Effects of Salinity Stress on Grasspea (Lathyrus sativus L.) and Its Wild Relatives: Morpho-Physiological Insights at the Seedling Stage
Abstract
1. Introduction
2. Results
2.1. Genotype, Treatment, and Their Interaction Effects on the Variation in Investigated Traits
2.2. Phenotypic Variation for Various Traits in Response to Salt Stress Treatments
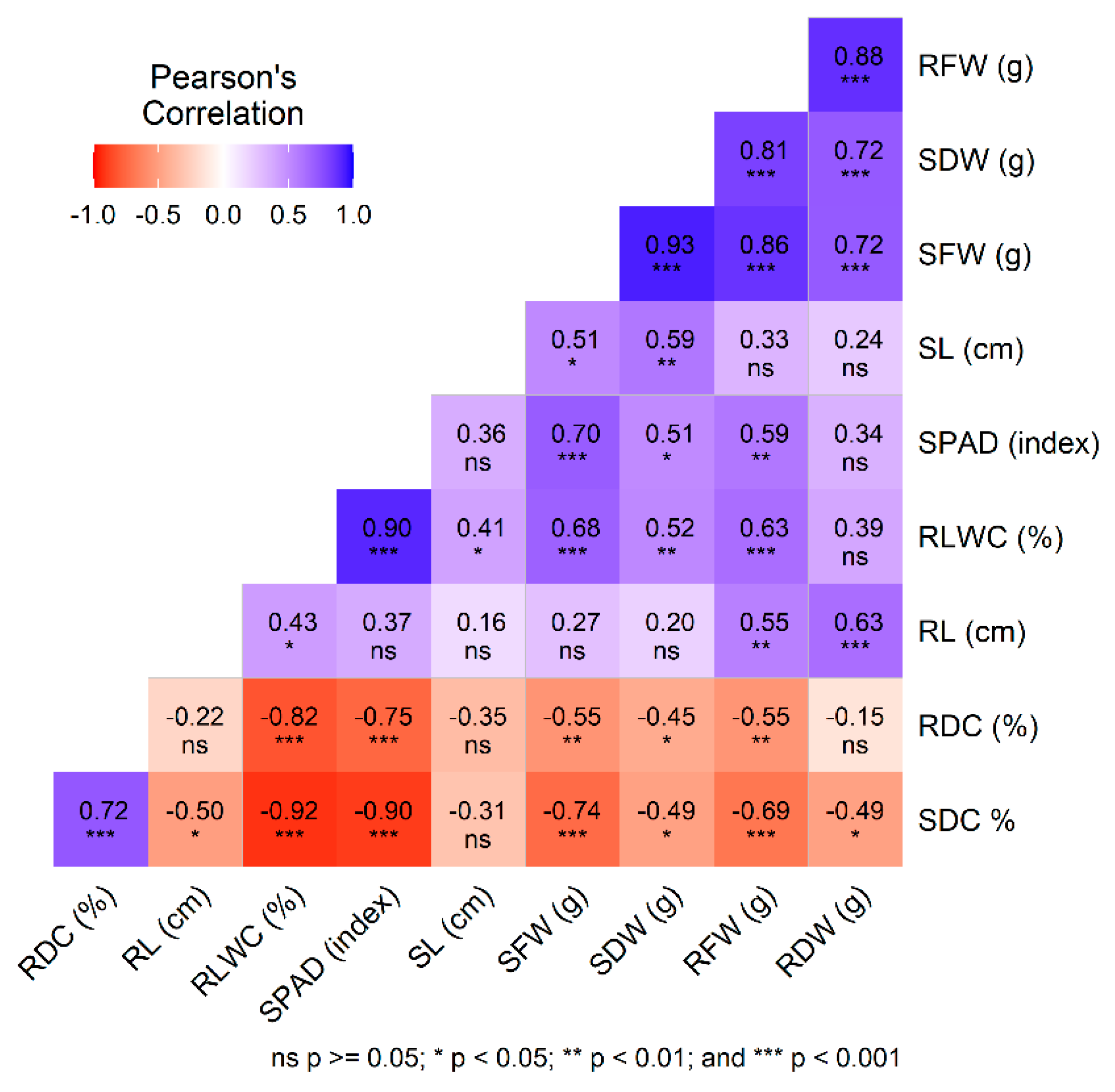
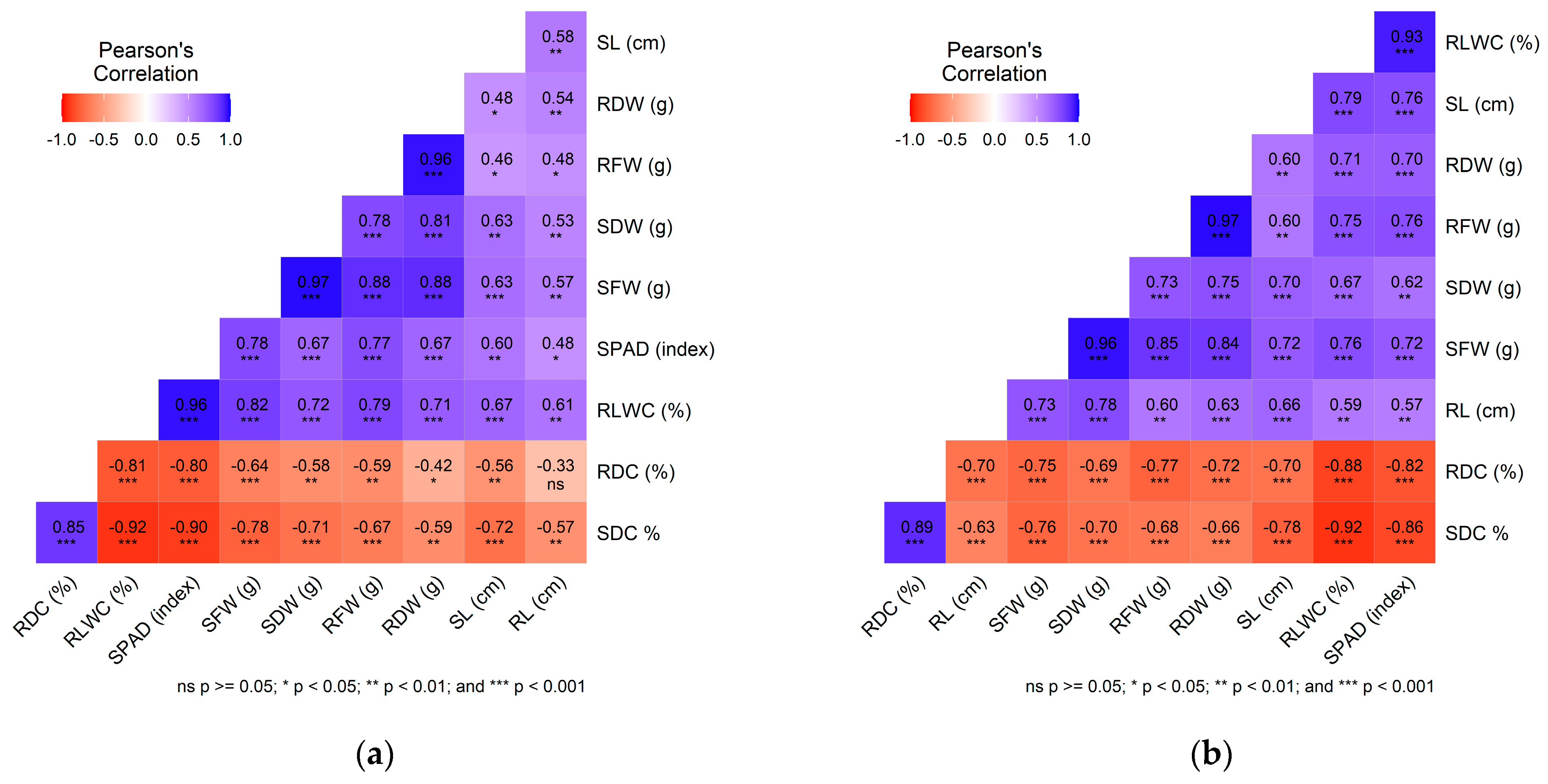
2.3. Correlation Assessment of Evaluated Traits Under Control and Salt Stress Conditions
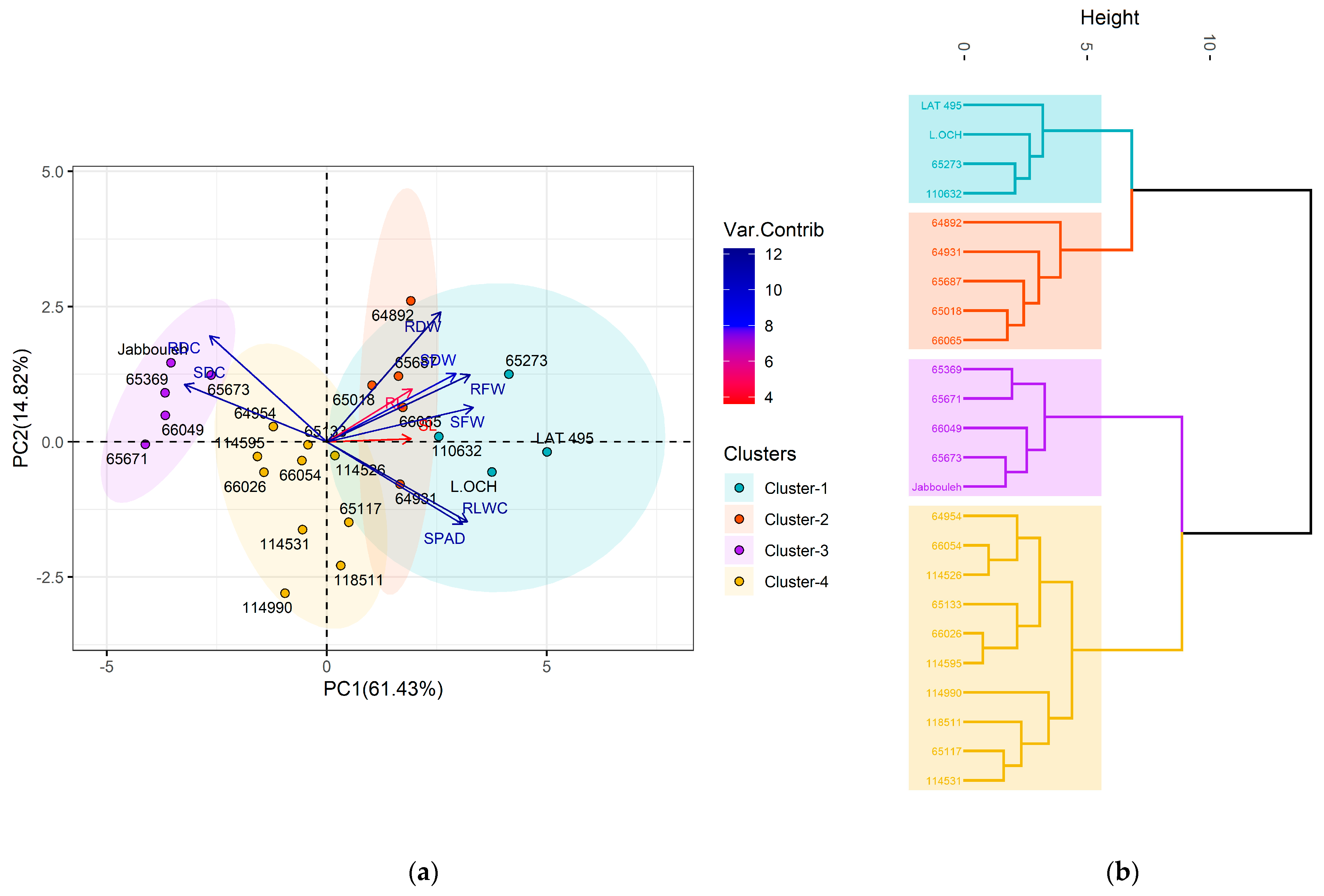
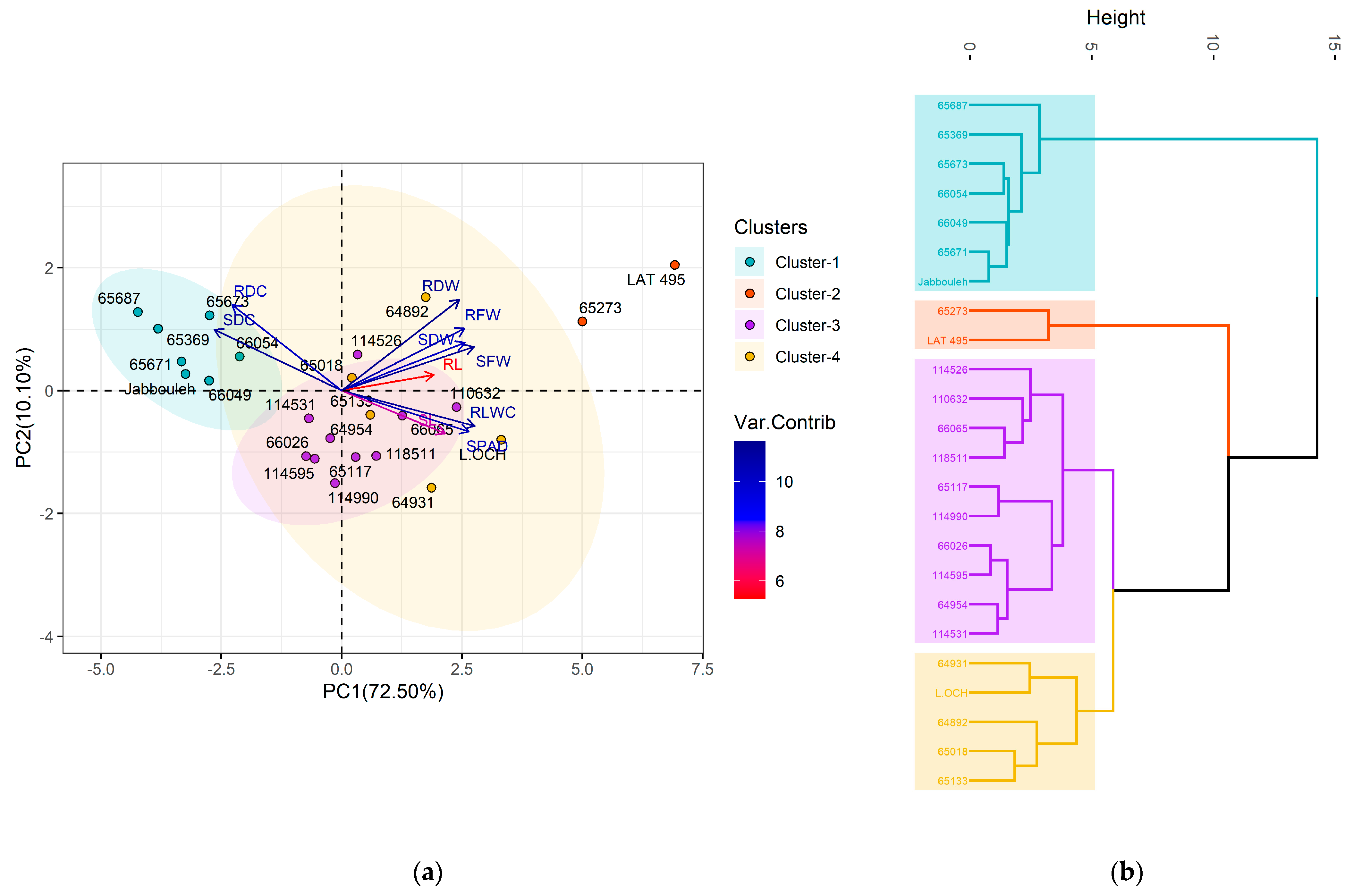
2.4. Multivariate Analysis
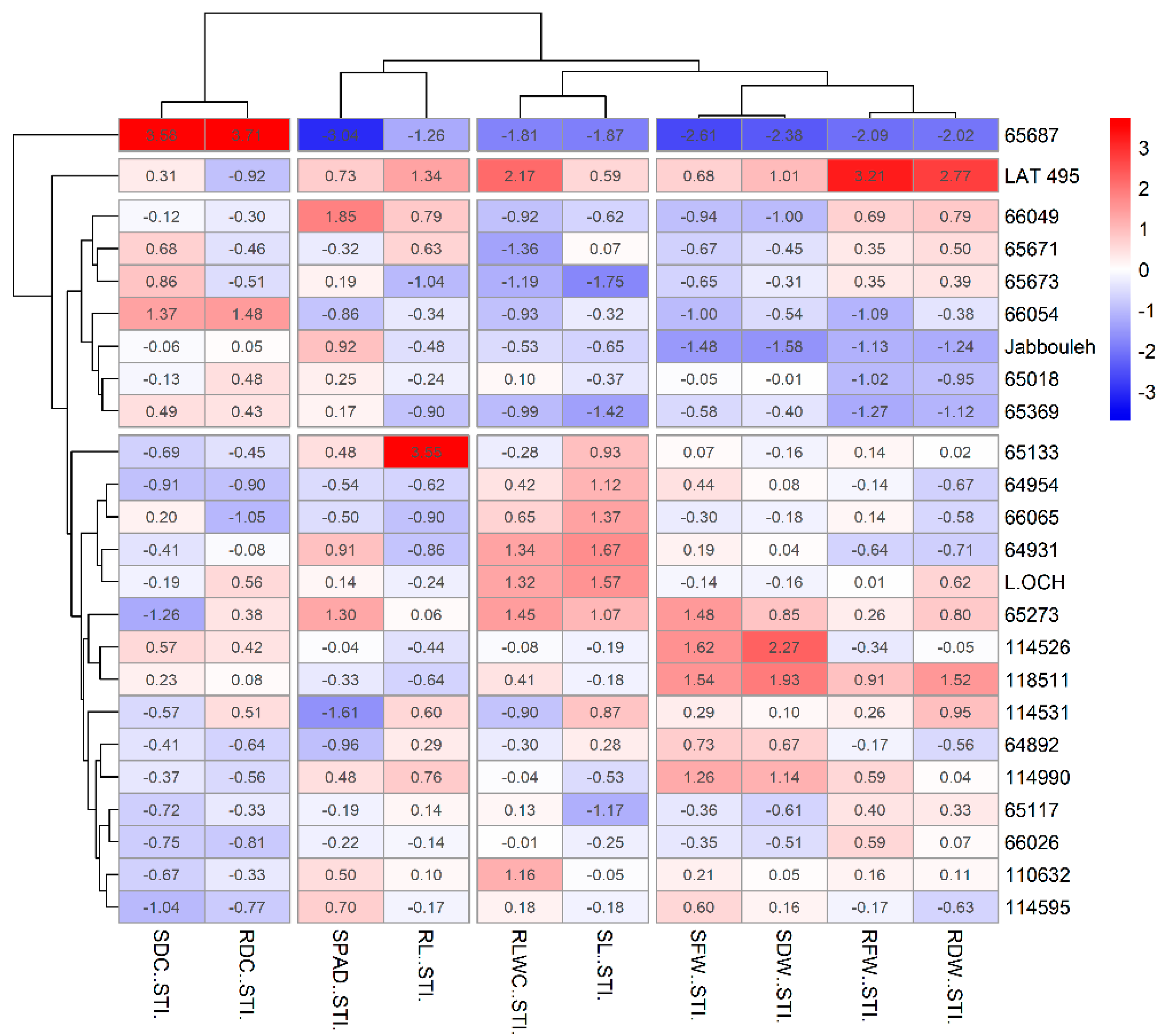
2.5. Salinity Tolerance
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Growth Conditions and Stress Treatments
4.3. Relative Leaf Water Content
4.4. Total Chlorophyll
4.5. Biometric Traits
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALA | 5-Aminolevulinic Acid |
| BLUPs | Best Linear Unbiased Predictions |
| BLUEs | Best Linear Unbiased Estimators |
| Cl− | Chloride Ion |
| CV | Coefficient of Variation |
| DC | Dry Content |
| DMRT | Duncan Multiple Range Test |
| FAO | Food and Agriculture Organization |
| FW | Fresh Weight (g) |
| G | Genotype |
| G × T | Genotype × Treatment Interaction |
| HCA | Hierarchical Cluster Analysis |
| ICARDA | International Center for Agricultural Research in the Dry Areas |
| Na+ | Sodium Ion |
| NaCl | Sodium Chloride |
| ODAP | 3-N-Oxalyl-L-α,β-diaminopropionic acid |
| PCA | Principal Component Analysis |
| PC1 | Principal Component 1 |
| PC2 | Principal Component 2 |
| RDC | Root Dry Content |
| RDW | Root Dry Weight |
| RL | Root Length |
| RLWC | Relative Leaf Water Content |
| ROS | Reactive Oxygen Species |
| RFW | Root Fresh Weight |
| SDW | Shoot Dry Weight |
| SDC | Shoot Dry Content |
| SFW | Shoot Fresh Weight |
| SL | Shoot Length |
| SPAD | Soil Plant Analysis Development (index) |
| STI | Salt Tolerance Index |
| T | Treatment |
| TW | Turgid Weight |
References
- Rathore, V.S.; Tanwar, S.P.S.; Kumar, P.; Yadav, O.P. Integrated Farming System: Key to Sustainability in Arid and Semi-Arid Regions. Indian J. Agric. Sci. 2019, 89, 181–192. [Google Scholar] [CrossRef]
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer Nature: Berlin/Heidelberg, Germany, 2018; 164p. [Google Scholar] [CrossRef]
- Musie, W.; Gonfa, G. Fresh Water Resource, Scarcity, Water Salinity Challenges and Possible Remedies: A Review. Heliyon 2023, 9, e18685. [Google Scholar] [CrossRef]
- Gupta, S.K.; Goyal, M.R.; Singh, A. Engineering Practices for Management of Soil Salinity: Agricultural, Physiological, and Adaptive Approaches; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Global Map of Salt Affected Soils. FAO. 2021. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/31be1fac-a057-4b6b-80ea-a4554910368c/content (accessed on 7 April 2025).
- Butcher, K.; Wick, A.F.; Desutter, T.; Chatterjee, A.; Harmon, J. Soil Salinity: A Threat to Global Food Security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the Physiological and Biochemical Impacts of Salt Stress on Plant Growth and Development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Sher, A.; Sarwar, T.; Nawaz, A.; Ijaz, M.; Sattar, A.; Ahmad, S. Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plant; Springer: Singapore, 2019; pp. 1–10. [Google Scholar] [CrossRef]
- Jamshidi Goharrizi, K.; Amirmahani, F.; Salehi, F. Assessment of Changes in Physiological and Biochemical Traits in Four Pistachio Rootstocks under Drought, Salinity and Drought + Salinity Stresses. Physiol. Plant. 2020, 168, 973–989. [Google Scholar] [CrossRef]
- Hu, D.; Li, R.; Dong, S.; Zhang, J.; Zhao, B.; Ren, B.; Ren, H.; Yao, H.; Wang, Z.; Liu, P. Maize (Zea mays L.) Responses to Salt Stress in Terms of Root Anatomy, Respiration and Antioxidative Enzyme Activity. BMC Plant Biol. 2022, 22, 602. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Baset Mia, M.A.; Quddus, M.A.; Sarker, K.K.; Rahman, M.; Skalicky, M.; Brestic, M.; Gaber, A.; Alsuhaibani, A.M.; Hossain, A. Salinity-Induced Physiological Changes in Pea (Pisum sativum L.): Germination Rate, Biomass Accumulation, Relative Water Content, Seedling Vigor and Salt Tolerance Index. Plants 2022, 11, 3493. [Google Scholar] [CrossRef] [PubMed]
- Bandeoǧlu, E.; Eyidoǧan, F.; Yücel, M.; Öktem, H.A. Antioxidant Responses of Shoots and Roots of Lentil to NaCl-Salinity Stress. Plant Growth Regul. 2004, 42, 69–77. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Kordrostami, M.; Zakir, A.; Zaki, H.; Saleh, O.M. Eustress with H2O2 Facilitates Plant Growth by Improving Tolerance to Salt Stress in Two Wheat Cultivars. Plants 2019, 8, 303. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant Response to Salt Stress and Role of Exogenous Protectants to Mitigate Salt-Induced Damages. In Ecophysiology and Responses of Plants Under Salt Stress; Springer: New York, NY, USA, 2013; pp. 25–87. [Google Scholar] [CrossRef]
- Rajabi Dehnavi, A.; Zahedi, M.; Piernik, A. Understanding Salinity Stress Responses in Sorghum: Exploring Genotype Variability and Salt Tolerance Mechanisms. Front. Plant Sci. 2024, 14, 1296286. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bejiga, G.; Ahmed, S.; Nakkoul, H.; Sarker, A. Genetic Improvement of Grass Pea for Low Neurotoxin (β-ODAP) Content. Food Chem. Toxicol. 2011, 49, 589–600. [Google Scholar] [CrossRef]
- Dixit, G.P.; Parihar, A.K.; Bohra, A.; Singh, N.P. Achievements and Prospects of Grass Pea (Lathyrus sativus L.) Improvement for Sustainable Food Production. Crop J. 2016, 4, 407–416. [Google Scholar] [CrossRef]
- Lambein, F.; Travella, S.; Kuo, Y.H.; Van Montagu, M.; Heijde, M. Grass Pea (Lathyrus sativus L.): Orphan Crop, Nutraceutical or Just Plain Food? Planta 2019, 250, 821–838. [Google Scholar] [CrossRef]
- Ramya, K.R.; Tripathi, K.; Pandey, A.; Barpete, S.; Gore, P.G.; Raina, A.P.; Khawar, K.M.; Swain, N.; Sarker, A. Rediscovering the Potential of Multifaceted Orphan Legume Grasspea- a Sustainable Resource with High Nutritional Values. Front. Nutr. 2022, 8, 826208. [Google Scholar] [CrossRef] [PubMed]
- Duranti, M. Grain Legume Proteins and Nutraceutical Properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef]
- Tamburino, R.; Guida, V.; Pacifico, S.; Rocco, M.; Zarelli, A.; Parente, A.; Di Maro, A. Nutritional Values and Radical Scavenging Capacities of Grass Pea (Lathyrus sativus L.) Seeds in Valle Agricola District, Italy. Aust. J. Crop Sci. 2012, 6, 149–156. [Google Scholar]
- Schulz, S.; Keatinge, J.D.H.; Wells, G.J. Productivity and Residual Effects of Legumes in Rice-Based Cropping Systems in a Warm-Temperate Environment: I. Legume Biomass Production and N Fixation. Field Crops Res. 1999, 61, 23–35. [Google Scholar] [CrossRef]
- Tripathi, K.; Gore, P.G.; Ramya, K.R.; Sarker, A. Grass Pea an Inherent Abiotic Stress-Tolerant Legume: Current Status and Future Scope under Changing Environment. In Developing Climate Resilient Grain and Forage Legumes; Springer: Berlin/Heidelberg, Germany, 2022; pp. 125–139. [Google Scholar] [CrossRef]
- Gonçalves, L.; Rubiales, D.; Bronze, M.R.; Vaz Patto, M.C. Grass Pea (Lathyrus sativus L.)—A Sustainable and Resilient Answer to Climate Challenges. Agronomy 2022, 12, 1324. [Google Scholar] [CrossRef]
- Sarkar, A.; Emmrich, P.M.F.; Sarker, A.; Zong, X.; Martin, C.; Wang, T.L. Grass Pea: Remodeling an Ancient Insurance Crop for Climate Resilience. In Genomic Designing of Climate-Smart Pulse Crops; Springer: Berlin/Heidelberg, Germany, 2019; pp. 425–469. [Google Scholar] [CrossRef]
- Piwowarczyk, B.; Tokarz, K.; Kamińska, I. Responses of Grass Pea Seedlings to Salinity Stress in in Vitro Culture Conditions. Plant Cell Tissue Organ Cult. 2016, 124, 227–240. [Google Scholar] [CrossRef]
- Haileselasie, T.H. The Effect of Salinity (NaCl) on Germination of Selected Grass Pea (Lathyrus sativus L.) Landraces of Tigray. Asian J. Agric. Sci. 2012, 4, 96–101. [Google Scholar]
- Mahdavi, B.; Sanavy, S.A.M.M. Germination and Seedling Growth in Grasspea (Lathyrus sativus) Cultivars under Salinity Conditions. Agron. Sustain. Dev. 2007, 10, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, B.; Wójtowicz, T.; Makowski, W.; Jedrzejczyk, R.J.; Tokarz, K.M. What Is the Difference between the Response of Grass Pea (Lathyrus sativus L.) to Salinity and Drought Stress?—A Physiological Study. Agronomy 2020, 10, 833. [Google Scholar] [CrossRef]
- Tokarz, K.M.; Wesołowski, W.; Tokarz, B.; Makowski, W.; Wysocka, A.; Jędrzejczyk, R.J.; Chrabaszcz, K.; Malek, K.; Kostecka-Gugała, A. Stem Photosynthesis—A Key Element of Grass Pea (Lathyrus sativus L.) Acclimatisation to Salinity. Int. J. Mol. Sci. 2021, 22, 685. [Google Scholar] [CrossRef]
- Hossain, A.; Maitra, S.; Pramanick, B.; Bhutia, K.L.; Ahmad, Z.; Moulik, D.; Syed, M.A.; Shankar, T.; Adeel, M.; Hassan, M.M.; et al. Wild Relatives of Plants as Sources for the Development of Abiotic Stress Tolerance in Plants. In Plant Perspectives to Global Climate Changes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 471–518. [Google Scholar] [CrossRef]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New Insights on Plant SaltTolerance Mechanisms and Their Potential Use for Breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Aloui, K.; Choukri, H.; El Haddad, N.; Gupta, P.; El Bouhmadi, K.; Emmrich, P.M.F.; Singh, A.; Edwards, A.; Maalouf, F.; Bouhlal, O.; et al. Impact of Heat and Drought Stress on Grasspea and Its Wild Relatives. Plants 2023, 12, 3501. [Google Scholar] [CrossRef]
- Chettri, P.; Atta, K.; Pal, A.K. Comparative Physiology of Drought and Salinity Stress in Grass Pea (Lathyrus sativus L.) Seedlings. Int. J. Environ. Clim. Chang. 2021, 11, 111–119. [Google Scholar] [CrossRef]
- Kenicer, G.J.; Kajita, T.; Pennington, R.T.; Murata, J. Systematics and Biogeography of Lathyrus (Leguminosae) Based on Internal Transcribed Spacer and CpDNA Sequence Data. Am. J. Bot. 2005, 92, 1199–1209. [Google Scholar] [CrossRef]
- Asadi, S.A.; Pourmohammad, A.; Aliloo, A.-A.; Alizadeh, K.; Hassanpouraghdam, M.B. Forage yield components of some tolerant and sensitive grass pea genotype seedlings affected by the salinity stress. Genetika 2021, 53, 79–92. [Google Scholar] [CrossRef]
- Amirjani, M.R. Effect of Salinity Stress on Growth, Mineral Composition, Proline Content, Antioxidant Enzymes of Soybean. Am. J. Plant Physiol. 2010, 5, 350–360. [Google Scholar] [CrossRef]
- Selvakumar, G.; Kim, K.; Hu, S.; Sa, T. Effect of Salinity on Plants and the Role of Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria in Alleviation of Salt Stress. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Springer: New York, NY, USA, 2014; Volume 1, pp. 115–144. [Google Scholar] [CrossRef]
- Shtaya, M.J.Y.; Yasin, A.; Fatoom, J.; Jebreen, M. The Effect of Salinity on Leaf Relative Water Content and Chlorophyll Content of Three Wheat (Triticum aestivum L.) Landraces from Palestine. Hebron Univ. Res. J. (A) 2013, 8, 57–65. [Google Scholar]
- Yan, G.; Fan, X.; Peng, M.; Yin, C.; Xiao, Z.; Liang, Y. Silicon Improves Rice Salinity Resistance by Alleviating Ionic Toxicity and Osmotic Constraint in an Organ-Specific Pattern. Front. Plant Sci. 2020, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Sanwal, S.K.; Sehrawat, N.; Kumar, A.; Kumar, N.; Mann, A. Assessing the Effect of Salinity Stress on Root and Shoot Physiology of Chickpea Genotypes Using Hydroponic Technique. Indian J. Genet. Plant Breed. 2021, 81, 586–589. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Subba, P.; Pandey, A.; Bhushan, D.; Kumar, R.; Datta, A.; Chakraborty, S.; Chakraborty, N. Analysis of the Grasspea Proteome and Identification of Stress-Responsive Proteins upon Exposure to High Salinity, Low Temperature, and Abscisic Acid Treatment. Phytochemistry 2011, 72, 1293–1307. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Pervez, M.A.; Abbas, T.; Ashfaq, M.; Ghazanfar, U.; Afzal, M.; Rashid, A.; Garcia-Sanchez, F.; Mattson, N.S. Differential Response of Pea (Pisum sativum L.) Genotypes to Salt Stress in Relation to the Growth, Physiological Attributes Antioxidant Activity and Organic Solutes. Aust. J. Crop Sci. 2012, 6, 828–838. [Google Scholar]
- Ahmad, P.; John, R.; Sarwat, M.; Umar, S. Responses of Proline, Lipid Peroxidation and Antioxidative Enzymes in Two Varieties of Pisum sativum L. under Salt Stress. Int. J. Plant Prod. 2012, 2, 353–366. [Google Scholar] [CrossRef]
- Radi, A.A.; Farghaly, F.A.; Hamada, A.M. Physiological and Biochemical Responses of Salt-Tolerant and Salt-Sensitive Wheat and Bean Cultivars to Salinity. J. Biol. Earth Sci. 2013, 3, 72–88. [Google Scholar]
- Dutta, P.; Bera, A.K. Effect of NaCl Salinity on Seed Germination and Seedling Growth of Mungbean Cultivars. Legum. Res. Int. J. 2014, 37, 161–164. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of Chlorophyll Biosynthesis and Degradation by Salt Stress in Sunflower Leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Phung, T.-H.; Jung, H.; Park, J.-H.; Kim, J.-G.; Back, K.; Jung, S. Porphyrin Biosynthesis Control under Water Stress: Sustained Porphyrin Status Correlates with Drought Tolerance in Transgenic Rice. Plant Physiol. 2011, 157, 1746–1764. [Google Scholar] [CrossRef]
- Dalal, V.K.; Tripathy, B.C. Modulation of Chlorophyll Biosynthesis by Water Stress in Rice Seedlings during Chloroplast Biogenesis. Plant. Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef] [PubMed]
- Güleç Şen, K.; Başaran, U.; Çopur Doğrusöz, M.; Gülümser, E.; Mut, H. Growth and Biochemical Responses of Grass Pea (Lathyrus sativus L.) Genotypes Under Salt (NaCl) Stress Generated by Irrigation Water, and Changes in Soil PH and EC. Gesunde Pflanz. 2023, 75, 667–675. [Google Scholar] [CrossRef]
- Hu, L.; Huang, Z.; Liu, S.; Fu, J. Growth Response and Gene Expression in Antioxidant-Related Enzymes in Two Bermudagrass Genotypes Differing in Salt Tolerance. J. Am. Soc. Hortic. Sci. 2012, 137, 134–143. [Google Scholar] [CrossRef]
- Manchanda, G.; Garg, N. Salinity and Its Effects on the Functional Biology of Legumes. Acta Physiol. Plant. 2008, 30, 595–618. [Google Scholar] [CrossRef]
- Rewald, B.; Shelef, O.; Ephrath, J.E.; Rachmilevitch, S. Adaptive Plasticity of Salt-Stressed Root Systems. In Ecophysiology and Responses of Plants Under Salt Stress; Springer: New York, NY, USA, 2013; pp. 169–201. [Google Scholar] [CrossRef]
- Saqib, M.; Zörb, C.; Rengel, Z.; Schubert, S. The Expression of the Endogenous Vacuolar Na+/H+ Antiporters in Roots and Shoots Correlates Positively with the Salt Resistance of Wheat (Triticum aestivum L.). Plant Sci. 2005, 169, 959–965. [Google Scholar] [CrossRef]
- Saqib, M.; Akhtar, J.; Qureshi, R.H. Sodicity Intensifies the Effect of Salinity on Grain Yield and Yield Components of Wheat. J. Plant Nutr. 2008, 31, 689–701. [Google Scholar] [CrossRef]
- Duan, L.; Sebastian, J.; Dinneny, J.R. Salt-Stress Regulation of Root System Growth and Architecture in Arabidopsis Seedlings. In Plant Cell Expansion. Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; pp. 105–122. [Google Scholar] [CrossRef]
- Munns, R. Genes and Salt Tolerance: Bringing Them Together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Piwowarczyk, B.; Tokarz, K.; Makowski, W.; Łukasiewicz, A. Different Acclimatization Mechanisms of Two Grass Pea Cultivars to Osmotic Stress in in Vitro Culture. Acta Physiol. Plant. 2017, 39, 96. [Google Scholar] [CrossRef]
- Gill, P.K.; Sharma, A.D.; Singh, P.; Bhullar, S.S. Changes in Germination, Growth and Soluble Sugar Contents of Sorghum bicolor (L.) Moench Seeds under Various Abiotic Stresses. Plant Growth Regul. 2003, 40, 157–162. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Snyder, W.C. Nutrition of Strawberry Plant under Controlled Conditions:(A) Effects of Deficiencies of Boron and Certain Other Elements:(B) Susceptibility to Injury from Sodium Salts. Proc. Am. Soc. Hortic. Sci. 1933, 30, 288–294. [Google Scholar]
- Hothem, S.D.; Marley, K.A.; Larson, R.A. Photochemistry in Hoagland’s Nutrient Solution. J. Plant Nutr. 2003, 26, 845–854. [Google Scholar] [CrossRef]
- Waheed, H.; Javaid, M.M.; Shahid, A.; Ali, H.H.; Nargis, J.; Mehmood, A. Impact of Foliar-Applied Hoagland’s Nutrient Solution on Growth and Yield of Mash Bean (Vigna mungo L.) under Different Growth Stages. J. Plant Nutr. 2019, 42, 1133–1141. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A Re-Examination of the Relative Turgidity Technique for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Islam, M.R.; Haque, K.S.; Akter, N.; Karim, M.A. Leaf chlorophyll dynamics in wheat based on SPAD meter reading and its relationship with grain yield. Sci. Agric. 2014, 8, 13–18. [Google Scholar] [CrossRef]
- Zhu, J.; Tremblay, N.; Liang, Y. Comparing SPAD and AtLEAF Values for Chlorophyll Assessment in Crop Species. Can. J. Soil Sci. 2012, 92, 645–648. [Google Scholar] [CrossRef]
- Roumet, C.; Urcelay, C.; Díaz, S. Suites of Root Traits Differ between Annual and PerennialSpecies Growing in the Field. New Phytol. 2006, 170, 357–368. [Google Scholar] [CrossRef]
- Bashan, Y.; De-Bashan, L.E. Fresh-Weight Measurements of Roots Provide Inaccurate Estimates of the Effects of Plant Growth-Promoting Bacteria on Root Growth: A Critical Examination. Soil Biol. Biochem. 2005, 37, 1795–1804. [Google Scholar] [CrossRef]
- De Boeck, P.; Bakker, M.; Zwitser, R.; Nivard, M.; Hofman, A.; Tuerlinckx, F.; Partchev, I. The Estimation of Item Response Models with the Lmer Function from the Lme4 Package in R. J. Stat. Softw. 2011, 39, 1–28. [Google Scholar] [CrossRef]
- Wu, H.; Guo, J.; Wang, C.; Li, K.; Zhang, X.; Yang, Z.; Li, M.; Wang, B. An Effective Screening Method and a Reliable Screening Trait for Salt Tolerance of Brassica Napus at the Germination Stage. Front. Plant Sci. 2019, 10, 530. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.C. Metan: An R Package for Multi-Environment Trial Analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘Ggplot2.’ Create Elegant Data Visualisations Using the Grammar of Graphics, R Package Version 2.1; the R Project for Statistical Computing: Vienna, Austria, 2016; Volume 2, pp. 1–189. Available online: https://cran.rproject.org/web/packages/ggplot2/index.html (accessed on 7 April 2025).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses; R Package Version 1.0.7; The R Project for Statistical Computing: Vienna, Austria, 2020; Available online: https://cran.r-project.org/web/packages/factoextra/index.html (accessed on 6 April 2025).
- Husson, F.; Josse, J.; Le, S.; Mazet, J. FactoMineR: Multivariate Exploratory Data Analysis and Data Mining; Version 2.11; The R Project for Statistical Computing: Vienna, Austria, 2019; Available online: https://cran.r-project.org/web/packages/FactoMineR/index.html (accessed on 7 April 2025).
- Galili, T. Dendextend: An R Package for Visualizing, Adjusting and Comparing Trees of Hierarchical Clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R.; Kolde, M.R. R Package; Version 1; Packag. ‘Pheatmap’; The R Project for Statistical Computing: Vienna, Austria, 2018; Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 15 March 2025).




| Trait | G | T | G × T | Residual | CV (%) | H2 |
|---|---|---|---|---|---|---|
| RLWC (%) | 54.851 * | 114.628 * | 32.169 * | 5.663 | 3.128 | 0.83 |
| SPAD (index) | 43.846 * | 56.867 * | 10.703 * | 5.627 | 6.634 | 0.92 |
| SL (cm) | 46.732 * | 80.04 * | 19.416 * | 13.058 | 11.633 | 0.86 |
| SFW (g) | 1.164 * | 0.455 * | 0.204 * | 0.054 | 12.116 | 0.94 |
| SDW (g) | 0.008 * | 0.002 * | 0.003 * | 0.001 | 12.974 | 0.90 |
| SDC (%) | 5.329 * | 6.305 * | 3.857 * | 0.459 | 5.086 | 0.80 |
| RL (cm) | 96.248 * | 223.002 * | 65.327 * | 19.826 | 9.912 | 0.80 |
| RFW (g) | 0.414 * | 0.189 * | 0.138 * | 0.042 | 19.929 | 0.89 |
| RDW (g) | 0.002 * | 0.001 * | 0.001 * | 0.0001 | 17.325 | 0.88 |
| RDC (%) | 5.096 * | 6.691 * | 2.863 * | 0.624 | 7.519 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloui, K.; Bouhlal, O.; Choukri, H.; Gupta, P.; El Bouhmadi, K.; El Haddad, N.; El Bargui, K.; Maalouf, F.; Kumar, S. Effects of Salinity Stress on Grasspea (Lathyrus sativus L.) and Its Wild Relatives: Morpho-Physiological Insights at the Seedling Stage. Plants 2025, 14, 1666. https://doi.org/10.3390/plants14111666
Aloui K, Bouhlal O, Choukri H, Gupta P, El Bouhmadi K, El Haddad N, El Bargui K, Maalouf F, Kumar S. Effects of Salinity Stress on Grasspea (Lathyrus sativus L.) and Its Wild Relatives: Morpho-Physiological Insights at the Seedling Stage. Plants. 2025; 14(11):1666. https://doi.org/10.3390/plants14111666
Chicago/Turabian StyleAloui, Khawla, Outmane Bouhlal, Hasnae Choukri, Priyanka Gupta, Keltoum El Bouhmadi, Noureddine El Haddad, Khadija El Bargui, Fouad Maalouf, and Shiv Kumar. 2025. "Effects of Salinity Stress on Grasspea (Lathyrus sativus L.) and Its Wild Relatives: Morpho-Physiological Insights at the Seedling Stage" Plants 14, no. 11: 1666. https://doi.org/10.3390/plants14111666
APA StyleAloui, K., Bouhlal, O., Choukri, H., Gupta, P., El Bouhmadi, K., El Haddad, N., El Bargui, K., Maalouf, F., & Kumar, S. (2025). Effects of Salinity Stress on Grasspea (Lathyrus sativus L.) and Its Wild Relatives: Morpho-Physiological Insights at the Seedling Stage. Plants, 14(11), 1666. https://doi.org/10.3390/plants14111666







