. 2007, 46, 56–87. [Google Scholar] [CrossRef]
- Tietz, S.; Leuenberger, M.; Höhner, R.; Olson, A.H.; Fleming, G.R.; Kirchhoff, H. A proteoliposome-based system reveals how lipids control photosynthetic light harvesting. J. Biol. Chem. 2020, 295, 1857–1866. [Google Scholar] [CrossRef]
- Kern, J.; Guskov, A. Lipids in photosystem II: Multifunctional cofactors. J. Photochem. Photobiol. B Biol. 2011, 104, 19–34. [Google Scholar] [CrossRef]
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Lipids in photosystem II: Interaction with protein and cofactors. Biochim. Biophys. Acta. 2007, 1767, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Mock, T.; Kroon, B.M.A. Photosynthetic energy conversion under extreme conditions-II: The significance of lipids under light limited growth in Antarctic sea ice diatoms. Phytochemistry 2002, 61, 53–60. [Google Scholar] [CrossRef]
- Alboresi, A.; Perin, G.; Vitulo, N.; Diretto, G.; Block, M.; Jouhet, J.; Meneghesso, A.; Valle, G.; Giuliano, G.; Maréchal, E.; et al. Light remodels lipid biosynthesis in Nannochloropsis gaditana by modulating carbon partitioning between organelles. Plant Physiol. 2016, 171, 2468–2482. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.; Hwang, Y.S.; Wang, B.; Kim, M.; Jeong, J.; Lee, C.G.; Hu, Q.; Han, D.; Jin, E. Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. J. Exp. Bot. 2014, 65, 4317–4334. [Google Scholar] [CrossRef]
- Kobayashi, K.; Endo, K.; Wada, H. Specific distribution of phosphatidylglycerol to photosystem complexes in the thylakoid membrane. Front. Plant Sci. 2017, 8, 1991. [Google Scholar] [CrossRef]
- Mizusawa, N.; Wada, H. The role of lipids in photosystem II. Biochim. Biophys. Acta. Bioenerg. 2012, 1817, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Wada, H. Lipid biosynthesis and its regulation in cyanobacteria. In Lipids in Photosynthesis Essential and Regulatory Functions; Wada, H., Murata, N., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 157–178. [Google Scholar]
- Kansy, M.; Wilhelm, C.; Goss, R. Influence of thylakoid membrane lipids on the structure and function of the plant photosystem II core complex. Planta 2014, 240, 781–796. [Google Scholar] [CrossRef]
- Pineau, B.; Girard-Bascou, J.; Eberhard, S.; Choquet, Y.; Tremolieres, A.; Gerard-Hirne, C.; Bennardo-Connan, A.; Decottignies, P.; Gillet, S.; Wollman, F.A. A single mutation that causes phosphatidylglycerol deficiency impairs synthesis of photosystem II cores in Chlamydomonas reinhardtii. Eur. J. Biochem. 2004, 271, 329–338. [Google Scholar] [CrossRef]
- Gray, G.R.; Ivanov, A.G.; Krol, M.; Williams, J.P.; Kahn, M.U.; Myscich, E.G.; Huner, N.P.A. Temperature and light modulate the trans- delta3-hexadecenoic acid content of phosphatidylglycerol: Light harvesting complex II organization and non- photochemical quenching. Plant Cell Physiol. 2005, 46, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Nagar, S.; Thakur, M.; Suriyakumar, P.; Kataria, S.; Shanker, A.K.; Landi, M.; Anand, A. Photosystems under high light stress: Throwing light on mechanism and adaptation. Photosynthetica 2023, 61, 250–263. [Google Scholar] [CrossRef]
- Han, D.; Jia, J.; Li, J.; Sommerfeld, M.; Xu, J.; Hu, Q. Metabolic remodeling of membrane glycerolipids in the microalga Nannochloropsis oceanica under nitrogen deprivation. Front. Mar. Sci. 2017, 4, 242. [Google Scholar] [CrossRef]
- Domonkos, I.; Kis, M.; Gombos, Z. Versatile roles of lipids and carotenoids in membranes. Acta Biol. Szeged. 2015, 59, 83–104. [Google Scholar]
- Brown, M.R.; Dunstan, G.A.; Norwood, S.J.; Miller, K.A. Effects of harvest stage and light on the biochemical composition of the diatom Thalassiosira pseudonana. J. Phycol. 1996, 32, 64–73. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Carreau, J.P.; Dubacq, J.P. Adaptation of macro-scale method to the micro-scale for fatty acid methyl transesterification of biological lipid extracts. J. Chromatogr. 1978, 151, 384–390. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll fluorescence as a non-intrusive indicator for rapid assessment of in vivo photosynthesis. In Ecophysiology of Photosynthesis. Ecological Studies; Schulze, E.D., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 49–70. [Google Scholar]
- Littler, M.M. Sources of variability in macroalgal primary productivity: Sampling and interpretative problems. Aquat. Bot. 1982, 8, 141–156. [Google Scholar] [CrossRef]
- Platt, T.; Gallegos, C.L.; Harrison, W.G. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 1980, 38, 687–701. [Google Scholar]
- Wheeler, W.N. Pigment content and photosynthetic rate of the fronds of Macrocystis pyrifera. Mar. Biol. 1980, 56, 97–102. [Google Scholar] [CrossRef]
- Seely, G.R.; Duncan, M.J.; Vidaver, W.E. Preparative and analytical extraction of pigments from brown algae with dimethylsulfoxide. Mar. Biol. 1972, 12, 184–188. [Google Scholar] [CrossRef]


| Fatty Acid | PC | PE | PI | TAG | ||||
|---|---|---|---|---|---|---|---|---|
| ML | HL | ML | HL | ML | HL | ML | HL | |
| 14:0 | 6.7 ± 0.4 | 8.9 ± 0.4 | 2.4 ± 0.4 | 2.2 ± 0.5 | 4.4 ± 0.2 | 3.4 ± 0.8 | 7.5 ± 1.0 | 7.5 ± 0.8 |
| 16:0 | 24.6 ± 1.2 | 27.8 ± 1.3 * | 15.0 ± 1.1 | 16.0 ± 1.0 | 56.3 ± 1.5 | 52.1 ± 1.0 | 34.8 ± 1.4 | 24.1 ± 1.8 * |
| 16:1n-7 | 0.4 ± 0.2 | 0.6 ± 0.1 | 0.8 ± 0.1 | 0.6 ± 0.2 | 1.7 ± 0.2 | 1.9 ± 0.8 | 3.4 ± 0.5 | 1.5 ± 0.6 * |
| 18:0 | 3.8 ± 0.5 | 3.6 ± 0.4 | 3.6 ± 0.8 | 3.5 ± 0.3 | 5.7 ± 0.7 | 6.7 ± 1.0 | 12.0 ± 1.1 | 7.1 ± 1.1 * |
| 18:1n-9 | 2.0 ± 0.7 | 2.0 ± 0.3 | 1.9 ± 0.3 | 2.9 ± 0.5 * | 20.4 ± 1.1 | 21.7 ± 1.1 | 11.8 ± 1.8 | 11.3 ± 1.0 |
| 18:1n-7 | – | – | 0.5 ± 0.2 | 0.6 ± 0.2 | – | – | 0.8 ± 0.7 | 0.2 ± 0.1 |
| 18:2n-6 | 9.4 ± 0.6 | 8.1 ± 0.8 | 2.3 ± 0.5 | 2.1 ± 0.4 | 8.0 ± 0.8 | 10.1 ± 1.1 | 7.9 ± 0.5 | 13.3 ± 1.2 * |
| 18:3n-6 | 0.4 ± 0.1 | 0.2 ± 0.2 | – | – | – | – | 0.2 ± 0.1 | 0.5 ± 0.5 |
| 18:3n-3 | 2.2 ± 0.1 | 2.4 ± 0.6 | 0.6 ± 0.1 | 0.4 ± 0.2 | 2.0 ± 0.8 | 2.1 ± 0.5 | 3.7 ± 0.5 | 6.2 ± 1.0 * |
| 18:4n-3 | 0.9 ± 0.2 | 0.3 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.1 | – | – | 5.1 ± 0.8 | 6.9 ± 0.8 * |
| 20:3n-6 | 5.9 ± 0.7 | 3.7 ± 0.8 * | 0.5 ± 0.2 | 0.7 ± 0.3 | – | – | – | – |
| 20:4n-6 | 34.5 ± 1.5 | 32.5 ± 1.7 | 59.5 ± 1.2 | 62.1 ± 1.1 * | 1.4 ± 0.4 | 2.0 ± 0.4 | 5.8 ± 1.1 | 10.9 ± 1.4 * |
| 20:5n-3 | 9.4 ± 1.1 | 10.0 ± 1.4 | 12.2 ± 1.4 | 8.5 ± 0.5 * | – | – | 7.2 ± 1.1 | 10.6 ± 1.2 * |
| SFA | 35.0 ± 2.1 | 40.3 ± 2.4 | 21.0 ± 2.3 | 21.7 ± 1.7 | 66.4 ± 2.4 | 62.2 ± 2.8 | 54.3 ± 3.5 | 38.7 ± 3.7 * |
| MUFA | 2.4 ± 0.9 | 2.6 ± 0.5 | 3.2 ± 0.6 | 4.1 ± 0.9 | 22.1 ± 1.3 | 23.6 ± 1.9 | 16.0 ± 3.0 | 13.0 ± 1.9 |
| PUFA n-3 | 12.4 ± 1.3 | 12.8 ± 2.3 | 13.5 ± 1.7 | 9.3 ± 0.8 * | 2.0 ± 0.8 | 2.1 ± 0.5 | 16.1 ± 2.9 | 23.7 ± 3.0 * |
| PUFA n-6 | 50.1 ± 3.0 | 44.4 ± 3.5 | 62.3 ± 2.0 | 64.9 ± 1.9 | 9.4 ± 1.4 | 12.0 ± 1.5 | 13.7 ± 2.1 | 24.6 ± 3.2 * |
| Light Treatment | ||
|---|---|---|
| Parameters | ML | HL |
| Area of chloroplast cross section, [µm2] a | 7.72 ± 0.05 A | 4.73 ± 0.17 B |
| Thylakoid stack, number per chloroplast cross section b | 6.55 ± 0.11 A | 4.07 ± 0.14 B |
| Distance between thylakoid stacks, [nm] c | 63.24 ± 0.12 A | 66.70 ± 0.14 B |
| Thylakoid membrane concentration, number per µm2 b | 4.99 ± 0.07 A | 4.12 ± 0.12 B |
| Light Treatment | ||
|---|---|---|
| Parameters | ML | HL |
| Fv/Fm (rel. units) | 0.602 ± 0.014 A | 0.336 ± 0.030 B |
| Pmax (mgO2 g−1 dw h−1) | 10.074 ± 0.433 A | 5.221 ± 0.138 B |
| Rd (mg O2 g−1 dw h−1) | −3.282 ± 0.378 A | −2.871 ± 0.279 A |
| Chl (a + c) (mg g−1 ww) | 0.409 ± 0.025 A | 0.177 ± 0.013 B |
| Ek (µmol photons m−2 s−1) | 177.5 ± 14.1 A | 251.6 ± 8.7 B |
| LPX–MDA (nmol g−1 ww) | 9.042 ± 0.560 A | 13.560 ± 0.551 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukova, N.V.; Yakovleva, I.M. Modulations of Photosynthetic Membrane Lipids and Fatty Acids in Response to High Light in Brown Algae (Undaria pinnatifida). Plants 2025, 14, 1818. https://doi.org/10.3390/plants14121818
Zhukova NV, Yakovleva IM. Modulations of Photosynthetic Membrane Lipids and Fatty Acids in Response to High Light in Brown Algae (Undaria pinnatifida). Plants. 2025; 14(12):1818. https://doi.org/10.3390/plants14121818
Chicago/Turabian StyleZhukova, Natalia V., and Irina M. Yakovleva. 2025. "Modulations of Photosynthetic Membrane Lipids and Fatty Acids in Response to High Light in Brown Algae (Undaria pinnatifida)" Plants 14, no. 12: 1818. https://doi.org/10.3390/plants14121818
APA StyleZhukova, N. V., & Yakovleva, I. M. (2025). Modulations of Photosynthetic Membrane Lipids and Fatty Acids in Response to High Light in Brown Algae (Undaria pinnatifida). Plants, 14(12), 1818. https://doi.org/10.3390/plants14121818






