Abstract
This study explores the interaction between Prymnesium parvum and Daphnia magna under low-salinity conditions. P. parvum showed reduced growth below 0.4 PSU and peaked at 1.0 PSU within the tested 0.2–1.0 PSU range. D. magna, exposed to P. parvum across 0.0–6.0 PSU, experienced increased mortality at 4.0 and 6.0 PSU, but tolerated 0.0–1.0 PSU well and grazed actively on P. parvum without significant vitality loss. This range reflects conditions observed in the Oder River during the 2022 fish die-off. The count of P. parvum cells did not vary significantly across the 0.2 to 1.0 PSU range of salinities in D. magna presence, except at 0.6 PSU. All daphnids survived even at P. parvum densities of 1 × 105 cells/mL, though increasing algal concentrations reduced juvenile growth rates. Direct observation under a microscope confirmed algal ingestion. Toxin accumulation in cells and medium likely reduced grazing efficiency via allelopathic effects. The study assessed whether D. magna can tolerate prymnesins while maintaining feeding under varying salinities. Results suggest that Daphnia magna could act as a biological suppressor of golden algae under certain environmental conditions, though further work is needed to quantify grazing efficiency and prymnesins concentrations.
1. Introduction
The proliferation of harmful algae blooms (HABs) has raised significant concerns in aquatic ecosystems. Prymnesium parvum is a mixotrophic microalgae capable of producing potent toxins (prymnesins) that lead to mass fish kills (MFK) and ecological imbalances. In the summer of 2022, the Oder River, which flows through Poland and Germany, experienced a significant environmental disaster characterized by a massive loss of aquatic life, including fish, mussels, and snails. Investigations [1] identified the primary cause as a harmful algal bloom of Prymnesium parvum, commonly known as “golden algae”. The ecological consequences were severe, with an estimated 1000 metric tons of fish and numerous mussels and snails perishing. The disaster highlighted the vulnerability of freshwater ecosystems to HABs, especially when exacerbated by human activities such as industrial discharges [2] Of the available traditional methods, chemical and physical mitigation strategies often have unintended negative environmental impacts [3], necessitating the exploration of biological control methods. A promising approach is the use of Daphnia spp. (water fleas) as a means of controlling P. parvum blooms through predation during the early stages of golden algae proliferation.
1.1. Human-Engineered Application of Daphnia in Algal Bloom Control
Daphnia sp. as an obligate filter feeder is capable of filtering small particulate matter as well as microorganisms, contributing to the clarification of water in natural water ecosystems [4]. The Cladocera family, to which this particular Daphnia type belongs, are commonly spread in freshwater, mainly in lakes, although their presence is also being reported in river estuaries [5]. These properties have made Daphnia an object of interest in freshwater biological control methods.
The natural efficiency of Daphnia in controlling algae has allowed us to develop strategies to enhance their effectiveness through biomanipulation [6,7]; This involves deliberate efforts to increase the populations of Daphnia and their grazing pressure on the algae. An approach is the introduction of large-bodied Daphnia species into lakes and reservoirs, particularly in nutrient-rich (eutrophic) environments.
Another key strategy involves managing predator populations, as fish that feed on Daphnia (such as planktivorous fish) can limit their grazing effectiveness. By reducing the population of these fish or introducing predatory fish (such as pike) that feed on smaller fish, Daphnia populations can increase, leading to greater control of algal blooms [8]. Habitat improvement, such as improving water quality and increasing aquatic plant coverage, also helps to support Daphnia survival and reproduction.
The efficiency of Daphnia in controlling algal blooms has been demonstrated in various field studies. For example, in Lake Vesijärvi (Finland), a large-scale biomanipulation project involving fish removal and Daphnia stocking led to a significant reduction in algal biomass and improved water clarity [9]. Similarly, in Lake Mendota (USA), the introduction of large-bodied Daphnia combined with predator management resulted in reduced chlorophyll a concentrations and increased water transparency [10].
1.2. Adaptation of Prymnesium parvum to Low Salinity: Proliferation Potential in Freshwater Ecosystems
P. parvum can thrive at salinity levels ranging from 0.5 to 30 PSU (Practical Salinity Units), with optimal growth often observed around 15 PSU. However, different strains may exhibit varying salinity tolerances. Adaptation mechanisms of P. parvum to varying salinity involve physiological adjustments. Algae produce compounds like dimethylsulfoniopropionate (DMSP) and other polyols, which are believed to play a role in osmoregulation, helping the organism cope with changes in salinity [11].
Salinity not only affects the growth of P. parvum, but also its toxicity. Lower salinity levels can lead to decreased growth rates and reduced toxin production [12]. In contrast, optimal salinity conditions can enhance both growth and toxicity, contributing to the formation of harmful algal blooms. Despite the well-documented osmotic tolerance and efficient osmoregulatory mechanisms [13,14,15],; its cells appear to be highly sensitive to abrupt shifts in osmotic pressure. Rapid fluctuations in salinity cause the lysis of the P. parvum cell, leading to the release of its toxic metabolites, including prymnesins [16].
1.3. Nutritional Quality of the Culture of P. parvum Cells as Feed for Daphnia
A single-celled Prymnesium parvum is characterized by an ellipsoid or oval shape. Its cell dimensions typically range from 8 to 11 µm in length and 4 to 6 µm in width [17]. However, at extremes, the reported size range may exceed lengths between 6 and 12 µm and widths from 3.5 to 8 µm. These variations may be attributed to environmental factors, as research indicates that changes in water salinity can affect the cell volume of P. parvum [16]. P. parvum cells fall within the size range suitable for the ingestion of Daphnia. However, if the cell density is too high, the cells are too toxic, or they are nutritionally deficient, they may hinder Daphnia’s ability to graze efficiently [18,19]. Overcoming these limitations could be the key to utilizing Daphnia as an effective method of suppression of the bloom of P. parvum [20].
Our research aimed to evaluate whether Daphnia magna can suppress the proliferation of Prymnesium parvum under environmentally relevant salinity conditions. To this end, we first analyzed the growth of P. parvum across a selected salinity range. We assessed the toxicity of P. parvum to D. magna at salinities ranging from 0.0 to 6.0 PSU, with particular focus on the 0.0 to 1.0 PSU range. Mortality rates and ephippia production were monitored as indicators of D. magna stress responses. Subsequently, within the selected range of 0.0 to 1.0 PSU, we conducted grazing experiments to determine whether D. magna could reduce P. parvum cell densities. The suppression of P. parvum was quantified by assessing its cell growth across a range of salinity conditions in the presence of daphnids. To determine the cell concentration of P. parvum that could serve as a sole food source for D. magna, we performed juvenile growth rate assays. Under the same salinity condition (1.0 PSU), D. magna individuals were fed varying concentrations of P. parvum, and their growth was measured to identify a potentially toxic threshold of algal density.
Overall, our objective was to explore the potential application of Daphnia magna as a biological control agent to reduce P. parvum populations under salinity conditions that are ecologically relevant and tolerable for both species.
2. Materials and Methods
2.1. Prymnesium parvum Culture
The Prymnesium parvum strain CCAP 946/6 (CCMP708) was obtained from the Culture Collection of Algae and Protozoa (CCAP, Scotland, UK) and maintained in standard f/2 medium with 1.75 PSU sea salt. For low-salinity experiments, the sea salt concentration was reduced accordingly. Growth light was provided by fluorescent tubes or LED sources, and was set to 100 μmol·m−2·s−1 during the growth phase and 20 μmol·m−2·s−1 for precultures. Cultures were either grown in stationary conditions or agitated by bubbling with filtered compressed air (Millex®-FH air filter, 0.45 µm pores, Millipore, Burlington, MA, USA).
2.2. Daphnia magna Culture
The Daphnia magna clone used in this study was a monoculture established from a single female, following the method described in [21]’s study. The clone was obtained from the clone library of the Department of Biochemistry and Microbiology at the Warsaw University of Life Sciences, Poland. It originated from a city park pond in Warsaw, Poland (52°12′42.0″ N, 21°00′01.0″ E). Before and during the experiment, daphnids were kept under constant conditions, maintained by a climate cabinet (Plant Growth Chambers Sanyo MLR-350H, Moriguchi, Japan) at a temperature of 20 °C ± 0.5 °C and a summer photoperiod (16L:8D; 0.30 ± 2 μmol s−1 m−2). Prior to the experiment, the daphnids were fed daily with the green algae Acutodesmus obliquus at a non-limiting growth concentration of 1 mg total organic carbon (TOC) L−1, following Lampert [22].
D. magna were cultured in “Aachener Daphnien Medium” (ADaM) according to the protocol described by [23]. To standardize pre-experimental conditions and assess the specific effect of Prymnesium parvum on D. magna, daphnids were maintained in an increased salt concentration (1 g L−1) for two generations. For the experiments, third-clutch offspring from the second generation, cultured under these conditions, were used.
2.3. Acclimation of Daphnia magna to Experimental Salinity Conditions
Three adult females (7–10 days old) were transferred into 50 mL of ADaM medium with salinity adjusted to one of six levels: 0.0, 0.2, 1.0, 2.0, 4.0, or 6.0 PSU. Salinity was modified using sterile-filtered NaCl solutions, and each salinity treatment was performed in triplicate (n = 3), totaling 18 experimental units.
The animals were maintained under standard conditions (20 ± 1 °C; 16:8 h light:dark photoperiod) for 72 h. Mortality was assessed every 24 h, and the timing of ephippia (resting egg) production was recorded as an indicator of environmental stress.
2.4. Grazing of Daphnia magna on Prymnesium parvum Cells
Following the initial salinity tolerance trials, a second experiment was conducted to evaluate the grazing response of Daphnia magna on Prymnesium parvum under low-salinity conditions. Salinity levels were adjusted to 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0 PSU, and each treatment was performed in triplicate (n = 3), resulting in 18 experimental units. Adult female D. magna (7–10 days old) were placed in 50 mL of ADaM medium supplemented with P. parvum as the sole food source. The algal density was standardized to 1.25 × 10⁴ cells/mL, corresponding to a non-limiting food concentration equivalent to 1 mg total organic carbon (TOC) per liter, as defined by Lampert [22]. The cell concentration of P. parvum was verified by optical density measurements at 800 nm (OD₈₀₀) using a UV-1900 spectrophotometer (Shimadzu, Kyoto, Japan) and by direct microscopic counting with a Neubauer hemocytometer (Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany). Over the 72-h test period, mortality and ephippia production of D. magna were recorded at 24 h intervals.
2.5. Assessment of the Influence of Prymnesium parvum on Daphnia magna Juvenile Growth Rate
To evaluate the effect of Prymnesium parvum on the somatic growth of Daphnia magna neonates, a controlled growth rate experiment was conducted at a salinity of 1.0 g L−1 (1.0 PSU). Three neonates, all derived from the third clutch of the second laboratory-cultured generation maintained at 1.0 g L−1 salinity, were placed in 50 mL of ADaM medium with the same salinity. Each treatment condition was tested in triplicate (n = 3).
At the beginning of the experiment, all neonates were synchronized in age (<16 h old). They were fed P. parvum cells from whole culture suspensions (i.e., without centrifugation), thus containing both intact cells and extracellular exotoxins. The algal density was adjusted to provide a total organic carbon (TOC) concentration of 1.0 mg L−1, corresponding to the non-limiting food concentration for Daphnia growth [22]. Additional treatment groups were prepared using elevated P. parvum concentrations equivalent to 2.0, 4.0, and 6.0 mg TOC L−1 to assess dose-dependent effects.
Algal concentrations were verified using both optical density readings at 800 nm (OD₈₀₀) with a UV-1900 spectrophotometer (Shimadzu, Kyoto, Japan) and direct cell counts with a Neubauer hemocytometer (Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany). A control group was included, in which D. magna were fed the green alga Acutodesmus obliquus at 1.0 mg TOC L−1.
The somatic growth rate of D. magna was determined by measuring body length at the start of the experiment and after four days of exposure, following the method [24]. Individuals were photographed using an optical microscope (Nexcope NE620; Ningbo Yongxin Optics Co., Ltd., Ningbo, China) equipped with a DLT-Cam PRO 8.3MP USB 3.0 camera (DLTA08300CMOSSEU3). Image analysis and length measurements were performed using DLT-Cam Viewer 3.7 software.
The specific growth rate (Gr) was calculated using the formula
where
Lt0 is the length of the neonate at the start of the experiment (<16 h old),
Lt1 is the length of the 4-day-old daphnid,
Δt is the time (in days) between the first and fourth day of the experiment.
The medium was replaced every two days, with an appropriate food supply provided.
2.6. Statistical Analysis
Data were analyzed using R software (version 4.0.3). The normality of data distribution was assessed with the Shapiro–Wilk test. For data following a normal distribution, an ANOVA was performed, followed by Tukey’s Honest Significant Difference (Tukey-HSD) post hoc test for multiple comparisons. For non-normally distributed data, the Kruskal–Wallis rank sum test was applied. In all tests, the significance level was set at α = 0.05.
3. Results
3.1. Growth Rates of Prymnesium parvum Cells in Selected Conditions of Salinity
Prymnesium parvum cells were cultured in f/2 medium for 120 h (5 days) using a multicultivator (PSI, Drásov, Czechia) under a light intensity of 80–100 μmol⋅m−2⋅s−1 in a 12 h/12 h light/dark cycle, in duplicate (Figure 1A). The fastest growth was observed at salinities between 0.6 and 1.0 PSU. Growth rates (inset A) in this range were 3–3.5 times higher compared to the 0.2–0.6 PSU range. Cell counts (Panel B) in the higher salinity range (0.6–1.0 PSU) were 6–7 times greater than those in the lower range (0.2–0.4 PSU, p < 0.05). No significant differences in cell counts were observed within the low-salinity range. Although cell growth in this range increased ~1.5-fold, it was not statistically significant (p > 0.05). Conductivity under the tested salinity conditions was measured after the growth period (inset B). The tested salinity range overlapped with the conditions associated with mass fish kills (MFK) in the Oder River in 2022 [25].
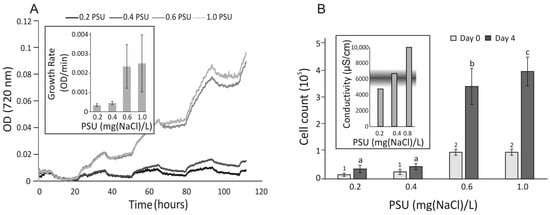
Figure 1.
Growth curves of Prymnesium parvum cells under selected salinity conditions. (Panel (A)) Cell growth was continuously monitored in duplicate cultures across a salinity range of 0.2–1.0 PSU. (Inset (A)) Growth rates were calculated as the mean increase in cell density during the light phase. (Panel (B)) Cell counts were recorded before and after cultivation. Mean values are shown, and statistical analysis was performed using ANOVA followed by Tukey’s post hoc test in R. Statistically significant differences were annotated in groups with different low-case letters at the end of experiment and numbers at the beginning of the experiment. (Inset (B)) Conductivity values corresponding to the tested salinity range are shown, with the shaded area indicating the range associated with mass fish kill (MFK) events in the Oder River.
3.2. Physiological Response of Daphnia magna to Prymnesium parvum
To assess whether P. parvum poses a threat or causes mortality in obligate filter-feeding organisms such as daphnids, a D. magna clone culture was used in this study. For each experimental variant, three adult daphnids were used in triplicate, totaling nine daphnids per condition. P. parvum served as the sole food source under different salt concentrations for 72 h. The physiological responses of D. magna under these experimental conditions are summarized in Figure 2. The mortality of water fleas fed P. parvum was confirmed only in 4.0 and 6.0 PSU conditions. The highest mortality was noted in D. magna fed P. parvum under the 6.0 PSU condition. D. magna also showed an ecological response to stress in their environment through the production of ephippia. The fastest ephippia production was noted in 1.0, 2.0, 6.0 PSU. Interestingly, even in a proportionally low concentration of salt (0.2 PSU), ephippia occurrence under daphnid carapax was confirmed after 48 h.
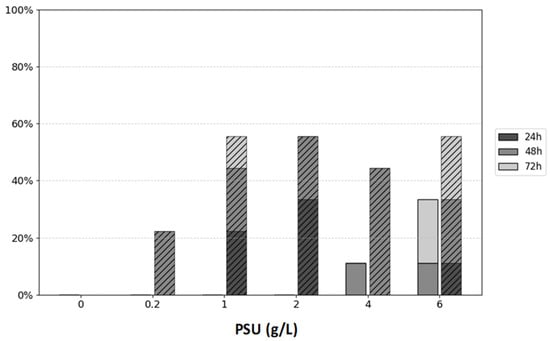
Figure 2.
The figure represents Daphnia’s mortality (solid bars) and ephippia production (hatched bars) at three time points (24, 48 and 72 h) under different salt concentrations ranging within 0.0–6.0 PSU (g/L). Mortality and ephippia production are presented as percentages [%] of the total number of daphnids (9 individuals) in each variant.
3.3. Prymnesium parvum Cell Count in the Presence of Filter-Feeding Daphnia magna
Cytotoxic Prymnesium parvum (containing exotoxins) was used as the sole food source for Daphnia magna, provided at a non-limiting growth concentration of 1.0 mg total organic carbon (TOC) per liter of medium. As shown in Figure 3, over the course of three days, in the presence of obligate filter-feeding D. magna, P. parvum cell density tended to increase. The highest increase in P. parvum cells during presence of D. magna was noted for the 0.6 PSU concentration of salt.
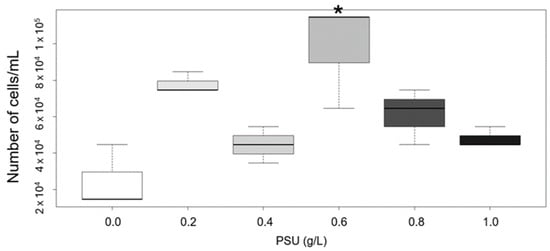
Figure 3.
Difference between Prymnesium parvum cell count at the start and after 72 h in the presence of Daphnia magna. At the beginning of the experiment, the cell number of P. parvum used as the sole food source for daphnids was measured and adjusted to fulfill the non-limiting concentration of total organic carbon (TOC), which is 1 mg L−1. The evaluation of cell number showed that 1 mg TOC concentration is equal to 1.25 × 104 cells/mL of P. parvum cells. For each variant, we used the same number of cells at the beginning and checked the cell number of P. parvum after 72 h of presence to filter feeding D. magna in different salinity concentrations. Change in cell number within that time is represented as boxplot. In each box, a central bold line represents the median. Whiskers in every box stand for minimal and maximal values. Asterisk indicate statistically significant difference between variants with different salinity in comparison to the control (1.0 PSU) (p < 0.01, one-way ANOVA and post hoc Tukey HSD). Each box represents P. parvum cells in the presence of D. magna at different salinity levels; white box—0.0 PSU, light gray—0.2 PSU, gray—0.4 PSU, dark gray—0.6 PSU, the darkest gray—0.8 PSU, and the black one—1.0 PSU, used as a control in this study.
A 1.0 PSU salt concentration was used as the control in this study to determine whether daphnids, which were primary adjusted to this salinity, could survive when P. parvum was provided as the sole food source. This salinity was also in the range within which P. parvum cells’ proliferation normally occurs. What is worth noticing is the fact that the experiment was conducted with a logarithmic increase in salt concentration, mimicking conditions observed during golden algae blooms. After 72 h, all daphnids remained alive under all the experimental conditions.
3.4. Impact of Prymnesium parvum on Daphnia magna Juvenile Growth Rate
One of the most significant vital parameters for D. magna is the somatic growth rate of juveniles [24]. The results shown in Figure 4 demonstrate that an increase in P. parvum cell density, when used as food for juvenile daphnids, leads to a decrease in their growth rate, as measured after four days of the study. During this study, all neonates survived in the presence of P. parvum cells. However, in the variant with 6 mg TOC L−1 from P. parvum cells, one juvenile daphnid became immobilized. These findings suggest that higher densities of P. parvum may disrupt certain aspects of D. magna development.
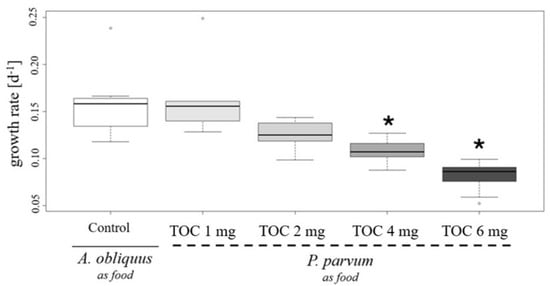
Figure 4.
Change in growth rate of D. magna caused by Prymnesium parvum influence as a nutrition source. Three neonates, previously adapted to salt concentration of 1 g L−1, were used in the study, with three replicates per variant, resulting in nine individuals per variant. The study was conducted at a salinity of 1.0 PSU. P. parvum was provided as the sole food source for the neonates, with concentrations of 1.0 mg (light gray box), 2.0 mg (gray box), 4.0 mg (dark gray box), and 6.0 mg total organic carbon (TOC) per liter (darkest gray box). As the control, neonates were fed green algae Acutodesmus obliquus at a concentration of 1.0 mg TOC per liter, representing the minimal non-limiting growth concentration (white box). The evaluation of TOC concentration indicates that for P. parvum, 1 mg TOC is equal to 1.25 × 104 cells/mL, and for A. obliquus, 1 mg TOC is represented by 9.85 × 105 of cells/mL. The boxplot shows the growth rates over time. The bold central line in each box represents the median value, while the whiskers indicate the minimum and maximum values. Outliers are marked as circular points. Stars indicate statistically significant differences between variants with P. parvum as the food source compared to the control (p < 0.001), based on the non-parametric Kruskal–Wallis test and post hoc Tukey HSD.
A single Daphnia specimen was isolated from a suspension containing Prymnesium parvum cells after 4 days of grazing for each image, where P. parvum cells were introduced for Daphnia magna at 6 mg L−1 TOC concentration in 1.0 PSU salinity. Characteristic filter-feeding behavior was observed (Panel A). The presence of P. parvum cells within the digestive tract of Daphnia (Panel B) confirms that Daphnia is capable of grazing on P. parvum. Additionally, P. parvum cells were clearly visible near the antennae and in the immediate surroundings, exhibiting their typical oval shape (8–10 µm in length) and green-yellow pigmentation (Panel B). Although visible in Panel C, the accumulation of P. parvum cells around the D. magna body may indicate that 6 mg L−1 TOC concentration of algae causes progressive proliferation of golden algae cells, followed by daphnid immobilization. This may partially disable Daphnia filtration, causing a limitation of the food source for daphnids, followed by further distractions in life parameters, which corresponds to the results obtained in neonates’ growth rate represented in Figure 5.
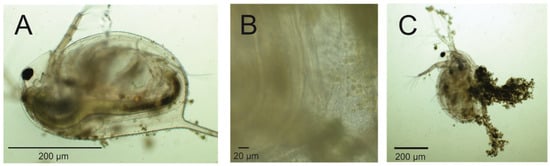
Figure 5.
Interaction between Daphnia magna and Prymnesium parvum cells observed with an optical microscope. Daphnia magna was collected from suspension with 6 mg TOC per liter, which is equal to a density of 7.51 × 104 cell/mL of P. parvum. The feeding behavior (i.e., rhythmic movements of the thoracic appendages) of the Daphnia specimen was observed under a microscope (Nexcope NE620). The presence of Prymnesium parvum cells within the gut of the specimen was confirmed (A,B), although an aggregation of algal cells around daphniid, resulting in reduced mobility, was also observed (C).
4. Discussion
4.1. Growth Conditions
Our results suggest that both Prymnesium parvum and Daphnia magna exhibit tolerance to the salinity levels selected for the experiment [12]. The salinity range of 0.2–1.0 PSU lies at the lower end of the tolerance spectrum for P. parvum, whose optimal range is reported to be between 5.0 and 20.0 PSU [26]. However, the selected range reflects conditions observed during the mass fish mortality event in the Oder River in 2022, where salinity levels, inferred from conductivity measurements (850–7000 µS/cm), ranged between 0.5 and 1.2 PSU [1,25].
The proliferation rate of P. parvum (Figure 1A) was highly dependent on salinity. Concentrations below 0.4 PSU were particularly inhibitory, reducing cell growth by approximately 3 to 3.5 times compared to 1.0 PSU. Cell density was also significantly reduced in conditions below 0.4 PSU (Figure 1B), whereas at 1.0 PSU, it reached approximately 4 × 105 cells/mL—almost double the mean levels recorded in the Oder River in 2022 and comparable to the highest levels reported by Sobieraj and Metelski [25]
Daphnia magna generally does not tolerate salinities above 6.0 PSU, although this is a species-specific trait. In our study, Daphnia displayed sensitivity at 4.0 PSU, with one individual dying after 48 h, and at 6.0 PSU, where one individual died within 48 h and two other individuals died after 72 h (Figure 2). In the test presented in Figure 2, we evaluated the sensitivity of Daphnia fed with P. parvum in a 0.0 to 6.0 PSU range of salinity. Results demonstrate that salinity concentrations above 1.0 PSU are responsible for ephippia (resting egg) production by around 50% of daphnids used in the test. Ephippium egg production is an important part of daphnids’ ecological strategy to survive under unfavorable conditions and indicate their physiological status. Usually, ephippium egg formation takes place when seasonal changes appear, but it may also be caused by biotic, e.g., predator stress or abiotic stressors, e.g., the pH of water or toxins [21,27,28].
The Daphnia magna mortality experiment depicted in Figure 2 suggests that at higher salinity levels (6.0–10.0 PSU), D. magna may be unable to graze efficiently on Prymnesium parvum due to the combined effects of osmotic stress and exposure to algal toxins. These cumulative stressors likely exceed the physiological tolerance of D. magna, leading to increased mortality. In contrast, the lower salinity range of 0.0–1.0 PSU was well tolerated, with minimal adverse effects observed. This range aligns with ecologically relevant conditions where both D. magna and P. parvum are known to.
4.2. Prymnesium parvum Cell Count in the Presence of Filter-Feeding Daphnia magna
In the presence of preculture medium likely containing residual P. parvum toxins, increased P. parvum proliferation was observed. This could be explained by allelopathic effects that compromised Daphnia vitality. Interestingly, the highest growth rate of P. parvum was observed at 0.6 PSU. This salinity is just below a critical threshold [16], where osmotic stress induces cells to adopt an inflated, spherical morphology. We propose that conditions around 0.6 PSU may induce prymnesin production due to cellular stress, without exceeding the cell’s ability to maintain membrane integrity. At lower salinities (<0.6 PSU), elevated mortality may hinder toxin production and prevent the environmental accumulation of lethal prymnesins concentrations, allowing for more effective Daphnia grazing. Conversely, at higher salinities, osmotic conditions may favor P. parvum cell integrity and reduce toxin production [29], thereby allowing Daphnia for better grazing.
The susceptibility of Daphnia to Prymnesium parvum toxins was evaluated by exposing individuals to a suspension equivalent to 1 mg L−1 total organic carbon (TOC), corresponding to approximately 1.25 × 104 cells/mL (Figure 3). However, in the grazing test (Figure 3), the observed susceptibility in our experiments was markedly lower than previously reported by Remmel et al. [30]; our test organisms survived at higher cell densities, corroborating the findings of the same study under specific conditions. These observations are more closely aligned with the findings of Cagle et al. [31], who also reported relatively low Daphnia mortality under high P. parvum cell densities.
In contrast, the actual toxicity of Oder River water during the harmful algal bloom (HAB) event was assessed on the day of peak bloom intensity using a Daphnia bioassay [32]. The results revealed acute toxicity, with Daphnia mortality exceeding 90% even after a fourfold dilution, within 24 and 48 h of exposure. These findings indicate that under bloom conditions, environmental samples may contain significantly higher levels or more bioavailable forms of prymnesins than those observed in laboratory-controlled cultures.
To verify the ingestion of P. parvum cells by Daphnia, microscopic examination was conducted. The presence of P. parvum cells within the digestive tracts of test animals was confirmed (Figure 5), providing direct evidence of active grazing under all tested conditions.
4.3. Life History Parameters
With a higher density of golden algae cells, corresponding to both 4 and 6 mg L−1 TOC concentrations, reduced growth rates of juvenile daphnids were noted in our study. Juvenile growth rate is an important parameter which evaluates D. magna life history. Reduced growth rate may be caused by many different factors, for example, chemicals, toxins, cyanobacteria presence, or worse quality and limited food [33]. As represented in Figure 5, Panel C, with higher cell density an accumulation of cells occurred which partially hindered daphnids in performing filtration, and might have probably caused worse food uptake, despite its higher accessibility. Another explanation is that with a higher density of P. parvum cells, a higher toxin concentration is noted, which causes a reduced growth rate of D. magna neonates.
When it comes to changes in water chemistry, phosphorus (P) and nitrogen (N) concentrations may also be impeded during harmful algal blooms. Both of those elements’ acquisitions are limitation factors, not only for P. parvum but also in Daphnia sp. physiology. During imbalanced stoichiometry in the N:P ratio in a high-density population of P. parvum cells, algae produce more toxins and chemical substances, which may exert an allelopathy effect of Daphnia, causing their higher mortality [31]. This may explain why, even with the high density of algae (6 mg L−1 TOC) in our study (Figure 5), daphnids survived during four days of investigation. Similarly to Cagle et al. [31]’s study, the P. parvum used in our experiment was cultured in a balanced N:P ratio before being introduced to Daphnia, and we did not obtain high mortality, especially in 0.2 to 1.0 PSU, as probably in these conditions the deleterious effects of toxins released from golden algae cells is low.
Thus, this study underlines the importance of freshwater ecosystems’ environmental monitoring, and the need to take action before HABs occur. We evaluated the usage of biological control methods by the application of D. magna, which may potentially help to reduce golden algae cells’ quantity. Nevertheless, this study indicates that action should take place before environmental catastrophes occur. In addition, interactions between the organisms described and presented here still demand further studies, both in the laboratory and environmental conditions.
5. Conclusions and Future Work
We have shown that Daphnia magna can coexist with and feed on Prymnesium parvum cells, potentially contributing to the suppression of algal blooms. Prymnesins in tested conditions do not appear to significantly hinder Daphnia magna growth, which contrasts with some previously published data. To address discrepancies in the literature, a more precise quantification of cell densities, salinity, and prymnesins concentrations is needed. Unfortunately, such analyses are constrained by the lack of standardized reference materials and the need for specialized analytical equipment. Further quantitative studies on Daphnia’s grazing efficiency are also warranted. We suggest that daphnids may survive at least the beginning of P. parvum blooms and could potentially serve as biological suppressors of harmful algal communities. As obligate filter feeders, they may partially consume P. parvum cells. However, higher daphnid densities, longer exposure times, and increased replication are necessary to draw definitive and statistically robust conclusions.
Author Contributions
Conceptualization, T.K., methodology, T.K. and M.G. (Marta Galas), execution of experiments T.K., M.G. (Marta Galas) and M.G. (Marta Grabska), validation and formal analysis T.K., M.G. (Marta Galas) and M.G. (Marta Grabska), resources M.Z., Writing- original draft preparation, T.K., M.G. (Marta Galas) and M.G. (Marta Grabska), project administration T.K. and M.Z., funding acquisition, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grant IDUB 2024 (501-D114-20-0004316), granted by BOB, University of Warsaw, to TK.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Free, G.; Van De Bund, W.; Gawlik, B.; Van Wijk, L.; Wood, M.; Guagnini, E.; Koutelos, K.; Annunziato, A.; Grizzetti, B.; Vigiak, O.; et al. An EU Analysis of the Ecological Disaster in the Oder River of 2022 (JRC132271); Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Köhler, J.; Varga, E.; Spahr, S.; Gessner, J.; Stelzer, K.; Brandt, G.; Mahecha, M.D.; Kraemer, G.; Pusch, M.; Wolter, C.; et al. Unpredicted ecosystem response to compound human impacts in a European river. Sci. Rep. 2024, 14, 16445. [Google Scholar] [CrossRef] [PubMed]
- Umphres, G.D.; Roelke, D.L.; Netherland, M.D. A chemical approach for the mitigation of Prymnesium parvum blooms. Toxicon 2012, 60, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Serra, T.; Soler, M.; Pous, N.; Colomer, J. Daphnia magna filtration, swimming and mortality under ammonium, nitrite, nitrate and phosphate. Sci. Total Environ. 2019, 656, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D. Daphnia as a versatile model system in ecology and evolution. EvoDevo 2022, 13, 16. [Google Scholar] [CrossRef]
- Urrutia-Cordero, P.; Ekvall, M.K.; Hansson, L.-A. Controlling harmful cyanobacteria: Taxa-specific responses of cyanobacteria to grazing by large-bodied Daphnia in a biomanipulation scenario. PLoS ONE 2016, 11, e0153032. [Google Scholar] [CrossRef]
- Lange, J.; Berges, A.C.; von Elert, E. Multiclonal study of Daphnia magna with respect to adaptation to toxic cyanobacteria. Limnol. Oceanogr. 2023, 68, 514–524. [Google Scholar] [CrossRef]
- Bernes, C.; Carpenter, S.R.; Gårdmark, A.; Larsson, P.; Persson, L.; Skov, C.; Speed, J.D.; Van Donk, E. What is the influence of a reduction of planktivorous and benthivorous fish on water quality in temperate eutrophic lakes? A systematic review. Env. Evid. 2015, 4, 7. [Google Scholar] [CrossRef]
- Salonen, K.; Sarvala, J.; Horppila, J.; Keto, J.; Malin, I.; Malinen, T.; Niemistö, J.; Ruuhijärvi, J. Development of Lake Vesijärvi through four decades of remediation efforts. Hydrobiologia 2020, 847, 4601–4619. [Google Scholar] [CrossRef]
- Lathrop, R.C.; Johnson, B.M.; Johnson, T.B.; Vogelsang, M.T.; Carpenter, S.R.; Hrabik, T.R.; Kitchell, J.F.; Magnuson, J.J.; Rudstam, L.G.; Stewart, R.S. Stocking piscivores to improve fishing and water clarity: A synthesis of the Lake Mendota biomanipulation project. Freshw. Biol. 2002, 47, 2410–2424. [Google Scholar] [CrossRef]
- Dickson, D.M.J.; Kirst, G.O. Osmotic adjustment in marine eukaryotic algae: The role of inorganic ions, quaternary ammonium, tertiary sulphonium and carbohydrate solutes: I. Diatoms and a rhodophyte. New Phytol. 1987, 106, 645–655. [Google Scholar] [CrossRef]
- Caron, D.A.; Lie, A.A.Y.; Buckowski, T.; Turner, J.; Frabotta, K. The effect of pH and salinity on the toxicity and growth of the golden alga, Prymnesium parvum. Protist 2023, 174, 125927. [Google Scholar] [CrossRef] [PubMed]
- Aure, J.; Rey, F. Oceanographic conditions in the sandsfjord system, Western Norway, after a bloom of the toxic prymnesiophyte Prymnesium parvum Carter in August 1990. Sarsia 1992, 76, 247–254. [Google Scholar] [CrossRef]
- Roelke, D.L.; Barkoh, A.; Brooks, B.W.; Grover, J.P.; Hambright, K.D.; LaClaire, J.W.; Moeller, P.D.R.; Patino, R. Erratum to: A chronicle of a killer alga in the west: Ecology, assessment, and management of Prymnesium parvum blooms. Hydrobiologia 2016, 764, 51. [Google Scholar] [CrossRef]
- Yin, J.; Sun, X.; Zhao, R.; Qiu, X.; Eeswaran, R. Application of uniform design to evaluate the different conditions on the growth of algae Prymnesium parvum. Sci. Rep. 2021, 11, 12672. [Google Scholar] [CrossRef]
- Woźnica, A.; Karczewski, J.; Lipowczan, M.; Tylko, G.; Jarosz, W.; Matysik, M.; Sierka, E.; Janczewska, N.; Bąk, M.; Prokopowicz, A.; et al. The reaction of Prymnesium parvum to a sudden salinity decrease. Ecohydrol. Hydrobiol. 2024; advance online publication. [Google Scholar] [CrossRef]
- Green, J.C.; Hibberd, D.J.; Pienaar, R.N. The taxonomy of Prymnesium (Prymnesiophyceae) including a description of a new cosmopolitan species, P. patellifera sp. nov., and further observations on P. parvum N. Carter. Br. Phycol. J. 1982, 17, 363–382. [Google Scholar] [CrossRef]
- Sellner, K.G. Physiology, ecology, and toxic properties of marine cyanobacteria blooms. Limnol. Oceanogr. 1997, 42 Pt 2, 1089–1104. [Google Scholar] [CrossRef]
- Manning, S.R.; La Claire, J.W. Prymnesins: Toxic metabolites of the golden alga Prymnesium parvum Carter (Haptophyta). Mar. Drugs 2010, 8, 678–704. [Google Scholar] [CrossRef]
- Tillmann, U. Kill and eat your predator: A winning strategy of planktonic flagellates. Aquat. Microb. Ecol. 2003, 32, 73–84. [Google Scholar] [CrossRef]
- Grzesiuk, M.; Grabska, M.; Malinowska, A.; Świderska, B.; Grzesiuk, E.; Garbicz, D.; Górecki, A. Daphnia stress response to environmental concentrations of chloramphenicol-multi-omics approach. Environ. Sci. Pollut. Res. Int. 2024, 49, 58876–58888. [Google Scholar] [CrossRef]
- Lampert, W. Feeding and nutrition in Daphnia. Mem. Dell’istituto Ital. Idrobiol. 1987, 45, 143–219. [Google Scholar]
- Klüttgen, B.; Dülmer, U.; Engels, M.; Ratte, H.T. ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 1994, 28, 743–746. [Google Scholar] [CrossRef]
- Lampert, W.; Trubetskova, I. Juvenile growth rate as a measure of fitness in Daphnia. Funct. Ecol. 1996, 10, 631–635. [Google Scholar] [CrossRef]
- Sobieraj, J.; Metelski, D. Insights into toxic Prymnesium parvum blooms as a cause of the ecological disaster on the Odra River. Toxins 2023, 15, 403. [Google Scholar] [CrossRef]
- Granéli, E.; Edvardsen, B.; Roelke, D.L.; Hagström, J.A. The ecophysiology and bloom dynamics of Prymnesium spp. Harmful Algae 2012, 14, 260–270. Harmful Algae 2012, 14, 260–270. [Google Scholar] [CrossRef]
- Ebert, D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2005. Available online: https://www.ncbi.nlm.nih.gov/books/NBK2036/ (accessed on 28 April 2025).
- Hiruta, C.; Tochinai, S. Formation and structure of the ephippium (resting egg case) in relation to molting and egg laying in the water flea Daphnia pulex De Geer (Cladocera: Daphniidae). J. Morphol. 2014, 275, 760–767. [Google Scholar] [CrossRef]
- Weissbach, A.; Legrand, C. Effect of different salinities on growth and intra- and extracellular toxicity of four strains of the haptophyte Prymnesium parvum. Aquat. Microb. Ecol. 2012, 67, 139–149. [Google Scholar] [CrossRef]
- Remmel, E.J.; Kohmescher, N.; Larson, J.H.; Hambright, K.D. An experimental analysis of harmful algae–zooplankton interactions and the ultimate defense. Limnol. Oceanogr. 2011, 56, 461–470. Available online: https://www.jstor.org/stable/26953880 (accessed on 28 April 2025). [CrossRef]
- Cagle, S.E.; Roelke, D.L.; Muhl, R.W. Allelopathy and micropredation paradigms reconcile with system stoichiometry. Ecosphere 2021, 12, e03372. [Google Scholar] [CrossRef]
- Szklarek, S.; Font-Nájera, A.; Mazur-Marzec, H.; Jurczak, T.; Sadowski, J.; Mankiewicz-Boczek, J. Prymnesium as a threat for planktonic communities—An ecotoxicological approach for the environmental disaster in the Oder River 2022. Ecohydrol. Hydrobiol. 2024, 24, 516–522. [Google Scholar] [CrossRef]
- Bednarska, A. Food Quantity and Quality Shapes Reproductive Strategies of Daphnia. Ecol. Evol. 2022, 12, e9163. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).