Genotypic, Environmental, and Processing Effects on Phenolic Content and Antioxidant Activity in Barley and Wheat
Abstract
1. Introduction
2. Results
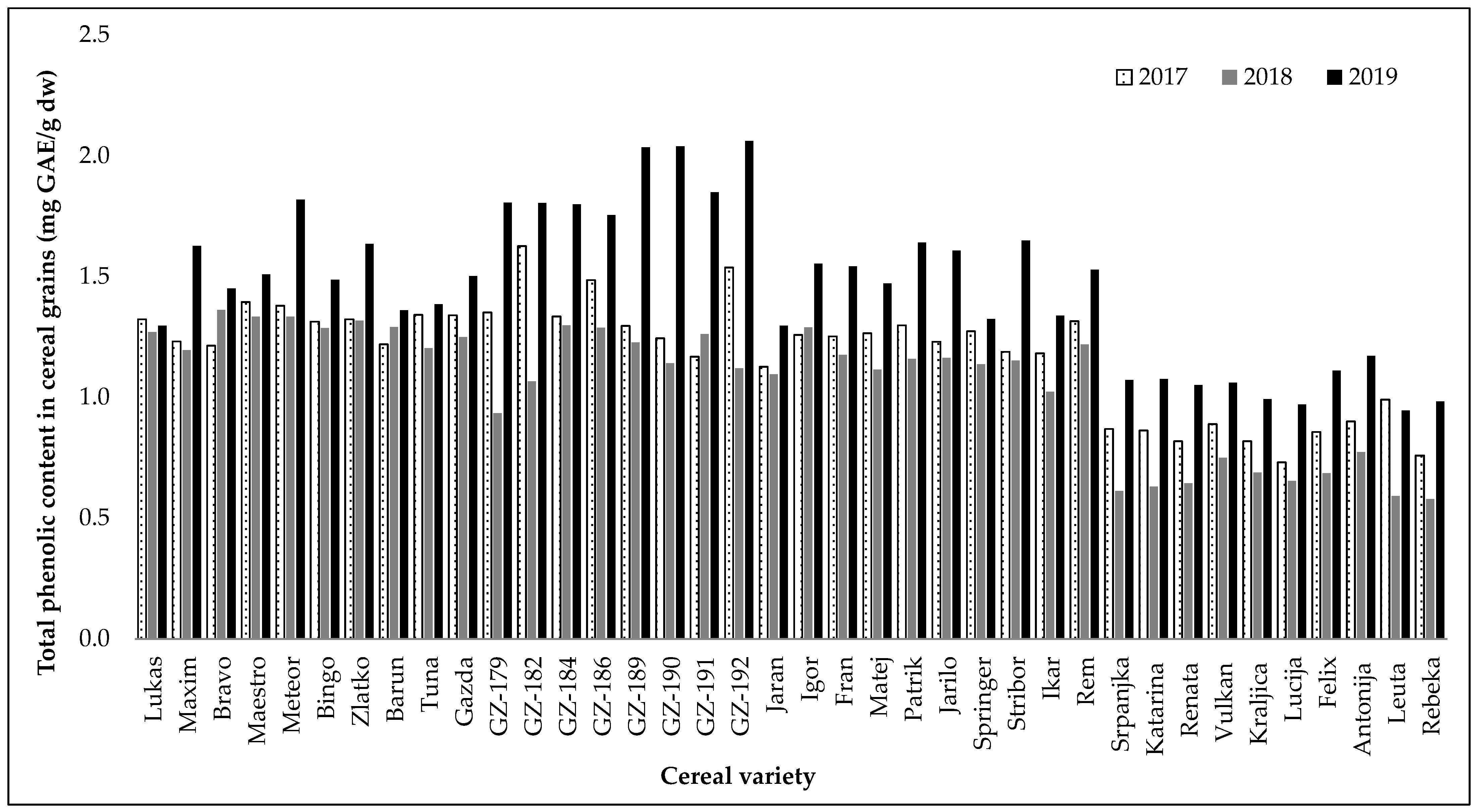
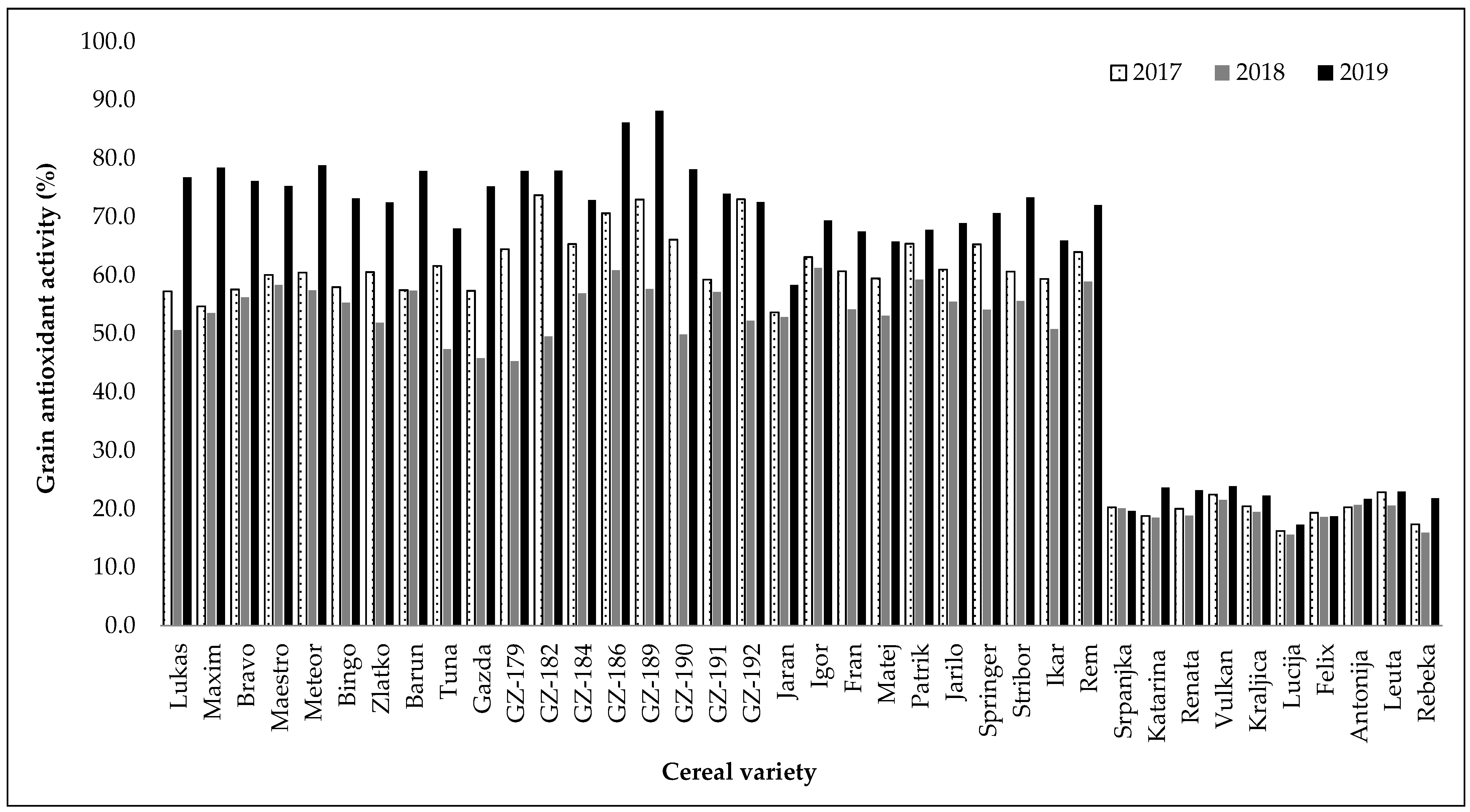
2.1. Evaluation of Total Phenolic Content and Antioxidant Activity of Extracts from Different Cereal Varieties
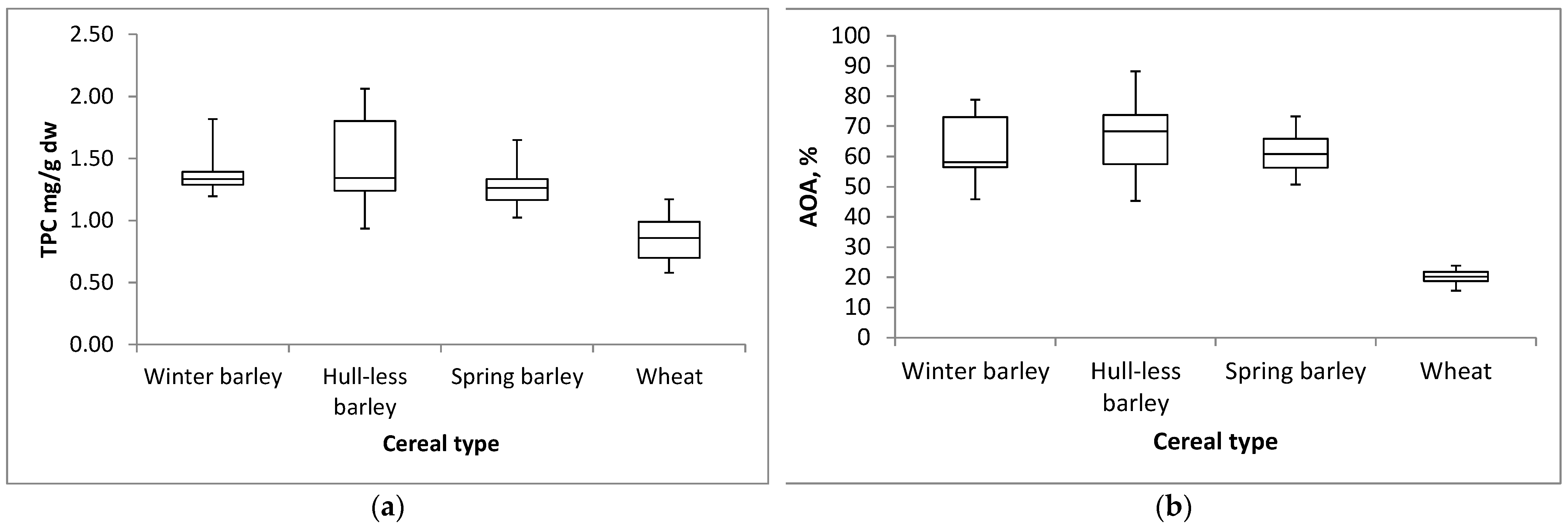
2.2. Effect of Cereal Type on Total Phenolic Content and Antioxidant Activity
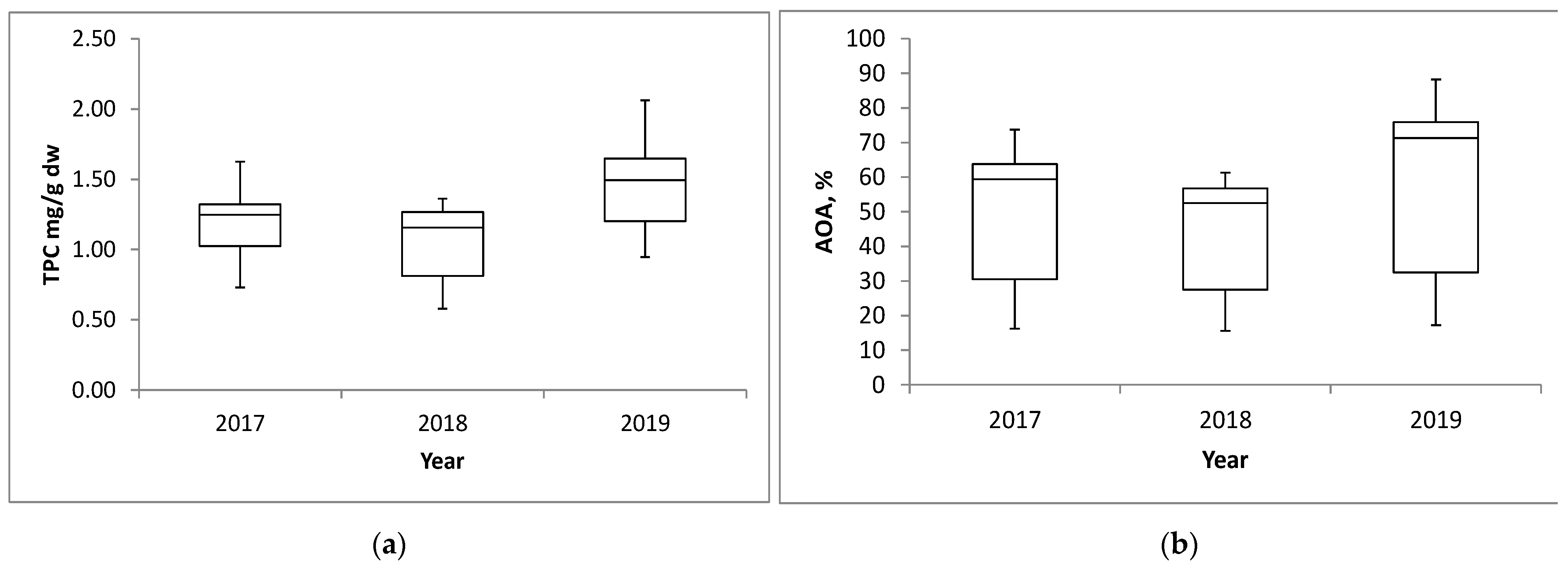
2.3. Variation in Total Phenolic Contents and Antioxidant Activities Between Growing Years
2.4. Effect of Malting on Total Phenolic Content and Antioxidant Activity of Barley Varieties
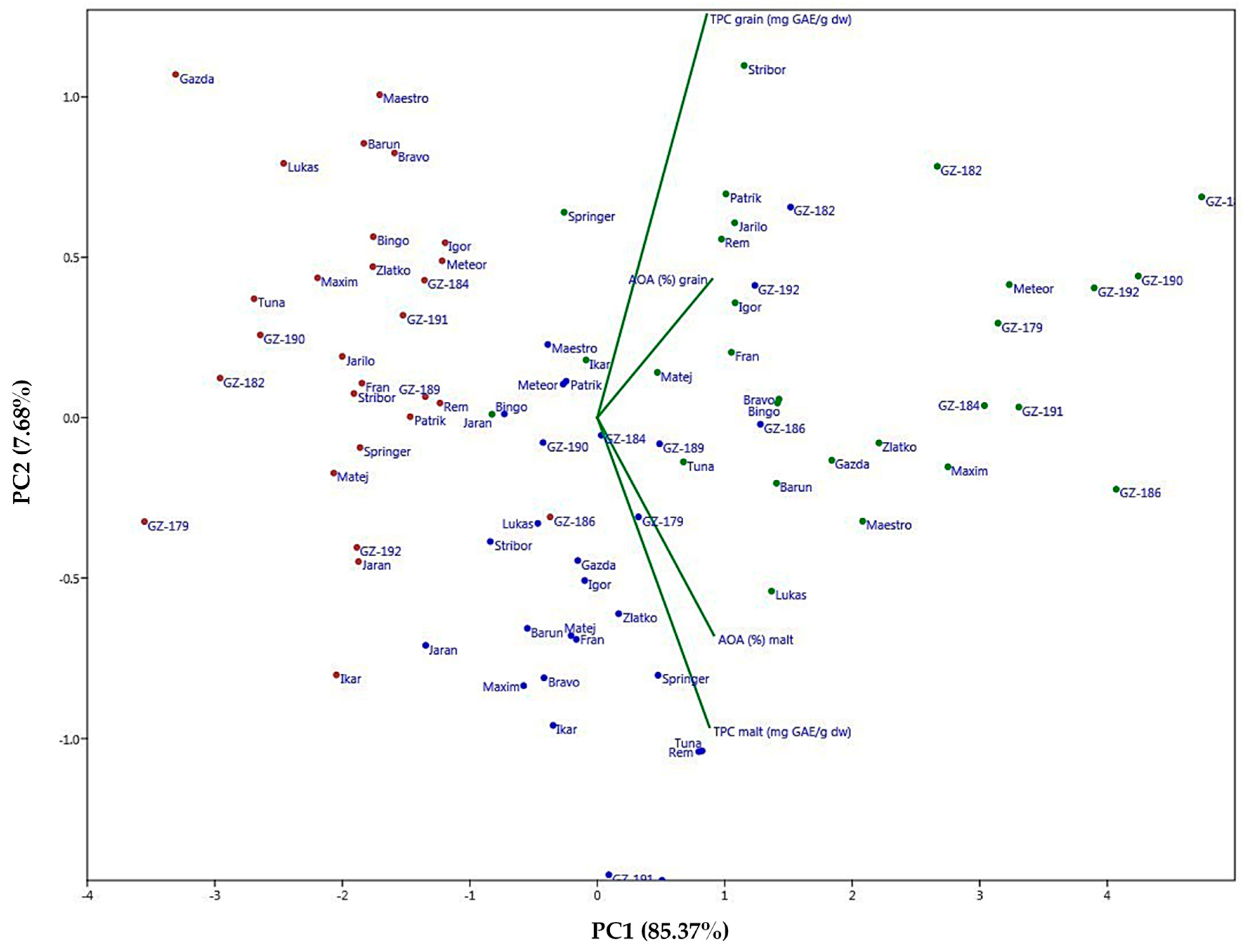
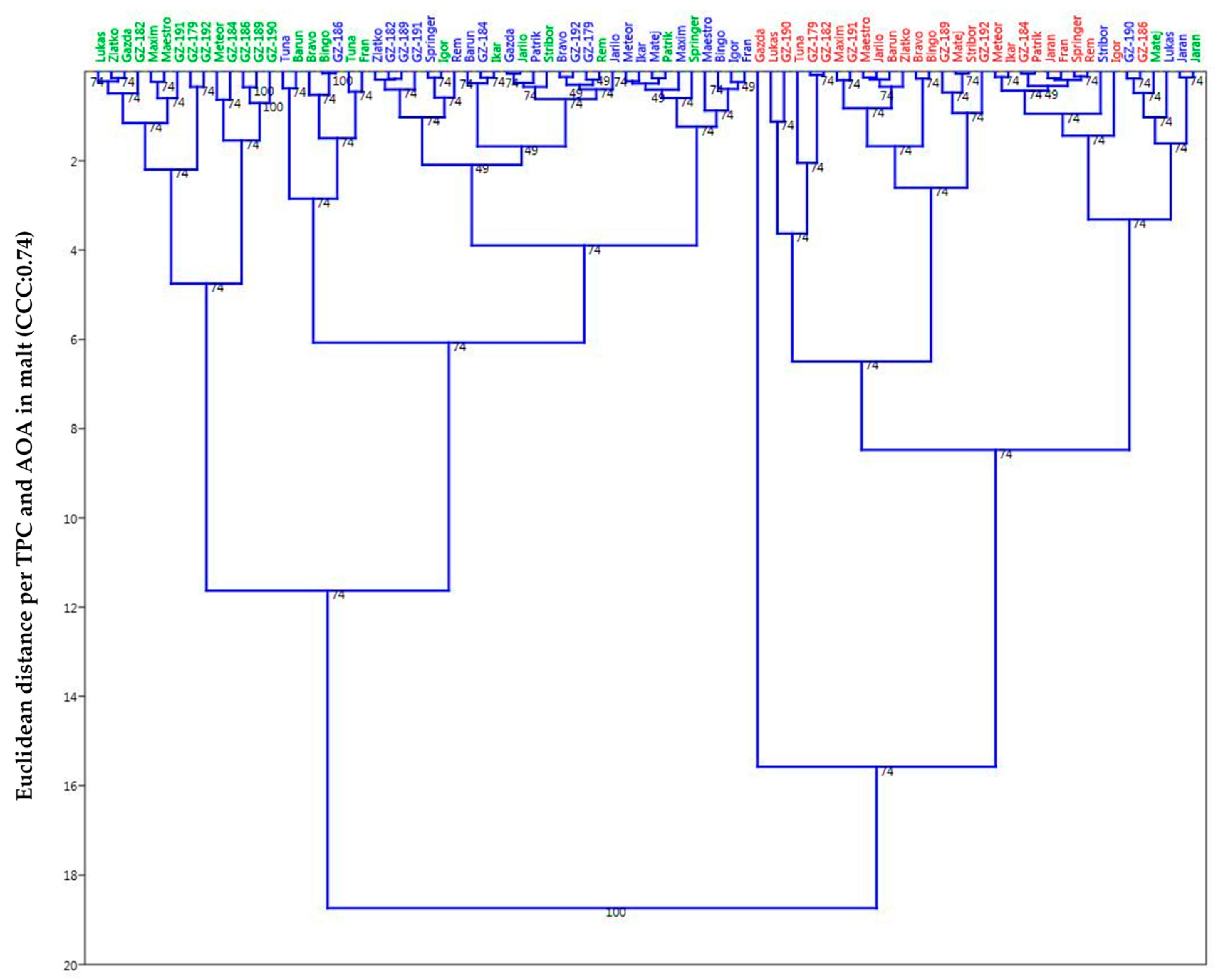
2.5. Principal Component Analysis (PCA) and Unweighted Pair Group Method Analysis (UPGMA)
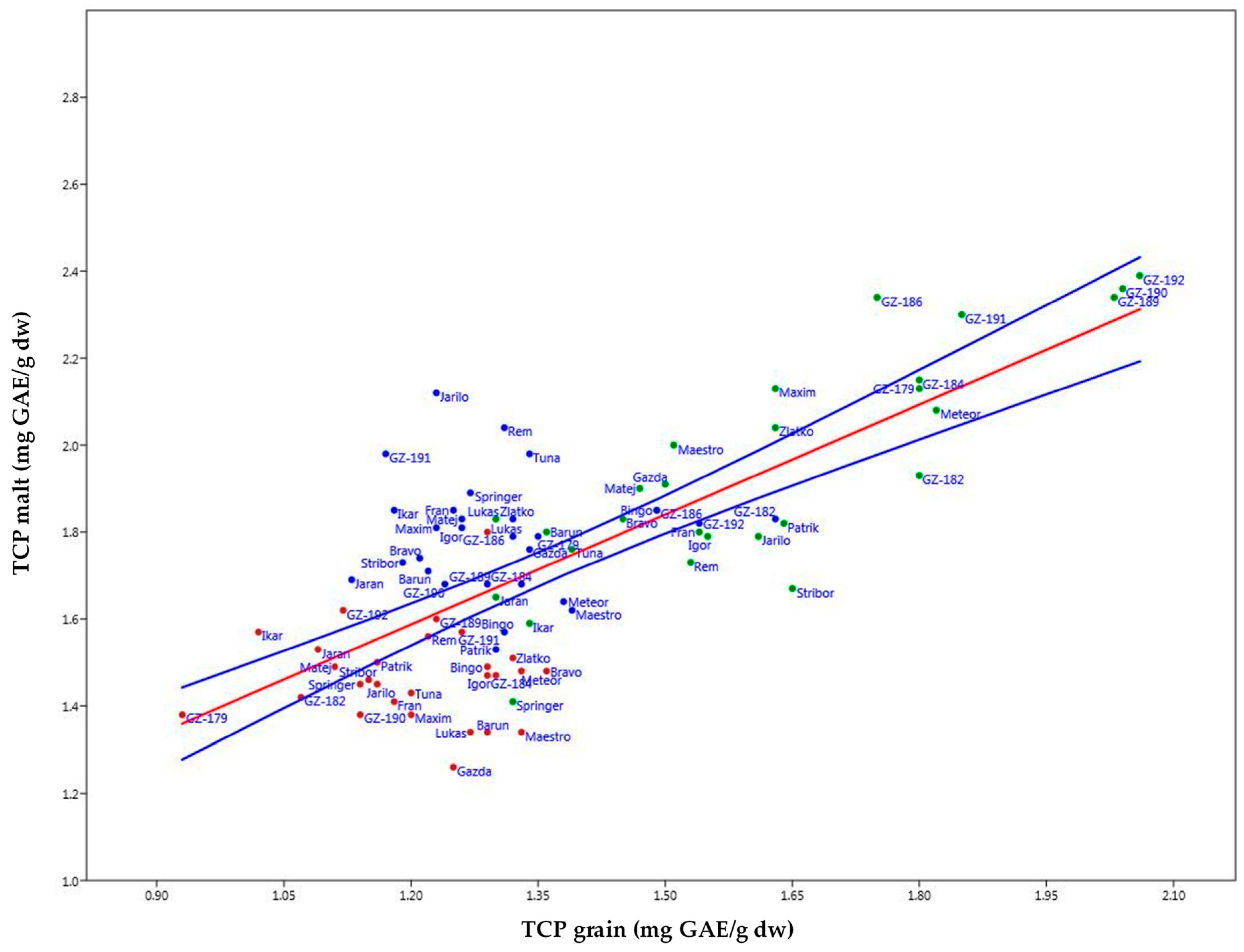
2.6. Bivariate Linear Model Methodology
3. Discussion
4. Materials and Methods
4.1. Plant Material and Field Trials
4.2. Micro-Malting
4.3. Analysis of Total Phenolic Content and Antioxidant Activity
4.3.1. Determination of Total Phenolic Content
4.3.2. Analysis of Antioxidant Activity
4.4. Statistical Analysis
4.4.1. Principal Component Analysis (PCA)
4.4.2. Unweighted Pair Group Method Analysis (UPGMA)
4.4.3. Results from Bivariate Linear Model Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| AOA | Antioxidant Activity |
| CUPRAC | Cupric Reducing Antioxidant Capacity |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EU | European Union |
| FRAP | Ferric Reducing Antioxidant Power |
| GAE | Gallic Acid Equivalent |
| PCA | Principal Component Analysis |
| PVPP | Polyvinylpolypyrrolidone |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| TPC | Total Phenolic Content |
| UPGMA | Unweighted Pair Group Method Analysis |
References
- Diekmann, F. Small Grain Cereals. J. Agric. Food Inf. 2009, 10, 182–197. [Google Scholar] [CrossRef]
- Žilić, S.; Šukalović, V.H.-T.; Dodig, D.; Maksimović, V.; Maksimović, M.; Basić, Z. Antioxidant activity of small grain cereals caused by phenolics and lipid soluble antioxidants. J. Cereal Sci. 2011, 54, 417–424. [Google Scholar] [CrossRef]
- Nignpense, B.E.; Latif, S.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Bioaccessibility and Antioxidant Activity of Polyphenols from Pigmented Barley and Wheat. Foods 2022, 11, 3697. [Google Scholar] [CrossRef]
- Kaur, A.; Purewal, S.S.; Phimolsiripol, Y.; Bangar, S.P. Unraveling the Hidden Potential of Barley (Hordeum vulgare): An Important Review. Plants 2024, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Eurostat (European Statistical Office). 2025. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_crops#Cereals (accessed on 7 April 2025).
- Euronews. Available online: https://www.euronews.com/my-europe/2024/11/20/europe-faces-growing-crisis-in-cereal-production (accessed on 3 December 2024).
- FAOSTAT (FAO Statistical Database). 2023. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 3 December 2024).
- The World of Food Grains; Wyatt, J. Encyclopedia of Food Grains, 2nd ed.; Academic Press: New York, NY, USA, 2016. [Google Scholar]
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Ibba, M.I.; Palacios-Rojas, N.; Rosales-Nolasco, A. Screening and use of nutritional and healthrelated benefits of the main crops. In Developing Sustainable and Health-Promoting Cereals and Pseudocereals: Conventional and Molecular Breeding, 1st ed.; Rakszegi, M., Papageorgiou, M., Rocha, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 25–55. [Google Scholar]
- Abebaw, G. Review on Structure, Functional and Nutritional Composition of Barley (Hordeum vulgare). J. Nutr. Food Process. 2021, 4, 1–8. [Google Scholar] [CrossRef]
- Lukinac, J.; Jukic, M. Barley in the Production of Cereal-Based Products. Plants 2022, 11, 3519. [Google Scholar] [CrossRef]
- Ragaee, S.; Guzar, I.; Abdel-Aal, E.-S.M.; Seetharaman, K. Bioactive components and antioxidant capacity of Ontario hard and soft wheat varieties. Can. J. Plant Sci. 2012, 92, 19–30. [Google Scholar] [CrossRef]
- Shahidi, F. Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Technol. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Shahidi, F.; Danielski, R.; Ikeda, C. Phenolic compounds in cereal grains and effects of processing on their composition and bioactivities: A review. J. Food Bioact. 2021, 15, 39–50. [Google Scholar] [CrossRef]
- Raj, R.; Shams, R.; Pandey, V.K.; Dash, K.K.; Singh, P.; Bashir, O. Barley phytochemicals and health promoting benefits: A comprehensive review. J. Agric. Food Res. 2023, 14, 100677. [Google Scholar] [CrossRef]
- Deng, J.; Yu, M.; Yang, Y.; Liu, T.; Xiang, Z.; Chen, J.; Yang, K.; Zhan, R.; Zhu, B.; Zhu, Y.; et al. Studies of phytochemical constituents by UPLC-QTOF-MS/MS of black hulless barley bran and its antioxidation and α-glucosidase inhibition effect. Arab. J. Chem. 2024, 17, 105644. [Google Scholar] [CrossRef]
- Niroula, A.; Khatri, S.; Khadka, D.; Timilsina, R. Total phenolic contents and antioxidant activity profile of selected cereal sprouts and grasses. Int. J. Food Prop. 2019, 22, 427–437. [Google Scholar] [CrossRef]
- Kruma, Z.; Tomsone, L.; Ķince, T.; Galoburda, R.; Senhofa, S.; Sabovics, M.; Straumite, E.; Sturite, I. Effects of germination on total phenolic compounds and radical scavenging activity in hull-less spring cereals and triticale. Agron. Res. 2016, 14 (Suppl. S2), 1372–1383. [Google Scholar]
- Šimić, G.; Horvat, D.; Lalić, A.; Komlenić, D.K.; Abičić, I.; Zdunić, Z. Distribution of β-Glucan, Phenolic Acids, and Proteins as Functional Phytonutrients of Hull-Less Barley Grain. Foods 2019, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Goudar, G.; Longvah, T.; Gour, V.S.; Kothari, S.L.; Wani, I.A. Fate of Polyphenols and Antioxidant Activity of Barley during Processing. Food Rev. Int. 2020, 38, 163–198. [Google Scholar] [CrossRef]
- Marcus, A.N. Application of Traditional Methods of Analysis and Modern Proteomics to Evaluate the Malting and Brewing Potential of the Novel Grain Kernza (Thinopyrum intermedium). Master’s Thesis, University of California, Davis, CA, USA, 2021. [Google Scholar]
- Carvalho, D.O.; Gonçalves, L.M.; Guido, L.F. Overall Antioxidant Properties of Malt and How They Are Influenced by the Individual Constituents of Barley and the Malting Process. Compr. Rev. Food Sci. Food Saf. 2016, 15, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, D.O.; Guido, L.F. A review on the fate of phenolic compounds during malting and brewing: Technological strategies and beer styles. Food Chem. 2022, 372, 131093. [Google Scholar] [CrossRef]
- Rani, H.; Bhardwaj, R.D. Quality attributes for barley malt: “The backbone of beer”. J. Food Sci. 2021, 86, 3322–3340. [Google Scholar] [CrossRef]
- Vingrys, K.; Mathai, M.; Ashton, J.F.; Stojanovska, L.; Vasiljevic, T.; McAinch, A.J.; Donkor, O.N. The effect of malting on phenolic compounds and radical scavenging activity in grains and breakfast cereals. J. Food Sci. 2022, 87, 4188–4202. [Google Scholar] [CrossRef]
- Koren, D.; Kun, S.; Vecseri, B.H.; Kun-Farkas, G. Study of antioxidant activity during the malting and brewing process. J. Food Sci. Technol. 2019, 56, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Jin, Z.; Schwarz, P.; Li, Y. Impact of Genotype, Environment, and Malting Conditions on the Antioxidant Activity and Phenolic Content in US Malting Barley. Fermentation 2020, 6, 48. [Google Scholar] [CrossRef]
- Ha, K.S.; Jo, S.H.; Mannam, V.; Kwon, Y.I.; Apostolidis, E. Stimulation of Phenolics, Antioxidant and α-Glucosidase Inhibitory Activities During Barley (Hordeum vulgare L.) Seed Germination. Plant Foods Hum. Nutr. 2016, 71, 211–217. [Google Scholar] [CrossRef]
- Bangar, S.P.; Sandhu, K.S.; Trif, M.; Manjunatha, V.; Lorenzo, J.M. Germinated Barley Cultivars: Effect on Physicochemical and Bioactive Properties. Food Anal. Methods 2022, 9, 2505–2512. [Google Scholar] [CrossRef]
- Özcan, M.M.; Aljuhaimi, F.; Uslu, N. Effect of malt process steps on bioactive properties and fatty acid composition of barley, green malt and malt grains. J. Food Sci. Technol. 2018, 55, 226–232. [Google Scholar] [CrossRef]
- Quan, M.; Li, Q.; Zhao, P.; Tian, C. Chemical composition and hepatoprotective effect of free phenolic extract from barley during malting process. Sci. Rep. 2018, 8, 4460. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Horvat, D.; Dvojković, K.; Abičić, I.; Viljevac Vuletić, M.; Tucak, M.; Lalić, A. Evaluation of total phenolic content and antioxidant activity of malting and hulless barley grain and malt extracts. Czech J. Food Sci. 2017, 35, 73–78. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Ledenčan, T.; Tucak, M.; Plavšić, H.; Andrić, L.; Zdunić, Z. Phenolic Acid Profiles and Antioxidant Activity of Major Cereal Crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Fu, X.; Abbasi, A.M.; Zheng, B.; Liu, R.H. Phenolics content, antioxidant and antiproliferative activities of dehulled highland barley (Hordeum vulgare L.). J. Funct. Foods 2015, 19, 439–450. [Google Scholar] [CrossRef]
- Fogarasi, A.-L.; Kun, S.; Tanko, G.; Stefanovits-Banyai, E.; Hegyesne-Vecseri, B. A comparative assessment of antioxidant properties, total phenolic content of einkorn, wheat, barley and their malts. Food Chem. 2015, 167, 1–6. [Google Scholar] [CrossRef]
- Bleidere, M.; Zute, S.; Jākobsone, I. Characterisation of Physical and Biochemical Traits of Hulless Spring Barley Grain in the Latvian Breeding Programme. Proc. Latvian Acad. Sci. Sect. B 2013, 67, 399–404. [Google Scholar] [CrossRef]
- Kruma, Z.; Tomsone, L.; Galoburda, R.; Straumite, E.; Kronberga, A.; Åssveen, M. Total phenols and antioxidant capacity of hull-less barley and hull-less oats. Agron. Res. 2016, 14 (Suppl. S2), 1361–1371. [Google Scholar]
- Ondrejovič, M.; Chmelová, D.; Ivanišová, E.; Dráb, Š.; Psota, V. Evaluation of Antioxidant Activities of Cereals and their Malts. Nova Biotechnol. Chim. 2014, 13, 172–181. [Google Scholar] [CrossRef]
- Edney, M.J.; Izydorczyk, M.S. MALT/Malt Types and Products. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Finglas, P., Toldra, F., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 3671–3677. [Google Scholar]
- Salmenkallio-Marttila, M.; Heiniö, R.-L.; Kaukovirta-Norja, A.; Poutanen, K. Flavor and Texture in Processing of New Oat Foods. In OATS: Chemistry and Technology; Webster, F.H., Wood, P.J., Eds.; Woodhead Publishing: Cambridge, UK; AACC International Press: St. Paul, MN, USA, 2011; pp. 333–346. [Google Scholar]
- Lu, J.; Zhao, H.; Chen, J.; Fan, W.; Dong, J.; Kong, W.; Sun, J.; Cao, Y.; Cai, G. Evolution of phenolic compounds and antioxidant activity during malting. J. Agric. Food Chem. 2007, 55, 10994–11001. [Google Scholar] [CrossRef] [PubMed]
- Shopska, V.; Denkova-Kostova, R.; Dzhivoderova-Zarcheva, M.; Teneva, D.; Denev, P.; Kostov, G. Comparative Study on Phenolic Content and Antioxidant Activity of Different Malt Types. Antioxidants 2021, 10, 1124. [Google Scholar] [CrossRef]
- Koren, D. Study of Health Promoting Components Throughout the Malting and Brewing Process. Ph.D. Thesis, Hungarian University of Agriculture and Life Sciences, Budapest, Hungary, 2021. [Google Scholar]
- Maillard, M.N.; Soum, M.H.; Boivin, P.; Berset, C. Antioxidant activity of barley and malt: Relationship with phenolic content. Lebensm. Wiss. Technol. 1996, 29, 238–244. [Google Scholar] [CrossRef]
- Horvat, D.; Dvojković, K.; Novoselović, D.; Tucak, M.; Andrić, L.; Magdić, D.; Drezner, G. Response of Wheat Yield and Protein-Related Quality on Late-Season Urea Application. Agronomy 2022, 12, 886. [Google Scholar] [CrossRef]
- Habschied, K.; Lalić, A.; Krstanović, V.; Dvojković, K.; Abičić, I.; Šimić, G.; Mastanjević, K. Comprehensive Comparative Study of the Malting Qualities of Winter Hull-Less and Hulled Barley (2016–2019). Fermentation 2021, 7, 8. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Romagnoli, J.; Thomas, M.C. Extracting knowledge from historical databases for process monitoring using feature extraction and data clustering. In Computer Aided Chemical Engineering; Kravanja, Z., Bogataj, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 859–864. [Google Scholar]
- Talabis, M.R.M.; McPherson, R.; Miyamoto, I.; Martin, J.L.; Kaye, D. Analytics Defined. In Information Security Analytics; Rearick, B., Katsaropoulos, C., Eds.; Syngress: Burlington, MA, USA, 2015; pp. 1–12. [Google Scholar]
- James, F.C.; McCulloch, C.E. Multivariate Analysis in Ecology and Systematic Panacea or Padora’s Box? Ann. Rev. Eco. Sys. 1990, 21, 129–166. [Google Scholar] [CrossRef]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy: The Principles and Practice of Numerical Classification; W.H. Freeman & Co.: San Francisco, CA, USA, 1973. [Google Scholar]
- Roldan-Ruiz, I.; Eeuwijk, F.A.; Gilliland, T.J.; Dubreuil, P.; Dillmann, C.; Lallemand, J.; De Loose, M.; Baril, C.P. A comparative study of molecular and morphological methods of describing relationships between perennial ryegrass (Lolium perenne L.) varieties. Theor. App. Gen. 2001, 103, 1138–1150. [Google Scholar] [CrossRef]
- Mejza, I.; Ambroży-Deręgowska, K.; Bocianowski, J.; Błażewicz, J.; Liszewski, M.; Nowosad, K.; Zalewski, D. On modeling and analyzing barley malt data in different years. Biom. Lett. 2019, 56, 45–57. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis (version 4.03, 2020). Palaeontol. Electron. 2001, 4, 9. [Google Scholar]


| Cereal Type | Genotype | Year of Registration | Field Trials |
|---|---|---|---|
| Winter hulled barley | Lukas | 2010 | 2016–2017 2017–2018 2018–2019 |
| Maxim | 2009 | ||
| Bravo | 2009 | ||
| Maestro | 2010 | ||
| Meteor | - | ||
| Bingo | 2005 | ||
| Zlatko | 1999 | ||
| Barun | 2001 | ||
| Tuna | 2014 | ||
| Gazda | 2007 | ||
| Winter hull-less barley | GZ-179 | Breeding line | 2016–2017 2017–2018 2018–2019 |
| GZ-182 | Breeding line | ||
| GZ-184 | Breeding line | ||
| GZ-186 | Breeding line | ||
| GZ-189 | Breeding line | ||
| GZ-190 | Breeding line | ||
| GZ-191 | Breeding line | ||
| GZ-192 | Breeding line | ||
| Spring hulled barley | Jaran | 1983 | 2017 2018 2019 |
| Igor | 1998 | ||
| Fran | 2002 | ||
| Matej | 2002 | ||
| Patrik | 2004 | ||
| Jarilo | - | ||
| Springer | 2009 | ||
| Stribor | 2009 | ||
| Ikar | 2010 | ||
| Rem | 2007 | ||
| Wheat | Srpanjka | 1989 | 2016–2017 2017–2018 2018–2019 |
| Katarina | 2006 | ||
| Renata | 2006 | ||
| Vulkan | 2009 | ||
| Kraljica | 2010 | ||
| Lucija | 2001 | ||
| Felix | 2007 | ||
| Antonija | 2011 | ||
| Leuta | 2011 | ||
| Rebeka | 2011 |
| TPC 1 (mg GAE/g dw) | DPPH 2 (% Inhibition) | |||
|---|---|---|---|---|
| Cereal Type | Mean 3 | SD 6 | Mean | SD |
| Winter hulled barley | 1.37 b | ±0.14 | 62.36 b | ±10.03 |
| Winter hull-less barley | 1.48 a | ±0.34 | 66.76 a | ±11.62 |
| Spring hulled barley | 1.30 b | ±0.17 | 61.58 b | ±6.26 |
| Winter wheat | 0.85 c | ±0.17 | 20.09 c | ±2.28 |
| Year | Mean 4 | SD | Mean | SD |
| 2017 | 1.18 b | ±0.23 | 51.07 b | ±19.51 |
| 2018 | 1.06 c | ±0.26 | 44.95 c | ±16.18 |
| 2019 | 1.46 a | ±0.32 | 59.84 a | ±23.85 |
| Cereal Crop | Mean 5 | SD | Mean | SD |
| Barley | 1.37 a | ±0.23 | 63.34 a | ±9.54 |
| Wheat | 0.85 b | ±0.17 | 20.09 b | ±2.28 |
| Variety/ Year | TPC 1 (mg GAE/g dw) | TPC Incr. 2 (%) | DPPH 3 (% Inhibition) | DPPH Incr. (%) | ||
|---|---|---|---|---|---|---|
| Grain | Malt | Grain | Malt | |||
| Winter hulled barley | ||||||
| Lukas | 1.30 4a ± 0.03 | 1.65 a ± 0.27 | 27.24 a ± 19.36 | 61.54 a ± 13.58 | 69.78 a ± 15.53 | 13.39 a ± 6.95 |
| Maxim | 1.35 a ± 0.24 | 1.77 a ± 0.38 | 31.22 a ± 15.92 | 62.21 a ± 14.05 | 72.66 a ± 14.10 | 17.66 a ± 13.66 |
| Bravo | 1.34 a ± 0.12 | 1.68 a ± 0.18 | 25.99 a ± 17.42 | 63.29 a ± 11.11 | 71.63 a ± 12.68 | 13.80 a ± 17.04 |
| Maestro | 1.41 a ± 0.09 | 1.65 a ± 0.33 | 16.46 a ± 15.95 | 64.55 a ± 9.32 | 72.36 a ± 13.63 | 11.77 a ± 9.17 |
| Meteor | 1.51 a ± 0.27 | 1.73 a ± 0.31 | 14.82 a ± 3.72 | 65.55 a ± 11.58 | 76.14 a ± 12.72 | 16.30 a ± 4.27 |
| Bingo | 1.36 a ± 0.11 | 1.64 a ± 0.19 | 20.15 a ± 4.30 | 62.13 a ± 9.66 | 70.31 a ± 12.15 | 13.17 a ± 10.54 |
| Zlatko | 1.42 a ± 0.18 | 1.79 a ± 0.27 | 25.94 a ± 11.78 | 61.64 a ± 10.32 | 73.95 a ± 13.57 | 19.81 a ± 7.35 |
| Barun | 1.29 a ± 0.07 | 1.61 a ± 0.24 | 25.19 a ± 19.17 | 64.24 a ± 11.80 | 72.66 a ± 12.30 | 13.76 a ± 14.50 |
| Tuna | 1.31 a ± 0.10 | 1.72 a ± 0.28 | 31.26 a ± 14.78 | 58.98 a ± 10.60 | 71.90 a ± 16.65 | 21.28 a ± 12.46 |
| Gazda | 1.36 a ± 0.13 | 1.64 a ± 0.34 | 19.98 a ± 16.39 | 59.45 a ± 14.81 | 69.19 a ± 20.60 | 15.63 a ± 16.37 |
| 2017 | 1.31 5b ± 0.06 | 1.74 b ± 0.12 | 33.75 a ± 11.88 | 58.49 b ± 2.10 | 74.79 b ± 3.94 | 27.92 a ± 6.03 |
| 2018 | 1.28 b ± 0.06 | 1.41 c ± 0.09 | 9.58 b ± 6.65 | 53.38 c ± 4.37 | 56.89 c ± 5.02 | 6.62 c ± 5.10 |
| 2019 | 1.51 a ± 0.15 | 1.92 a ± 0.13 | 28.15 a ± 7.00 | 75.21 a ± 3.28 | 84.50 a ± 3.14 | 12.43 b ± 3.78 |
| Winter hull-less barley | ||||||
| GZ-179 | 1.36 a ± 0.44 | 1.77 a ± 0.38 | 32.57 a ± 14.52 | 62.53 a ± 16.38 | 71.81 a ± 19.15 | 14.69 a ± 3.65 |
| GZ-182 | 1.50 a ± 0.38 | 1.73 a ± 0.27 | 17.66 a ± 13.97 | 67.04 a ± 15.32 | 71.44 a ± 18.36 | 6.06 a ± 3.92 |
| GZ-184 | 1.48 a ± 0.28 | 1.77 a ± 0.35 | 19.53 a ± 6.32 | 65.04 a ± 8.00 | 76.61 a ± 13.21 | 17.34 a ± 6.15 |
| GZ-186 | 1.51 a ± 0.23 | 2.00 a ± 0.30 | 32.54 a ± 7.53 | 72.54 a ± 12.75 | 80.65 a ± 12.07 | 11.51 a ± 4.14 |
| GZ-189 | 1.52 a ± 0.45 | 1.88 a ± 0.41 | 25.21 a ± 8.66 | 72.92 a ± 15.28 | 77.05 a ± 15.16 | 5.85 a ± 1.41 |
| GZ-190 | 1.47 a ± 0.49 | 1.81 a ± 0.50 | 23.90 a ± 10.08 | 64.70 a ± 14.20 | 71.71 a ± 18.17 | 10.51 a ± 7.02 |
| GZ-191 | 1.43 a ± 0.37 | 1.95 a ± 0.36 | 39.71 a ± 26.15 | 63.43 a ± 9.14 | 74.44 a ± 14.65 | 17.14 a ± 14.62 |
| GZ-192 | 1.57 a ± 0.47 | 1.94 a ± 0.40 | 26.17 a ± 15.86 | 65.91 a ± 11.89 | 75.21 a ± 14.03 | 14.29 a ± 8.32 |
| 2017 | 1.38 b ± 0.16 | 1.79 b ± 0.11 | 31.04 a ± 17.31 | 68.18 b ± 5.19 | 76.35 b ± 3.84 | 12.49 a ± 9.57 |
| 2018 | 1.17 c ± 0.13 | 1.53 c ± 0.15 | 31.82 a ± 11.82 | 53.66 c ± 5.27 | 58.77 c ± 6.22 | 9.56 a ± 5.05 |
| 2019 | 1.89 a ± 0.13 | 2.24 a ± 0.16 | 18.63 a ± 7.71 | 78.45 a ± 5.87 | 89.47 a ± 2.42 | 14.47 a ± 7.03 |
| Spring hulled barley | ||||||
| Jaran | 1.17 a ± 0.11 | 1.62 a ± 0.08 | 39.01 a ± 11.34 | 54.94 b ± 2.97 | 66.25 a ± 1.20 | 20.77 a ± 5.41 |
| Igor | 1.37 a ± 0.16 | 1.69 a ± 0.19 | 24.31 a ± 17.34 | 64.57 a ± 4.25 | 71.39 a ± 7.55 | 10.40 a ± 6.00 |
| Fran | 1.32 a ± 0.19 | 1.69 a ± 0.24 | 28.25 a ± 17.10 | 60.75 ab ± 6.65 | 72.44 a ± 7.61 | 19.28 a ± 0.58 |
| Matej | 1.28 a ± 0.18 | 1.74 a ± 0.22 | 36.08 a ± 7.88 | 59.43 ab ± 6.37 | 67.73 a ± 6.14 | 14.36 a ± 9.64 |
| Patrik | 1.37 a ± 0.25 | 1.62 a ± 0.18 | 19.44 a ± 9.60 | 64.10 a ± 4.40 | 71.37 a ± 6.16 | 11.29 a ± 4.51 |
| Jarilo | 1.33 a ± 0.24 | 1.79 a ± 0.33 | 36.38 a ± 32.07 | 61.80 a ± 6.76 | 70.67 a ± 9.89 | 14.29 a ± 9.97 |
| Springer | 1.24 a ± 0.10 | 1.58 a ± 0.26 | 27.27 a ± 20.86 | 63.35 a ± 8.44 | 72.30 a ± 7.03 | 14.70 a ± 9.00 |
| Stribor | 1.33 a ± 0.28 | 1.62 a ± 0.14 | 24.28 a ± 22.20 | 63.17 a ± 9.14 | 67.56 a ± 7.52 | 7.29 a ± 3.44 |
| Ikar | 1.18 a ± 0.16 | 1.67 a ± 0.16 | 42.85 a ± 21.02 | 58.69 ab ± 7.64 | 70.95 a ± 5.18 | 21.53 a ± 7.68 |
| Rem | 1.35 a ± 0.16 | 1.77 a ± 0.24 | 31.93 a ± 21.23 | 64.97 a ± 6.59 | 73.37 a ± 7.65 | 13.14 a ± 9.13 |
| 2017 | 1.24 b ± 0.06 | 1.83 a± 0.17 | 48.21 a ± 13.66 | 61.25 b ± 3.48 | 73.44 a ± 4.45 | 20.00 a ± 5.47 |
| 2018 | 1.15 c ± 0.07 | 1.49 b ± 0.05 | 29.76 b ±10.91 | 55.53 c ± 3.30 | 63.35 b ± 2.05 | 14.44 ab ± 7.90 |
| 2019 | 1.49 a ± 0.13 | 1.71 a ± 0.14 | 14.97 c ± 8.63 | 67.95 a ± 4.17 | 74.42 a ± 4.06 | 9.67 b ± 5.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimić, G.; Lalić, A.; Horvat, D.; Viljevac Vuletić, M.; Dvojković, K.; Jukić, M.; Lukinac, J.; Abičić, I. Genotypic, Environmental, and Processing Effects on Phenolic Content and Antioxidant Activity in Barley and Wheat. Plants 2025, 14, 1664. https://doi.org/10.3390/plants14111664
Šimić G, Lalić A, Horvat D, Viljevac Vuletić M, Dvojković K, Jukić M, Lukinac J, Abičić I. Genotypic, Environmental, and Processing Effects on Phenolic Content and Antioxidant Activity in Barley and Wheat. Plants. 2025; 14(11):1664. https://doi.org/10.3390/plants14111664
Chicago/Turabian StyleŠimić, Gordana, Alojzije Lalić, Daniela Horvat, Marija Viljevac Vuletić, Krešimir Dvojković, Marko Jukić, Jasmina Lukinac, and Ivan Abičić. 2025. "Genotypic, Environmental, and Processing Effects on Phenolic Content and Antioxidant Activity in Barley and Wheat" Plants 14, no. 11: 1664. https://doi.org/10.3390/plants14111664
APA StyleŠimić, G., Lalić, A., Horvat, D., Viljevac Vuletić, M., Dvojković, K., Jukić, M., Lukinac, J., & Abičić, I. (2025). Genotypic, Environmental, and Processing Effects on Phenolic Content and Antioxidant Activity in Barley and Wheat. Plants, 14(11), 1664. https://doi.org/10.3390/plants14111664










