Identification and Comprehensive Evaluation of Drought Tolerance in Sorghum During Germination and Seedling Stages
Abstract
1. Introduction
2. Results
2.1. Germination Potential and Germination Rate
2.2. Phenotypic Attributes
2.3. Single Drought Tolerance Coefficient and Correlation Analysis
2.4. Principal Component Analysis of Drought Tolerance Attributes
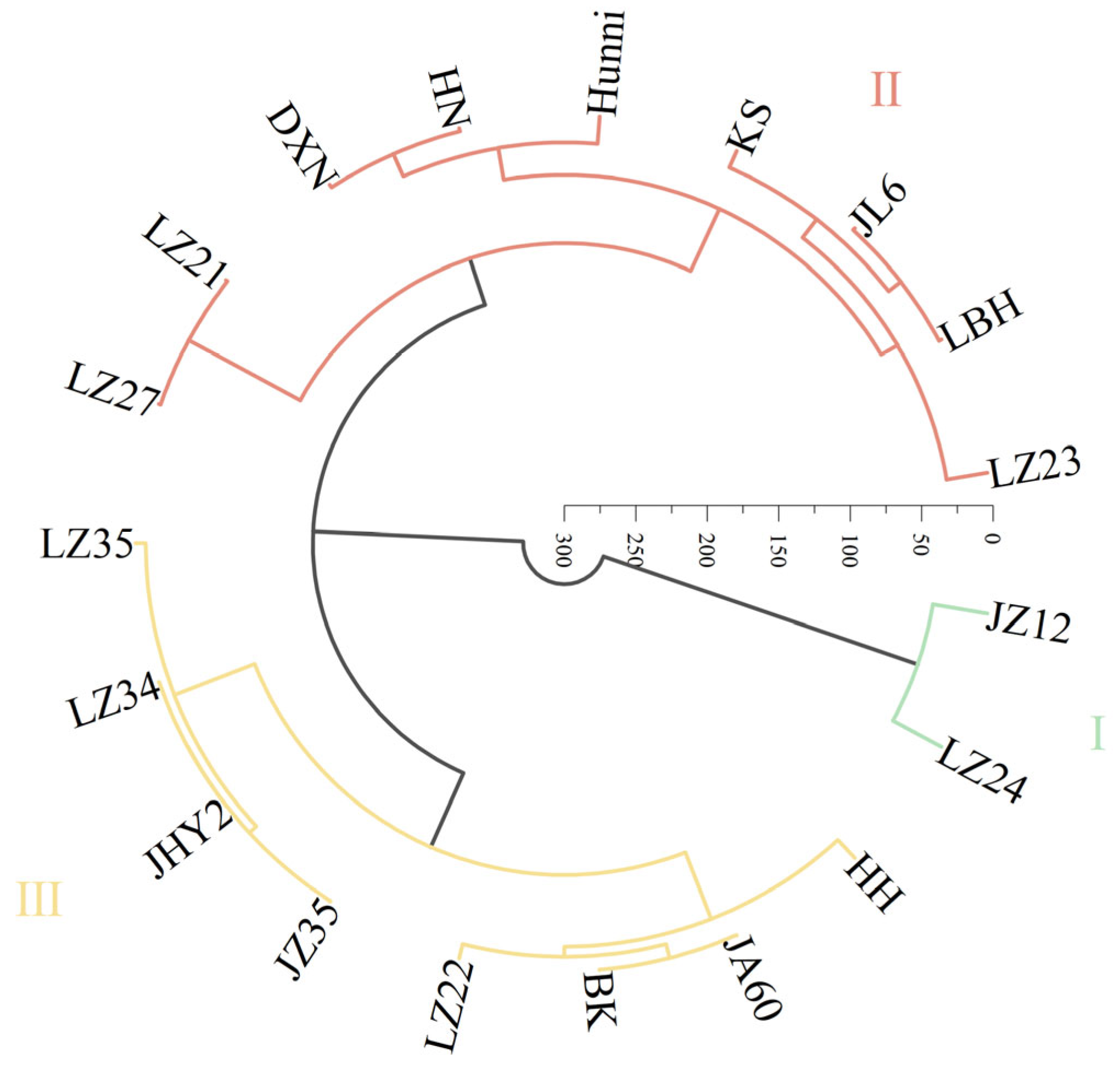
2.5. Comprehensive Evaluation and Cluster Analysis of Drought Tolerance
3. Discussion
3.1. Screening of Drought Resistance Indicators in Sorghum Under Drought Stress
3.2. Methods for Screening Drought Tolerance in Sorghum
4. Materials and Methods
4.1. Sorghum Varieties
4.2. Experimental Methods
4.3. Measurements
4.3.1. Sampling and Measurement
4.3.2. Calculation Methods for Indicators
4.4. Data Statistical and Analytical Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Yuan, F.; He, X. Probabilistic assessment of global drought recovery and its response to precipitation changes. Geophys. Res. Lett. 2024, 51, e2023GL106067. [Google Scholar] [CrossRef]
- Dietz, K.J.; Zörb, C.; Geilfus, C.M. Drought and crop yield. Plant Biol. J. 2021, 23, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Yin, G.; He, W.; Liu, W.; Liu, X.; Xia, Y.; Zhang, H. Drought stress and its characteristics in China from 2001 to 2020 considering vegetation response and drought creep effect. J. Hydrol. Reg. Stud. 2024, 53, 101763. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y. Temporal and spatial variations in drought and its impact on agriculture in China. Water 2024, 16, 1713. [Google Scholar] [CrossRef]
- Zhang, Q.; Yao, Y.; Li, Y.; Huang, J.; Ma, Z.; Wang, Z.; Ying, W.; Yu, Z. Progress and prospect on the study of causes and variation regularity of droughts in China. Acta Meteorol. Sin. 2020, 78, 500–521. [Google Scholar] [CrossRef]
- Sarshad, A.; Talei, D.; Torabi, M.; Rafiei, F.; Nejatkhah, P. Morphological and biochemical responses of Sorghum bicolor (L.) Moench under drought stress. SN Appl. Sci. 2021, 3, 81–93. [Google Scholar] [CrossRef]
- Hossain, M.S.; Islam, M.N.; Rahman, M.M.; Mostofa, M.G.; Khan, M.A.R. Sorghum: A prospective crop for climatic vulnerability, food and nutritional security. J. Agric. Food Res. 2022, 8, 100300. [Google Scholar] [CrossRef]
- Hou, X.; Dan, Y.; Qi, N.; Li, C.; Li, Y.; Yao, Y.; Liao, W. Nitric oxide promotes adventitious rooting in cucumber under drought stress through regulating ascorbate glutathione cycle and protein S-nitrosylation. J. Plant Growth Regul. 2024, 43, 2771–2784. [Google Scholar] [CrossRef]
- El-Naggar, H.M.; Osman, A.R. Enhancing growth and bioactive metabolites characteristics in Mentha pulegium L. via silicon nanoparticles during in vitro drought stress. BMC Plant Biol. 2024, 24, 657–673. [Google Scholar] [CrossRef]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Abreha, K.B.; Enyew, M.; Carlsson, A.S.; Vetukur, R.R.; Feyissa, T.; Motlhaodi, T.; Ng′uni, D.; Geleta, M. Sorghum in dryland: Morphological, physiological, and molecular responses of sorghum under drought stress. Planta 2021, 255, 20–43. [Google Scholar] [CrossRef] [PubMed]
- Umair, H.M.; Aamer, M.; Umer, C.M. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef]
- Bao, W.W.; Chen, X.; Li, R.N.; Li, M.; Xie, C.J.; Dou, M.R.; Zhang, K.Z.; Wang, J.; Gao, Z.X.; Liu, Z.D.; et al. Comprehensive assessment of drought resistance and recovery in kiwifruit genotypes using multivariate analysis. Plant J. 2024, 119, 100–114. [Google Scholar] [CrossRef]
- Alarmelu, S.; Sheelamary, S.; Anusheela, V.; Kumar, R.A.; Vasantha, S.; Swamy, H.K.M.A.; Anna, D. Identification of drought tolerance indicators in interspecific hybrid derivatives of saccharum spp using membership function value of drought tolerance (MFVD). Sugar Tech 2025, 27, 860–872. [Google Scholar] [CrossRef]
- Negarestani, M.; Tohidi-Nejad, E.; Khajoei-Nejad, G.; Nakhoda, B.; Mohammadi-Nejad, G. Comparison of Different Multivariate Statistical Methods for Screening the Drought Tolerant Genotypes of Pearl Millet (Pennisetum americanum L.) and Sorghum (Sorghum bicolor L.). Agronomy 2019, 9, 645. [Google Scholar] [CrossRef]
- Vijayalakshmi, D.; Jeevitha, R.; Gowsiga, S.; Vinitha, A.; Soumya, R. Evaluation of chlorophyll index as indicators to screen sorghum genotypes for drought stress tolerance. Cereal Res. Commun. 2024, 52, 1511–1525. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, J.; Lee, J.; Lee, S.; Jung, H.; Park, G.-H. Drought Tolerance Evaluation and Growth Response of Chinese Cabbage Seedlings to Water Deficit Treatment. Agronomy 2024, 14, 279. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, X.; Naseer, M.A.; Zhou, H.; Cheng, W.; Xie, H.; Qin, L.; Yang, X.; Jiang, Y.; Zhou, X. Screening and Physiological Responses of Maize Inbred Lines to Drought Stress in South China. Sustainability 2024, 16, 7366. [Google Scholar] [CrossRef]
- Gupta, C.; Salgotra, R.K. Epigenetics and its role in effecting agronomical traits. Front. Plant Sci. 2022, 13, 925688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.L.; Gan, Y.J.; Le, L.; Pu, L. Epigenetic variation in maize agronomical traits for breeding and trait improvement. J. Genet. Genom. 2025, 52, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ma, L.; Li, J.; Hou, D.; Zeng, B.; Zhang, L.; Liu, C.; Bi, Q.; Tan, J.; Yu, X.; et al. Factors Influencing Seed Dormancy and Germination and Advances in Seed Priming Technology. Plants 2024, 13, 1319. [Google Scholar] [CrossRef] [PubMed]
- Hamouzová, K.; Šuk, J.; Bhattacharya, S.; Valičková, V.; Kolářová, M.; Soukup, J. The effect of various factors (light, temperature, salt, and drought) on germination of Bromus sterilis L. Plant Soil Environ. 2024, 70, 287–295. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Sun, F.; Chen, Q.; Chen, Q.; Jiang, M.; Gao, W.; Qu, Y. Screening of key drought tolerance indices for cotton at the flowering and boll setting stage using the dimension reduction method. Front. Plant Sci. 2021, 12, 619926. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Dodd, I.C.; Veselov, D.S.; Rothwell, S.A.; Veselov, S.Y. Common and specific responses to availability of mineral nutrients and water. J. Exp. Bot. 2015, 66, 21–33. [Google Scholar] [CrossRef]
- Hartmann, H. Will a 385 million year-struggle for light become a struggle for water and for carbon?—How trees may cope with more frequent climate change-type drought events. Glob. Change Biol. 2011, 17, 642–655. [Google Scholar] [CrossRef]
- Sofi, P.A.; Rehman, K.; Gull, M.; Kumari, J.; Prasad, P. Integrating root architecture and physiological approaches for improving drought tolerance in common bean (Phaseolus vulgaris L.). Plant Physiol. Rep. 2021, 26, 4–22. [Google Scholar] [CrossRef]
- De Vienne, D. What is a phenotype? History and new developments of the concept. Genetica 2022, 150, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Y.; Ahmad, S.; Wang, Y.; Wang, B.W.; Huang, J.H.; Jahan, M.S.; Zhou, X.B.; Shi, C.Q. Multivariate analysis compares and evaluates drought and flooding tolerances of maize germplasm. Plant Physiol. 2023, 193, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Abbas, K.; Wang, L.; Gong, B.; Hou, S.; Wang, W.; Dai, B.; Xia, H.; Wu, X.; Lv, G.; et al. Drought resistance index screening and evaluation of lettuce under water deficit conditions on the basis of morphological and physiological differences. Front. Plant Sci. 2023, 14, 1228084. [Google Scholar] [CrossRef]
- Liu, T.; Díaz-Pachón, D.A.; Rao, J.S.; Dazard, J.E. High Dimensional Mode Hunting Using Pettiest Components Analysis. IEEE Trans. Pattern Anal. Mach. Intell. 2023, 45, 4637–4649. [Google Scholar] [CrossRef]
- Maheswari, M.; Tekula, V.L.; Yellisetty, V.; Sarkar, B.; Yadav, S.K.; Singh, J. Functional mechanisms of drought tolerance in maize through phenotyping and genotyping under well-watered and water stressed conditions. Eur. J. Agron. 2016, 79, 43–57. [Google Scholar] [CrossRef]
- Javaid, A.; Razzaq, H.; Khan, F.A.; Awan, F. Evaluation of maize accessions for drought tolerance through principal component analysis. Sabrao J. Breed. Genet. 2023, 55, 476–484. [Google Scholar] [CrossRef]
- Zou, J.; Hu, W.; Li, Y.X.; He, J.Q.; Zhu, H.H.; Zhou, Z.G. Screening of drought resistance indices and evaluation of drought resistance in cotton (Gossypium hirsutum L.). J. Integr. Agric. 2020, 19, 495–508. [Google Scholar] [CrossRef]
- Lapuimakuni, S.; Khumaida, N.S.W. Evaluation of drought tolerance indices for genotype selection of foxtail millet (Setaria italica). Trop. Drylands 2018, 2, 37–40. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, Z.X.; Chen, G.Q.; Liu, Z.; Xin, Z.X.; Kong, F.X. Evaluation of drought tolerance in different genotypes of foxtail millet during the entire growth period. Agron. J. 2021, 114, 340–355. [Google Scholar] [CrossRef]
- Jones, M.M.; Turner, N.C. Osmotic adjustment in leaves of sorghum in response to water deficits. Plant Physiol. 1978, 61, 122–126. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, W.; He, J.; Zhang, L.; Wei, Y.; Yang, M. Nitric oxide alleviates salt stress in seed germination and early seedling growth of pakchoi (Brassica chinensis L.) by enhancing physiological and biochemical parameters. Ecotoxicol. Environ. Saf. 2020, 187, 109785. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.H.; Han, K.N.; Du, X.F.; Wang, Z.L.; Li, Y.X.; Lian, S.C.; Zhang, L.Y.; Wang, J. Identification and application of salt-tolerant germplasms at germination stage in foxtail millet (Setaria italica L.). Plant Sci. J. 2024, 42, 75–84. [Google Scholar]
- Bao, X.; Hou, X.; Duan, W.; Yin, B.; Ren, J.; Wang, Y.; Liu, X.; Gu, L.; Zhen, W. Screening and evaluation of drought resistance traits of winter wheat in the north China plain. Front. Plant Sci. 2023, 14, 1194759. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Min, D.; Yasir, T.A.; Hu, Y.G. Evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD). Field Crops Res. 2012, 137, 195–201. [Google Scholar] [CrossRef]
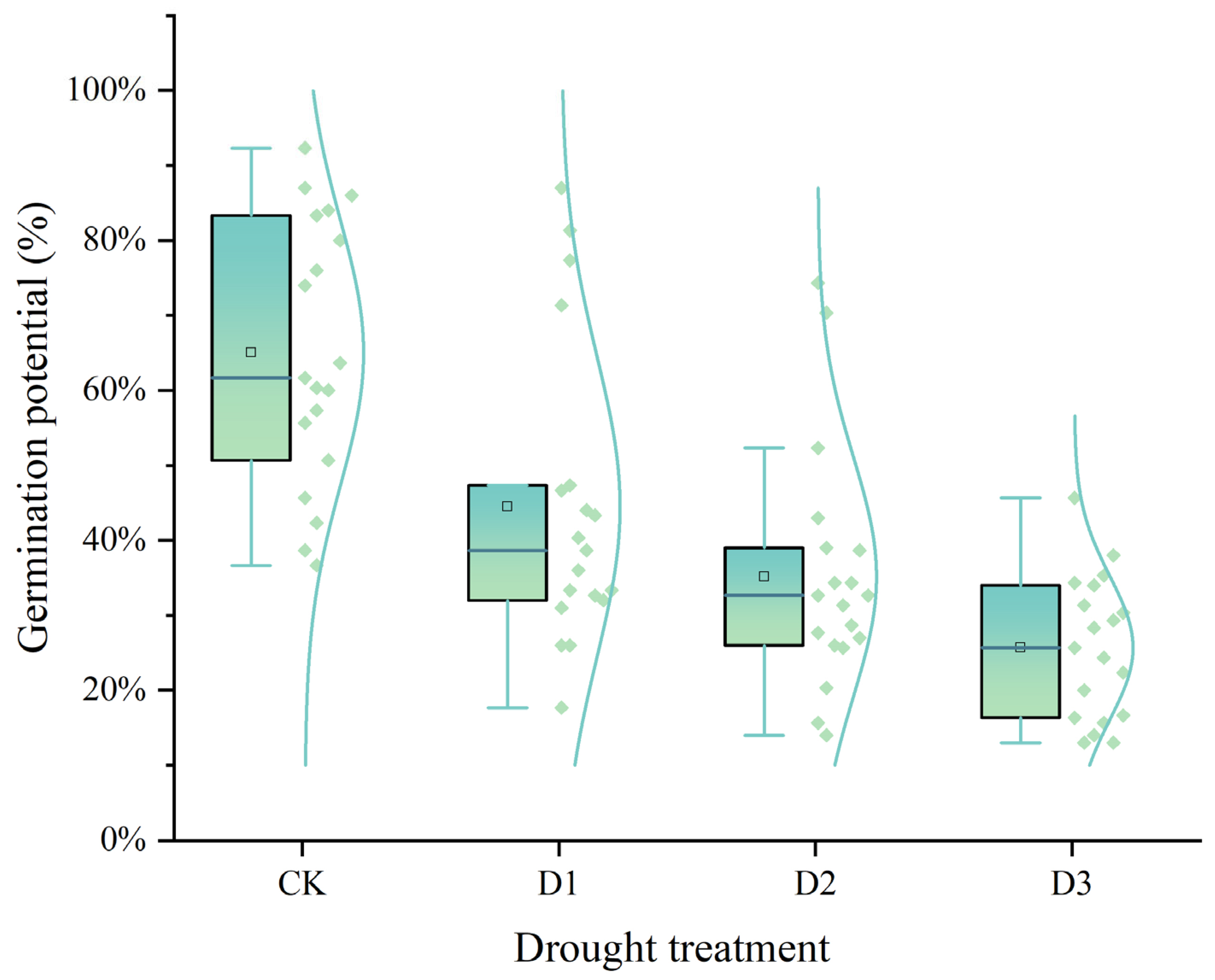
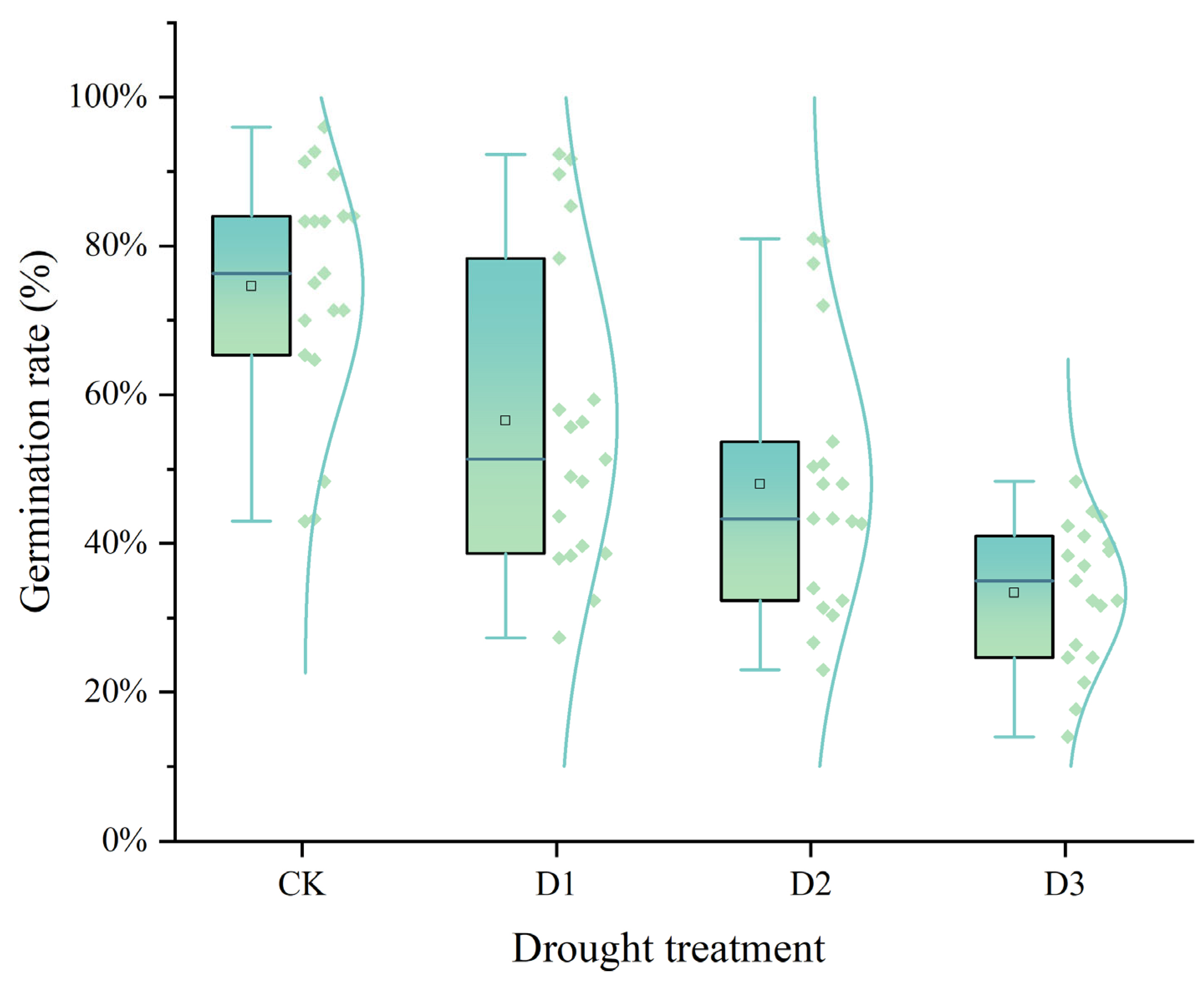
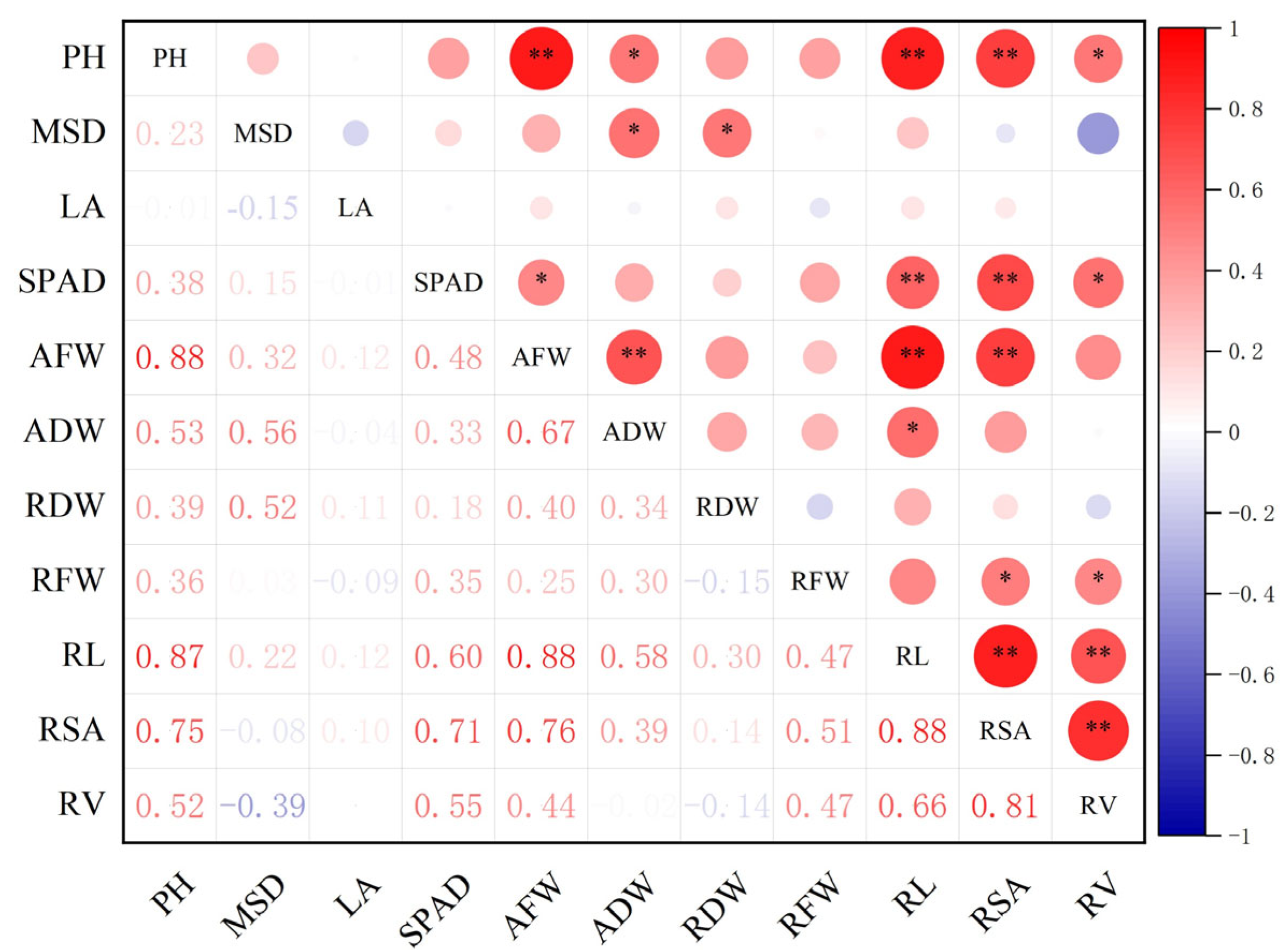

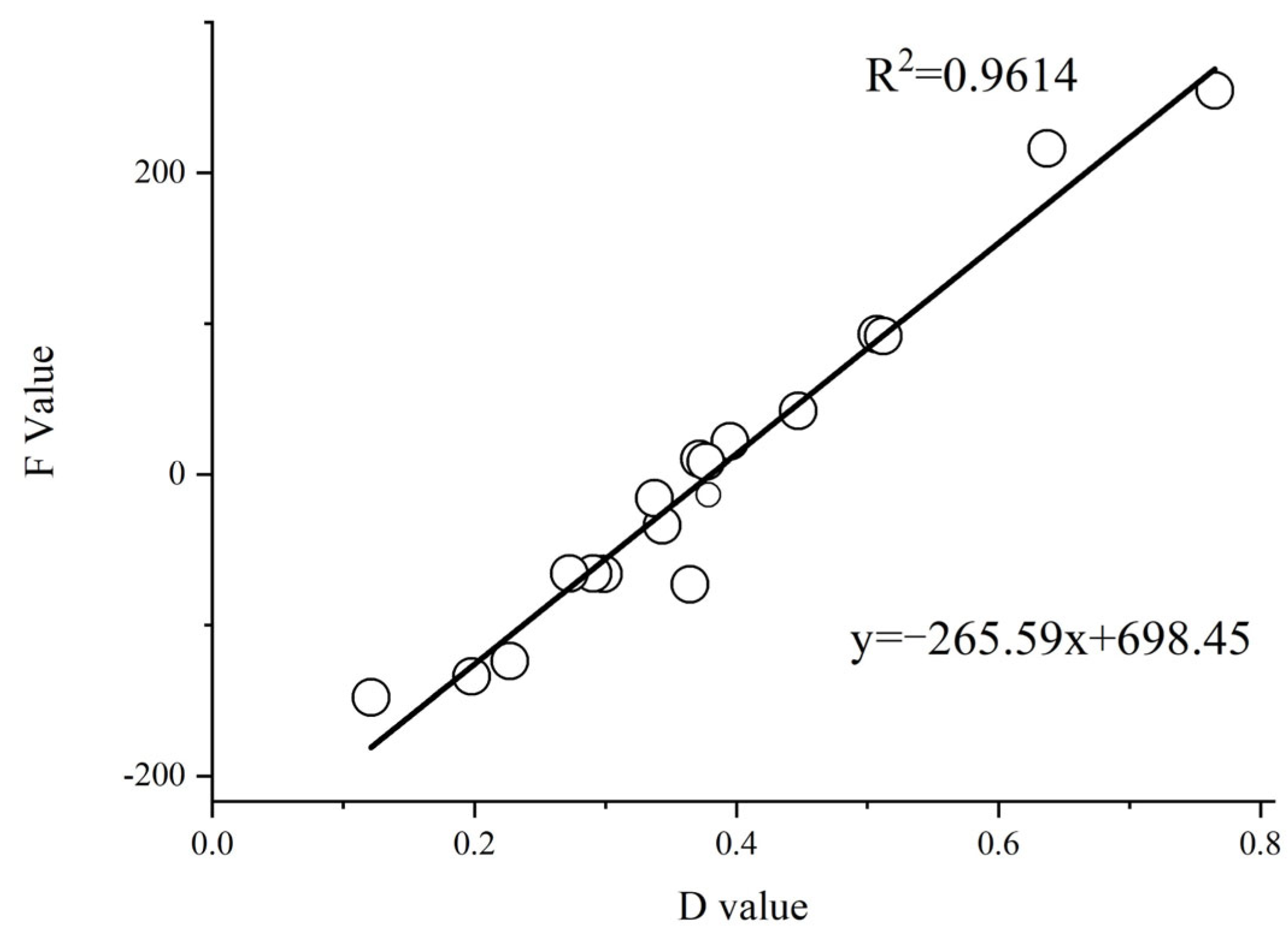
| Cultivars | Germination Potential (%) | Germination Rate (%) | Relative Germination Potential | Relative Germination Rate | Ranking | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | D1 | D2 | D3 | CK | D1 | D2 | D3 | D1 | D2 | D3 | D1 | D2 | D3 | ||
| LZ23 | 55.67 a | 31.00 b | 27.67 b | 16.33 c | 70.00 a | 58.00 b | 43.33 bc | 38.33 c | 0.56 a | 0.50 a | 0.29 b | 0.83 a | 0.62 ab | 0.55 b | 13 |
| LZ24 | 87.00 a | 87.00 a | 74.33 b | 25.67 c | 91.33 a | 92.33 a | 81.00 a | 42.33 b | 1.00 a | 0.85 b | 0.30 c | 1.01 a | 0.89 a | 0.46 b | 3 |
| LZ35 | 83.33 a | 33.33 b | 20.33 c | 13.00 d | 83.33 a | 38.00 a | 34.00 a | 24.67 b | 0.40 a | 0.24 b | 0.16 c | 0.46 a | 0.41 a | 0.30 b | 18 |
| LZ34 | 74.00 a | 36.00 b | 32.67 bc | 20.00 c | 83.33 a | 38.33 a | 26.67 ab | 26.33 b | 0.49 a | 0.44 a | 0.27 b | 0.46 a | 0.32 a | 0.32 a | 17 |
| LZ21 | 57.33 a | 26.00 b | 15.67 b | 14.00 b | 65.33 a | 39.67 a | 31.33 ab | 21.33 c | 0.45 a | 0.27 b | 0.24 b | 0.61 a | 0.48 a | 0.33 b | 16 |
| LZ22 | 84.00 a | 46.67 b | 26.00 c | 15.67 c | 92.67 a | 55.67 ab | 48.00 ab | 24.67 b | 0.56 a | 0.31 b | 0.19 b | 0.60 a | 0.52 a | 0.27 b | 15 |
| LZ27 | 50.67 a | 17.67 b | 14.00 b | 13.00 b | 64.67 a | 27.33 a | 23.00 b | 14.00 b | 0.35 a | 0.28 a | 0.26 a | 0.42 a | 0.36 ab | 0.22 b | 19 |
| Hunni | 92.33 a | 71.33 b | 70.33 c | 45.67 c | 96.00 a | 78.33 ab | 77.67 b | 48.33 c | 0.77 a | 0.76 a | 0.49 b | 0.82 a | 0.81 a | 0.50 b | 4 |
| Bk | 80.00 a | 77.33 b | 39.00 c | 34.33 c | 89.67 a | 83.33 a | 50.33 a | 41.00 b | 0.97 a | 0.49 b | 0.43 b | 0.93 a | 0.60 b | 0.49 b | 8 |
| HN | 86.00 a | 81.33 b | 52.33 c | 31.33 c | 89.67 a | 91.67 a | 72.00 a | 35.00 b | 0.95 a | 0.61 b | 0.36 c | 0.98 a | 0.80 b | 0.39 c | 6 |
| LBH | 61.67 a | 38.67 b | 34.33 c | 34.00 c | 84.00 a | 56.33 ab | 50.67 b | 37.00 c | 0.63 a | 0.56 a | 0.55 a | 0.67 a | 0.60 ab | 0.44 b | 12 |
| KS | 38.67 a | 26.00 ab | 25.67 bc | 16.67 c | 43.00 a | 32.33 a | 30.33 b | 17.67 b | 0.67 a | 0.66 a | 0.43 b | 0.75 a | 0.71 a | 0.41 b | 10 |
| JZ12 | 76.00 a | 47.33 b | 43.00 bc | 35.33 c | 85.33 a | 84.00 a | 80.67 a | 44.33 b | 0.62 a | 0.57 ab | 0.46 b | 0.98 a | 0.96 a | 0.53 b | 5 |
| JZ35 | 45.67 a | 32.67 a | 31.33 a | 28.33 a | 43.67 a | 43.33 a | 43.33 a | 32.33 b | 0.72 a | 0.69 a | 0.62 a | 0.99 a | 0.99 a | 0.75 a | 2 |
| JA60 | 60.33 a | 32.00 b | 28.67 bc | 24.33 c | 75.00 a | 38.67 ab | 32.33 b | 31.67 c | 0.53 a | 0.48 a | 0.40 a | 0.52 a | 0.43 a | 0.42 a | 14 |
| JHY2 | 60.00 a | 40.33 b | 34.33 b | 29.33 b | 76.33 a | 49.00 ab | 48.00 b | 43.67 c | 0.67 a | 0.57 a | 0.49 a | 0.64 a | 0.63 a | 0.57 a | 11 |
| HH | 63.67 a | 44.00 b | 38.67 bc | 38.00 c | 71.33 a | 59.33 ab | 53.67 b | 40.00 c | 0.69 a | 0.61 a | 0.60 a | 0.83 a | 0.75 a | 0.56 b | 7 |
| DXN | 36.67 a | 33.33 ab | 32.67 b | 30.33 b | 51.33 a | 48.33 a | 43.00 b | 39.00 b | 0.91 a | 0.89 a | 0.83 a | 0.94 a | 0.84 a | 0.81 a | 1 |
| JL6 | 43.33 a | 42.33 a | 27.00 a | 22.33 a | 71.33 a | 48.33 a | 42.67 a | 32.33 b | 0.98 a | 0.64 b | 0.53 b | 0.68 a | 0.60 a | 0.45 b | 9 |
| Parameters | PEG Treatment | Mean | Standard Deviation | CV (%) |
|---|---|---|---|---|
| PH (cm) | CK | 28.09 | 10.51 | 0.37 |
| D1 | 20.02 | 4.11 | 0.21 | |
| D2 | 12.48 | 3.60 | 0.29 | |
| D3 | 5.96 | 2.47 | 0.41 | |
| SD (mm) | CK | 2.26 | 0.78 | 0.35 |
| D1 | 1.69 | 0.42 | 0.25 | |
| D2 | 1.42 | 0.30 | 0.21 | |
| D3 | 1.16 | 0.27 | 0.24 | |
| LA (cm2) | CK | 22.80 | 20.44 | 0.90 |
| D1 | 10.28 | 4.30 | 0.42 | |
| D2 | 5.99 | 3.67 | 0.61 | |
| D3 | 1.99 | 2.38 | 1.20 | |
| AFW (mg) | CK | 542.84 | 592.97 | 1.09 |
| D1 | 202.43 | 120.25 | 0.59 | |
| D2 | 116.45 | 80.30 | 0.69 | |
| D3 | 33.95 | 19.91 | 0.59 | |
| ADW (mg) | CK | 44.91 | 30.10 | 0.67 |
| D1 | 30.26 | 24.65 | 0.81 | |
| D2 | 20.44 | 15.09 | 0.74 | |
| D3 | 7.38 | 3.24 | 0.44 | |
| RFW (mg) | CK | 322.00 | 319.95 | 0.99 |
| D1 | 139.19 | 66.42 | 0.48 | |
| D2 | 106.96 | 54.16 | 0.51 | |
| D3 | 57.05 | 12.76 | 0.22 | |
| RDW (mg) | CK | 62.55 | 66.44 | 1.06 |
| D1 | 19.73 | 8.11 | 0.41 | |
| D2 | 14.90 | 4.94 | 0.33 | |
| D3 | 9.85 | 4.36 | 0.44 | |
| SPAD | CK | 28.26 | 5.48 | 0.19 |
| D1 | 28.27 | 2.67 | 0.09 | |
| D2 | 21.51 | 4.17 | 0.19 | |
| D3 | 14.21 | 5.56 | 0.39 | |
| RL (cm) | CK | 204.15 | 205.84 | 1.01 |
| D1 | 69.45 | 37.32 | 0.54 | |
| D2 | 41.07 | 23.17 | 0.56 | |
| D3 | 14.13 | 7.69 | 0.54 | |
| RSA (cm2) | CK | 21.22 | 24.84 | 1.17 |
| D1 | 8.15 | 4.92 | 0.60 | |
| D2 | 4.12 | 2.53 | 0.61 | |
| D3 | 1.49 | 0.70 | 0.47 | |
| RV (mm3) | CK | 163.12 | 227.54 | 1.39 |
| D1 | 99.07 | 80.72 | 0.81 | |
| D2 | 33.25 | 20.93 | 0.63 | |
| D3 | 14.65 | 9.03 | 0.62 |
| Cultivars | Single Drought Resistance Coefficient (a) | Comprehensive Drought Resistance Coefficient (B) | Ranking | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH (cm) | SD (mm) | LA (cm2) | AFW (mg) | ADW (mg) | RFW (mg) | RDW (mg) | SPAD | RL (cm) | RSA (cm2) | RV (mm3) | |||
| LZ23 | 0.31 | 0.58 | 0.14 | 0.48 | 0.13 | 0.17 | 0.14 | 0.35 | 0.09 | 0.09 | 0.08 | 0.21 | 12 |
| LZ24 | 0.43 | 0.51 | 0.17 | 0.57 | 0.17 | 0.17 | 0.22 | 0.30 | 0.19 | 0.17 | 0.18 | 0.26 | 8 |
| LZ35 | 0.12 | 0.43 | 0.55 | 0.42 | 0.04 | 0.07 | 0.16 | 0.18 | 0.05 | 0.06 | 0.09 | 0.18 | 13 |
| LZ34 | 0.17 | 0.76 | 0.07 | 0.25 | 0.05 | 0.09 | 0.11 | 0.21 | 0.03 | 0.07 | 0.06 | 0.16 | 15 |
| LZ21 | 0.22 | 0.83 | 0.06 | 0.75 | 0.10 | 0.13 | 0.19 | 0.28 | 0.08 | 0.09 | 0.08 | 0.23 | 11 |
| LZ22 | 0.09 | 0.57 | 0.20 | 0.39 | 0.03 | 0.03 | 0.13 | 0.28 | 0.04 | 0.04 | 0.07 | 0.16 | 14 |
| LZ27 | 0.26 | 0.66 | 0.13 | 0.81 | 0.12 | 0.21 | 0.17 | 0.44 | 0.06 | 0.08 | 0.09 | 0.25 | 9 |
| Hunni | 0.14 | 0.38 | 0.02 | 0.27 | 0.02 | 0.12 | 0.06 | 0.07 | 0.02 | 0.01 | 0.01 | 0.09 | 17 |
| Bk | 0.07 | 0.27 | 0.003 | 0.18 | 0.01 | 0.09 | 0.04 | 0.04 | 0.01 | 0.01 | 0.00 | 0.06 | 18 |
| HN | 0.14 | 0.39 | 0.01 | 0.39 | 0.02 | 0.25 | 0.11 | 0.06 | 0.02 | 0.01 | 0.02 | 0.12 | 16 |
| LBH | 0.20 | 0.81 | 0.09 | 0.59 | 0.13 | 0.58 | 0.58 | 0.49 | 0.12 | 0.11 | 0.42 | 0.34 | 6 |
| KS | 0.25 | 0.72 | 0.17 | 0.64 | 0.24 | 0.92 | 0.45 | 0.35 | 0.19 | 0.28 | 0.51 | 0.39 | 3 |
| JZ12 | 0.50 | 0.71 | 0.34 | 0.72 | 0.32 | 0.55 | 0.59 | 0.30 | 0.30 | 0.24 | 0.85 | 0.45 | 1 |
| JZ35 | 0.18 | 0.67 | 0.12 | 0.47 | 0.11 | 0.23 | 0.57 | 0.09 | 0.08 | 0.17 | 0.23 | 0.24 | 10 |
| JA60 | 19 | ||||||||||||
| JHY2 | 0.23 | 0.89 | 0.04 | 0.27 | 0.09 | 0.38 | 0.48 | 0.42 | 0.27 | 0.33 | 0.43 | 0.32 | 7 |
| HH | 0.15 | 0.51 | 0.04 | 0.81 | 0.08 | 0.43 | 0.48 | 0.22 | 0.43 | 0.61 | 0.81 | 0.38 | 5 |
| DXN | 0.35 | 0.66 | 0.19 | 0.48 | 0.21 | 0.13 | 0.71 | 0.23 | 0.55 | 0.60 | 0.53 | 0.39 | 4 |
| JL6 | 0.29 | 0.62 | 0.16 | 0.57 | 0.23 | 0.71 | 0.52 | 0.20 | 0.61 | 0.58 | 0.47 | 0.41 | 2 |
| Attribute | Principal Component | ||
|---|---|---|---|
| F1 | F2 | F3 | |
| PH (cm) | 0.88 | 0.10 | 0.06 |
| SD (mm) | 0.25 | 0.84 | −0.26 |
| LA (cm2) | 0.06 | −0.04 | 0.85 |
| AFW (mg) | 0.69 | −0.15 | −0.09 |
| ADW (mg) | 0.90 | 0.21 | 0.15 |
| RFW (mg) | 0.63 | 0.52 | −0.21 |
| RDW (mg) | 0.52 | −0.33 | −0.45 |
| SPAD | 0.35 | 0.68 | 0.31 |
| RL (cm) | 0.97 | −0.02 | 0.07 |
| RSA (cm2) | 0.91 | −0.33 | 0.07 |
| RV (mm3) | 0.66 | −0.68 | 0.03 |
| Characteristic value | 5.13 | 2.18 | 1.18 |
| Contribution rate% | 46.59 | 19.84 | 10.74 |
| Cumulative contribution rate% | 46.59 | 66.43 | 77.18 |
| Factor weight | 0.60 | 0.26 | 0.14 |
| Cultivars | F Value | Ranking |
|---|---|---|
| LZ23 | 42.28 | 5 |
| LZ24 | 254.61 | 1 |
| LZ35 | −72.83 | 15 |
| LZ34 | −66.01 | 14 |
| LZ21 | 93.16 | 3 |
| LZ22 | −123.81 | 16 |
| LZ27 | 91.95 | 4 |
| Hunni | −33.84 | 11 |
| BK | −133.95 | 17 |
| HN | −13.60 | 9 |
| LBH | 10.29 | 7 |
| KS | 21.95 | 6 |
| JZ12 | 216.13 | 2 |
| JZ35 | −65.57 | 12 |
| JA60 | 19 | |
| JHY2 | −65.82 | 13 |
| HH | −147.79 | 18 |
| DXN | −15.70 | 10 |
| JL6 | 8.55 | 8 |
| Cultivars | Membership Function Value (μ) | Drought Tolerance Measure (D) | |||||
|---|---|---|---|---|---|---|---|
| μ1 | μ2 | μ3 | μ4 | μ5 | Value | Ranking | |
| LZ23 | 0.38 | 0.80 | 0.52 | 0.17 | 0.10 | 0.45 | 5 |
| LZ24 | 0.95 | 0.74 | 0.52 | 0.70 | 0.00 | 0.77 | 1 |
| LZ35 | 0.13 | 0.47 | 1.00 | 1.00 | 0.65 | 0.36 | 10 |
| LZ34 | 0.14 | 0.70 | 0.46 | 0.22 | 0.20 | 0.30 | 13 |
| LZ21 | 0.49 | 1.00 | 0.26 | 0.09 | 1.00 | 0.51 | 4 |
| LZ22 | 0.00 | 0.53 | 0.82 | 0.18 | 0.64 | 0.23 | 16 |
| LZ27 | 0.51 | 0.83 | 0.46 | 0.18 | 0.65 | 0.51 | 3 |
| Hunni | 0.24 | 0.70 | 0.28 | 0.38 | 0.18 | 0.34 | 11 |
| BK | 0.01 | 0.62 | 0.30 | 0.28 | 0.09 | 0.20 | 17 |
| HN | 0.32 | 0.68 | 0.12 | 0.63 | 0.27 | 0.38 | 7 |
| LBH | 0.47 | 0.39 | 0.15 | 0.37 | 0.95 | 0.37 | 9 |
| KS | 0.48 | 0.38 | 0.34 | 0.34 | 0.49 | 0.40 | 6 |
| JZ12 | 1.00 | 0.16 | 0.68 | 0.10 | 0.42 | 0.64 | 2 |
| JZ35 | 0.33 | 0.25 | 0.00 | 0.85 | 0.57 | 0.29 | 14 |
| JA60 | 19 | ||||||
| JHY2 | 0.26 | 0.41 | 0.19 | 0.33 | 0.20 | 0.27 | 15 |
| HH | 0.15 | 0.00 | 0.38 | 0.00 | 0.37 | 0.12 | 18 |
| DXN | 0.34 | 0.46 | 0.48 | 0.07 | 0.25 | 0.34 | 12 |
| JL6 | 0.44 | 0.37 | 0.41 | 0.29 | 0.17 | 0.38 | 8 |
| Cultivars | Breeding Country | Abbreviation | Source |
|---|---|---|---|
| Longza23 | China | LZ23 | Heilongjiang Academy of Agricultural Sciences |
| Longza24 | China | LZ24 | Heilongjiang Academy of Agricultural Sciences |
| Longza35 | China | LZ35 | Heilongjiang Academy of Agricultural Sciences |
| Longza34 | China | LZ34 | Heilongjiang Academy of Agricultural Sciences |
| Longza21 | China | LZ21 | Heilongjiang Academy of Agricultural Sciences |
| Longza22 | China | LZ22 | Heilongjiang Academy of Agricultural Sciences |
| Longza27 | China | LZ27 | Heilongjiang Academy of Agricultural Sciences |
| Hunnigreen | USA | Hunni | Fuyuanlai Limited |
| Big Kahuna | USA | BK | Barenbrug |
| Hainiu | Australian | HN | Fuyuanlai Limited |
| Lvbaohong | China | LBH | Shouhe Agriculture |
| Kangsi | China | KS | Shouhe Agriculture |
| Jingza12 | China | JZ12 | Shouhe Agriculture |
| Jingza35 | China | JZ35 | Shouhe Agriculture |
| Jiaai60 | China | JA60 | Shouhe Agriculture |
| Jinghongying2 | China | JHY2 | Shouhe Agriculture |
| Huanghe | China | HH | Shouhe Agriculture |
| Dingxinnuo | China | DXN | Shouhe Agriculture |
| Jiliang6 | China | JL6 | Shouhe Agriculture |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Ahmad, I.; Qin, B.; Chen, L.; Bu, W.; Zhu, G.; Zhou, G. Identification and Comprehensive Evaluation of Drought Tolerance in Sorghum During Germination and Seedling Stages. Plants 2025, 14, 1793. https://doi.org/10.3390/plants14121793
Wang M, Ahmad I, Qin B, Chen L, Bu W, Zhu G, Zhou G. Identification and Comprehensive Evaluation of Drought Tolerance in Sorghum During Germination and Seedling Stages. Plants. 2025; 14(12):1793. https://doi.org/10.3390/plants14121793
Chicago/Turabian StyleWang, Manhong, Irshad Ahmad, Bin Qin, Lei Chen, Weicheng Bu, Guanglong Zhu, and Guisheng Zhou. 2025. "Identification and Comprehensive Evaluation of Drought Tolerance in Sorghum During Germination and Seedling Stages" Plants 14, no. 12: 1793. https://doi.org/10.3390/plants14121793
APA StyleWang, M., Ahmad, I., Qin, B., Chen, L., Bu, W., Zhu, G., & Zhou, G. (2025). Identification and Comprehensive Evaluation of Drought Tolerance in Sorghum During Germination and Seedling Stages. Plants, 14(12), 1793. https://doi.org/10.3390/plants14121793








