Comparative Transcriptome and Metabolome Profiling Revealed Molecular Cascade Events During the Enzymatic Browning of Potato Tubers After Cutting
Abstract
1. Introduction
2. Results
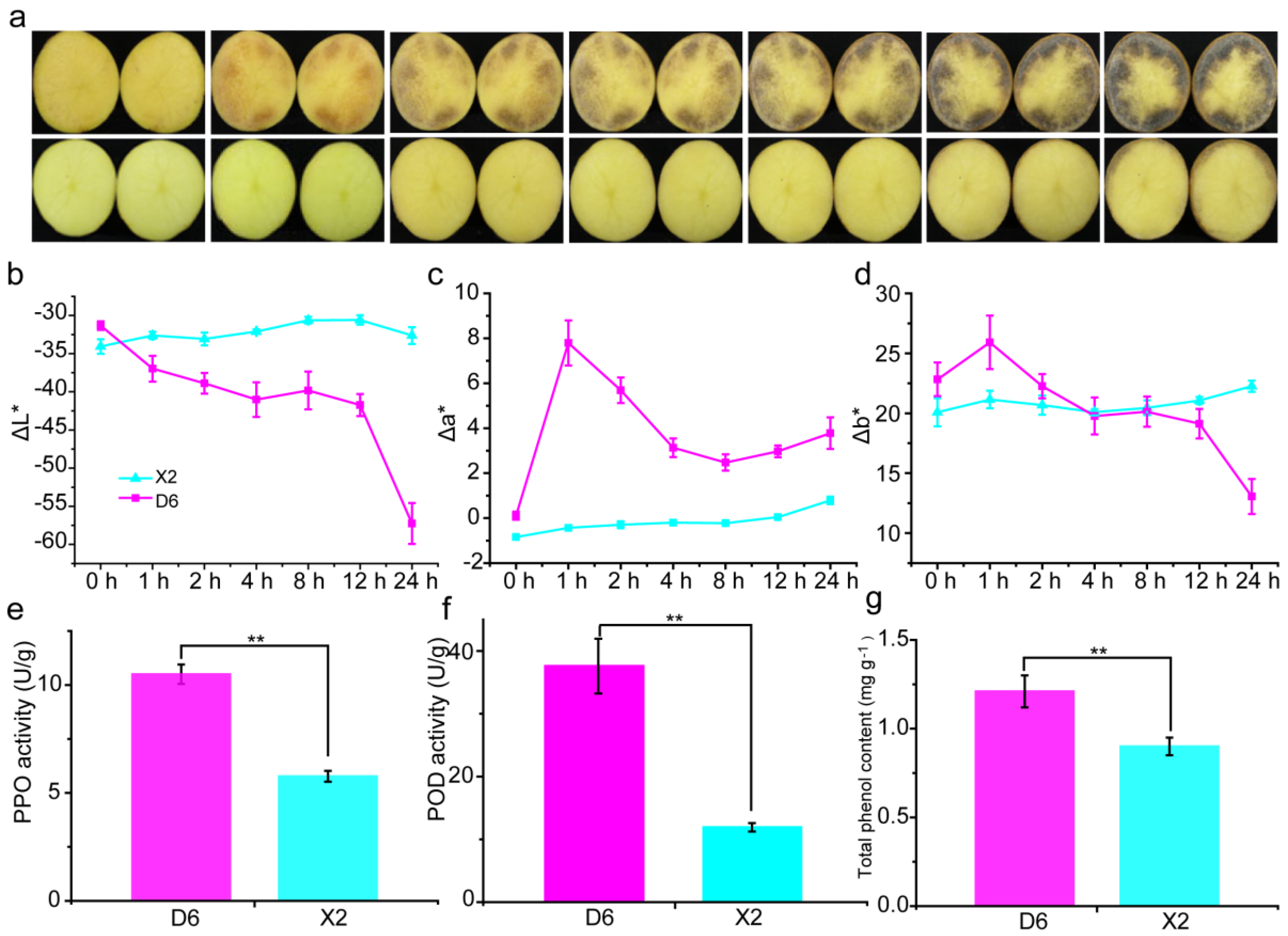
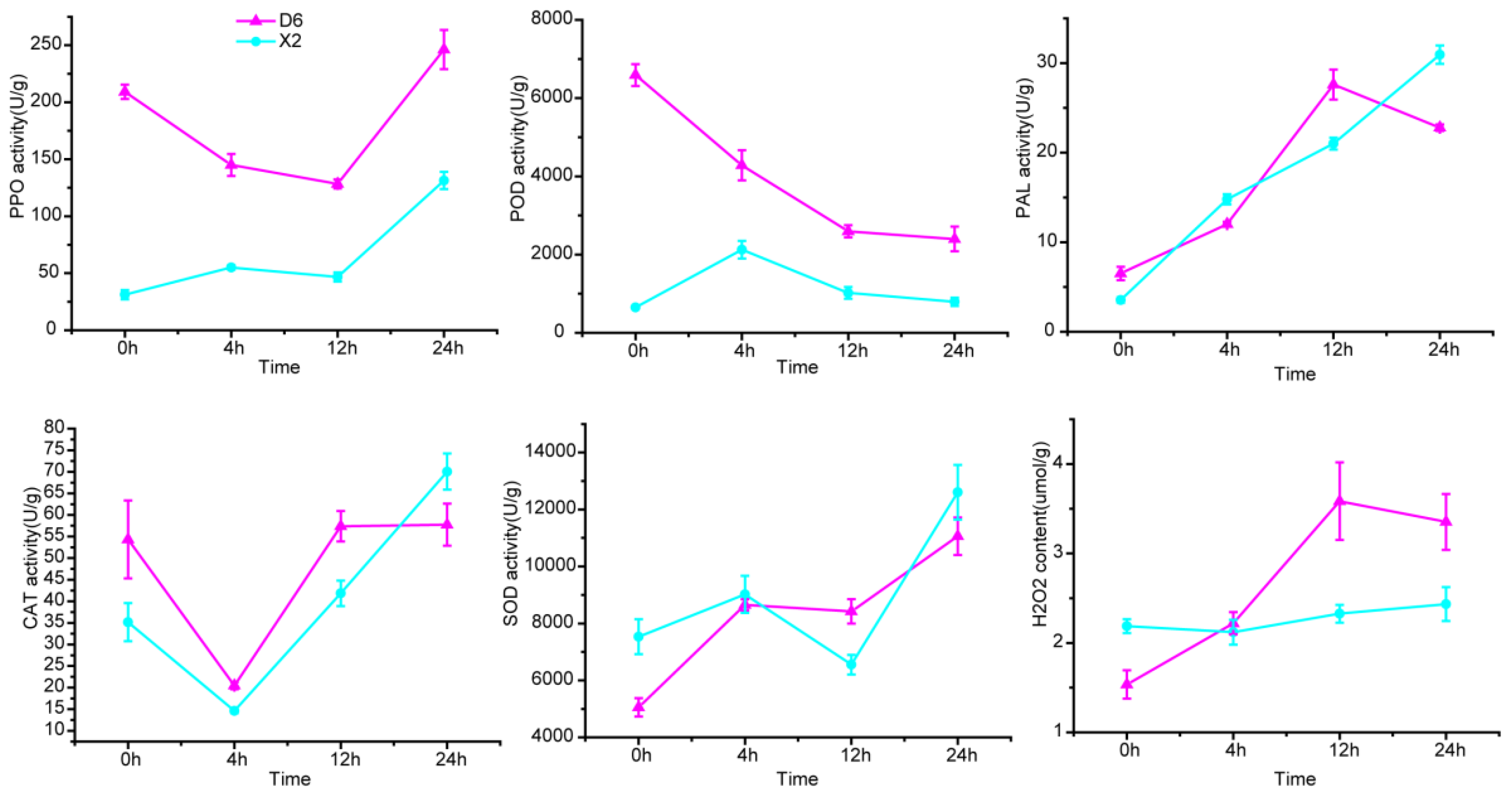
2.1. Enzymatic Browning and Activities of Total Phenols, PPO, and POD in Potato Tubers
2.2. RNA-Seq and De Novo Assembly of Potato Transcriptome
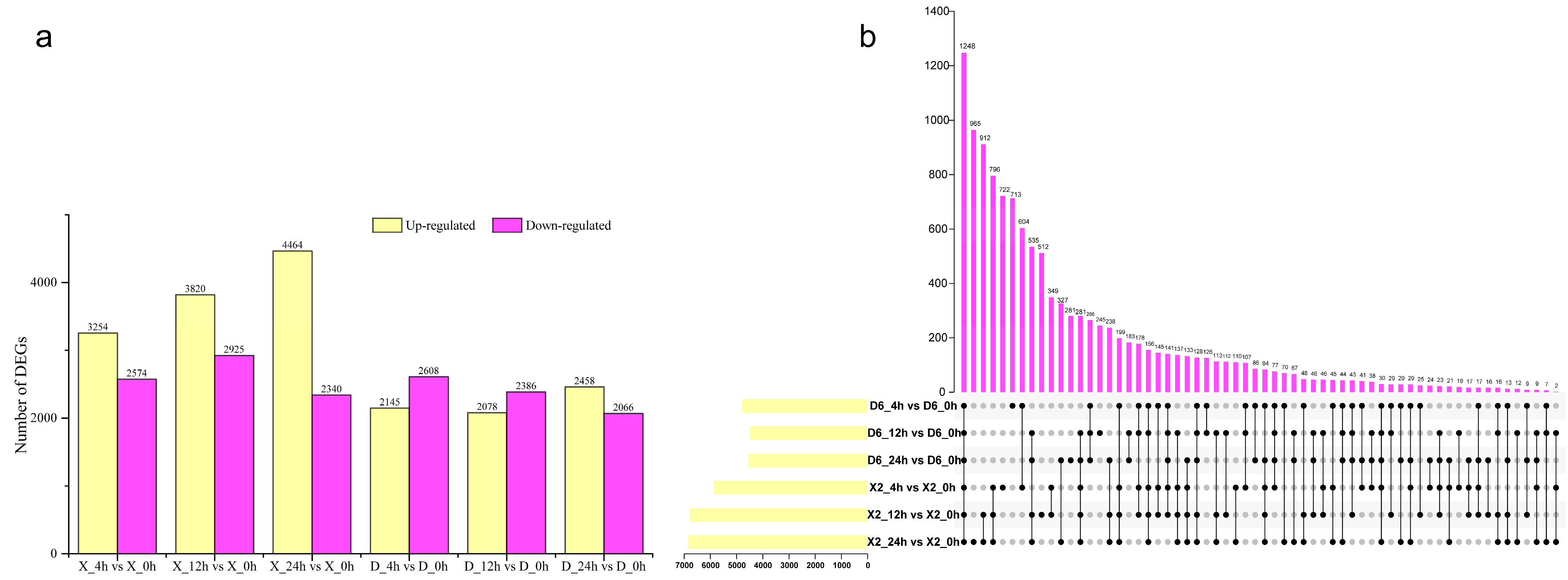
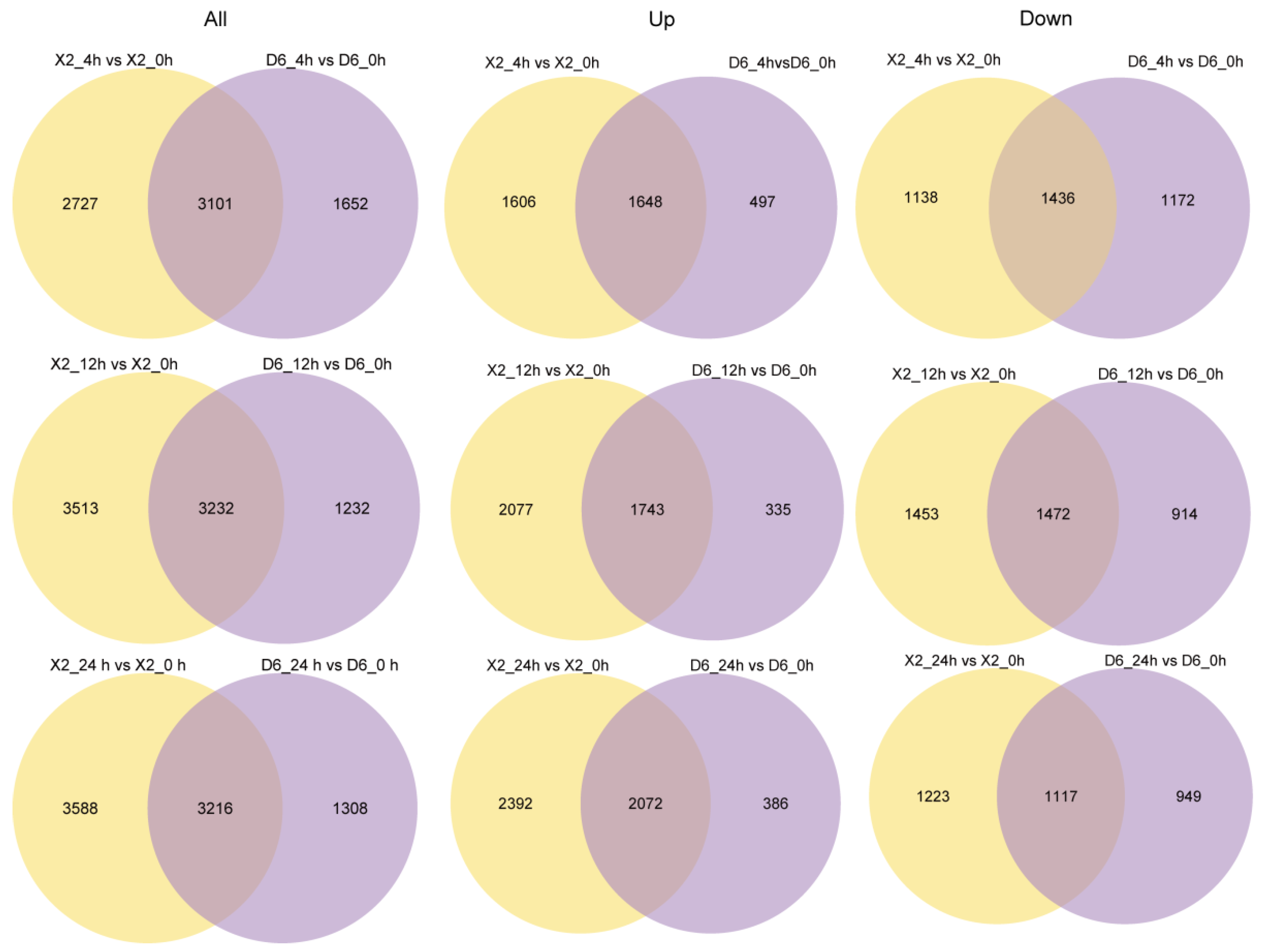
2.3. Differentially Expressed Genes (DEGs) of Potato Tubers at Different Time Points After Cutting
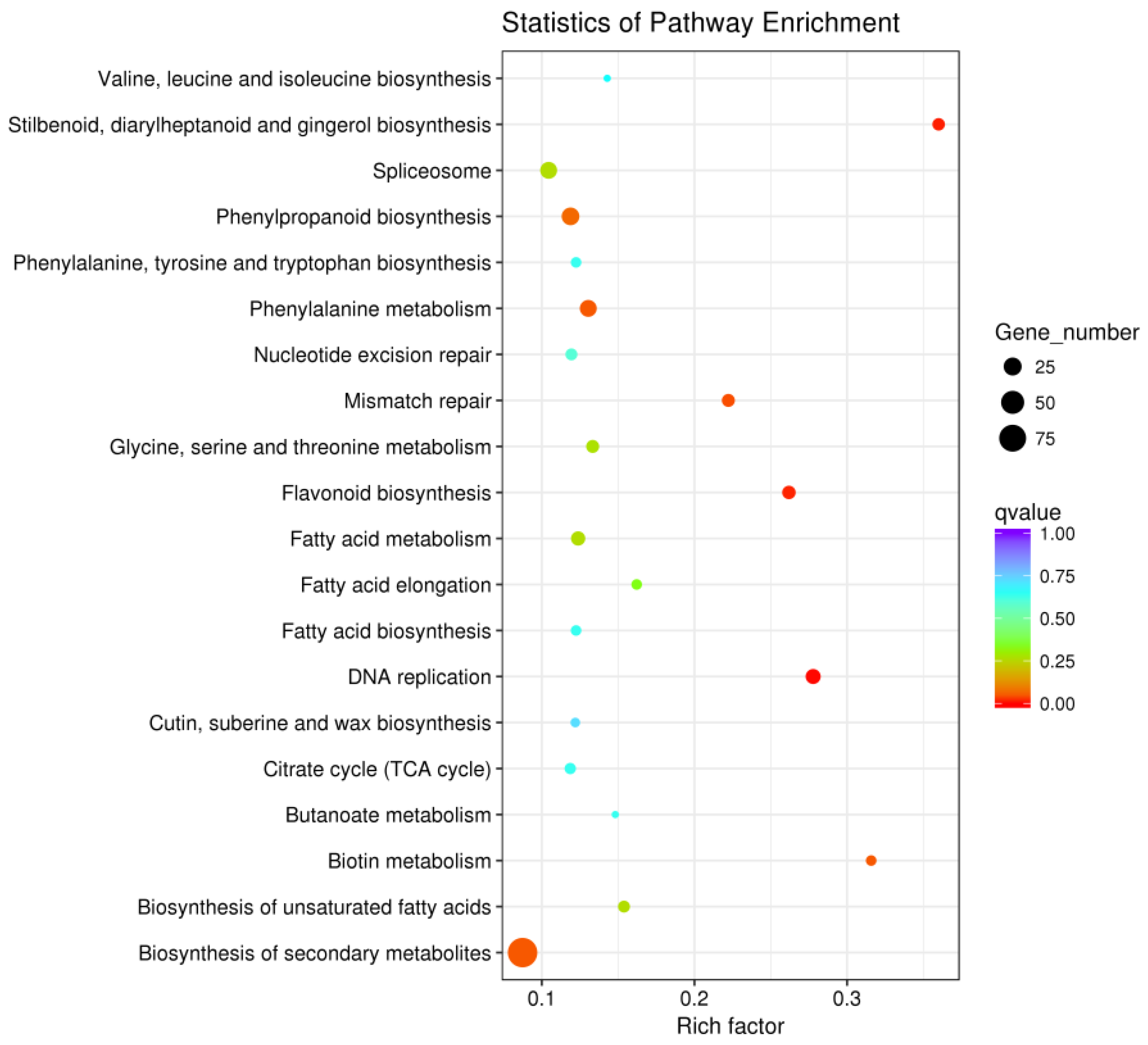
2.4. Critical Metabolic Pathways and Genes Related to Enzymatic Browning
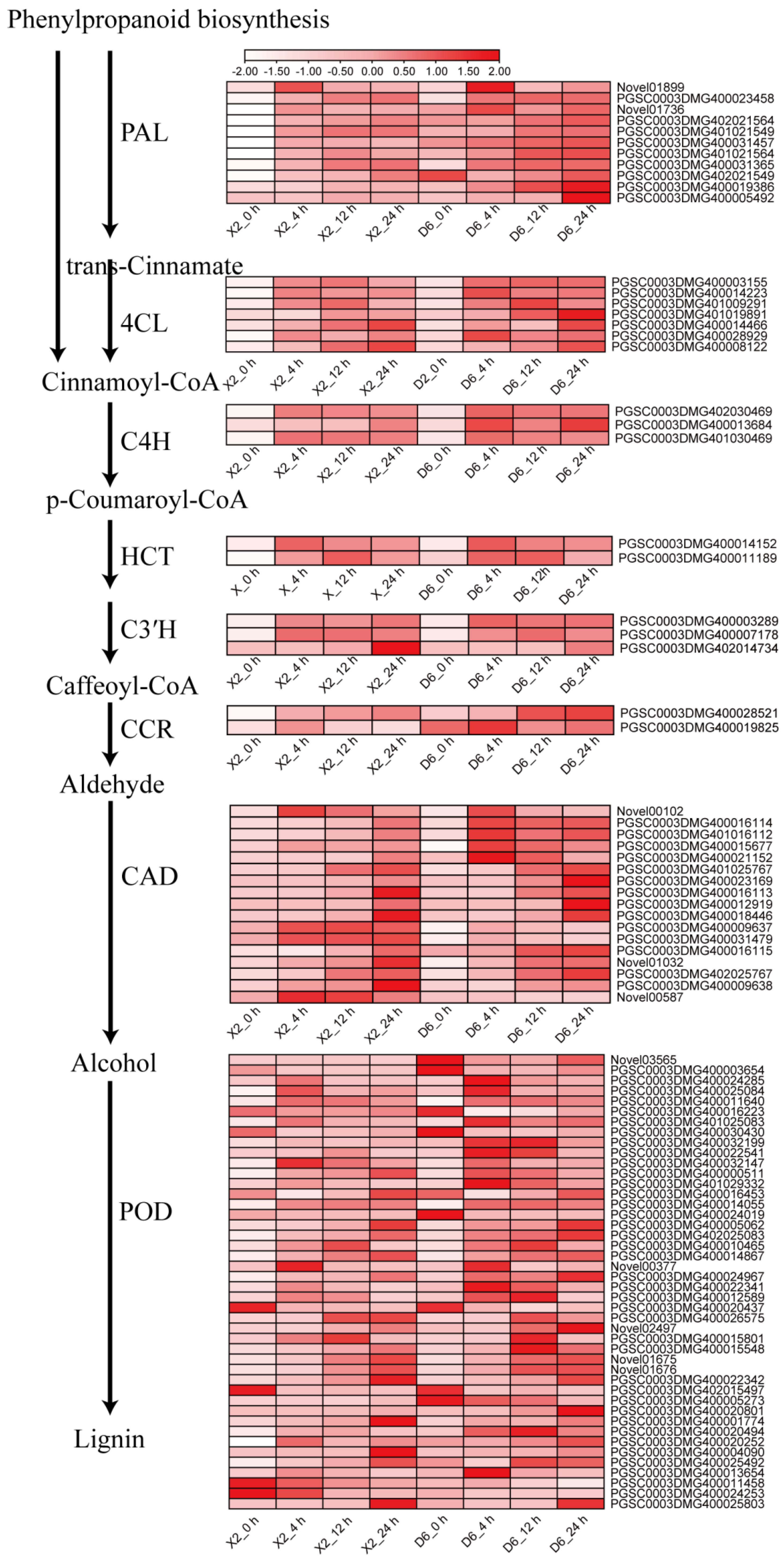
2.4.1. Phenylpropanoid Biosynthesis
2.4.2. Antioxidant Enzyme-Related Genes
2.5. Redox-Related Enzyme Activity and H2O2 Content at Different Time After Cutting
2.6. Changes in DEG-Related Plant Hormone Signal Transduction During Enzymatic Browning After Cutting
2.7. WGCNA Reveals Gene Modules Associated with Enzymatic Browning of Potato Tubers
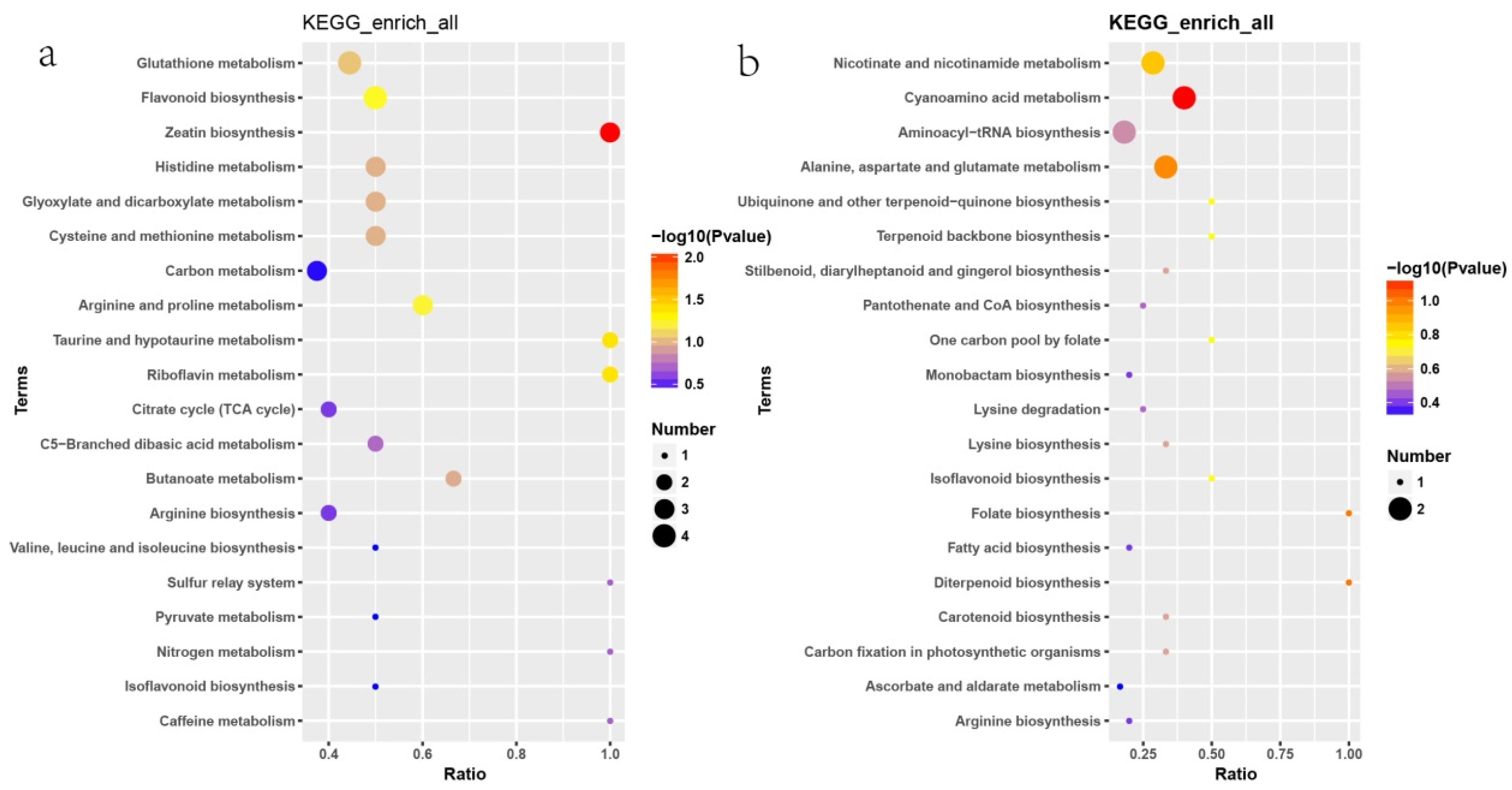
2.8. Changes in Metabolites in Potato Tubers After Browning
3. Discussion
3.1. Response of DEGs to Cut-Wounding and the Differences Between BR and BS Cultivars
3.2. The Cut-Wounding Promotes the Biosynthesis of Phenolics, Flavonoids, and Lipids
3.3. Higher PPO, POD Activity and H2O2 Content Were the Main Reasons Why D6 Was More Prone to Browning than X2
3.4. LAC May Play an Important Role in Enzymatic Browning of Potato Tubers
3.5. Genes Related to Plant Hormone Signal Transduction May Be Involved in the Enzymatic Browning of Potato Tubers
4. Materials and Methods
4.1. Plant Materials
4.2. Determination of Enzyme Activity, Total Phenol and H2O2 Content
4.3. RNA Extraction, RNA Sequencing (RNA-Seq), and Functional Annotations
4.4. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.5. Weighted Gene Co-Expression Network Analysis (WGCNA)
4.6. Metabolome Profiling
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Walker, J.R. Enzymatic browning in fruits: Its biochemistry and control. In Enzymatic Browning and Its Prevention, 1st ed.; Lee, C.Y., Whitaker, J.R., Eds.; ACS Publications: Washington, DC, USA, 1995; Volume 600, pp. 8–22. [Google Scholar]
- He, Q.; Luo, Y. Enzymatic browning and its control in fresh-cut produce. Stewart Postharvest Rev. 2007, 6, 3. [Google Scholar] [CrossRef]
- Nicolas, J.J.; Richard-Forget, F.C.; Goupy, P.M.; Amiot, M.J.; Aubert, S.Y. Enzymatic browning reactions in apple and apple products. Crit. Rev. Food Sci. Nutr. 1994, 34, 109–157. [Google Scholar] [CrossRef]
- Richard-Forget, F.C.; Gauillard, F.A. Oxidation of chlorogenic acid, catechins, and 4-Methylcatechol in model solutions by combinations of pear (Pyrus communis Cv. Williams) polyphenol oxidase and peroxidase: A Possible involvement of peroxidase in enzymatic browning. J. Agric. Food Chem. 1997, 45, 2472–2476. [Google Scholar] [CrossRef]
- Ciou, J.Y.; Lin, H.H.; Chiang, P.Y.; Wang, C.C.; Charles, A.L. The role of polyphenol oxidase and peroxidase in the browning of water caltrop pericarp during heat treatment. Food Chem. 2011, 127, 523–527. [Google Scholar] [CrossRef]
- Singh, B.; Suri, K.; Shevkani, K.; Kaur, A.; Kaur, A.; Singh, N. Enzymatic browning of fruit and vegetables: A review. In Enzymes in Food Technology, 1st ed.; Kuddus, M., Ed.; Springer: Singapore, 2018; pp. 63–78. [Google Scholar]
- Paudel, P.; Seong, S.H.; Wagle, A.; Min, B.S.; Jung, H.A.; Choi, J.S. Antioxidant and anti-browning property of 2-arylbenzofuran derivatives from Morus alba Linn root bark. Food Chem. 2020, 309, 125739. [Google Scholar] [CrossRef]
- Steffens, J.C.; Harel, E.; Hunt, M.D. Polyphenol oxidase. In Genetic Engineering of Plant Secondary Metabolism. Recent Advances in Phytochemistry, 1st ed.; Ellis, B.E., Kuroki, G.W., Stafford, H.A., Eds.; Springer: Boston, MA, USA, 1994; Volume 28, pp. 275–312. [Google Scholar]
- Murata, M.; Tsurutani, M.; Hagiwara, S.; Homma, S. Subcellular location of polyphenol oxidase in apples. Biosci. Biotechnol. Biochem. 1997, 61, 1495–1499. [Google Scholar] [CrossRef]
- Rasool, F.; Uzair, M.; Naeem, M.K.; Rehman, N.; Afroz, A.; Shah, H.; Khan, M.R. Phenylalanine ammonia-lyase (PAL) genes family in wheat (Triticum aestivum L.): Genome-wide characterization and expression profiling. Agronomy 2021, 11, 2511. [Google Scholar] [CrossRef]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Effect of wounding on phenolic enzymes in six minimally processed lettuce cultivars upon storage. J. Agric. Food Chem. 2001, 49, 322–330. [Google Scholar] [CrossRef]
- Taylor, M. Routes to genetic gain in potato. Nat. Plants 2018, 4, 631–632. [Google Scholar] [CrossRef]
- Hoopes, G.; Meng, X.; Hamilton, J.P.; Achakkagari, S.R.; de Alves Freitas Guesdes, F.; Bolger, M.E.; Coombs, J.J.; Esselink, D.; Kaiser, N.R.; Kodde, L.; et al. Phased, chromosome-scale genome assemblies of tetraploid potato reveal a complex genome, transcriptome, and predicted proteome landscape underpinning genetic diversity. Mol. Plant 2022, 15, 520–536. [Google Scholar] [CrossRef]
- Liu, P.; Xu, N.; Liu, R.; Liu, J.; Peng, Y.; Wang, Q. Exogenous proline treatment inhibiting enzymatic browning of fresh-cut potatoes during cold storage. Postharvest Biol. Technol. 2022, 184, 111754. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Zeng, L.; Suo, H.; Li, C.; Shan, J.; Liu, J.; Luo, H.; Li, X.; Xiong, X. Characteristics and differences of polyphenol oxidase, peroxidase activities and polyphenol content in different potato (Solanum tuberosum) tubers. Appl. Ecol. Environ. Res. 2020, 18, 8171–8187. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Fu, Q.Q.; Wang, Z.D.; Liu, X.J.; Sun, L.L.; Zhao, Z.Y. Transcriptomic and metabolomic analysis reveals a protein module involved in preharvest apple peel browning. Plant Physiol. 2023, 192, 2102–2122. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Rodriguez-Buey, M.; Karlsson, J.; Campbell, M.M. The response of the poplar transcriptome to wounding and subsequent infection by a viral pathogen. New Phytol. 2004, 164, 123–136. [Google Scholar] [CrossRef]
- Qiao, L.P.; Gao, M.; Wang, Y.S.; Tian, X.J.; Lu, L.F.; Liu, X. Integrated transcriptomic and metabolomic analysis of cultivar differences provides insights into the browning mechanism of fresh-cut potato tubers. Postharvest Biol. Technol. 2022, 188, 111905. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Ma, L.; Ren, Y.Y.; Bi, Y.; Prusky, D. Transcriptome sequencing and diferential expression analysis of natural and BTH-treated wound healing in potato tubers (Solanum tuberosum L.). BMC Genom. 2022, 23, 263. [Google Scholar] [CrossRef]
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin biosynthesis and its integration into metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef]
- Saltveit, M.E. Wound induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol. Technol. 2000, 21, 61–69. [Google Scholar] [CrossRef]
- Solekha, R.; Susanto, F.A.; Joko, T.; Nuringtyas, T.R.; Purwestri, Y.A. Phenylalanine ammonia lyase (PAL) contributes to the resistance of black rice against Xanthomonas oryzae pv. oryzae. J. Plant Pathol. 2020, 102, 359–365. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Yuan, D.; Duan, M.; Liu, Y.; Shen, Z.; Yang, C.; Qiu, Z.; Liu, D.; Wen, P.; et al. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271. [Google Scholar] [CrossRef]
- Nag, S.; Kumaria, S. In silico characterization and transcriptional modulation of phenylalanine ammonia lyase (PAL) by abiotic stresses in the medicinal orchid Vanda coerulea Griff. ex Lindl. Phytochemistry 2018, 156, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Khakdan, F.; Alizadeh, H.; Ranjbar, M. Molecular cloning, functional characterization and expression of a drought inducible phenylalanine ammonia-lyase gene (ObPAL) from Ocimum basilicum L. Plant Physiol. Biochem. 2018, 130, 464–472. [Google Scholar]
- Tang, T.T.; Xie, X.F.; Ren, X.; Wang, W.J.; Tang, X.M.; Zhang, J.; Wang, Z.D. A difference of enzymatic browning unrelated to PPO from physiology, targeted metabolomics and gene expression analysis in Fuji apples. Postharvest Biol. Technol. 2020, 170, 111323. [Google Scholar] [CrossRef]
- Hussain, S.; Rao, M.J.; Anjum, M.A.; Ejaz, S.; Zakir, I.; Ali, M.A.; Ahmad, N.; Ahmad, S. Oxidative stress and antioxidant defense in plants under drought conditions. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Hakeem, K., Nahar, K., Alharby, H., Eds.; Springer: Cham, Switzerland, 2019; pp. 207–219. [Google Scholar]
- Minibayeva, F.; Kolesnikov, O.; Chasov, A.; Beckett, R.P.; LÜThje, S.; Vylegzhanina, N.; Buck, F.; BÖTtger, M. Wound-induced apoplastic peroxidase activities: Their roles in the production and detoxification of reactive oxygen species. Plant Cell Environ. 2009, 32, 497–508. [Google Scholar] [CrossRef]
- Qiao, L.; Han, X.; Wang, H.; Gao, M.; Tian, J.; Lu, L.; Liu, X. Novel alternative for controlling enzymatic browning: Catalase and its application in fresh-cut potatoes. J. Food Sci. 2021, 86, 3529–3539. [Google Scholar] [CrossRef]
- Boeckx, T.; Winters, A.; Webb, K.J.; Kingston-Smith, A.H. Detection of potential chloroplastic substrates for polyphenol oxidase suggests a role in undamaged leaves. Front. Plant Sci. 2017, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.X.; Suo, H.C.; Hu, X.X.; Qin, Y.Z.; Zeng, L.; Li, X.B.; Xiong, X.Y. The relationship between enzymatic browning and relevant physiological index of potato tubers. Acta Hortic. Sin. 2019, 46, 1519–1530. [Google Scholar]
- Fang, F.; Zhang, X.L.; Luo, H.H.; Zhou, J.J.; Gong, Y.H.; Li, W.J.; Shi, Z.W.; He, Q.; Wu, Q.; Lu, L. An intracellular laccase is responsible for epicatechinmediated anthocyanin degradation in litchi fruit pericarp. Plant Physiol. 2015, 169, 2391–2408. [Google Scholar]
- Legay, S.; Guerriero, G.; Deleruelle, A.; Lateur, M.; Evers, D.; André, C.M.; Hausman, J.F. Apple russeting as seen through the RNA-seq lens: Strong alterations in the exocarp cell wall. Plant Mol. Biol. 2015, 88, 21–40. [Google Scholar] [CrossRef]
- Gong, Y.H.; Song, J.; Du, L.N.; Vinqvist, M.; Palmer, L.C.; Fillmore, S.; Pang, X.Q.; Zhang, Z.Q. Characterization of laccase from apple fruit during postharvest storage and its response to diphenylamine and 1-methylcyclopropene treatments. Food Chem. 2018, 253, 314–321. [Google Scholar]
- Lonegan, G.; Baker, W.L. Comparative study of substrates of fungal laccase. Lett. Appl. Microbiol. 1995, 21, 31–33. [Google Scholar] [CrossRef]
- Zhao, Q.; Nakashima, J.; Chen, F.; Yin, Y.B.; Fu, C.X.; Yun, J.F.; Shao, H.; Wang, X.Q.; Wang, Z.Y.; Dixon, R.A. Laccase is necessary and nonredundant with peroxidase for lignin polymerization during vascular development in Arabidopsis. Plant Cell 2013, 25, 3976–3987. [Google Scholar] [CrossRef]
- Suttle, J.C.; Lulai, E.C.; Huckle, L.L.; Neubauer, J.D. Wounding of potato tubers induces increases in ABA biosynthesis and catabolism and alters expression of ABA metabolic genes. J. Plant Physiol. 2013, 170, 560–566. [Google Scholar] [CrossRef]
- Jia, M. Jasmonic acid transport in wound-induced systemic immunity. Mol. Plant 2020, 13, 1673. [Google Scholar] [CrossRef]
- Kumar, G.N.M.; Knowles, N.R. Wound-induced superoxide production and PAL activity decline with potato tuber age and wound healing ability. Physiol. Plant. 2003, 117, 108–117. [Google Scholar] [CrossRef]
- Romero, P.; Lafuente, M.T. Ethylene-driven changes in epicuticular wax metabolism in citrus fruit. Food Chem. 2022, 372, 131320. [Google Scholar] [CrossRef] [PubMed]
- Tatsuki, M.; Nakajima, N.; Fujii, H.; Shimada, T.; Nakano, M.; Hayashi, K.-i.; Hayama, H.; Yoshioka, H.; Nakamura, Y. Increased levels of IAA are required for system 2 ethylene synthesis causing fruit softening in peach (Prunus persica L. Batsch). J. Exp. Bot. 2013, 64, 1049–1059. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Xu, Y.; Ji, Y.; Yang, X.; Feng, K. Proteomic analysis validates previous findings on wounding-responsive plant hormone signaling and primary metabolism contributing to the biosynthesis of secondary metabolites based on metabolomic analysis in harvested broccoli (Brassica oleracea L. var. italica). Food Res. Int. 2021, 145, 110388. [Google Scholar]
- Kumar, G.; Lulai, E.C.; Suttle, J.C.; Knowles, N.R. Age-induced loss of wound-healing ability in potato tubers is partly regulated by ABA. Planta 2010, 232, 1433–1445. [Google Scholar] [CrossRef]
- Han, X.; Wei, X.; Lu, W.; Wu, Q.; Mao, L.; Luo, Z. Transcriptional regulation of KCS gene by bZIP29 and MYB70 transcription factors during ABA-stimulated wound suberization of kiwifruit (Actinidia deliciosa). BMC Plant Biol. 2022, 22, 23. [Google Scholar] [CrossRef]
- Cui, F.; Brosché, M.; Sipari, N.; Tang, S.; Overmyer, K. Regulation of ABA dependent wound induced spreading cell death by MYB108. New Phytol. 2013, 200, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Hillwig, M.S.; LeBrasseur, N.D.; Green, P.J.; MacIntosh, G.C. Impact of transcriptional, ABA-dependent, and ABA-independent pathways on wounding regulation of RNS1 expression. Mol. Genet. Genom. 2008, 280, 249–261. [Google Scholar] [CrossRef]
- Tao, X.; Mao, L.; Li, J.; Chen, J.; Lu, W.; Huang, S. Abscisic acid mediates wound-healing in harvested tomato fruit. Postharvest Biol. Technol. 2016, 118, 128–133. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; He, C.; Zhu, S. Postharvest exogenous application of abscisic acid reduces internal browning in pineapple. J. Agric. Food Chem. 2015, 63, 5313–5320. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, F.; Na, R.; Zhang, X.; Yang, S.; Gao, J.; Fan, M.; Zhao, Y.; Zhao, J. AtROP1 negatively regulates potato resistance to Phytophthora infestans via NADPH oxidase-mediated accumulation of H2O2. BMC Plant Biol. 2014, 14, 392. [Google Scholar] [CrossRef] [PubMed]
- Si, T.; Wang, X.; Zhao, C.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. The role of hydrogen peroxide in mediating the mechanical wounding-induced freezing tolerance in wheat. Front. Plant Sci. 2018, 9, 327. [Google Scholar] [CrossRef]
- Zhou, F.; Jiang, A.; Feng, K.; Gu, S.; Xu, D.; Hu, W. Effect of methyl jasmonate on wound healing and resistance in fresh-cut potato cubes. Postharvest Biol. Technol. 2019, 157, 110958. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Q.H.; Yang, H.R.; Liu, Y.Y.; Huang, W.D. Relationship between H2O2 and jasmonic acid in pea leaf wounding response. Russ. J. Plant Physiol. 2008, 55, 765–775. [Google Scholar] [CrossRef]
- Qu, S.; Li, M.; Wang, G.; Zhu, S. Application of ABA and GA3 alleviated browning of litchi (Litchi chinensis sonn) via different strategies. Postharvest Biol. Technol. 2021, 181, 111672. [Google Scholar] [CrossRef]
- Kuchi, V.S.; Kabir, J.; Siddiqui, M.W. Gibberellins: The roles in pre- and postharvest quality of horticultural produce. In Postharvest Management of Horticultural Crops, 1st ed.; Siddiqui, M.W., Ali, A., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2017; pp. 181–230. [Google Scholar]
- Aly, E.Z.; Ismail, H.A. Effect of preharvest GA3, CaCl2 and boron treatments on quality and enzymatic browning in Balady guava fruits. Ann. Agric. Sci. 2000, 38, 1101–1108. [Google Scholar]
- Ali, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Hussain, S.; Ejaz, S. Effects of brassinosteroids on postharvest physiology of horticultural crops: A concise review. J. Hortic. Sci. Technol. 2019, 2, 62–68. [Google Scholar] [CrossRef]
- MohammadrRezakhani, S.; Pakkish, Z. Influences of brassinosteroide and hot water on postharvest enzyme activity and lipid peroxidaion of lime (Citrus aurantifolia L.) fruit during storage at cold temperature. Int. J. Hortic. Sci. Technol. 2017, 4, 57–65. [Google Scholar]
- Gao, H.; Chai, H.; Cheng, N.; Cao, W. Effects of 24-epibrassinolide on enzymatic browning and antioxidant activity of fresh-cut lotus root slices. Food Chem. 2017, 217, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, R.; Zhang, X.; Wang, Q.; Wang, B.; Zheng, X.; Li, Y.; Prusky, D.; Bi, Y. Brassinosteroid accelerates wound healing of potato tubers by activation of reactive oxygen metabolism and phenylpropanoid metabolism. Foods 2022, 11, 906. [Google Scholar] [CrossRef]
- Xu, X.; Pan, S.K.; Cheng, S.F.; Zhang, B.; Bachem, C.W.B.; Boer, J.M.d.; Borm, T.J.A.; Kloosterman, B.A.; Eck, H.J.v.; Datema, E.; et al. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Du, Z.; Zhou, X.; Ling, Y.; Zhang, Z.; Su, Z. agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010, 38, 64–70. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenführer, J. topGO: Enrichment Analysis for Gene Ontology, R package version 2.24.0; R Team: Vienna, Austria, 2019. [Google Scholar]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef]



| GO_Accession | Description | Term_Type | DEG Number | qValue |
|---|---|---|---|---|
| GO:0016491 | oxidoreductase activity | MF | 181 | 8.04 × 10−5 |
| GO:0016614 | oxidoreductase activity, acting on CH-OH group of donors | MF | 35 | 0.015521 |
| GO:0016616 | oxidoreductase activity, acting on the CH-OH group of donors, NAD or NADP as acceptor | MF | 32 | 0.019845 |
| GO:0015238 | drug transmembrane transporter activity | MF | 14 | 0.041072 |
| GO:0090484 | drug transporter activity | MF | 14 | 0.041072 |
| GO:0055114 | oxidation-reduction process | BP | 172 | 0.000327 |
| GO:0042493 | response to drug | BP | 14 | 0.041072 |
| GO:0044699 | single-organism process | BP | 494 | 0.041072 |
| The Type of DEGs | Samples | GO Enrichment |
|---|---|---|
| 4 h after cut-wounding | ||
| Up regulation | X2_specific | protein phosphorylation, cellular protein, modification process, protein modification process, transferase activity, transferring hexosyl groups, transferase activity, transferring glycosyl groups, transferase activity |
| D6_specific | oxidation-reduction process, single-organism metabolic process, oxidoreductase activity | |
| Down regulation | X2_specific | ADP binding, transition metal ion binding, cation binding, metal ion binding |
| D6_specific | ADP binding, transition metal ion binding, cation binding, metal ion binding | |
| 12 h after cut-wounding | ||
| Up regulation | X2_specific | catalytic activity, transferase activity, oxidoreductase activity |
| D6_specific | metabolic process, organic substance metabolic process, cellular metabolic process, metabolic process, organic substance metabolic process, cellular metabolic process, nitrogen compound metabolic process, cellular nitrogen compound metabolic process, gene expression, cytoplasmic part, intracellular organelle, organelle cytoplasm | |
| Down regulation | X2_specific | intracellular organelle part, organelle part, monovalent inorganic cation transport, monovalent inorganic cation transmembrane transporter activity |
| D6_specific | cation binding, metal ion binding, ion binding, regulation of RNA biosynthetic process, regulation of RNA metabolic process, transcription, DNA-templated, regulation of transcription, DNA-templated, regulation of nucleic acid-templated transcription | |
| 24 h after cut-wounding | ||
| Up regulation | X2_specific | catalytic activity, single-organism metabolic process, oxidation-reduction process, oxidoreductase activity, transferase activity, metabolic process |
| D6_specific | oxidoreductase activity, oxidation-reduction process, catalytic activity, metabolic process, single-organism metabolic process, single-organism process | |
| Down regulation | X2_specific | cation binding, metal ion binding, nucleic acid binding |
| D6_specific | ion binding, regulated-related | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Shan, J.; Liu, J.; An, K.; Yang, K.; Li, C.; Li, X.; Xiong, X. Comparative Transcriptome and Metabolome Profiling Revealed Molecular Cascade Events During the Enzymatic Browning of Potato Tubers After Cutting. Plants 2025, 14, 1817. https://doi.org/10.3390/plants14121817
Wang L, Shan J, Liu J, An K, Yang K, Li C, Li X, Xiong X. Comparative Transcriptome and Metabolome Profiling Revealed Molecular Cascade Events During the Enzymatic Browning of Potato Tubers After Cutting. Plants. 2025; 14(12):1817. https://doi.org/10.3390/plants14121817
Chicago/Turabian StyleWang, Li, Jianwei Shan, Jitao Liu, Kang An, Kun Yang, Chengchen Li, Xiaobo Li, and Xingyao Xiong. 2025. "Comparative Transcriptome and Metabolome Profiling Revealed Molecular Cascade Events During the Enzymatic Browning of Potato Tubers After Cutting" Plants 14, no. 12: 1817. https://doi.org/10.3390/plants14121817
APA StyleWang, L., Shan, J., Liu, J., An, K., Yang, K., Li, C., Li, X., & Xiong, X. (2025). Comparative Transcriptome and Metabolome Profiling Revealed Molecular Cascade Events During the Enzymatic Browning of Potato Tubers After Cutting. Plants, 14(12), 1817. https://doi.org/10.3390/plants14121817




